Abstract
We report risk factors, clinical manifestations, and treatment course of 2 lung transplant recipients diagnosed with coronavirus disease 2019 (COVID-19) pneumonia. Both patients underwent an initial hospitalization and discharged home, followed by readmission several days later with significant worsening of respiratory status and infectious symptoms. The first patient underwent prolonged hospitalization requiring tracheostomy and feeding tube placement. The second patient declined intubation and expired. The early documented experiences of COVID-19 pneumonia in lung transplant recipients suggest that although recovery is achievable, the high rate of comorbid conditions and immunocompromised state may place these patients at higher risk for poor outcomes.
Coronavirus disease 2019 (COVID-19) caused by the novel SARS-CoV-2 virus is a global pandemic, with up to 15% of those infected suffering from severe disease and 5% classified as critical.1 Patients with a history of lung transplantation may be particularly vulnerable to SARS-CoV-2 infection and at higher risk for severe complications due to chronic immunosuppression and underlying comorbid conditions. The manifestations, clinical course, and outcomes after SARS-CoV-2 infection in lung transplant recipients is not well defined, however. We report the clinical presentations and management of 2 lung transplant recipients who presented with COVID-19 pneumonia several years after transplantation.
Case Reports
Patient 1
The first case is a 62-year-old woman who underwent bilateral lung transplant in November 2016 for tobacco-induced end-stage emphysema. The patient’s postoperative course was uneventful. She was discharged home on immunosuppressive therapy consisting of prednisone, cyclosporine, and azathioprine. Over the years her regimen was adjusted to prednisone, azathioprine, tacrolimus, and everolimus. Pulmonary function testing 2 months prior to diagnosis of COVID-19 demonstrated a forced expiratory volume at 1 second of 2.5 L (112% predicted) and a forced vital capacity of 2.8 L (95% predicted).
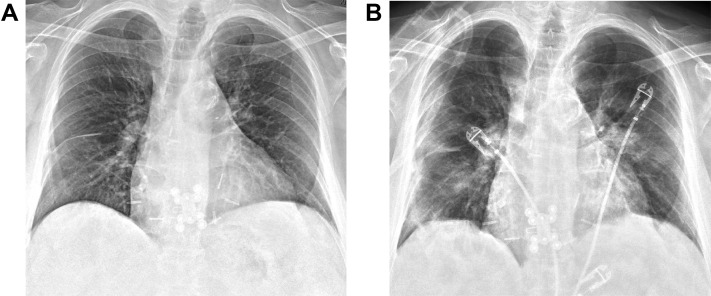
Approximately 3.5 years after transplantation, the patient presented to her primary care physician after exposure to several family members who tested positive for COVID-19. Her symptoms included chills, rhinorrhea, and a mild dry cough. A nasopharyngeal swab was positive for SARS-CoV-2 RNA. Two days later, the patient was admitted to the hospital with fevers, shortness of breath, and hypoxia requiring nasal cannula supplementation. Chest radiography revealed no abnormalities (Figure 1A). The patient was treated with a 5-day course of remdesivir and 10-day course of dexamethasone 6 mg. The patient was weaned to room air and discharged home on hospital day 6.
Figure 1.
Patient 1. (A) Chest radiograph at initial hospital admission demonstrating no acute pulmonary abnormalities. (B) On readmission to the hospital 5 days later, chest radiograph revealed new bilateral multifocal patchy opacities.
Five days after discharge she returned to the hospital with recurrent fevers and chills. Oxygen saturation was 84% on room air with chest radiograph demonstrating new bilateral multifocal patchy opacities (Figure 1B). The patient’s oxygenation and work of breathing continued to worsen requiring intubation on hospital day 2. She was started on empiric vancomycin and cefepime, completing a 7-day course. She was paralyzed and proned on hospital day 3 with only temporary improvement in oxygenation levels during proning episodes. She was also administered high-dose pulse steroids for empiric treatment of acute allograft rejection. She developed acute renal failure requiring initiation of hemodialysis on hospital day 5. Extubation was attempted on hospital day 16, but the patient required reintubation later that day. She underwent tracheostomy and gastrostomy tube placement on hospital day 19. She tolerated a ventilator wean over the ensuing weeks and was eventually decannulated on hospital day 38. She was discharged to an acute rehabilitation facility prior to returning home.
Patient 2
The second case is a 56-year-old woman who underwent bilateral lung transplant in September 2014 due to interstitial lung disease and secondary pulmonary hypertension. Her postoperative course was complicated by reintubation, atrial tachyarrhythmias, and delirium. The patient’s initial immunosuppressive regimen consisted of prednisone, cyclosporine, and azathioprine. Several months after discharge she developed posttransplant lymphoproliferative disorder which was treated with rituximab and discontinuation of azathioprine. Over the subsequent years, the patient experienced continual decline in her pulmonary function. She was eventually diagnosed with grade 3 restrictive chronic lung allograft dysfunction requiring 4 L/min nasal cannula at rest and 8 L/min with exertion. Pulmonary function testing 1 month prior to infection demonstrated a forced expiratory volume at 1 second of 1.1 L (43% predicted) and forced vital capacity of 1.3 L (42% predicted).
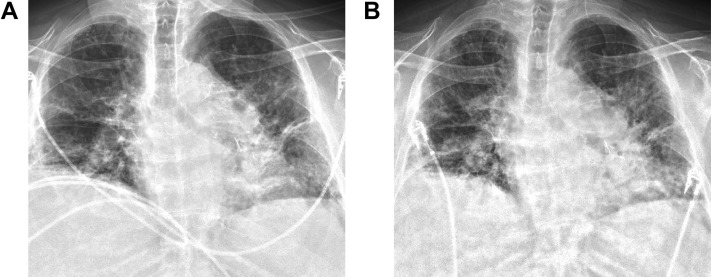
Approximately 5.5 years post-transplant, the patient presented to the emergency department with cough, fevers, and shortness of breath after close contact with family members who tested positive for COVID-19. The patient required 8 L/min oxygen to maintain adequate saturations. Chest radiograph demonstrated an ill-defined hazy opacity over the left lung (Figure 2A). A nasopharyngeal swab was positive for SARS-CoV-2 RNA. The patient was treated with 5 days of remdesivir and empiric cefepime. She was eventually weaned to 4 L/min nasal cannula at rest and 10 L/min with exertion. She was discharged home on hospital day 6.
Figure 2.
Patient 2. (A) Chest radiograph at initial hospital admission demonstrating an ill-defined hazy opacity over the left lung. (B) On readmission to the hospital 3 days later, chest radiograph revealed stable to slightly worsening pulmonary opacities.
Three days later, the patient presented with worsening hypoxia and work of breathing. Repeat chest radiograph demonstrated slightly worsening pulmonary opacities (Figure 2B). The patient was started on broad spectrum antibiotics including vancomycin, meropenem, and azithromycin, as well as high-dose steroids for empiric treatment of acute allograft rejection. The patient did not wish to be intubated and was transitioned to comfort care. On hospital day 3, the patient expired.
Comment
Lung transplant recipients are at high risk for infectious complications due to a variety of patient- and transplant-related factors. Patient risk factors include frequent comorbid conditions as well as regular contact with medical care, which may increase exposure risk. Transplant-mediated factors include need for chronic immunosuppression, continuous exposure of the transplanted allograft to the external environment, and impaired pulmonary host defenses due to loss of lymphatics and decreased mucociliary clearance following transplantation.2 Taken together, these factors have generated widespread concern for increased disease severity and likelihood of poor outcomes among lung transplant patients diagnosed with COVID-19 pneumonia.
The cases presented in this report largely validate this concern, demonstrating an unusually similar clinical course in 2 patients with distinctly different baseline health and respiratory function. Although both patients presented with a relatively benign disease course initially, this was followed by readmission with severe symptoms approximately 10 days after diagnosis. This reflects disease patterns seen in the non-transplant population with evolution of symptoms and admission frequently occurring over a week after symptom onset. Although the outcome for the second patient may be expected given her tenuous respiratory status at the onset infection, the first patient experienced a comparably challenging clinical course despite significantly better allograft function. This is likely explained by her multiple preexisting medical conditions, all of which are known risk factors for severe disease. This again mirrors the pathogenesis of COVID-19 among non-transplant patients, emphasizing the importance of comorbidities in predicting poor and, in some cases, fatal outcomes. Although refractory hypoxemia and multiorgan failure may ensue regardless of transplant status, the ability to recover from such complications could be further compromised following transplant.
The early documented experiences of COVID-19 in lung transplant recipients suggest that although recovery is achievable, the high rate of comorbid conditions and immunocompromised state may place these patients at higher risk for poor outcomes. Regardless of disease severity at presentation, providers should have a low threshold for intubation and initiation of advanced therapies. Further investigation into the effects of immunosuppression on SARS-CoV-2 infection and host response is critical to understanding the optimal management strategy of immunosuppressive therapy in these patients. For patients who recover, it remains to be seen how infection with COVID-19 pneumonia will affect future lung allograft function. For now, a cautious and vigilant approach should be pursued for all lung transplant recipients regardless of baseline health or functional status, with collective goals of reducing exposure risk, detecting infection early, and anticipating potential complications.
References
- 1.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2.Hachem R.R. The role of the immune system in lung transplantation: towards improved long-term results. J Thorac Dis. 2019;11(suppl 14):S1721–S1731. doi: 10.21037/jtd.2019.04.25. [DOI] [PMC free article] [PubMed] [Google Scholar]