Abstract
Background
. Teriflunomide is an immunomodulatory drug approved for Multiple Sclerosis (MS) treatment that inhibits dihydroorotate dehydrogenase, a mitochondrial enzyme involved in the de novo pyrimidine synthesis pathway. This mechanism can produce antiviral effects, thus teriflunomide has gained attention during COVID-19 pandemic. Moreover, in the last months, some case-reports have been published describing MS patients treated with teriflunomide who developed mild and self-limiting forms of COVID-19.
Methods
Here, we describe the case of a 57-year-old man affected by MS, and treated with teriflunomide, who developed a mild form of SARS-CoV-2 infection. Moreover, we provide a detailed literature review about the available cases of COVID-19 in MS patients treated with teriflunomide. We report clinical features, disease course and outcome, and we discuss similarities and differences among patients.
Results
Apart from the present report, since February 2020, five papers have been published describing 14 MS patients who developed SARS-CoV-2 infection during teriflunomide treatment. Patients were mostly female (53%), with an average age of 50.5 (±11.3) years. Median EDSS was 2.25 (range 0–6). The average time on treatment with teriflunomide was 3.7 (± 1.6) years. Relevant comorbidities were present in 4 patients (27%). Regarding SARS-CoV-2 infection, the most common symptom was fever (100%) followed by gastrointestinal disturbances (67%), fatigue (55%) and cough (55%). 5 patients were hospitalized and 2 required oxygen support. In patient hospitalized (n=5) compared to the others (n=10), age was significantly higher (59.6 vs 45.9 years, p=0.025) while gender, EDSS, duration of teriflunomide therapy and comorbidities were not significantly different. Outcome was good for all patients with a variable recovery time, ranging from few days to some weeks. Teriflunomide was continued during the entire course of SARS-CoV-2 infection in all patients except for two. Compared to the patients already described, our patient was 7 years older, average time on teriflunomide treatment was about 2.5 years shorter, and median EDSS was 1.5 point lower. Despite significant comorbidities, the outcome was good since our patient was hospitalized but he did not require oxygen supplementation nor intensive care and was able to return at home after only 10 days. Teriflunomide therapy was continued throughout the period.
Conclusion
Available data suggest that teriflunomide therapy should not be discontinued in MS patients who develop SARS-CoV-2 infection, also in presence of significant comorbidities or clinical conditions requiring hospitalization. Additional studies are necessary to assess if the drug can also have a protective role against SARS-CoV-2.
Keywords: Multiple sclerosis, Teriflunomide, COVID-19, SARS-CoV-2
1. Introduction
It is well known that people with multiple sclerosis (PwMS) present an increased risk of infections and hospitalization (Marrie et al., 2014; Wijnands et al., 2017) compared to general population.
In this regard, it is still not clear the specific risks related to the outbreak of SARS-Coronavirus disease 19 (COVID-19) in PwMS. The use of disease-modifying therapies (DMTs) is considered the most important factor for determining such risk (Brownlee et al., 2020) and there is wide discussion about the opportunity of continuing or stopping DMTs in MS patients with a diagnosis of COVID-19.
DMTs could possibly increase the COVID-19 risks in PwMS because of their effects on immune system. Available data suggest that the risk is higher for second-generation DMTs (Zheng et al., 2020; Sormani et al., 2020). On the other side, it has been hypothesized that DMTs could also have a protective effect against COVID-19 because they could modulate the immune response against SARS‐CoV‐2, one of the factors associated with the clinical severity of COVID‐19, and in some cases, they could exert a direct antiviral effect (Baker et al., 2020; Rostami Mansoor and Ghasemi‐Kasman, 2020).
The specific mechanisms of action of the various DMTs and patients’ clinical characteristics should be taken into account to stratify the COVID-19-related risks in PwMS, although reassuring data from literature have highlighted that DMTs do not seem to increase the risk of acquiring COVID-19 (Thakolwiboon et al., 2020). Indeed, the specific effect of DMT on immune response could be beneficial for blunting the consequences of the more severe forms of SARS-CoV-2 infection (Ciotti et al., 2020). In this perspective, teriflunomide has recently gained attention during COVID-19 pandemic because of its peculiar mechanism of action based on the selective and reversible inhibition of the dihydroorotate dehydrogenase, a mitochondrial enzyme involved in the de novo pyrimidine synthesis pathway (Bar-Or et al., 2014).
Indeed, pharmacological studies have demonstrated that such mechanism can produce antiviral effects (Coelho and Oliveira, 2020). Besides, in the last months, some case-reports have been published describing MS patients treated with teriflunomide who developed mild and self-limiting forms of COVID-19 (Bollo et al., 2020; Ciardi et al., 2020; Maghzi et al., 2020; Mantero et al., 2020; Möhn et al., 2020)
Here, we describe a further case of a 57-year old MS patient treated with teriflunomide who developed a mild form of SARS-CoV-2 infection. Moreover, we provide a detailed literature review about the available cases of COVID-19 in MS patients treated with teriflunomide. We describe clinical features, disease course and outcome, and we discuss similarities and differences among patients, highlighting future directions for research in this field (Fig. 1 ).
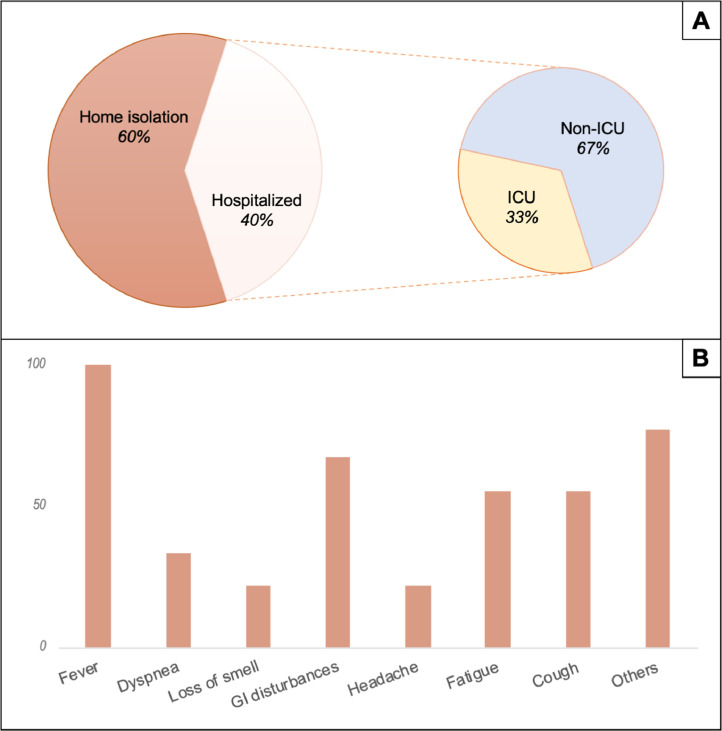
Fig. 1.
Clinical presentation and management in teriflunomide-treated MS patients with COVID-19. Panel A: Hospitalization rate. ICU: intensive care unit. Panel B: symptoms distribution among patients.
2. Case report
A 57-year old male patient was diagnosed with relapsing-remitting MS in June 2018 according to the revised McDonald criteria (Thompson et al., 2018). Brain MRI showed multiple supratentorial and infratentorial FLAIR hyperintense lesions while spine MRI was normal. Cerebrospinal Fluid (CSF) analysis revealed a blood-brain barrier damage (QAlb CSF/Serum 21.7 × 103) and the presence of CSF-restricted IgG oligoclonal bands (Pattern II).
In April 2019, he started therapy with teriflunomide (14 mg/day). Since then, he did not experience clinical relapses or MRI worsening.
His medical history was remarkable for obesity, hypertension treated with ramipril 5 mg/day, and depressive and anxiety disorder in treatment with citalopram 10 mg/die and brotizolam 0.25 mg/die.
Since the first days of September 2020, he developed severe and progressive fatigue. For this reason, he was admitted to our outpatient MS clinic on 9 September 2020. Neurological examination was normal except for increased reflexes in lower limbs. EDSS was 1. His last brain and spine MRI, performed on March 2020, was stable. His last available blood test, performed on September 3, 2020, showed normal blood count.
On September 10, 2020, he developed fever (39.5°) and cough. For this reason, he was admitted to Emergency Department of a local hospital. Chest CT scan revealed interstitial pneumonia and a nasopharyngeal swab was positive for COVID-19. Blood test showed high levels of C-reactive protein (CRP) (95 mg/dL) and lactate dehydrogenase (LDH) (260 UI/L); the partial pressure of oxygen was 65 mmHg.
On September 11, 2020, patient was moved to the COVID-19 ward (Covid Centre 3, Regione Lazio). At the admission, temperature was 38.5°C. Blood gas analysis showed that the partial pressure of oxygen was 76 mmHg, with a PaO2/FiO2 (P/F) ratio of 361. CRP was 84 mg/dl. Other lab tests, including a complete blood count, were normal.
The patient was started on ceftriaxone 2 g/die, clarithromycin 500 mg bid, enoxaparin 4000 UI bid, dexamethasone 4 mg/die and pantoprazole 40 mg/die. Teriflunomide was continued.
During hospitalization, he never developed respiratory distress and no oxygen supplementation was needed.
Clinical condition and laboratory tests rapidly improved. On September 13, he was afebrile. On September 20, CRP was normal (1.3 mg/dl). After two consecutive negative nasopharyngeal swabs, the patient was discharged at home in good health.
3. Discussion
Apart from the present report, five papers have been published since February 2020, describing 14 MS patients who developed SARS-CoV-2 infection during teriflunomide treatment (Bollo et al., 2020; Ciardi et al., 2020; Maghzi et al., 2020; Mantero et al., 2020; Möhn et al., 2020). Patients’ clinical characteristics are reported in Table 1 .
Table 1.
Clinical characteristics of SARS-CoV-2 infection in teriflunomide-treated MS patients. EDSS: Expanded Disability Status Scale. MS: Multiple Sclerosis. ICU: Intensive Care Unit. RR: Relapsing-Remitting. RIS: Radiologically Isolated Syndrome. SP: Secondary Progressive. Not reported: data non reported from authors.
| Paper | Patient ID | Age | Gender | EDSS | MS type | Years on Teriflunomide | Teriflunomide Discontinuation during Covid-19 infection | COVID-19 Symptoms | COVID-19 diagnosis | Comorbidities | Covid-19 therapy | Hospitalization | Oxygen support | ICU care | Outcome |
| Bollo et al. | #1 | 58 | F | 2.5 | RR | 4 | Yes (reinitiated at hospital discharge) | Fever, slight dyspnea, diarrhea | nasopharyngeal swab | Not reported | antibiotics, chloroquine, lopinavir/ ritonavir, enoxaparin | Yes | Yes | No | Good |
| Ciardi et al. | #2 | 62 | F | 6 | RR | 5 | Yes (reinitiated at hospital discharge) | Fever, dyspnea, fatigue, dry cough, diarrhea | nasopharyngeal swab | Not reported | antibiotics, chloroquine, lopinavir/ ritonavir | Yes | No | No | Good |
| Maghzi et al. | #3 | 52 | M | 0 | RIS | 3 | No | Fatigue, headache, nasal congestion, loss of smell, myalgia, fever, rigors, rhinorrhea, diarrhea | nasal swab | Obstructive sleep apnea, hyperlipidemia, attention deficit hyperactivity disorder, depression | None | No | No | No | Good (complete recovery in 25 days) |
| #4 | 52 | F | 2.5 | RR | 4 | No | Headache, nausea and vomiting, fever, asthenia, loss of smell | nasopharyngeal swab | No | None | No | No | No | Good (complete recovery in 20 days) | |
| #5 | 47 | M | 1 | RR | 4 | No | Sore throat, diarrhea, fever, cough, mild dyspnea | nasal swab | No | none | No | No | No | Good (complete recovery in 38 days) | |
| #6 | 38 | M | 3 | RR | 2 | No | Fever, dry cough | reported by patient | Attention deficit disorder, anxiety, alcohol abuse | none | No | No | No | Good (complete recovery in few days) | |
| #7 | 79 | F | 6 | SP | 3 | Yes (discontinued for 12 days before Covid-19 infection and taken at reduced dosage during Covid-19 infection) | Fever, dry cough | nasopharyngeal swab | Hypertension, urinary tract infections. | none | Yes | Not reported | Yes | Good (complete recovery in 8 days) | |
| Mantero et al. | #8 | 37 | F | 1.5 | RR | 0.5 | No | Not reported | nasopharyngeal swab | No | none | No | No | No | Good |
| #9 | 54 | F | 3.5 | RR | 1 | No | Not reported | nasopharyngeal swab | No | none | No | No | No | Good | |
| #10 | 57 | F | 1 | RR | 0.5 | No | Not reported | nasopharyngeal swab | No | none | No | No | No | Good | |
| #11 | 40 | M | 1 | RR | 3 | No | Not reported | clinical criteria | No | none | No | No | No | Good | |
| #12 | 48 | M | 2 | RR | 2.5 | No | Not reported | clinical criteria | No | none | No | No | No | Good | |
| #13 | 34 | F | 4.5 | RR | 5 | No | Not reported | clinical criteria | No | none | No | No | No | Good | |
| Mohn et al. | #14 | 42 | M | Not reported | RR | 7 | No | Nausea, vomiting, general weakness, sore throat, gait disturbance, fever | nasopharyngeal swab | No | none | Yes | No | No | Good (complete recovery in 7 days) |
| Our case | #15 | 57 | M | 1 | RR | 1.2 | No | Fatigue, fever, cough | nasopharyngeal swab | Hypertension, obesity, anxiety, depression | antibiotics, corticosteroids, enoxaparin | Yes | Yes | No | Good (complete recovery in 10 days) |
Patients were mostly female (53%), with an average age of 50.5 (±11.3) years. Median EDSS was 2.25 (range 0–6). Regarding MS phenotype, 13 patients were affected by relapsing-remitting form, 1 patient by radiologically isolated syndrome and 1 patient by secondary progressive form. The average time on treatment with teriflunomide was 3.7 (± 1.6) years. Relevant comorbidities were present in 4 patients (27%).
Regarding SARS-CoV-2 infection, diagnosis was based on nasal or nasopharyngeal swab in 11 patients, on clinical criteria in 3 patients and only reported in one patient. A detailed description of COVID-19 patient's clinical condition was available for 9 patients; the most common symptom was fever (100%) followed by gastrointestinal disturbances (67%), fatigue (55%) and cough (55%).
5 patients were hospitalized and 2 required oxygen support. Only one patient (#7) required intensive care hospitalization but this occurred some days before COVID-19 diagnosis for a different clinical reason (Klebsiella pyelonephritis/sepsis).
In patient hospitalized (n=5) compared to the others (n=10), age was significantly higher (59.6 vs 45.9 years, p=0.025) while gender, EDSS, duration of teriflunomide therapy and comorbidities were not significantly different.
Specific pharmacological treatments for COVID-19 were performed in 3 patients, including antibiotics (3/3), corticosteroids (1/3), enoxaparin (2/3) and lopinavir/ ritonavir (2/3).
Outcome was good for all patients with a variable recovery time, ranging from few days to some weeks. However, it should be noted that this item is hardly comparable among patients because it is reported with scarce details and in different manners (e.g. hospital discharge, swab negativity, symptoms resolution, return to work or usual activities).
Teriflunomide was continued during the entire course of SARS-CoV-2 infection in all patients except for two (#1 and #2). Both of them required hospitalization for symptomatic interstitial pneumonia and this could have contributed to the clinical decision to stop teriflunomide. In both cases, therapy was started again at hospital discharge. In patient #7, teriflunomide was discontinued for 12 days before COVID-19 diagnosis (because of Klebsiella systemic infection) and then reintroduced at reduced dosage (14 mg every other day) during SARS-CoV-2 infection.
Compared with to the patients already described, our patient was 7 years older, average time on teriflunomide treatment was about 2.5 years shorter, and median EDSS was 1.5 point lower.
Our patient required hospitalization, however despite significant comorbidities (hypertension and obesity), the outcome was good since he did not require oxygen supplementation nor intensive care and was able to return at home after 10 days. Teriflunomide therapy was continued throughout the period.
Taken together, available data suggest that teriflunomide does not require to be discontinued in MS patients who develop SARS-CoV-2 infection. In addition to literature data, our case report suggests that continuing therapy might be safe also in patients with significant comorbidities, who develop SARS-CoV-2 related pneumonia requiring hospitalization.
Moreover, review of the available literature indicates that, as expected, age is a risk factor for a more aggressive course of COVID-19, also in MS patients. Indeed, Louapre et al. (2020) observed that age, together with neurological disability and obesity, are the main risk factors for severe forms of COVID-19 in PwMS, while DMTs did not seem to influence the risk of getting SARS-CoV-2 infection nor the clinical outcome. In this paper we found that hospitalized patients were significantly older compared to others, while the lack of significant differences between groups in terms of disability level (EDSS) and comorbidities could be due to the low number of patients.
Some authors hypothesized that teriflunomide, not only does not increase the COVID-19 related risks but it could also have a protective role against SARS‐CoV‐2 (Ciardi et al., 2020; Maghzi et al., 2020).
This hypothesis is based on different findings. First of all, it is well known that the inhibition of dihydroorotate dehydrogenase by teriflunomide can induce antiviral effects by two different mechanisms: a) the depletion of pyrimidine pool interferes with the transcription and replication of RNA viruses (such as SARS‐CoV‐2) causing a halt in infection; b) the expression of antiviral genes, such as interferon-simulated genes, which foster innate immune responses against viruses (Cheung et al., 2017; Lucas-Hourani et al., 2013; Luthra et al., 2018). Moreover, teriflunomide has an immunomodulatory activity because it reduces the proliferation of rapidly dividing cells, including activated T and B lymphocytes, without causing cell death (Bar-Or et al., 2014). Accordingly, teriflunomide could reduce the severity of COVID-19 by preventing an excessive immunological host response to virus.
Recently, Ciardi et al. (Ciardi et al., 2020) described the peripheral blood immune cell profile in a teriflunomide-treated MS patient, before and during SARS-COV-2 infection. They found out no changes in the rates of immune activation and immunosenescence of T-cells. They hypothesized that chronic treatment with teriflunomide prevented an excessive activation of the immune system against the SARS‐CoV‐2 and this positively influences patient prognosis.
However, although intriguing, this hypothesis is not yet supported by robust and reliable clinical data. Indeed, although all reported MS patients treated with teriflunomide developed mild forms of COVID-19, we cannot rule out that the positive outcome may have been unrelated to teriflunomide, given that most patients recover spontaneously without treatment. Similar conclusions can be drawn for other DMTs (Sormani, 2020). In this regard, Sormani and colleagues collected data of PwMS who developed COVID-19 in the first half of 2020 (Sormani et al., 2020). Overall, they found out that DMTs were not associated with a worse outcome, apart from B-cell-depleting agents and methylprednisolone (administered in the month preceding the onset of the symptoms). Additionally, Interferon seems to decrease the risk of SARS-CoV-2 infection, presumably through a direct antiviral effect (Sormani et al., 2020). However, randomized, controlled, clinical trials are warranted to clarify the role of teriflunomide and other DMTs during SARS-CoV-2 infection.
Along similar lines, some concern exists for vaccination of PwMS against SARS-CoV-2. However, most of COVID-19 candidates are non-live vaccines (Jeyanathan et al., 2020), thus they could be administered with reasonable safety to people treated with DMTs too (Riva et al., 2020).
4. Conclusion
In conclusion, available data suggest that teriflunomide therapy should not be discontinued in MS patients who develop SARS-CoV-2 infection, also in presence of significant comorbidities or clinical conditions requiring hospitalization. Additional studies are necessary to assess if the drug can also have a protective role against SARS-CoV-2.
Author disclosures
FC has received travel grants from Biogen, Merck, Sanofi-Genzyme, and Roche. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
Authors deny any funding source for the present study.
CRediT authorship contribution statement
Fioravante Capone: Conceptualization, Writing - original draft. Francesco Motolese: Investigation, Writing - review & editing. Tiziano Luce: Investigation, Writing - review & editing. Mariagrazia Rossi: Writing - review & editing. Alessandro Magliozzi: Writing - review & editing. Vincenzo Di Lazzaro: Writing - review & editing, Supervision.
Declaration of Competing Interest
None.
Acknowledgment
We thank our patient and his family for their disposability.
References
- Baker D., Amor S., Kang A.S., Schmierer K., Giovannoni G. The underpinning biology relating to multiple sclerosis disease modifying treatments during the COVID-19 pandemic. Mult. Scler. Relat. Disord. 2020;43 doi: 10.1016/j.msard.2020.102174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Or A., Pachner A., Menguy-Vacheron F., Kaplan J., Wiendl H. Teriflunomide and its mechanism of action in multiple sclerosis. Drugs. 2014;74:659–674. doi: 10.1007/s40265-014-0212-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollo L., Guerra T., Bavaro D.F., Monno L., Saracino A., Angarano G., Paolicelli D., Trojano M., Iaffaldano P. Seroconversion and indolent course of COVID-19 in patients with multiple sclerosis treated with fingolimod and teriflunomide. J. Neurol. Sci. 2020;416 doi: 10.1016/j.jns.2020.117011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee W., Bourdette D., Broadley S., Killestein J., Ciccarelli O. Treating multiple sclerosis and neuromyelitis optica spectrum disorder during the COVID-19 pandemic. Neurology. 2020;94:949–952. doi: 10.1212/WNL.0000000000009507. [DOI] [PubMed] [Google Scholar]
- Cheung N.N., Lai K.K., Dai J., Kok K.H., Chen H., Chan K.-H., Yuen K.-Y., Kao R.Y.T. Broad-spectrum inhibition of common respiratory RNA viruses by a pyrimidine synthesis inhibitor with involvement of the host antiviral response. J. Gen. Virol. 2017;98:946–954. doi: 10.1099/jgv.0.000758. [DOI] [PubMed] [Google Scholar]
- Ciardi M.R., Zingaropoli M.A., Pasculli P., Perri V., Tartaglia M., Valeri S., Russo G., Conte A., Mastroianni C.M. The peripheral blood immune cell profile in a teriflunomide-treated multiple sclerosis patient with COVID-19 pneumonia. J. Neuroimmunol. 2020;346 doi: 10.1016/j.jneuroim.2020.577323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciotti J.R., Grebenciucova E., Moss B.P., Newsome S.D. Multiple sclerosis disease-modifying therapies in the COVID-19 era. Ann. Neurol. 2020;88:1062–1064. doi: 10.1002/ana.25907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho A.R., Oliveira P.J. Dihydroorotate dehydrogenase inhibitors in SARS-CoV-2 infection. Eur. J. Clin. Invest. 2020:50. doi: 10.1111/eci.13366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyanathan M., Afkhami S., Smaill F., et al. Immunological considerations for COVID-19 vaccine strategies. Nat. Rev. Immunol. 2020;20(10):615–632. doi: 10.1038/s41577-020-00434-6. https://www.nature.com/articles/s41577-020-00434-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louapre C., Collongues N., Stankoff B., et al. Clinical characteristics and outcomes in patients with coronavirus disease 2019 and multiple sclerosis. JAMA Neurol. 2020;77(9):1079–1088. doi: 10.1001/jamaneurol.2020.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas-Hourani M., Dauzonne D., Jorda P., Cousin G., Lupan A., Helynck O., Caignard G., Janvier G., André-Leroux G., Khiar S., Escriou N., Desprès P., Jacob Y., Munier-Lehmann H., Tangy F., Vidalain P.-O. Inhibition of pyrimidine biosynthesis pathway suppresses viral growth through innate immunity. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthra P., Naidoo J., Pietzsch C.A., De S., Khadka S., Anantpadma M., Williams C.G., Edwards M.R., Davey R.A., Bukreyev A., Ready J.M., Basler C.F. Inhibiting pyrimidine biosynthesis impairs Ebola virus replication through depletion of nucleoside pools and activation of innate immune responses. Antiviral Res. 2018;158:288–302. doi: 10.1016/j.antiviral.2018.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maghzi A.H., Houtchens M.K., Preziosa P., Ionete C., Beretich B.D., Stankiewicz J.M., Tauhid S., Cabot A., Berriosmorales I., Schwartz T.H.W., Sloane J.A., Freedman M.S., Filippi M., Weiner H.L., Bakshi R. COVID-19 in teriflunomide-treated patients with multiple sclerosis. J. Neurol. 2020;267:2790–2796. doi: 10.1007/s00415-020-09944-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantero V., Baroncini D., Balgera R., Guaschino C., Basilico P., Annovazzi P., Zaffaroni M., Salmaggi A., Cordano C. Mild COVID-19 infection in a group of teriflunomide-treated patients with multiple sclerosis. J. Neurol. 2020 doi: 10.1007/s00415-020-10196-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrie R.A., Elliott L., Marriott J., Cossoy M., Blanchard J., Tennakoon A., Yu N. Dramatically changing rates and reasons for hospitalization in multiple sclerosis. Neurology. 2014;83:929–937. doi: 10.1212/WNL.0000000000000753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möhn N., Saker F., Bonda V., Respondek G., Bachmann M., Stoll M., Wattjes M.P., Stangel M., Skripuletz T. Mild COVID-19 symptoms despite treatment with teriflunomide and high-dose methylprednisolone due to multiple sclerosis relapse. J. Neurol. 2020;267:2803–2805. doi: 10.1007/s00415-020-09921-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva A., Barcella V., Benatti S.V., et al. Vaccinations in patients with multiple sclerosis: a Delphi consensus statement. Mult. Scler. 2020 doi: 10.1177/1352458520952310. Sep 17Online ahead of print. 10.1177/1352458520952310. [DOI] [PubMed] [Google Scholar]
- Rostami Mansoor S., Ghasemi-Kasman M. Impact of disease-modifying drugs on the severity of COVID-19 infection in multiple sclerosis patients. J. Med. Virol. 2020:26593. doi: 10.1002/jmv.26593. jmv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani M.P., Italian Study Group on COVID-19 infection in multiple sclerosis An Italian programme for COVID-19 infection in multiple sclerosis. Lancet Neurol. 2020;19(6):481–482. doi: 10.1016/S1474-4422(20)30147-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani, M.P., De Rossi, N., Schiavetti, I., Carmisciano, L., Cordioli, C., Moiola, L., Radaelli, M., Immovilli, P., Capobianco, M., Trojano, M., Zaratin, P., Tedeschi, G., Comi, G., Battaglia, M.A. and Patti, F., Salvetti, M., Group, Musc-19 study, disease modifying therapies and COVID-19 severity in multiple sclerosis (2020). Available at SSRN: https://ssrn.com/abstract=3631244 or 10.2139/ssrn.3631244.
- Thakolwiboon S., Zhao-Fleming H., Pan J., Scott J.K., Shoji E., Sohn G., Avila M. Disease-modifying therapies during the COVID-19 outbreak: a narrative review of international and national recommendations. Int. J. MS Care. 2020;22(4):151–157. doi: 10.7224/1537-2073.2020-037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson A.J., Banwell B.L., Barkhof F., Carroll W.M., Coetzee T., Comi G., Correale J., Fazekas F., Filippi M., Freedman M.S., Fujihara K., Galetta S.L., Hartung H.P., Kappos L., Lublin F.D., Marrie R.A., Miller A.E., Miller D.H., Montalban X., Mowry E.M., Sorensen P.S., Tintoré M., Traboulsee A.L., Trojano M., Uitdehaag B.M.J., Vukusic S., Waubant E., Weinshenker B.G., Reingold S.C., Cohen J.A. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17:162–173. doi: 10.1016/S1474-4422(17)30470-2. [DOI] [PubMed] [Google Scholar]
- Wijnands J.M., Kingwell E., Zhu F., Zhao Y., Fisk J.D., Evans C., Marrie R.A., Tremlett H. Infection-related health care utilization among people with and without multiple sclerosis. Mult. Scler. J. 2017;23:1506–1516. doi: 10.1177/1352458516681198. [DOI] [PubMed] [Google Scholar]
- Zheng C., Kar I., Chen C.K., Sau C., Woodson S., Serra A., Abboud H. Multiple sclerosis disease-modifying therapy and the COVID-19 pandemic: implications on the risk of infection and future vaccination. CNS Drugs. 2020;34:879–896. doi: 10.1007/s40263-020-00756-y. [DOI] [PMC free article] [PubMed] [Google Scholar]