Abstract
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection carries a substantial risk of severe and prolonged illness; treatment options are currently limited. We assessed the efficacy and safety of inhaled nebulised interferon beta-1a (SNG001) for the treatment of patients admitted to hospital with COVID-19.
Methods
We did a randomised, double-blind, placebo-controlled, phase 2 pilot trial at nine UK sites. Adults aged 18 years or older and admitted to hospital with COVID-19 symptoms, with a positive RT-PCR or point-of-care test, or both, were randomly assigned (1:1) to receive SNG001 (6 MIU) or placebo by inhalation via a mouthpiece daily for 14 days. The primary outcome was the change in clinical condition on the WHO Ordinal Scale for Clinical Improvement (OSCI) during the dosing period in the intention-to-treat population (all randomised patients who received at least one dose of the study drug). The OSCI is a 9-point scale, where 0 corresponds to no infection and 8 corresponds to death. Multiple analyses were done to identify the most suitable statistical method for future clinical trials. Safety was assessed by monitoring adverse events for 28 days. This trial is registered with Clinicaltrialsregister.eu (2020-001023-14) and ClinicalTrials.gov (NCT04385095); the pilot trial of inpatients with COVID-19 is now completed.
Findings
Between March 30 and May 30, 2020, 101 patients were randomly assigned to SNG001 (n=50) or placebo (n=51). 48 received SNG001 and 50 received placebo and were included in the intention-to-treat population. 66 (67%) patients required oxygen supplementation at baseline: 29 in the placebo group and 37 in the SNG001 group. Patients receiving SNG001 had greater odds of improvement on the OSCI scale (odds ratio 2·32 [95% CI 1·07–5·04]; p=0·033) on day 15 or 16 and were more likely than those receiving placebo to recover to an OSCI score of 1 (no limitation of activities) during treatment (hazard ratio 2·19 [95% CI 1·03–4·69]; p=0·043). SNG001 was well tolerated. The most frequently reported treatment-emergent adverse event was headache (seven [15%] patients in the SNG001 group and five [10%] in the placebo group). There were three deaths in the placebo group and none in the SNG001 group.
Interpretation
Patients who received SNG001 had greater odds of improvement and recovered more rapidly from SARS-CoV-2 infection than patients who received placebo, providing a strong rationale for further trials.
Funding
Synairgen Research.
Introduction
COVID-19, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), leads to a spectrum of clinical presentations, ranging from asymptomatic infection to life-threatening illness.1 More severe disease is characterised by infection of the lower respiratory tract, pneumonia, and respiratory failure, which results in death in about 0·5% of confirmed cases.2
In view of the rapid and relentless spread of the COVID-19 pandemic around the world, there is a pressing need to develop new treatments. Evidence-based therapy is currently limited to remdesivir, an antiviral agent that has shown benefits in the hospital discharge rate,3 and dexamethasone, a broad-spectrum anti-inflammatory drug offering benefit in patients already requiring respiratory support.4
In all viral infections, especially those caused by novel strains where there is little to no established adaptive immunity to the pathogen, the infected host is dependent on innate immune responses to limit the severity of illness once infection occurs. Essential to this innate response is the action of interferons, key orchestrators of the antiviral immune response with both potent antiviral and immunomodulatory functions.5 The type I interferon (interferon-β) is one of the first cytokines induced by viral infection of a cell and is a primary driver of innate immune responses in the human lung.6 SARS-CoV-2 directly suppresses the release of interferon-β in vitro,7 and a recent clinical study of patients with COVID-19 showed significantly decreased interferon activity in patients who developed more severe disease.8 At-risk groups, such as those with comorbidities, older people, and recipients of immunosuppressive medication, produce less interferon-β, which contributes to the risk of more severe lung disease.9, 10
Research in context.
Evidence before this study
Interferons are cytokines that modulate immune responses to viral infection. The type I interferons (interferon-α and interferon-β) have been tested against coronavirus infections in vitro, with encouraging results. Antiviral responses mediated by interferon-β have been shown to be compromised in people susceptible to COVID-19, such as older people or those with chronic airway diseases. Furthermore, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) suppresses cellular interferon production, thus limiting the strength of the initial innate immune response. Exogenous use of inhaled interferon beta-1a in patients with asthma and respiratory viral infections has previously been shown to improve antiviral responses and improve lung function. These properties might facilitate improvement or recovery in patients with SARS-CoV-2 infection—a severe, viral, respiratory disease where the need for an effective therapeutic intervention is paramount. We aimed to evaluate the potential effects of an inhaled interferon beta-1a formulation (SNG001) in patients admitted to hospital with confirmed SARS-CoV-2 infection.
Added value of this study
In this randomised, double-blind, placebo-controlled, multicentre pilot trial in 98 patients, SNG001 increased the odds of improvement in clinical status (based on the 9-point WHO Ordinal Scale for Clinical Improvement [OSCI]) and enhanced the likelihood of recovery to a score of 1 on the OSCI (no limitation of activities). SNG001 was also well tolerated compared with placebo.
Implications of all the available evidence
The present study serves as a proof of concept that inhaled interferon beta-1a could attenuate the clinical consequences of COVID-19. Larger studies in patients with COVID-19 are needed to further investigate the therapeutic potential of SNG001 in this setting.
SNG001 is a formulation of recombinant interferon beta for inhaled delivery by nebuliser that is in development for the treatment of virus-induced lower respiratory tract illnesses. The route of delivery was selected with the aim of achieving sufficiently high concentrations of interferon-β in the lungs that would result in a robust local antiviral response while limiting systemic exposure to interferon-β, which is associated with flu-like symptoms.11, 12 Inhaled SNG001 has been well tolerated in clinical studies to date involving 230 patients with asthma or chronic obstructive pulmonary disease (COPD), or both.11, 13 In these patients, SNG001 has been shown to boost lung antiviral defences, as assessed by sputum cell antiviral biomarkers, in patients with and without respiratory viral infections.11, 14 In two phase 2 trials, SNG001 had a significantly greater beneficial effect on lung function than placebo in patients with asthma who had symptoms of respiratory viral infection.11, 15
The existing clinical data for inhaled SNG001, coupled with the known suppression of interferon-β by SARS-CoV-2, provided the rationale for a randomised, double-blind, placebo-controlled, phase 2 pilot trial to determine whether inhaled SNG001 has the potential to reduce the severity of lower respiratory tract illness and accelerate recovery in patients diagnosed with COVID-19. The design of this trial was based on the WHO R&D Blueprint Novel Coronavirus COVID-19 Therapeutic Trial Synopsis16 issued in February, 2020.
Methods
Study design and participants
This randomised, double-blind, placebo-controlled, multicentre, phase 2, pilot clinical trial was done in patients with confirmed SARS-CoV-2 infection and compared SNG001 and placebo given once daily for up to 14 days, with post-treatment follow-up for a maximum of 28 days.
The trial was done at nine UK sites in accordance with the protocol (appendix p 2) and all applicable laws and regulations including, but not limited to, the International Council for Harmonisation Guideline for Good Clinical Practice, the standards set out by the Research Governance Framework, the Medicines for Human Use (Clinical Trials) Regulations 2004, and the ethical principles that have their origin in the Declaration of Helsinki. All patients provided either written or verbal informed consent. Safety data were reviewed and monitored by an independent data safety monitoring committee. The trial protocol was reviewed and approved by the UK Medicines and Healthcare products Regulatory Agency and an independent ethical committee and site hospital boards where the trial was done (Research Ethics Committee reference number 20/NW/0168).
Eligible participants were adults aged 18 years or older, admitted to hospital with COVID-19 symptoms. All eligible participants had to have a confirmed SARS-CoV-2 test result in a UK National Health Service (NHS) diagnostic, qualitative RT-PCR assay or a positive point-of-care test (FebriDx, Lumos Diagnostics, Sarasota, FL, USA) within the previous 24 h.17 Patients were required to understand the information provided in the consenting process and to be willing to give consent. Exclusion criteria included inability to use a nebuliser with a mouthpiece (eg, ventilated patients and patients in intensive care); and pregnancy or intention to become pregnant and breastfeeding (a complete list of inclusion and exclusion criteria is provided in the appendix pp 2–3). During the trial, in version 3 of the protocol, the inclusion criteria were amended on April 16, 2020, to allow additional inclusion based on a positive point-of-care viral infection test in the presence of a strong clinical suspicion of SARS-CoV-2 infection. This change was made to prevent a delay in patient enrolment while waiting for RT-PCR results.
Randomisation and masking
After eligibility was confirmed and consent obtained, patients were allocated a unique patient identification number (appendix p 3) and assigned to one of two treatment groups according to a double-blind randomisation (1:1) schedule: active treatment (SNG001) or placebo, administered along with local standard-of-care treatment. Simple randomisation was done manually by use of sealed envelopes, with trained clinical research staff assigning the patient the next available randomisation number on the randomisation list. Study investigators, all research and analysis teams, and patients were masked to treatment allocation.
SNG001 (containing the active substance, recombinant interferon beta-1a) and placebo (with the same formulation as SNG001, excluding the active substance) were identical in appearance. The study medications were presented as ready-to-use aqueous solutions in pre-labelled syringes according to regulatory requirements. Further information about the investigational medicinal product is provided in the appendix (p 4).
Procedures
Medical history and demographic data were collected before dosing. Patients underwent evaluation in the domains of clinical frailty (mobility, energy, physical activity, and function); vital signs (temperature, respiratory rate, heart rate, systolic blood pressure, and oxygen saturation); physical examination (including chest auscultation) with clinical assessment; assessments for pneumonia; the WHO Ordinal Scale for Clinical Improvement (OSCI; table 1 ); and the Breathlessness, Cough And Sputum Scale (BCSS);18 as well as blood haematology and chemistry. Patients also had a 12-lead electrocardiogram. Chest x-rays were done if clinically required.
Table 1.
WHO Ordinal Scale for Clinical Improvement
| Score | |
|---|---|
| Uninfected | |
| No clinical or virological evidence of infection | 0 |
| Ambulatory | |
| No limitation of activities | 1 |
| Limitation of activities | 2 |
| Hospitalised (mild disease) | |
| Hospitalised (no oxygen therapy) | 3 |
| Oxygen by mask or nasal prongs | 4 |
| Hospitalised (severe disease) | |
| Non-invasive ventilation or high-flow oxygen | 5 |
| Intubation and mechanical ventilation | 6 |
| Ventilation plus additional organ support: pressors, renal replacement therapy, extracorporeal membrane oxygenation | 7 |
| Dead | |
| Death | 8 |
SNG001 (6 MIU interferon beta-1a) or placebo were delivered via the I-neb nebuliser (Philips Respironics, Murrysville, PA, USA) once daily for up to 14 days (appendix p 4). During the 14-day treatment period and while patients were in hospital, vital signs and levels of consciousness or evidence of confusion or agitation, or both, were recorded twice daily. Additionally, assessments for pneumonia by chest auscultation (and other forms of physical examination if deemed necessary by the investigator), and OSCI and BCSS were done daily. OSCI assessments, blood sampling, and 12-lead electrocardiogram assessments (and chest x-rays, if required) were done 24 h (±1 day) after the patient's last dose. The use of concomitant medications was recorded throughout the study. Patients were also regularly assessed for signs or symptoms that might be considered adverse events related to the investigational medicinal product. If a patient was discharged from hospital during the study, the assessments were done by telephone or video link or by email, where feasible. Patients underwent a final outcome assessment 14 days (±3 days) after the last dose of SNG001 or placebo.
Outcomes
The primary outcome, as defined in the protocol, was the change in clinical condition on the OSCI (table 1) during the dosing period in the intention-to-treat population. Secondary outcomes included the change in BCSS score and the safety and tolerability of the investigational drug. The full set of objectives and endpoints are provided in the protocol in the appendix (p 16). Throughout the study period, patients frequently underwent non-suggestive questioning about their wellbeing and any adverse events they experienced were noted. The onset, duration, intensity, and potential causal relationship of any adverse event with the study drug were noted.
Statistical analysis
The sample size of 100 patients (50 per group) in this pilot study was based on WHO recommendations that a pilot phase with 100 patients would be sufficient to inform follow-on clinical research.16 No formal power calculations were done. Analysis followed a prespecified statistical analysis plan on an intention-to-treat basis (ie, including all randomised patients who received at least one dose of the study drug); informal hypothesis testing was done at the 5% α-level with 95% CIs presented in all analyses. As no previous clinical data had been collected with the OSCI in the study population, the most appropriate way of analysing and interpreting the OSCI was unknown. It was therefore considered inappropriate to select a single primary analysis method for this pilot study. Multiple analyses were done to explore death from disease, worsening of disease, improvement and recovery from disease, both at fixed timepoints and over the dosing period, with the aim of identifying the most appropriate statistical method for future clinical trials. There was no hierarchy across analyses and no adjustments were made for multiplicity.
All analyses described below were adjusted for age, sex, comorbidity, OSCI score at baseline (categorised as ≤3 or ≥4), race (categorised as White or non-White) and duration of previous symptoms (categorised as <10 days or ≥10 days). Analyses of OSCI data were done over the treatment period or at each individual visit, as appropriate. For OSCI, the treatment period was defined as 16 days, which included the 14-day dosing period and an end-of-treatment visit on day 15 or day 16.
Analysis of improvement on the OSCI scale was done with an ordered logistic regression model assuming proportional odds.
Severe disease or death (OSCI ≥5), intubation or death (OSCI ≥6), and death (OSCI=8) were analysed with logistic regression models. Post-hoc analyses of these endpoints with Firth logistic regression were also done, due to data separation issues observed after study unblinding. Time to first severe disease or death, time to first intubation or death, and time to death were analysed with Cox proportional hazards models. Time to OSCI recovery (defined as OSCI ≤1 without rebound) and time to hospital discharge (defined as OSCI ≤2 without rebound) were analysed with Fine and Gray's sub-distribution hazard model fitted with death as a competing event. Sustained recovery and sustained hospital discharge were also analysed with logistic regression models.
A last-observation-carried-forward approach was used to impute missing data for analyses of sustained recovery, sustained hospital discharge, and improvement.
Breathlessness and cough scores and total BCSS score were analysed with mixed models for repeated measures. Data from the follow-up visits or early withdrawal visits after day 16 were not included in the analysis. Missing breathlessness symptom scores were imputed as 4 (severe) for patients who were intubated or who received non-invasive ventilation or high-flow oxygen (OSCI ≥5). Analysis of sputum scores was not planned because COVID-19 is characterised by a dry, non-productive cough.
Safety endpoints were described as frequencies (%). Due to the exploratory nature of this phase of the study, statistical determinations of p values and confidence intervals were not adjusted for multiple testing. All analyses were done with SAS, version 9.4.
This trial is registered with Clinicaltrialsregister.eu (2020-001023-14) and ClinicalTrials.gov (NCT04385095).
Role of the funding source
The funder of the study had a role in study design, data collection, data analysis, data interpretation, and writing of the report. The corresponding author and coauthors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
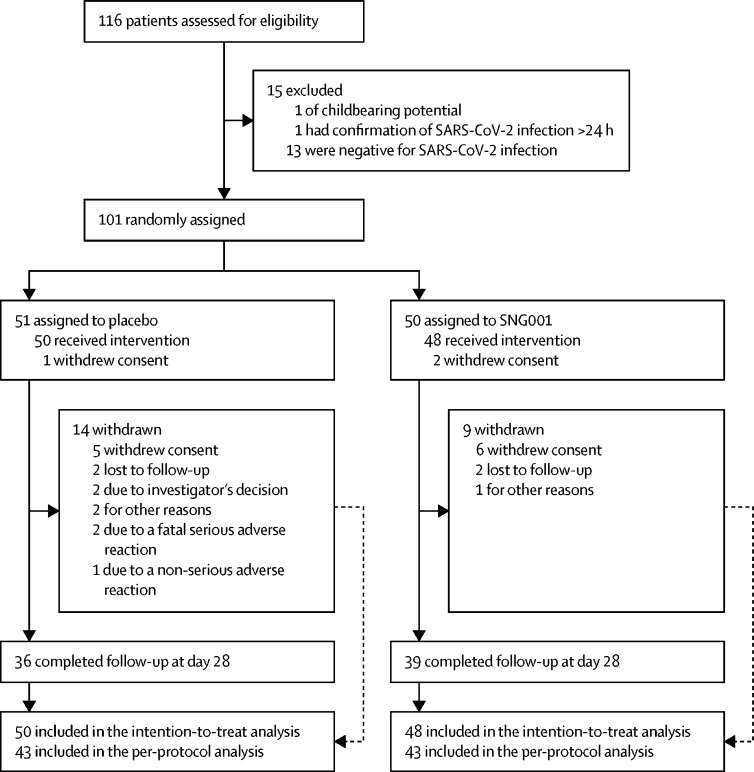
Between March 30 and May 30, 2020, 116 patients were screened, of whom 101 were enrolled into the study (51 in the placebo group and 50 in the SNG001 group; figure 1 ). Of these, 48 patients received SNG001 and 50 received placebo, and were included in the intention-to-treat population. All but one patient had a positive RT-PCR test for SARS-CoV-2. This patient initially had a positive point-of-care test and was therefore eligible for inclusion into the study, but was negative in a subsequent RT-PCR test. 43 patients in each treatment group were included in the per-protocol population (all patients who had SARS-CoV-2 infection confirmed by RT-PCR test, took at least two of their first three scheduled doses, and had no protocol deviations affecting efficacy). 39 (81%) patients in the SNG001 group and 36 (72%) in the placebo group were followed up for the full duration of the study. Results are presented for the intention-to-treat population.
Figure 1.
Trial profile
Eligible patients were randomly assigned (1:1) to receive inhaled nebulised interferon beta-1a (SNG001) or placebo. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
Demographic and baseline characteristics, including comorbidities, disease severity, duration of symptoms, and smoking status are presented in table 2 . Patients' mean age was 57·1 (SD 13·26) years; 58 (59%) were male, and 78 (80%) were White. 53 (54%) patients had baseline comorbidities of hypertension, cardiovascular disease, diabetes, chronic lung condition, or cancer. In general, patients in the treatment groups were well matched by baseline characteristics, apart from disease severity measured with the OSCI and the frequencies of specific comorbidities. Patients in the SNG001 group had more severe disease as judged by 37 (77%) patients receiving oxygen therapy (OSCI ≥4) compared with 29 (58%) in the placebo group (table 2). More patients in the placebo group than those in the SNG001 group had diabetes (nine [33%] vs three [12%]) or cardiovascular disease (eight [30%] vs five [19%]), and fewer had hypertension (11 [41%] vs 18 [69%]).
Table 2.
Demographic and baseline characteristics of participants
| Placebo (n=50) | SNG001 (n=48) | |||
|---|---|---|---|---|
| Age at inclusion, years | 56·5 (11·9) | 57·8 (14·6) | ||
| Sex | ||||
| Male | 31 (62%) | 27 (56%) | ||
| Female | 19 (38%) | 21 (44%) | ||
| Ethnicity | ||||
| White | 39 (78%) | 39 (81%) | ||
| Non-White | 11 (22%) | 9 (19%) | ||
| Comorbidities | ||||
| All | 27 | 26 | ||
| Hypertension | 11/27 (41%) | 18/26 (69%) | ||
| Chronic lung condition | 12/27 (44%) | 11/26 (42%) | ||
| Cardiovascular disease | 8/27 (30%) | 5/26 (19%) | ||
| Diabetes | 9/27 (33%) | 3/26 (12%) | ||
| Cancer | 1/27 (4%) | 0 | ||
| Severity of disease at baseline* | ||||
| No limitation of activities | 1 (2%) | 0 | ||
| Limitation of activities | 1 (2%) | 0 | ||
| Hospitalised (no oxygen therapy) | 19 (38%) | 11 (23%) | ||
| Oxygen by mask or nasal prongs | 28 (56%) | 36 (75%) | ||
| Non-invasive ventilation or high-flow oxygen | 1 (2%) | 1 (2%) | ||
| Duration of symptoms, days† | 9·5 (7·0–12·0) | 10·0 (8·0–11·0) | ||
| Current smoking status | ||||
| Currently uses tobacco | 1 (2%) | 1 (2%) | ||
| Former smoker | 16 (32%) | 11 (23%) | ||
| Never smoked | 33 (66%) | 36 (75%) | ||
Data are n (%) or mean (SD), unless otherwise indicated, and are presented for the intention-to-treat population.
Severity of disease at baseline followed the WHO Ordinal Scale for Clinical Improvement.
Duration of symptoms is presented as median (IQR).
The median duration of COVID-19 symptoms before initiation of treatment was 10 days (IQR 7–11).
Patients' OSCI scores could have changed from the time of randomisation to the time of baseline assessment, which might have occurred later in the day. At baseline, two (2%) patients were receiving non-invasive ventilation or high-flow oxygen (OSCI=5), 64 (65%) were receiving oxygen by mask or nasal prongs (OSCI=4), 30 (31%) were not receiving oxygen therapy (OSCI=3), and two were admitted to hospital for reasons of isolation or quarantine and not because of the severity of their disease; one (1%) patient had a baseline OSCI score of 2 (limitation of activities), and one (1%) had a baseline OSCI score of 1 (no limitation of activities).
The results for the primary outcome of change in OSCI over the dosing period are described below and summarised in table 3 . Results for the follow-up visit on day 28 are also presented.
Table 3.
Analysis of the WHO OSCI during the dosing period
|
Intention-to-treat population |
Per-protocol population |
|||
|---|---|---|---|---|
| Ratio (95% CI) | p value | Ratio (95% CI) | p value | |
| Odds of improvement on the OSCI | OR 2·32 (1·07–5·04) | 0·033 | OR 2·80 (1·21–6·52) | 0·017 |
| Time to severe disease or death (OSCI ≥5) | HR 0·50 (0·18–1·38) | 0·179 | Not calculated | .. |
| Odds of severe disease or death (OSCI ≥5) | OR 0·28 (0·07–1·08) | 0·064* | OR 0·18 (0·04–0·93) | 0·041* |
| Time to intubation or death (OSCI ≥6) | HR 0·38 (0·09–1·65) | 0·198 | Not calculated | .. |
| Odds of intubation or death (OSCI ≥6) | OR 0·42 (0·09–1·83) | 0·246* | OR 0·31 (0·05–1·79) | 0·189* |
| Time to recovery | HR 2·19 (1·03–4·69) | 0·043 | HR 2·29 (1·07–4·91) | 0·033 |
| Odds of recovery | OR 3·19 (1·24–8·24) | 0·017 | OR 3·18 (1·21–8·39) | 0·019 |
| Time to hospital discharge | HR 1·37 (0·85–2·20) | 0·196 | HR 1·53 (0·96–2·42) | 0·072 |
| Odds of hospital discharge | OR 1·63 (0·61–4·35) | 0·330 | OR 2·14 (0·64–7·12) | 0·215 |
ORs relate to the end-of-treatment visit on day 15 or 16. Time-to-event analyses include all data up to and including the end-of-treatment visit. Recovery was defined as a post-baseline OSCI score of 0 or 1, which does not rise above 1 at any subsequent visits. Hospital discharge was defined as a post-baseline OSCI score of 2 or less, which does not rise above 2 at any subsequent visits. Three patients died during the study; all deaths occurred in patients randomly assigned to placebo, so no modelling analysis was done. OSCI=Ordinal Scale for Clinical Improvement. OR=odds ratio. HR=hazard ratio.
Post-hoc analysis done by use of Firth logistic regression analysis.
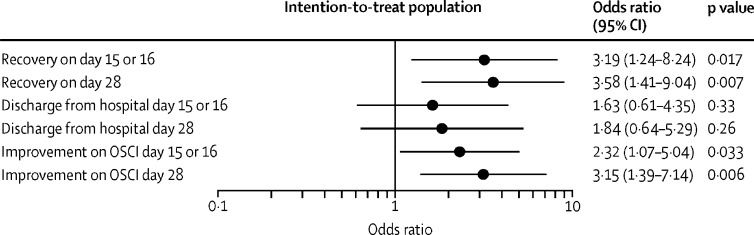
The odds of improvement on the OSCI scale were more than two-fold greater in the SNG001 group than in the placebo group on day 15 or 16 (odds ratio [OR] 2·32 [95% CI 1·07–5·04]; p=0·033; table 3; figure 2 ) and more than three-fold greater on day 28 (3·15 [1·39–7·14]; p=0·006; figure 2; appendix p 4).
Figure 2.
Odds ratios of recovery (OSCI ≤1), hospital discharge, and improvement
Odds ratios of recovery (defined as unchanged post-baseline OSCI score of 0 or 1), hospital discharge, and improvement on the WHO OSCI on days 15 or 16 (end-of-treatment visit) and on day 28 (follow-up visit) are shown. Comparisons were made between the SNG001 group (n=48) and placebo group (n=50) in the intention-to-treat population. OSCI=Ordinal Scale for Clinical Improvement.
Three patients died during the study; all deaths occurred in patients in the placebo group, so no modelling analysis was done. 11 (22%) patients in the placebo group developed severe disease or died (OSCI ≥5) between the first dose and day 16 compared with six (13%) patients in the SNG001 group. SNG001 reduced the odds of developing severe disease or dying by 79% (OR 0·21 [95% CI 0·04–0·97]; p=0·046) in the prespecified logistic regression analysis. As quasi-complete separation of data was observed in some model covariates, an additional, post-hoc Firth logistic regression analysis was done in which the difference between groups was not significant (72·0% reduction; OR 0·28 [95% CI 0·07–1·08]; p=0·064; table 3).
In the placebo group, five (10%) patients either underwent intubation or died (OSCI ≥6) between the first dose and day 15 or 16 versus three (6%) in the SNG001 group. There was no significant difference between treatment groups in the odds of intubation or the time to intubation or death (table 3).
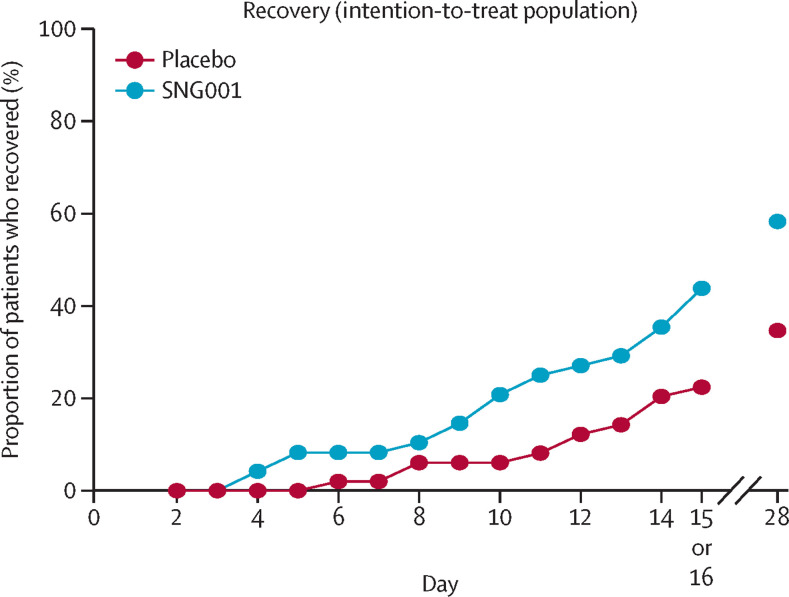
Over the 14-day treatment period, patients in the SNG001 group were more than twice as likely to recover as those in the placebo group (21 [44%] of 48 with SNG001 vs 11 [22%] of 49 with placebo; hazard ratio [HR] 2·19 [95% CI 1·03–4·69]; p=0·043; table 3; figure 3 ). On day 28 (the final outcome assessment visit), 28 (58%) of 48 patients in the SNG001 group and 17 (35%) of 49 in the placebo group had recovered (figure 3; appendix p 5). The odds of recovery on day 28 were more than three-fold greater in the SNG001 group than in the placebo group (OR 3·58 [95% CI 1·41–9·04]; p=0·007; figure 2).
Figure 3.
Patient recovery (OSCI ≤1) during the study
The proportion of patients who recovered (defined as having an unchanged post-baseline OSCI score of 0 or 1) up to day 15 or 16 (end-of-treatment visit) and on day 28 (follow-up visit) is presented for the intention-to-treat population (SNG001: n=48; placebo: n=50). OSCI=Ordinal Scale for Clinical Improvement.
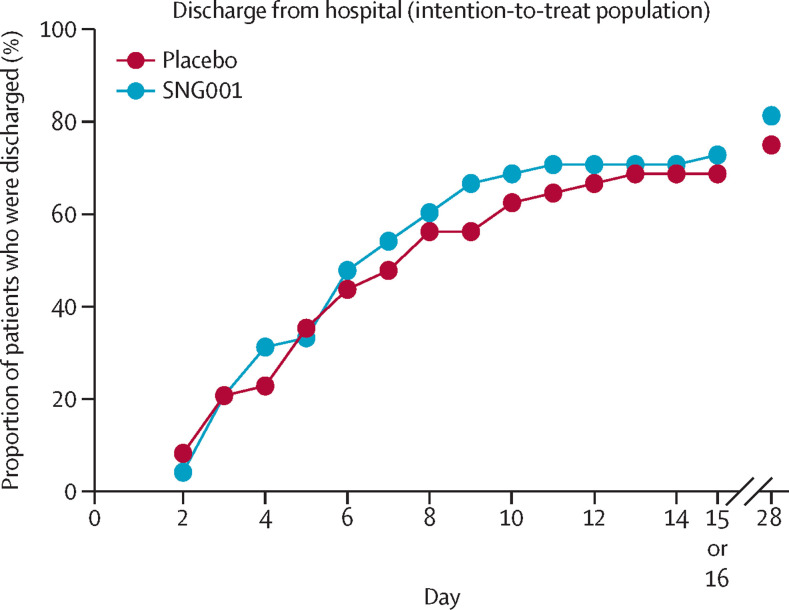
By day 16, 33 (69%) of 48 patients in the placebo group and 35 (73%) of 48 patients in the SNG001 group had been discharged from hospital (appendix p 5). By day 28, 39 (81%) of 48 patients had been discharged in the SNG001 group compared with 36 (75%) of 48 in the placebo group. There was no significant difference between treatment groups in the odds of hospital discharge or time to hospital discharge (Figure 2, Figure 4 ; table 3).
Figure 4.
Hospital discharge
The proportion of patients who were discharged from hospital up to day 15 or 16 (end-of-treatment visit) and on day 28 (follow-up visit) is presented for the intention-to-treat population (SNG001: n=48; placebo: n=50).
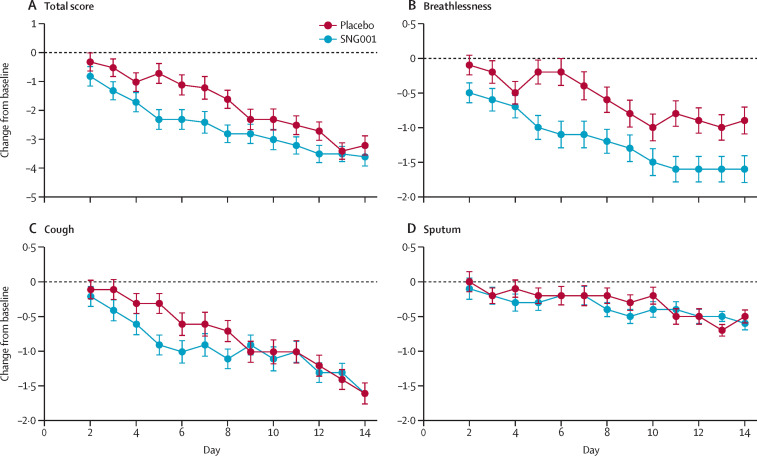
Patients in the SNG001 group showed a greater improvement in the secondary outcome analysis of total BCSS score compared with placebo over the 14-day treatment period (difference between SNG001 and placebo –0·8 [95% CI –1·5 to –0·1]; p=0·026; figure 5A ). The improvement in patient-reported breathlessness on a scale of 0 to 4 (with 0 corresponding to no symptoms and 4 corresponding to severe symptoms) was greater in the SNG001 group than in the placebo group over the treatment period (difference –0·6 [95% CI –1·0 to –0·2]; p=0·007; figure 5B). The improvement in patient-reported cough over the same period was not significant for patients receiving SNG001 versus those receiving placebo (difference –0·2 [95% CI –0·5 to 0·1]; p=0·285; figure 5C). The BCSS scores indicated that sputum production was not a problematic symptom for patients in this study (figure 5D).
Figure 5.
Breathlessness, Cough, and Sputum Scale evaluation
Least squares mean (95% CI) change from the baseline score up to day 14 is presented for total BCSS scores (A) and individual scores for breathlessness (B), cough (C), and sputum (D) for the intention-to-treat population (SNG001: n=48; placebo: n=50). BCSS=Breathlessness, Cough, and Sputum Scale.
Results in the per-protocol population were generally better than those in the intention-to-treat population (table 3); significant effects were observed with SNG001 versus placebo for improvement on the OSCI on day 15 or 16 (OR 2·80 [95% CI 1·21–6·52]; p=0·017), odds of severe disease or death (OSCI ≥5) on day 15 or 16 (OR 0·18 [95% CI 0·04–0·93]; p=0·041; post-hoc Firth logistic regression analysis), time to recovery over the treatment period (HR 2·29 [95% CI 1·07–4·91]; p=0·033), odds of recovery by day 15 or 16 (OR 3·18 [95% CI 1·21–8·39]; p=0·019), and a non-significant improvement in earlier hospital discharge over the treatment period (HR 1·53 [95% CI 0·96–2·42]; p=0·072).
26 (54%) patients in the SNG001 group and 30 (60%) in the placebo group had treatment-emergent adverse events (table 4 ). The most frequently reported treatment-emergent adverse event was headache, which was reported in seven (15%) patients in the SNG001 group and five (10%) patients in the placebo group (appendix p 6). Fewer patients had serious adverse events in the SNG001 group than in the placebo group (seven [15%] vs 14 [28%]). The most common serious adverse events were related to COVID-19: respiratory failure (three [6%] patients in the SNG001 group vs six [12%] in the placebo group) and pneumonia (three [6%] vs three [6%]). All serious adverse events were considered either unlikely be related to study treatment or not related to study treatment. Treatment-emergent adverse events related to treatment were more common in the SNG001 group than the placebo group (seven [15%] vs two [4%]); cough, reported by two (4%) patients, was the most frequently occurring treatment-related treatment-emergent adverse event in the SNG001 group. Other treatment-emergent adverse events related to SNG001, each occurring in one patient, included decreased oxygen saturation, diarrhoea, dry throat, oral pain, night sweats, and tremor. Three treatment-emergent adverse events led to study withdrawal in three patients in the placebo group: nausea, multiple organ dysfunction syndrome (fatal), and pulmonary embolism (fatal). In addition to the fatal treatment-emergent adverse events described above, a third patient in the placebo group died, with the cause of death recorded as COVID-19 pneumonia.
Table 4.
Summary of treatment-emergent adverse events
| Placebo (n=50) | SNG001 (n=48) | |
|---|---|---|
| Any treatment-emergent adverse event | 30 (60%) | 26 (54%) |
| Any treatment-emergent adverse event during treatment period | 25 (50%) | 23 (48%) |
| Any serious treatment-emergent adverse event | 14 (28%) | 7 (15%) |
| Any treatment-related treatment-emergent adverse event | 2 (4%) | 7 (15%) |
| Any fatal treatment-emergent adverse event | 3 (6%) | 0 |
| Any treatment-emergent adverse event that led to study withdrawal | 3 (6%) | 0 |
Data are n (%), where n corresponds to number of patients with adverse reactions.
Discussion
This study was a randomised, placebo-controlled multicentre, phase 2 trial evaluating the safety and efficacy of inhaled SNG001, an interferon beta-1a nebuliser solution, in patients admitted to hospital with COVID-19. The results of this pilot trial have shown that SNG001, given as a daily inhaled dose of 6 MIU via nebuliser for 14 days, was associated with greater odds of improvement versus placebo on the WHO OSCI and more rapid recovery to a point where patients were no longer limited in their activity, with a greater proportion of patients recovering during the 28-day study period. There was a non-significant reduction in the odds of progression to severe disease or death in the intention-to-treat population that became significant in the per-protocol population. Secondary outcome analysis of symptoms revealed a greater improvement in breathlessness and total BCSS over the treatment period with SNG001 than with placebo.
In keeping with previous observations in patients with asthma11 and COPD,13 nebulised SNG001 was well tolerated. More patients had serious adverse events in the placebo group than in the SNG001 group. The most common serious adverse events in both treatment groups were related to COVID-19. The most common treatment-emergent adverse event in the SNG001 group was headache. Treatment-emergent adverse events considered to be related to treatment were infrequent, with cough being the only treatment-related treatment-emergent adverse event in the SNG001 group that occurred in more than one (two) patients. Three patients died in the placebo group; there were no deaths in the SNG001 group.
The findings of this trial suggest the potential utility of SNG001 in treating patients admitted to hospital with COVID-19, although SNG001 should be explored further in a phase 3 trial. Currently, treatment options for COVID-19 remain limited, with the only evidence-based therapies being remdesivir and dexamethasone.3, 4 Despite the use of these therapies, clinical outcomes remain poor. Dexamethasone is only indicated in patients who already require respiratory support (oxygen with or without mechanical ventilation), with a numerically poorer outcome seen in patients with less severe disease.4 In the ACTT-1 trial, remdesivir shortened the time to hospital discharge by 5 days and increased the odds of improvement in clinical status, assessed with the OSCI.3 By contrast, remdesivir had little or no effect on patients admitted to hospital with COVID-19 in the WHO SOLIDARITY trial, as assessed by overall mortality, initiation of ventilation, or duration of hospital stay.19 There is clearly a need for additional therapeutic options for patients with COVID-19 and the results presented here suggest that inhaled interferon-β might be effective in this setting, although our results require further investigation in future trials.
Injectable drug products containing interferon-α and interferon-β have been used in the clinic for many years. In the context of highly pathogenic coronavirus strains including SARS-CoV-2, interferon-β has been shown to be more potent than interferon-α.20, 21, 22 Two forms of recombinant interferon beta are available. SNG001 contains interferon beta-1a, which is produced in mammalian cells, shares the same amino acid sequence as naturally occurring interferon-β, and has a higher specific activity than interferon beta-1b, which is produced in non-mammalian cells.23 SNG001 is formulated at pH 6·5 (as low pH is known to cause cough) and does not contain excipients (such as mannitol, human serum albumin, and arginine) present in injectable forms of interferon beta. SNG001 is inhaled via a mesh nebuliser, which maintains drug activity after aerosolisation.11 The rationale for this route of administration is to enable maximal delivery of the active drug to the biological focus of SARS-CoV-2 infection, the respiratory epithelium.24 Pragmatic studies25 and ongoing randomised controlled trials are seeking to explore the effects of injected type I interferons in COVID-19. The REMAP-Cap study, focusing on patients in the intensive care setting,26 and the SOLIDARITY19 and DisCoVeRy trials27 in patients admitted to hospital are evaluating injected interferon beta-1a, but have yet to show whether and to what extent systemic delivery is effective and well tolerated. Data from the SOLIDARITY study appear to indicate that injected interferon beta is ineffective and given the greater bioavailability of inhaled drugs at the lung epithelium, such concentrations could be matched only by giving extremely high doses of the injected drug with the risk of unacceptable intolerability, suggesting that the inhaled route is likely to provide better antiviral outcomes. Such an approach was taken in a recent analysis of an uncontrolled exploratory study of nebulised interferon alfa-2b, reported in a hospital setting in Wuhan, China, indicating a positive effect on viral load and the inflammatory biomarkers interleukin-6 and C-reactive protein, although there was an imbalance in age between treated patients and controls.28, 29 More recently, a similar, pragmatic, open-label study reported clinical benefits with aerosol inhalation of interferon kappa plus trefoil factor 2, showing reductions in viral shedding and duration of symptoms and hospital stay in patients with SARS-CoV-2 infection.30 Taken together with our study, these results suggest a potential antiviral benefit of aerosolised interferon therapy in COVID-19. The value of this approach might be driven, in part, by the evasive nature of SARS-CoV-2 on natural type I interferon production, which is more profound than with other comparable respiratory pathogens.8, 31 The optimal route and specific type of interferon might depend on the stage of disease, the spread of viral infection beyond the lung, and the ease of administration in different clinical settings.
The timing of interferon treatment for COVID-19 has been the subject of considerable debate, with concerns being raised that later interferon treatment might cause or exacerbate the cytokine storm observed in the later stages of severe disease. For example, to avoid concerns over potential pro-inflammatory effects of interferon, in an open-label study of injected interferon beta-1b in a triple combination with lopinavir–ritonavir and ribavirin for the treatment of COVID-19, investigators limited treatment with interferon to within 7 days of onset of symptoms.25 It was concluded that triple antiviral therapy was safe and superior to the lopinavir–ritonavir combination in alleviating symptoms and shortening the duration of viral shedding and hospital stay in patients with mild to moderate COVID-19. In our study, patients had a median duration of symptoms of 10 days at recruitment and SNG001 was given daily for 14 days. Importantly, we found that SNG001 was well tolerated and showed clinical benefit, suggesting that there is a substantially greater window for effective treatment.
Further trials in different clinical settings, including in ventilated patients or in patients with mild to moderate COVID-19 not requiring treatment in hospital, are warranted or underway.32 With the second wave of COVID-19 coinciding with the winter incidence of cold and influenza in the northern hemisphere, the development of broad-spectrum antiviral agents, such as interferon-β, that boost local lung defences rather than targeting specific viral mechanisms, might carry substantial additional benefits to patients and to overburdened health-care systems alike; this will also require investigation.5, 21
Our clinical trial had a number of limitations. As a pilot study of a relatively novel treatment in an inpatient population, the sample size was limited, making the generalisability of the findings to wider populations and health-care systems, where standard of care might vary, challenging. The OSCI at the time of the study was a new tool and its performance in randomised controlled trials of COVID-19 was uncertain. In this pilot study, we elected to assess this outcome in multiple statistical analyses and to report them without hierarchy. This approach enabled exploration of this new tool with the aim of identifying the most appropriate statistical method for future clinical trials. However, this approach introduces the issue of repeat analyses of this outcome, which requires consideration in the interpretation of the results and highlights the need for larger-scale, formal testing of a selected approach in the next phase of development. The nebuliser used in this study is not suitable for use in patients requiring ventilation; further studies of SNG001 with alternative delivery devices to include these critically ill patients are warranted. In any pilot study, not all factors can be evenly balanced during randomisation. In this study, SNG001 and placebo groups were well matched for age, sex, and overall comorbidities, but were less well matched for disease severity at recruitment and for specific comorbid conditions—particularly diabetes, cardiovascular disease, and hypertension. However, these factors were considered in the statistical model used and beneficial signals for therapy were enhanced when a priori adjustments were made. Phase 3 trials will address these issues through randomisation of larger and more heterogeneous groups. Due to an urgency to deliver a trial result in the setting of the COVID-19 pandemic, patient follow-up was completed at 28 days, a timepoint at which, interestingly, some of the greatest treatment effects of SNG001 on recovery were observed. Recent evidence suggests that the long-term sequelae of severe COVID-19 are significant,33 so it will be important to track the effects of SNG001 on the prevention and resolution of these symptoms in future trials. Additional sampling beyond core safety parameters was limited by the increased acute care burden due to the COVID-19 pandemic. Additional capture of exploratory inflammatory and virological endpoints could have provided useful information about the mechanism of therapeutic response but were not feasible in this setting. Future studies in different clinical settings will enable these analyses to be done.
Five patients withdrew consent in the placebo group, as did six in the SNG001 group. Although there was no indication that the treatment was associated with differential withdrawal, since similar numbers of patients withdrew in both groups, it is important to understand for future studies that substantial withdrawal rates might occur in the setting of acute COVID-19 care, perhaps due to associated complexities of care and uncertainties in outcome.
In conclusion, SNG001, a treatment already studied and shown to be well tolerated in patients with asthma and COPD, seems to also be well tolerated in patients admitted to hospital with COVID-19, with a range of clinical outcomes displaying a beneficial pattern of response to SNG001 therapy. These encouraging data provide a strong rationale for larger, international studies in the context of the ongoing clinical burden of COVID-19. In addition to a phase 3 trial of SNG001 in patients admitted to hospital with COVID-19 requiring no more than supplementary oxygen, it might be appropriate to also assess the safety and efficacy of SNG001 in ventilated, critically ill patients with COVID-19 who have evidence of active viral infection in the lungs. In view of the broad antiviral effects of interferon-β, the results of this pilot trial suggest that the efficacy of SNG001 should also be assessed in the hospital setting against other seasonal respiratory viruses, which cause considerable morbidity and mortality every year, including cases of SARS-CoV-2 co-infection, which could overwhelm health-care systems during the coming months in the northern hemisphere.
Data sharing
The data analysed and presented in this study are available from the corresponding author on reasonable request, providing the request meets local ethical and research governance criteria after publication. Patient-level data will be anonymised and study documents will be redacted to protect the privacy of trial participants. The study protocol is provided in the appendix (p 5).
Acknowledgments
Acknowledgments
This study was funded by Synairgen Research, UK. TMAW is supported in part by the NIHR Southampton Biomedical Research Centre. L-PH is supported in part by the NIHR Oxford Biomedical Research Centre. We thank all patients who volunteered for the study and clinical and research teams at all the participating clinical centres and the NIHR Respiratory Translational Research Collaboration for support in trial delivery. We are grateful to Antigoni Ekonomou of Niche Science & Technology for medical writing services and editorial assistance with the manuscript. We thank also Alastair Watson for helping with the preparation of the manuscript and coordination of the submission.
Contributors
PDM, RJM, VJT, JB, TNB, MM, FJG, DED, STH, TC, RD, and TMAW made substantial contributions to the conception and design of the work. PDM, VJT, JB, TNB, MM, TC, L-PH, and TMAW contributed to the acquisition of the study data. Data were analysed by PDM, RJM, VJT, JB, TNB, MM, FJG, RD, and TMAW. TNB was responsible for the statistical analysis. FJG was the Chair of the data safety monitoring committee during the study. PDM, RJM, VJT, JB, TNB, MM, FJG, DED, L-PH, STH, TC, RD, and TMAW contributed to the interpretation of the data, and to drafting and revising of the manuscript. VJT and TNB have accessed and verified the data. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors have read and approved the manuscript.
Declaration of interests
PDM is a Director of Synairgen Research (a University of Southampton spin-out company), is an employee and Director of Synairgen (the parent company of Synairgen Research), and own shares and holds share options in Synairgen. RJM is a Director of Synairgen Research, is an employee and Director of Synairgen, and own shares and holds share options in Synairgen. VJT is an employee of Synairgen Research and holds share options in Synairgen. PDM and VJT also have a patent (GB 2011216.5: Use of inhaled interferon-beta to improve outcome in SARS-CoV-2 infected patients) pending. JB is an employee of Synairgen Research and holds share options in Synairgen. MM reports fees as a consultant from Synairgen Research, outside of the submitted work. FJG is a Managing Partner of Transcrip Partners and reports personal fees from TranScrip Partners who supported Synairgen for the study, and personal fees from TranScrip Partners outside of this work. FJG was also a member of the independent data safety monitoring committee. DED is a co-founder of Synairgen Research, owns shares in Synairgen, and has received personal fees and other fees as a consultant to Synairgen Research during and outside of the conduct of the trial. DED has also received a grant from Boehringer Ingelheim for fibrosis studies unrelated to the trial and has patents relating to antiviral therapy for respiratory diseases (PCT/GB2005/050031) and the use of interferon-beta as a form of antiviral therapy for respiratory diseases (WO2005087253A3), with royalties paid to the University of Southampton and the inventors. STH is a co-founder of Synairgen Research, owns shares in the company, and has received personal fees as a Non-Executive Director and Consultant of Synairgen Research outside of the submitted work. STH also has patents for the use of interferon-beta as a form of antiviral therapy for respiratory diseases (WO2005087253A3 WIPO [PCT]; issued), antiviral therapy for respiratory diseases (US20090257980A1; issued), and inhaled interferon beta for COVID-19 (pending); STH is also Chair of the Academy of Medical Sciences for the report Preparing for a challenging winter 2020/21, published July 14, 2020. L-PH reports receiving funds from Synairgen Research to carry out a phase 1 clinical trial at her institution during the conduct of the study and grants from Celgene for a research study in fibrosis, outside of the submitted work. TC reports receipt of personal fees from BioMerieux and BioFire, Roche, Janssen, Cidara Therapeutics, Synairgen Research, and Randox; non-financial support from BioMerieux and BioFire, and Qiagen; and other fees from Synairgen Research outside of the submitted work. RD reports receiving fees for lectures at symposia organised by Novartis, AstraZeneca, and Teva, consultation fees for Teva and Novartis as a member of advisory boards, and participation in a scientific discussion about asthma organised by GlaxoSmithKline. RD is a co-founder, current consultant, and owns shares in Synairgen Research. TMAW reports receipt of personal fees and travel support from My mHealth, grants and personal fees from GlaxoSmithKline; grants and personal fees from AstraZeneca; personal fees from Boehringer Ingelheim; and grants and personal fees from Synairgen Research, outside of the submitted work. TNB declares no competing interests.
Contributor Information
Inhaled Interferon Beta COVID-19 Study Group:
Michael G Crooks, Davinder PS Dosanjh, Salman Siddiqui, Najib M Rahman, Jacklyn A Smith, Alexander Horsley, Timothy W Harrison, Dinesh Saralaya, Lorcan McGarvey, Alastair Watson, Edmund Foster, Adam Fleet, Dave Singh, Sophie Hemmings, Sandra Aitken, Sarah Dudley, Rona Beegan, Angela Thompson, and Pedro MB Rodrigues
Supplementary Material
References
- 1.Guan W-j, Ni Z-y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salje H, Tran Kiem C, Lefrancq N, et al. Estimating the burden of SARS-CoV-2 in France. Science. 2020;369:208–211. doi: 10.1126/science.abc3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19—final report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2007764. published online Oct 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with Covid-19—preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021436. published online July 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li S, Gong M, Zhao F, et al. Type I interferons: distinct biological activities and current applications for viral infection. Cell Physiol Biochem. 2018;51:2377–2396. doi: 10.1159/000495897. [DOI] [PubMed] [Google Scholar]
- 6.Watson A, Spalluto CM, McCrae C, et al. Dynamics of IFN-β responses during respiratory viral infection: insights for therapeutic strategies. Am J Respir Crit Care Med. 2020;201:83–94. doi: 10.1164/rccm.201901-0214OC. [DOI] [PubMed] [Google Scholar]
- 7.Yuen C-K, Lam J-Y, Wong W-M, et al. SARS-CoV-2 nsp13, nsp14, nsp15 and orf6 function as potent interferon antagonists. Emerg Microbes Infect. 2020;9:1418–1428. doi: 10.1080/22221751.2020.1780953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hadjadj J, Yatim N, Barnabei L, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agrawal A. Mechanisms and implications of age-associated impaired innate interferon secretion by dendritic cells: a mini-review. Gerontology. 2013;59:421–426. doi: 10.1159/000350536. [DOI] [PubMed] [Google Scholar]
- 10.Singanayagam A, Loo S-L, Calderazzo M, et al. Antiviral immunity is impaired in COPD patients with frequent exacerbations. Am J Physiol Lung Cell Mol Physiol. 2019;317:L893–L903. doi: 10.1152/ajplung.00253.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Djukanović R, Harrison T, Johnston SL, et al. The effect of inhaled IFN-β on worsening of asthma symptoms caused by viral infections. A randomized trial. Am J Respir Crit Care Med. 2014;190:145–154. doi: 10.1164/rccm.201312-2235OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Filipi ML, Beavin J, Brillante RT, et al. Nurses' perspective on approaches to limit flu-like symptoms during interferon therapy for multiple sclerosis. Int MS Care. 2014;16:55–60. doi: 10.7224/1537-2073.2013-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Synairgen Press release: Synairgen announces positive data from interim analysis of SNG001 trial in COPD patients. Sept 8, 2020. https://www.synairgen.com/wp-content/uploads/2020/09/200908-SG015-Press-Release-Final.pdf
- 14.Reynolds S, Lunn K, Beegan R, Tear V, Monk PD. Antiviral biomarkers are upregulated in sputum cells following administration of inhaled interferon beta to COPD patients. Eur Respir J. 2019;54(suppl 63):OA263. (abstr). [Google Scholar]
- 15.McCrae C, Olsson M, Aurell M, et al. On-demand inhaled interferon-beta 1a for the prevention of severe asthma exacerbations: results of the INEXAS phase 2a study. In D12. Immunotherapy in lung disease. Am J Respir Critical Care Med. 2018;201 (abstr). [Google Scholar]
- 16.WHO R&D Blueprint. COVID-19 therapeutic trial synopsis. Feb 16, 2020. https://www.who.int/publications/i/item/covid-19-therapeutic-trial-synopsis
- 17.NHS England and NHS Improvement Guidance and standard operating procedure. COVID-19 virus testing in NHS laboratories. March 16, 2020. https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2020/03/guidance-and-sop-covid-19-virus-testing-in-nhs-laboratories-v1.pdf
- 18.Leidy NK, Rennard SI, Schmier J, Jones MK, Goldman M. The Breathlessness, Cough, and Sputum Scale: the development of empirically based guidelines for interpretation. Chest. 2003;124:2182–2191. doi: 10.1378/chest.124.6.2182. [DOI] [PubMed] [Google Scholar]
- 19.WHO Solidarity trial consortium Repurposed antiviral drugs for COVID-19 – interim WHO SOLIDARITY trial results. medRxiv. 2020 doi: 10.1101/2020.10.15.20209817. published online Oct 15. (preprint) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hart BJ, Dyall J, Postnikova E, et al. Interferon-b and mycophenolic acid are potent inhibitors of Middle East respiratory syndrome coronavirus in cell-based assays. J Gen Virol. 2014;95:571–577. doi: 10.1099/vir.0.061911-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mantlo E, Bukreyeva N, Maruyama J, Paessler S, Huang C. Antiviral activities of type I interferons to SARS-CoV-2 infection. Antiviral Res. 2020;179 doi: 10.1016/j.antiviral.2020.104811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cinatl J, Morgenstern B, Bauer G, Chandra P, Rabenau H, Doerr HW. Treatment of SARS with human interferons. Lancet. 2003;362:293–294. doi: 10.1016/S0140-6736(03)13973-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Runkel L, Meier W, Pepinsky RB, et al. Structural and functional differences between glycosylated and non-glycosylated forms of human interferon-β (IFN-β) Pharm Res. 1998;15:641–649. doi: 10.1023/a:1011974512425. [DOI] [PubMed] [Google Scholar]
- 24.Schaefer IM, Padera RF, Solomon IH, et al. In situ detection of SARS-CoV-2 in lungs and airways of patients with COVID-19. Mod Pathol. 2020 doi: 10.1038/s41379-020-0595-z. published online June 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hung IF, Lung KC, Tso EY, et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395:1695–1704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Angus DC, Berry S, Lewis RJ, et al. The REMAP-CAP (Randomized Embedded Multifactorial Adaptive Platform for Community-acquired Pneumonia) Study. Rationale and design. Ann Am Thorac Soc. 2020;17:879–891. doi: 10.1513/AnnalsATS.202003-192SD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sallard E, Lescure FX, Yazdanpanah Y, Mentre F, Peiffer-Smadja N. Type 1 interferons as a potential treatment against COVID-19. Antiviral Res. 2020;178 doi: 10.1016/j.antiviral.2020.104791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou Q, Chen V, Shannon CP, et al. Interferon-α2b treatment for COVID-19. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.01061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hao SR, Yan R, Zhang SY, et al. Interferon-α2b spray inhalation did not shorten virus shedding time of SARS-CoV-2 in hospitalized patients: a preliminary matched case-control study. J Zhejiang Univ Sci B. 2020;21:628–636. doi: 10.1631/jzus.B2000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fu W, Liu Y, Xia L, et al. A clinical pilot study on the safety and efficacy of aerosol inhalation treatment of IFN-κ plus TFF2 in patients with moderate COVID-19. EClinicalMedicine. 2020;25 doi: 10.1016/j.eclinm.2020.100478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blanco-Melo D, Nilsson-Payant BE, Liu W-C, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036. doi: 10.1016/j.cell.2020.04.026. 45.e1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.University Hospital Southampton NHS Foundation Trust Clinical Research in Southampton. People with early COVID-19 symptoms sought for at home treatment trial. July 17, 2020. https://www.uhs.nhs.uk/ClinicalResearchinSouthampton/Research/News-and-updates/Articles/People-with-early-COVID-19-symptoms-sought-for-at-home-treatment-trial.aspx
- 33.Rovere Querini P, De Lorenzo R, Conte C, et al. Post-COVID-19 follow-up clinic: depicting chronicity of a new disease. Acta Biomed. 2020;91:22–28. doi: 10.23750/abm.v91i9-S.10146. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data analysed and presented in this study are available from the corresponding author on reasonable request, providing the request meets local ethical and research governance criteria after publication. Patient-level data will be anonymised and study documents will be redacted to protect the privacy of trial participants. The study protocol is provided in the appendix (p 5).