Abstract
Background and aim
There is increasing interest regarding SARS-CoV-2 infection in patients with autoimmune and immune-mediated inflammatory diseases (AI/IMID) with some discrepancies in different cohorts about their risk and outcomes. The aim was to describe a multidisciplinary cohort of patients with AI/IMID and symptomatic SARS-CoV-2 infection in a single tertiary center and analyze sociodemographic, clinical, and therapeutic factors associated with poor outcomes.
Methods
A retrospective observational study was conducted from the 1st of March until May 29th, 2020 in a University tertiary hospital in Barcelona, Spain. Patients with an underlying AI/IMID and symptomatic SARS-CoV-2 infection were identified in our local SARS-CoV-2 infection database. Controls (2:1) were selected from the same database and matched by age and gender. The primary outcome was severe SARS-CoV-2 infection, which was a composite endpoint including admission to the intensive care unit (ICU), need for mechanical ventilation (MV), and/or death. Several covariates including age, sex, and comorbidities among others were combined into a multivariate model having severe SARS-CoV-2 as the dependent variable. Also, a sensitivity analysis was performed evaluating AID and IMID separately.
Results
The prevalence of symptomatic SARS-CoV-2 infection in a cohort of AI/IMID patients was 1.3%. Eighty-five patients with AI/IMID and symptomatic SARS-CoV-2 were identified, requiring hospitalization in 58 (68%) cases. A total of 175 patients admitted for SARS-CoV-2 (58 with AI/IMID and 117 matched-controls) were analyzed. In logistic regression analysis, a significant inverse association between AI/IMID group and severe SARS-CoV-2 (OR 0.28; 95% CI 0.12–0.61; p = 0.001), need of MV (OR 0.20; IC 95% 0.05–0.71; p = 0.014), and ICU admission (OR 0.25; IC 95% 0.10–0.62; p = 0.003) was found.
Conclusions
Patients with AI/IMID who require admission for SARS-CoV-2 infection have a lower risk of developing severe disease, including the need to stay in the ICU and MV.
Keywords: Autoimmune diseases, Severe acute respiratory syndrome coronavirus 2, COVID-19, Immunosuppression, Adverse outcome
1. Introduction
The first cases of coronavirus disease 2019 (COVID-19) were reported from Wuhan, China in early December 2019 [1], now known to be caused by a novel beta-coronavirus, named as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [2]. COVID-19 has attained a global dimension, having been declared a pandemic by the World Health Organization (WHO) on March 11, 2020 [3].
More than 39 million confirmed cases of SARS-CoV-2 infection and 1 101 298 deaths (mortality rate 2.8%), have been reported worldwide as of October 17, 2020 [4]. Our center admitted the first case of SARS-CoV-2 in the Spanish peninsula at the end of February [5]. At the end of May, around 2500 cases including health workers at the front line had an infection confirmed by positive Real-Time PCR-based Severe Acute Respiratory Syndrome Coronavirus (RT-PCR SARS-CoV-2) [6].
The analysis of patients with COVID-19 allowed the identification of conditions that increase the risk of acquiring the infection, as well as worse outcomes including admission to the intensive care unit (ICU) and death. The most consistent factors associated with poor prognosis include older age, male gender, and preexisting comorbidities (e.g., hypertension, diabetes mellitus, chronic pulmonary disease) [[7], [8], [9]]. Nevertheless, there has not been an adequate representation of patients with autoimmune/immune-mediated inflammatory diseases (AI/IMID) in the first reported cohorts.
Although the presence of comorbidities and immunosuppressive therapies are associated with an increased risk of viral infections, it is not clear whether patients with AI/IMID are more susceptible to SARS-CoV-2 infection, or, when they are infected, whether they have more severe disease or a poorer outcome [[10], [11], [12], [13]]. Therefore, there is an increasing interest regarding SARS-CoV-2 infection in patients with AI/IMID with some discrepancies in different studies about their risk of hospitalization, complications development and death.
The aim of our study was to describe a multidisciplinary cohort of patients with AI/IMID and symptomatic SARS-CoV-2 infection in a single tertiary center and analyze sociodemographic, clinical and therapeutic factors associated with poor outcomes.
2. Methods
2.1. Study population
The study was conducted in a public tertiary hospital, Hospital Clínic de Barcelona (HCB), in Catalonia, Spain. The inclusion criteria were patients ≥18 years, symptomatic SARS-CoV-2 infection (either confirmed by RT-PCR SARS-CoV-2 positive test or probable cases), and a medical diagnosis of at least one well-characterized AI disease (AID) or IMID. Probable case of SARS-CoV-2 infection corresponds to patient that meets the clinical criteria for severe respiratory illness (i.e., temperature of >38 °C, one or more clinical findings of lower respiratory illness, and radiographic evidence of pneumonia, or acute respiratory distress syndrome) as well as the epidemiologic criteria for likely exposure to SARS-CoV-2 (i.e., close contact with a confirmed case of SARS-CoV-2 infection) [14]. The results of RT-PCR SARS-CoV-2 diagnostic assays were obtained from the Microbiology department of HCB.
2.2. Study design
A retrospective observational study was conducted from the 1st of March until May 29th. First, we identified all patients with an underlying AI/IMID and symptomatic SARS-CoV-2 infection. We classified as AID diseases (AID) patients with rheumatoid arthritis (RA), systemic lupus erythematosus, multiple sclerosis, autoimmune hepatitis, autoimmune kidney disease (membranous nephropathy, IgA nephropathy, and other primary nephropathies), myasthenia gravis, systemic vasculitis, Sjögren syndrome, systemic sclerosis, antiphospholipid syndrome, pemphigus, and vitiligo; and as IMID patients with inflammatory bowel disease (IBD, either ulcerative colitis or Crohn's disease), psoriasis, spondyloarthritis (SpA), psoriatic arthritis (PsA), Behçet's disease, sarcoidosis, polymyalgia rheumatica, and seronegative arthritis.
A comparative analysis between admitted patients with AI/IMID and controls (2:1) matched by age (±1 year), and sex without an underlying AI/IMID disease admitted during the same period was conducted. Patients with a previous history of solid or hematological transplantation that were under immunosuppressive treatment were excluded.
2.3. Outcome definition
The primary outcome evaluated was severe SARS-CoV-2 infection, which was a composite endpoint including admission to intensive care unit (ICU), need for mechanical ventilation (MV), development of cardiovascular complications, thrombosis, kidney failure requiring renal replacement therapy, and/or death as previously used in other studies [1,11]. Cardiovascular events included congestive heart failure, new-onset heart arrhythmias, and acute myocardial infarction. Thromboembolic events included venous thromboembolic disease (either deep vein thrombosis or pulmonary embolism), or arterial thrombosis. Severe outcomes including ICU admission, need of MV and death were analyzed separately also as secondary outcomes.
2.4. Variables
The information on patient socio-demographic and cumulative clinical and laboratory data were obtained by electronic medical records review. The data collected from each individual were stored in a secure survey platform (REDCap) hosted by HCB and designed for the current study. The database was audited by two independent reviewers from the research group.
Several sociodemographic and clinical variables were assessed including age, sex, AI/IMID diagnosis, treatment background, comorbidities, smoking status, and need for hospital and ICU admission. The age-adjusted Charlson comorbidity index (ACCI) was calculated for each individual [15] at the time of admission. A high ACCI was defined as a score equal to or greater than 2. The need for hospital admission depended on the criteria established by the hospital protocol. Overall, it depended on the clinical status of the patient (i.e., need for supplementary oxygen, evidence of pneumonia on chest X-ray, presence of serological factors associated with poor prognosis), and their comorbidities, among others. The criteria for judging admission to the ICU depended on the clinical situation of the patient and the therapeutic threshold established by the treating medical team.
All patients were classified based on their treatment background: Prednisone-equivalent glucocorticoid use (1–9 mg/day, ≥ 10 mg/day); conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs; hydroxychloroquine (HCQ), methotrexate, leflunomide, sulfasalazine, azathioprine, mycophenolate mofetil/mycophenolic acid, cyclophosphamide, cyclosporine, and tacrolimus); biologic therapy categorized as: Tumour Necrosis Factor inhibitors (anti-TNF), non-anti-TNF agents (including IL-6, IL-12/IL-23, and IL-17 inhibitors, B-cell-depleting therapy, Vedolizumab, and Natalizumab); and Janus Kinase (JAK) inhibitors (Tofacitinib and Baricitinib). Disease activity prior to SARS-CoV-2 infection was collected and categorized as a binary variable (remission or active) when applied according to the type of AI/IMID.
Variables related with SARS-CoV-2 infection were collected until the end of admission (either discharge or death) in order to describe the progression of the disease and included duration of symptoms before hospital admission, main symptoms, evidence of pneumonia by plain X-ray, pharmacological treatments, duration of hospital stay, and clinical outcomes (including ICU admission, MV and death). The clinical status was determined according to a 7-category ordinal scale that ranges from 1 (discharged with normal activity) to 7 (death), which has been used as the primary endpoint in several clinical trials including COVID-19 patients [[16], [17], [18], [19]]. The highest value of the scale reached during admission was recorded. This ordinal scale was also grouped into two categories. Non-severe corresponds to categories 2 and 3 (no oxygen requirement or low requirement) and the severe corresponds to categories 4 to 7 (high oxygen requirement, need for additional organic support and death).
Laboratory data were collected at baseline and during follow-up. The cut-off point of normality of the HCB laboratory was taken into account in order to classify abnormal values: C-reactive protein (CRP, ≥ 1 mg/dL), ferritin (>400 ng/mL), D-dimer (>500 ng/mL), lactate dehydrogenase (LDH, > 234 U/L), lymphopenia (<900 109/L), and procalcitonin (PCT, > 0.50 ng/mL). Hyperinflammation score was defined as lymphocyte counts lower than 1000 cells per mL, and 2 of the following three criteria: serum ferritin higher than 500 ng/mL, LDH higher than 300 U/L, and D-dimer higher than 1000 ng/mL. The cutoffs had been defined in the context of an ongoing clinical trial that is exploring the efficacy and safety of targeted therapies in reducing hyperinflammation in patients with SARS-CoV-2 [20], and included in two recently published studies [21,22]. Finally, we registered AI/IMID flares according to clinician's opinion, either during admission or after discharge as well as immunosuppressive treatment interruption due to SARS-CoV-2 infection.
2.5. Statistical analysis
Continuous variables are reported as median and interquartile range (IQR). Categorical variables are reported as count and percentage (%). In bivariate analyses, differences in demographic, clinical and therapeutic factors according to severe SARS-CoV-2 outcome were compared using chi square tests or Fisher's exact tests for categorical variables and Mann-Whitney U tests for ordinal or continuous variables.
Multivariate logistic regression models (adjusted by age and comorbidities) were run in a stepwise manner (backward elimination) having severe SARS-CoV-2 as the dependent variable using the likelihood ratio test with a p value > 0.1. Additionally, other models with secondary outcomes (ICU admission, need of MV and death) were analyzed using the same approach. Odds ratios (OR) were calculated with 95% confidence intervals (CI). P-values less than 0.05 were considered significant. Statistical analyses were done by SPSS, v.22, Chicago, IL, USA).
To assess the impact of AID and IMID categories in primary and secondary outcomes, a sensitivity analyses were performed. Additionally, a stratified analysis was conducted including only PCR-confirmed cases. Odds ratios (OR) were calculated with 95% confidence intervals (CI). P-values less than 0.05 were considered significant. Statistical analyses were done by SPSS, v.22, Chicago, IL, USA).
2. 6 Ethical considerations
The study was conducted in accordance with the Declaration of Helsinki and was approved by the local Ethical Committee (HCB/2020/0582).
3. Results
3.1. Baseline characteristics of AI/IMID cohort
More than 6100 patients with AI/IMID are under follow-up in our institution. During the study period, 989 patients with SARS-CoV-2 infection were admitted to our hospital. Fig. 1 shows a flowchart for selection of cases and matched controls in the present study. Eighty-five patients with AI/IMID and symptomatic SARS-CoV-2 were identified (prevalence of 1.3%), and 58 (68%) of them required hospitalization. Eight (9%) patients had more than one underlying disease. Therefore, there were a total of 93 AI/IMID diagnoses (see Supplementary Table A1). The proportion of patients with AID (57%) or IMID (43%) were similar. IBD, RA and psoriasis were the most common diseases in 14%, 11%, and 9% of patients, respectively.
Fig. 1.
Flowchart for selection of patients for matched-cohort study.
The median duration of the AI/IMID was 8 years (IQR 4–16). Disease activity was evaluated at the moment of admission in 62 patients with an AID or IMID with a clinical course characterized by flares or remission. Most cases were in remission prior to SARS-CoV-2 infection (45/62, 72%). Regarding baseline immunomodulatory treatment for AI/IMID, most patients were on glucocorticoids (29%, median prednisone-equivalent dose of 5 mg), followed by csDMARDs (31%, mainly methotrexate), and targeted therapies (mostly non-anti-TNF agents). During admission, some patients stopped their background therapies following onset of SARS-CoV-2 symptoms: glucocorticoids in 1/19 (5%), immunosuppressive therapies in 6/17 (35%), and targeted therapies in 1/3 (33%) cases, but treatments were resumed after hospital discharge in the majority of them. Nine (10%) patients presented a clinical flare (5 during admission and 4 patients within one month after discharge). There were no differences regarding immunomodulatory treatment between AID and IMID categories: prednisone-equivalent doses per day (8 mg vs 5.6 mg; p = 0.642), csDMARDs (34% vs 22%; p = 0.212), or targeted therapies (14% vs 25%; p = 0.212).
3.2. Hospital admission
In patients with AI/IMID, the comparison of hospitalized and non-hospitalized cases demonstrated a number of significant differences. Most hospitalized patients had hypertension (50 vs 18%; p = 0.08); likewise, most ambulatory patients were female (81 vs 48%; p = 0.006), had IBD (29 vs 8%; p = 0.018), and received non-anti-TNF anti-Cytokine therapy in monotherapy at baseline (11 vs 1%; p = 0.012). General characteristics of symptomatic SARS-CoV-2 patients with AI/IMID stratified by hospitalization status are summarized in Supplementary Table A2.
A total of 175 patients admitted for SARS-CoV-2 (58 with AI/IMID and 117 matched-controls) were included. In general terms, we had a well-balanced comparison between groups (AI/IMID vs non-AI/IMID) including demographics characteristics, comorbidities, and duration of hospital stay. As expected, there were more prevalence of glucocorticoids (32% vs 1%; p = 0.000), and HCQ (8% vs 0%; p = 0.008) use before SARS-CoV-2 infection in AI/IMID cohort. Only 2 patients in the non-AI/IMID cohort were taking glucocorticoids (prednisone-equivalent median dose 5 mg/day). Just minor differences in general COVID-19 symptoms were found among groups. Specific treatment for SARS-CoV-2 including antiviral therapies and anti-IL-6 agents were more common used in controls. General characteristics of admitted SARS-CoV-2 patients are summarized in Table 1 .
Table 1.
Baseline demographics and clinical characteristics and treatment of admitted SARS-CoV-2 patients.
| Variable | Total N = 175 n (%) |
AI/IMID (n = 58) | Non-AI/IMID (n = 117) | P value |
|---|---|---|---|---|
| Male gender | 90 (51.4) | 30 (51.7) | 60 (51.3) | 0.956 |
| Age, median (IQR) years ≤65 years |
61 (50–70) 109 (62.3) |
61 (48–71) 36 (62.1) |
62 (51–69) 73 (62.4) |
0.612 0.967 |
| PCR-confirmed SARS-CoV-2 infection | 137 (78.3) | 41 (70.7) | 96 (82.1) | 0.086 |
| Pneumonia at admission | 144 (82.3) | 42 (72.4) | 102 (87.2) | 0.016 |
| Duration of hospital stay, days | 10 (6–18) | 8 (5–14) | 10 (6–20) | 0.215 |
| Most common comorbidities | 142 (81.1) | 50 (86.2) | 92 (78.6) | 0.228 |
| Hypertension | 79 (45.1) | 29 (50.0) | 50 (42.7) | 0.363 |
| Dyslipidemia | 46 (26.3) | 11 (19.0) | 35 (29.9) | 0.121 |
| Chronic pulmonary diseasea | 44 (25.1) | 12 (20.7) | 32 (27.4) | 0.339 |
| Charlson comorbidity index (high ≥2) | 130 (74.3) | 39 (67.2) | 91 (77.8) | 0.133 |
| Ever smoking | 54 (30.8) | 16 (27.6) | 38 (32.4) | 0.525 |
| Baseline treatment | ||||
| ACE inhibitor or ARB | 51 (29.1) | 16 (27.6) | 35 (29.9) | 0.259 |
| Statins | 44 (25.1) | 12 (20.7) | 32 (27.4) | 0.339 |
| Glucocorticoids | 21 (12.0) | 19 (32.8) | 2 (1.7) | 0.000 |
| Hydroxychloroquine | 5 (2.8) | 5 (8.6) | 0 (0.0) | 0.008 |
| Main SARS-CoV-2 symptoms | ||||
| Duration of symptoms before hospital admission, days | 7 (4–9) | 6 (4–9) | 7 (4–10) | 0.374 |
| Fever | 138 (78.9) | 39 (67.2) | 99 (84.6) | 0.008 |
| Cough | 125 (71.4) | 37 (63.8) | 88 (75.2) | 0.115 |
| Dyspnea | 85 (48.6) | 20 (34.5) | 65 (55.6) | 0.009 |
| Prognostic factorsb | ||||
| CRP, ≥ 1 mg/dL (n = 157) | 147 (84.0) | 47 (81.0) | 100 (85.5) | 0.451 |
| Ferritin, > 400 ng/mL (n = 81) | 42 (24.0) | 12 (20.7) | 30 (25.6) | 0.470 |
| D-dimer, > 500 ng/mL (n = 126) | 75 (42.9) | 25 (43.1) | 50 (42.7) | 0.963 |
| LDH, > 234 U/L (n = 136) | 116 (66.3) | 35 (60.3) | 81 (69.2) | 0.242 |
| Lymphopenia, < 900 109/L (n = 159) | 78 (44.6) | 24 (41.4) | 54 (46.2) | 0.550 |
| Hyperinflammation score | 29 (16.6) | 10 (17.2) | 19 (16.2) | 0.867 |
| SARS-CoV-2 treatment | ||||
| Lopinavir/ritonavir | 144 (82.3) | 38 (65.5) | 106 (90.6) | 0.000 |
| Hydroxychloroquine | 157 (89.7) | 43 (74.1) | 114 (97.4) | 0.000 |
| Azithromycin | 148 (84.6) | 42 (72.4) | 106 (90.6) | 0.002 |
| Remdesivir | 17 (9.7) | 3 (5.2) | 14 (12.0) | 0.153 |
| High-dose glucocorticoids | 75 (42.9) | 21 (36.2) | 54 (46.2) | 0.211 |
| Anti-IL-6c | 74 (42.3) | 18 (35.0) | 56 (47.9) | 0.034 |
| Anakinra | 10 (5.7) | 4 (6.9) | 6 (5.1) | 0.635 |
| Baricitinib | 1 (0.6) | 0 (0.0) | 1 (0.9) | 0.480 |
AI/IMID: Autoimmune or immune-mediated inflammatory disease; ACE: angiotensin-converting enzyme; ARB: angiotensin receptor blocker; CRP: C-reactive protein; IQR: Interquartile range; LDH: Lactate dehydrogenase.
Chronic obstructive pulmonary disease, Asthma, Bronchiectasis and/or Sleep apnea.
Proportion of patients with abnormal values.
Anti-IL-6 include (n): Tocilizumab (63), Siltuximab (11), and Sarilumab (1).
3.3. Complications and severe SARS-CoV-2 infection
Nearly half of the patients developed complications during hospitalization, the most frequent being acute respiratory distress syndrome (ARDS). Main complications during hospitalization of SARS-CoV-2 patients are shown in Table 2 . Regarding severe outcomes, significant differences were found between groups (AI/IMID vs non-AI/IMID): Severe SARS-CoV-2 (20% vs 44%; p = 0.002), ICU admission (12% vs 35%; p = 0.003), ARDS (12% vs 44%; p = 0.000), MV (5% vs 20%; p = 0.008), and need of prone ventilation (0% vs 7%; p = 0.003). Of the total of 59 patients who developed ARDS, 25 were admitted to the ICU and required MV, 23 needed high-flow oxygen, and 8 non-invasive ventilation. A total of 13 patients died during admission (AI/IMID 4, 6.9%; Controls 9, 7.7%), being the majority older than 65 years and all having at least one comorbidity. The main cause of death corresponds to SARS-CoV-2-associated respiratory insufficiency. A thrombotic event during hospital admission occurred in 7% of cases but none of these patients had positivity for antiphospholipid antibodies.
Table 2.
Complications during hospitalization of SARS-CoV-2 patients.
| Variable | Total (N = 175) n (%) | AI/IMID (n = 58) | Non-AI/IMID (n = 117) | P value |
|---|---|---|---|---|
| Severe SARS-CoV-2a | 64 (36.6) | 12 (20.7) | 52 (44.4) | 0.002 |
| ARDS | 59 (33.7) | 7 (12.1) | 52 (44.4) | 0.000 |
| ICU admission | 50 (28.6) | 8 (13.8) | 42 (35.9) | 0.003 |
| Invasive mechanical ventilation | 29 (16.6) | 4 (6.7) | 25 (21.3) | 0.008 |
| Non-invasive ventilation | 10 (5.7) | 4 (6.9) | 6 (5.1) | 0.635 |
| High-flow oxygen | 29 (16.6) | 2 (3.4) | 27 (23.1) | 0.119 |
| Prone ventilation | 9 (5.1) | 0 (0.0) | 9 (7.7) | 0.003 |
| Organizing Pneumonia | 32 (18.3) | 6 (10.3) | 26 (22.2) | 0.056 |
| ECMO | 2 (1.1) | 1 (1.7) | 1 (0.9) | 0.317 |
| Vasoactive/inotropic drugs | 11 (6.3) | 4 (6.9) | 7 (6.0) | 0.080 |
| Hospital-acquired infection | 21 (12.0) | 3 (5.2) | 18 (15.4) | 0.050 |
| Acute kidney injury Without hemodialysis/hemofiltration With hemodialysis/hemofiltration |
17 (9.7) 4 (2.3) |
3 (5.2) 1 (1.7) |
14 (12.0) 3 (2.6) |
0.153 0.726 |
| Thromboembolic eventb | 13 (7.4) | 2 (3.4) | 11 (9.4) | 0.157 |
| Cardiovascular eventc | 11 (6.3) | 3 (5.2) | 8 (6.8) | 0.669 |
| Hemorrhagic complications | 8 (4.6) | 3 (5.2) | 5 (4.3) | 0.789 |
| Mortality | 13 (7.4) | 4 (6.9) | 9 (7.7) | 0.850 |
AI/IMID: autoimmune or immune-mediated inflammatory disease; ARDS: Acute Respiratory Distress Syndrome; ECMO: Extracorporeal membrane oxygenation; ICU: Intensive care unit.
Defined as: intensive care unit admission, intratracheal intubation, cardiovascular complications (heart failure or myocarditis), thrombosis, kidney failure requiring hemodialysis, and death.
Venous thromboembolic disease (either deep vein thrombosis or pulmonary embolism), or arterial thrombosis.
Include congestive heart failure, new-onset heart arrhythmias, and acute myocardial infarction.
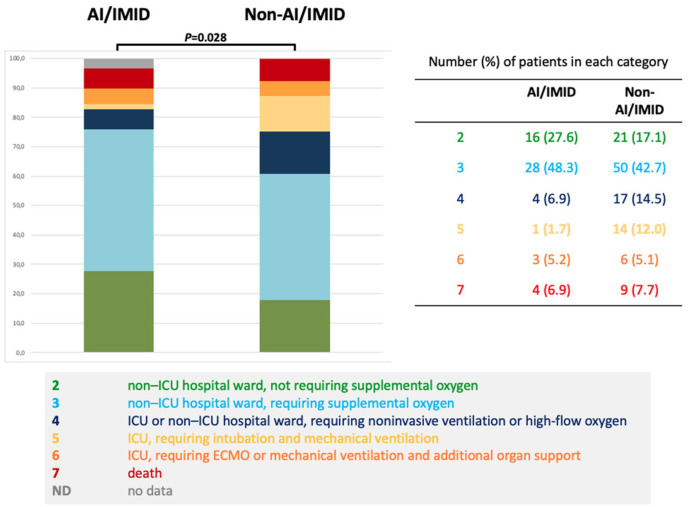
There were statistically significant differences in the 7-category ordinal scale between AI/IMID and non-AI/IMID groups (3.00 [IQR 2.00–3.00] vs 3.00 [IQR 3.00–4.00]; p = 0.028), AUC = 0.6 95% CI 0.51–0.68. The non-severe subgroup (i.e., 2 and 3 categories) was statistically significantly more common in the AI/IMID compared to the non-AI/IMID group (OR 2.35; 95% CI 1.14–5.11; p = 0.003). For details see Fig. 2 .
Fig. 2.
Highest value of clinical status using 7-category ordinal scale during admission.
The results of the bivariable analysis by severe SARS-CoV-2 status are shown in Supplementary Table A3. Older age (67 [IQR 56–73] vs 59 [IQR 49–67]; p = 0.002), and certain comorbidities including hypertension (OR 2.49; 95% CI 1.32–4.68; p = 0.004), and dyslipidemia (OR 2.42; 95% CI 1.21–4.83; p = 0.012), were associated with a greater risk of severe SARS-CoV-2. Patients with severe SARS-CoV-2 had higher baseline biomarkers than patients with non-severe disease, including CRP (10 [IQR 6–16] vs 6 [IQR 3–9]; p = 0.000), serum ferritin (998 [IQR 421–2495] vs 357 [IQR 172–1015]; p = 0.001), and LDH (419 [IQR 292–501] vs 280 [IQR 244–384]; p = 0.001). Additionally, those with severe phenotype had lower lymphocyte count (800 [IQR 600–900] vs 1000 [IQR 700–1200]; p = 0.000) than non-severe group.
Compared with non-severe SARS-CoV-2, those with severe phenotype were more likely to received treatment with high-dose glucocorticoids (OR 5.67; 95% CI 2.90–11.11; p = 0.000), and Tocilizumab (OR 2.58; 95% CI 1.35–4.90; p = 0.004) for systemic inflammatory response due to COVID-19. Patients with AI/IMID had a significant lower proportion of severe infection (OR 0.32; 95% CI 0.15–0.67; p = 0.003). There were no differences in gender, the ACCI, and the presence of hyperinflammation comparing AI/IMID vs non-AI/IMID.
A significant inverse association between AI/IMID group and severe SARS-CoV-2 (OR 0.28; 95% CI 0.12–0.61; p = 0.001), MV (OR 0.20; IC 95% 0.05–0.71; p = 0.014), and ICU admission (OR 0.25; IC 95% 0.10–0.62; p = 0.003) was found in the multivariable model (Table 3 ).
Table 3.
Multivariate logistic analysis for Severe SARS-CoV-2 and secondary outcomes.
| Outcome Variable |
Adjusted Odds ratio (95% confidence interval) |
|||
|---|---|---|---|---|
| Severe SARS-CoV-2 (n = 64) | ICU admission (n = 49) | MV (n = 27) | Mortality (n = 13) | |
| Age | 1.02 (0.99–1.06) | 0.99 (0.95–1.02) | 0.99 (0.95–1.04) | 1.07 (0.98–1.16) |
| AI/IMID | 0.28 (0.12–0.61)* | 0.25 (0.10–0.62)* | 0.20 (0.05–0.71)* | 0.75 (0.18–3.16) |
| Hypertension | 1.77 (0.80–3.94) | 1.48 (0.63–3.49) | 2.92 (0.99–8.57) | 8.67 (0.93–80.64) |
| CHD | 1.64 (0.66–4.02) | |||
| Dyslipidemia | 1.58 (0.70–3.57) | 1.33 (0.50–3.55) | 1.41 (0.37–5.42) | |
| COPD | 1.78 (0.57–5.51) | 1.34 (0.38–4.73) | ||
| Diabetes | 1.25 (0.33–4.74) | |||
*P < 0.005.
AI/IMID: autoimmune or immune-mediated inflammatory disease; CHD: chronic heart disease; COPD: chronic obstructive pulmonary disease; GC: glucocorticoids; ICU: intensive care unit; MV: mechanical ventilation.
A sensitivity analysis evaluating our primary outcome in AID and IMID separately, showed an attenuation of the effect but towards to the same direction of association that the main analysis for primary outcome (AID OR 0.36; 95% CI 0.14–0.89; p = 0.028 - IMID OR 0.31; 95% CI 0.09–0.99; p = 0.05). The stratified analysis limited to PCR-confirmed cases shows similar results (OR 0.33; 95% CI 0.14–0.79; p = 0.013).
4. Discussion
This single-center study reports factors associated with poor prognosis, clinical features related to hospital admission, and the prevalence of symptomatic SARS-CoV-2 infection in a wide spectrum of AID and IMID. Our study found that patients with AI/IMID who required admission for SARS-CoV-2 infection had a lower risk of developing severe disease, including the need to stay in the ICU and MV. However, no differences with mortality were found among groups. Different from other similar cohorts, we had a low mortality rate in the whole group due to severe SARS-CoV-2 infection [11,12,21,[23], [24], [25], [26]].
Some reports from Italy described only a few cases of COVID-19 infection in patients with inflammatory rheumatic diseases, in most cases with favorable outcomes [27,28]. In a German study, patients with inflammatory rheumatic diseases did not have a more severe course of SARS-CoV-2 infection [29]. In a population with sustained immunosuppressive treatment such as liver transplantation standardized mortality rates were similar to general matched population. Only those patients with mycophenolate doses greater than 1000 mg/day had a higher risk of severe SARS-CoV-2 infection [30].
Compared with the control group, patients with AI/IMID had a significantly lower risk to meet our primary outcome (severe COVID-19), but not other surrogates of severe infection. We hypothesize that the protective effect of AI/IMID may be related to several aspects: 1) a potential protective benefit of chronic use of immunomodulatory agents and low doses of glucocorticoids; 2) possible immunomodulatory effects of Vitamin D taking into account that a large proportion of these patients take continuous Vitamin D supplements (despite we were unable to quantified this factor) [31,32]; 3) a better baseline vaccination status preventing superinfections [33]; and, 4) a tighter control during hospitalization from health workers due to their baseline condition.
In the current study, 58 (68%) out of 85 patients required hospitalization due to SARS-CoV-2 infection. Different reports from Spain, informed a rate of admission between 44% and 71% [11,12,24]. Contrarily to our results where no differences were observed, one study [12] showed that patients with an autoimmune systemic condition have a higher risk of hospital admission related to COVID-19 compared with those with chronic inflammatory arthritis. These results must be interpreted with caution because there is no standard classification of AI/IMID diagnosis in different published studies and no homogeneous criteria for admission are used in different centers.
We observed several differences by gender, primary AI/IMID diagnosis, comorbidities, and baseline treatment according to hospital admission status. Among all the identified AI/IMID diagnoses, only IBD showed a lower proportion (5/13, 38%) of hospital admission. Similar results were found in a large cohort of 6000 IBD patients from France and Italy [34]. A study including 40 patients with IBD and a positive test for SARS-CoV-2 in the Basque Country (Spain) showed that although half of them required hospital admission, none were admitted to the ICU or required MV [35].
In the present cohort, patients receiving non-anti-TNF anti-cytokine therapy in monotherapy (i.e., IL-6, IL-12/IL-23, and IL-17 inhibitors) at baseline were less likely to require hospitalization. Recent data suggest that patients with IMID receiving cytokine inhibitors may not have an enhanced but at a lower risk for SARS-CoV-2 infection compared with the general population and IMID patients not receiving such drugs [36]. Our data show that baseline anti-TNF treatment had no impact on the need for hospital admission in patients with AI/IMID.
In a cohort-based on medical records from a health insurance in Detroit, USA, patients with IMID were analyzed according to COVID-19 status, admission rates, need for MV and dead. Those patients under glucocorticoid treatment had a 5-fold higher risk of admission, while patients under biologic DMARDs and specifically, those receiving anti-TNF had lower rates of admission [37]. In a national registry for patients with inflammatory rheumatic diseases in Germany, hospitalized patients were more often treated with glucocorticoids while bDMARDs were used less often [38]. A cohort from New York [39] including patients with inflammatory arthritis reported higher admission rates in patients receiving glucocorticoids after adjusting for body mass index and comorbidities. No differences were found for patients under anti-TNF therapies or IL-17 blockers. By the other hand, in general population the RECOVERY trial has shown that treatment with dexamethasone at a dose of 6 mg once daily for up to 10 days reduces 28-day mortality in patients with COVID-19 who are receiving respiratory support [40].
The prevalence of symptomatic SARS-CoV-2 infection in this cohort of patients with AI/IMID was low (1.3%). The risk of SARS-CoV-2 infection in patients with AI/IMID remains unclear. A case-control study from Milano [23] reported a negative association between AID and positivity for COVID-19 test. Some studies suggested a higher risk in patients on immunosuppressive or immuno-modulatory treatment [41], while other studies go in the opposite direction [42]. A recent systematic review [43] demonstrated a higher prevalence of COVID-19 in patients with AID (0.011; 95% CI 0.005–0.025) relative to a reference population. The meta-analysis demonstrated that the risk of COVID-19 in AID was significantly higher than in control patients (OR 2.19; 95% CI 1.05–4.58; p = 0.038). Meta-regression analysis showed glucocorticoids were significantly associated with the risk of COVID-19. Glucocorticoids, csDMARDs and biologic/targeted synthetic DMARDs–csDMARDs combination therapy increased the risk of severe outcomes, whereas anti-TNF agents in monotherapy were associated with a lower risk of hospitalization and death.
National health authorities reported a prevalence of SARS-CoV-2 infection around 7% of the general population in Barcelona province during the first wave [44]. A study focused on AI/IMID in Spain reported a slightly higher prevalence (0.76% vs 0.58%) of hospital PCR-confirmed COVID-19 cases than in the general population, especially in patients under targeted therapies and patients with systemic disease [45]. In another referral center in Catalonia, only 11 out of 959 patients (1.1%) with rheumatic diseases who completed a telephonic survey were confirmed as SARS-CoV-2 positive cases [46]. The incidence rates of the rheumatic patients in this cohort were very similar to that of the general population (0.48% vs 0.58%) [46]. Since many patients with AI/IMID remained confined during the COVID-19 outbreak, a protective effect related to self-care and distancing may explain this lower prevalence respect to the general population [13,47].
Several findings reported in this work were in agreement with the observations of the largest collection of COVID-19 cases among patients with rheumatic diseases, the COVID-19 Global Rheumatology Alliance (C19-GRA) [48]. The C19-GRA has collected more than 2000 patients as of September 2020 worldwide. The last report containing data for 600 patients from 40 countries [10]. Nearly half of the cases were hospitalized (46%) and 55 (9%) died. After multivariable analysis, comorbidities including hypertension were associated with higher odds of hospitalization. On the other hand, treatment with any anti-TNF agent in monotherapy just prior to COVID-19 diagnosis was significantly associated with lower odds of hospitalization compared with no DMARD therapy.
Information about the impact of SARS-CoV-2 infection over clinical disease activity is limited. A minor proportion of patients included in our study had a flare either during SARS-CoV-2 infection or 1 month later. In most patients, the disease flare was not related to the discontinuation of baseline immunosuppressive treatment. Identification of flares is not a simple task after COVID-19 infection since a significant number of patients persist with symptoms even after 60 days of initiation of infection, including fatigue, dyspnea, joint and chest pain [49].
Our study has some limitations. Since we included a heterogeneous group of AI/IMID and targeted therapies, an individual effect by class or by therapy is difficult to assess. Another limitation of our data is the fact that we were only able to capture symptomatic patients given the restrictions of local health authorities to test for SARS-CoV-2 infection in asymptomatic patients during the first outbreak. Additionally, we did not account for obesity as co-variate given a lot of cases with missing data. However, the present work also has remarkable strengths including a well-balanced 1:2 control group with a database designed for the same purpose, identification of disease flare during or after SARS-CoV-2 infection, a broad spectrum of AI/IMID analyzed, and the analysis of different severity tools for SARS-CoV-2 infection including hyperinflammation score and the 7-category ordinal scale.
5. Conclusions
Most of the information coming from referral centers reporting series of patients (including our current series) are toward the direction that patients with AI/IMID are not at a higher risk of SARS-CoV-2 infection or are more susceptible to suffer severe disease. The prevalence of symptomatic SARS-CoV-2 infection in patients with AI/IMID is low. Some sociodemographic factors, certain comorbidities, and the use of targeted anti-cytokine therapies have an impact on hospitalization. Patients with AI/IMID who require hospital admission for SARS-CoV-2 infection have a lower risk of developing severe disease, including the need for admission to the ICU and MV. Many questions remain unresolved, including the real protective effect of a sustained immunosuppressive status over inflammatory response secondary to SARS-CoV-2 infection as well as the long-term effect over disease activity during disease course of patients with an underlying AI/IMID. Long term follow-up, information from second waves, and bigger data will provide us with more light to optimize the approach to this type of patients.
Declaration of competing interest
None declared.
Acknowledgements
Research supported by Crowfunding from Hospital Clínic and IDIBAPS.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jaut.2020.102580.
AI/IMID: Autoimmune or immune-mediated inflammatory disease.
AI/IMID: autoimmune or immune-mediated inflammatory disease; ECMO: extracorporeal membrane oxygenation; ICU: intensive care unit.
Author statement
Juan C. Sarmiento-Monroy Conceptualization, Methodology, editing, Formal analysis and writing draft and original, Gerard Espinosa: Conceptualization, Methodology and data collection. Editing and supervision, Maria-Carlota Londoño: Conceptualization, Methodology, Formal analysis, editing and writing draft, Fernanda Meira Conceptualization, Methodology and software support, Berta Caballol: Data collection and writing draft, Sara Llufriu: Conceptualization, Methodology and data collection, Josep Lluis Carrasco: Methodology and formal analysis, Aina Moll-Udina: Data collection and writing draft, Luis F. Quintana: Data collection and writing draft, Priscila Giavedoni: Data collection and writing draft, Julio Ramírez: Data collection, editing and writing draft, Jose Inciarte-Mundo: Data collection and writing draft, Elisabeth Solana: Software support and analysis, Yolanda Blanco: Data collection and writing draft, Eugenia Martinez-Hernandez: Data collection and writing draft, Maria Sepúlveda: Data collection and writing draft, Victor Llorenç;: Data collection and writing draft, Sergio Prieto-González: Data collection and writing draft, Georgina Espígol-Frigolé: Data collection and writing draft, Jose C. Milisenda: Data collection and writing draft, Maria C Cid: Conceptualization, Supervision and writing draft, Jose M. Mascaró; Jr: Conceptualization, Supervision and writing draft, Isabel Blanco: Data collection and writing draft, Joan Albert Barberá;: Conceptualization, Methodology and supervision, Oriol Sibila: Conceptualization, Methodology and data collection, Jordi Gratacos-Ginès: Data collection and writing draft, Alfredo Adán: Conceptualization, Methodology and supervision, Alvaro Agustí;: Conceptualization, Methodology, editing and supervision, Raimon Sanmartí;: Conceptualization and Supervision, Julian Panés: Conceptualization, Methodology and supervision, Ricard Cervera: Conceptualization, editing, Methodology and supervision, Jordi Vila: Conceptualization, Methodology and supervision, Alex Soriano: Conceptualization, Methodology, Supervision and funding acquisition, José A. Gómez-Puerta: Conceptualization, Methodology, data collection, formal analysis, writing original and review, editing and supervision.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Guan W.-J., Ni Z.-Y., Hu Y.H.Y., Liang W.-H., Ou C.-Q., He J.-X., Liu L., Shan H., Lei C.-L., Hui D.S., Du B., Li L.-J., Zeng G., Yuen K.-Y.Y., Chen R.-C., Tang C.-L., Wang T., Chen P.-Y., Xiang J., Li S.-Y., Wang J.-M.M., Liang Z.-J., Peng Y.-X., Wei L., Liu Y., Hu Y.-H., Peng P., Wang J.-L., Liu J.-Y., Chen Z., Li G., Zheng Z.-J., Qiu S.-Q., Luo J., Ye C.-J., Zhu S.-Y., Zhong N.-S. China medical treatment expert group for covid-19, clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhimraj A., Morgan R.L., Shumaker A.H., Lavergne V., Baden L., Cheng V.C.-C., Edwards K.M., Gandhi R., Muller W.J., O'Horo J.C., Shoham S., Murad M.H., Mustafa R.A., Sultan S., Falck-Ytter Y. Infectious diseases society of America guidelines on the treatment and management of patients with COVID-19. Clin. Infect. Dis. 2020:1–32. doi: 10.1093/cid/ciaa478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020;109:102433. doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization WHO coronavirus disease (COVID-19) dashboard. https://covid19.who.int/ (n.d.)
- 5.Nacional de Epidemiología Centro, de Sanidad Ministerior, de España Gobierno. COVID-19 en España. https://cnecovid.isciii.es/covid19/ (n.d.)
- 6.Pericàs J.M., Hernandez-Meneses M., Sheahan T.P., Quintana E., Ambrosioni J., Sandoval E., Falces C., Marcos M.A., Tuset M., Vilella A., Moreno A., Miro J.M. Hospital clínic cardiovascular infections study group, COVID-19: from epidemiology to treatment. Eur. Heart J. 2020;41:2092–2112. doi: 10.1093/eurheartj/ehaa462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet (London, England) 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cummings M.J., Baldwin M.R., Abrams D., Jacobson S.D., Meyer B.J., Balough E.M., Aaron J.G., Claassen J., Rabbani L.E., Hastie J., Hochman B.R., Salazar-Schicchi J., Yip N.H., Brodie D., O'Donnell M.R. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet (London, England) 2020;395:1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun Y., Dong Y., Wang L., Xie H., Li B., Chang C., sheng Wang F. Characteristics and prognostic factors of disease severity in patients with COVID-19: the Beijing experience. J. Autoimmun. 2020;112:102473. doi: 10.1016/j.jaut.2020.102473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gianfrancesco M., Hyrich K.L., Hyrich K.L., Al-Adely S., Al-Adely S., Carmona L., Danila M.I., Gossec L., Gossec L., Izadi Z., Jacobsohn L., Katz P., Lawson-Tovey S., Lawson-Tovey S., Mateus E.F., Rush S., Schmajuk G., Simard J., Strangfeld A., Trupin L., Wysham K.D., Bhana S., Costello W., Grainger R., Hausmann J.S., Hausmann J.S., Liew J.W., Sirotich E., Sirotich E., Sufka P., Wallace Z.S., Wallace Z.S., Yazdany J., MacHado P.M., MacHado P.M., MacHado P.M., Robinson P.C., Robinson P.C. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann. Rheum. Dis. 2020;79:859–866. doi: 10.1136/annrheumdis-2020-217871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pablos J.L., Galindo M., Carmona L., Lledó A., Retuerto M., Blanco R., Gonzalez-Gay M.A., Martinez-Lopez D., Castrejón I., Alvaro-Gracia J.M., Fernández Fernández D., Mera-Varela A., Manrique-Arija S., Mena Vázquez N., Fernandez-Nebro A. Clinical outcomes of hospitalised patients with COVID-19 and chronic inflammatory and autoimmune rheumatic diseases: a multicentric matched cohort study. Ann. Rheum. Dis. 2020 doi: 10.1136/annrheumdis-2020-218296. annrheumdis-2020-218296. [DOI] [PubMed] [Google Scholar]
- 12.Freites Nuñez D.D., Leon L., Mucientes A., Rodriguez-Rodriguez L., Font Urgelles J., Madrid García A., Colomer J.I., Jover J.A., Fernandez-Gutierrez B., Abasolo L. Risk factors for hospital admissions related to COVID-19 in patients with autoimmune inflammatory rheumatic diseases. Ann. Rheum. Dis. 2020:1–7. doi: 10.1136/annrheumdis-2020-217984. [DOI] [PubMed] [Google Scholar]
- 13.Zen M., Fuzzi E., Astorri D., Saccon F., Padoan R., Ienna L., Cozzi G., Depascale R., Zanatta E., Gasparotto M., Benvenuti F., Bindoli S., Gatto M., Felicetti M., Ortolan A., Campaniello D., Larosa M., Lorenzin M., Ramonda R., Sfriso P., Schiavon F., Iaccarino L., Doria A. SARS-CoV-2 infection in patients with autoimmune rheumatic diseases in northeast Italy: a cross-sectional study on 916 patients. J. Autoimmun. 2020;112:102502. doi: 10.1016/j.jaut.2020.102502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention . SARS; 2020. Public Health Guidance for Community-Level Preparedness and Response to Severe Acute Respiratory Syndrome.https://www.cdc.gov/sars/guidance/b-surveillance/app1.html [Google Scholar]
- 15.Charlson M.E., Charlson R.E., Peterson J.C., Marinopoulos S.S., Briggs W.M., Hollenberg J.P. The Charlson comorbidity index is adapted to predict costs of chronic disease in primary care patients. J. Clin. Epidemiol. 2008;61:1234–1240. doi: 10.1016/j.jclinepi.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Rosas I., Bräu N., Waters M., Go R.C., Hunter B.D., Bhagani S., Skiest D., Aziz M.S., Cooper N., Douglas I.S., Savic S., Youngstein T., Del Sorbo L., Cubillo Gracian A., De La Zerda D.J., Ustian A., Malhotra A. 2020. Tocilizumab in Hospitalized Patients with COVID-19 Pneumonia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., Ruan L., Song B., Cai Y., Wei M., Li X., Xia J., Chen N., Xiang J., Yu T., Bai T., Xie X., Zhang L., Li C., Yuan Y., Chen H., Li H., Huang H., Tu S., Gong F., Liu Y., Wei Y., Dong C., Zhou F., Gu X., Xu J., Liu Z., Zhang Y., Li H., Shang L., Wang K., Li K., Zhou X., Dong X., Qu Z., Lu S., Hu X., Ruan S., Luo S., Wu J., Peng L., Cheng F., Pan L., Zou J., Jia C., Wang J., Liu X., Wang S., Wu X., Ge Q., He J., Zhan H., Qiu F., Guo L., Huang C., Jaki T., Hayden F.G., Horby P.W., Zhang D., Wang C. A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19. N. Engl. J. Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deftereos S.G., Giannopoulos G., Vrachatis D.A., Siasos G.D., Giotaki S.G., Gargalianos P., Metallidis S., Sianos G., Baltagiannis S., Panagopoulos P., Dolianitis K., Randou E., Syrigos K., Kotanidou A., Koulouris N.G., Milionis H., Sipsas N., Gogos C., Tsoukalas G., Olympios C.D., Tsagalou E., Migdalis I., Gerakari S., Angelidis C., Alexopoulos D., Davlouros P., Hahalis G., Kanonidis I., Katritsis D., Kolettis T., Manolis A.S., Michalis L., Naka K.K., Pyrgakis V.N., Toutouzas K.P., Triposkiadis F., Tsioufis K., Vavouranakis E., Martinèz-Dolz L., Reimers B., Stefanini G.G., Cleman M., Goudevenos J., Tsiodras S., Tousoulis D., Iliodromitis E., Mehran R., Dangas G., Stefanadis C. Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: the GRECCO-19 randomized clinical trial. JAMA Netw. Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.13136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Somers E.C., Eschenauer G.A., Troost J.P., Golob J.L., Gandhi T.N., Wang L., Zhou N., Petty L.A., Baang J.H., Dillman N.O., Frame D., Gregg K.S., Kaul D.R., Nagel J., Patel T.S., Zhou S., Lauring A.S., Hanauer D.A., Martin E., Sharma P., Fung C.M., Pogue J.M. Tocilizumab for treatment of mechanically ventilated patients with COVID-19. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Efficacy and safety of emapalumab and anakinra in reducing hyperinflammation and respiratory distress in patients with COVID-19 infection. https://clinicaltrials.gov/ct2/show/NCT04324021?term=NCT04324021&cond=COVID-19&draw=2&rank=1
- 21.Fredi M., Cavazzana I., Moschetti L., Andreoli L., Franceschini F. Brescia Rheumatology COVID-19 Study Group, COVID-19 in patients with rheumatic diseases in northern Italy: a single-centre observational and case-control study. Lancet Rheumatol. 2020;2:e549–e556. doi: 10.1016/S2665-9913(20)30169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santos C.S., Morales C.M., Álvarez E.D., Castro C.Á., Robles A.L., Sandoval T.P. Determinants of COVID-19 disease severity in patients with underlying rheumatic disease. Clin. Rheumatol. 2020;39:2789–2796. doi: 10.1007/s10067-020-05301-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murtas R., Andreano A., Gervasi F., Guido D., Consolazio D., Tunesi S., Andreoni L., Greco M.T., Gattoni M.E., Sandrini M., Riussi A., Russo A.G. Association between autoimmune diseases and COVID-19 as assessed in both a test-negative case-control and population case-control design. Auto- Immun. Highlights. 2020;11:15. doi: 10.1186/s13317-020-00141-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montero F., Martínez-Barrio J., Serrano-Benavente B., González T., Rivera J., Molina Collada J., Castrejón I., Álvaro-Gracia J. Coronavirus disease 2019 (COVID-19) in autoimmune and inflammatory conditions: clinical characteristics of poor outcomes. Rheumatol. Int. 2020;40:1593–1598. doi: 10.1007/s00296-020-04676-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D'Silva K.M., Serling-Boyd N., Wallwork R., Hsu T., Fu X., Gravallese E.M., Choi H.K., Sparks J.A., Wallace Z.S. Clinical characteristics and outcomes of patients with coronavirus disease 2019 (COVID-19) and rheumatic disease: a comparative cohort study from a US “hot spot. Ann. Rheum. Dis. 2020;2019:1–7. doi: 10.1136/annrheumdis-2020-217888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faye A.S., Lee K.E., Laszkowska M., Kim J., Blackett J.W., McKenney A.S., Krigel A., Giles J.T., Wang R., Bernstein E.J., Green P.H.R., Krishnareddy S., Hur C., Lebwohl B. Risk of adverse outcomes in hospitalized patients with autoimmune disease and COVID-19: a matched cohort study from New York city. J. Rheumatol. 2020:1–32. doi: 10.3899/jrheum.200989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Favalli E.G., Ingegnoli F., De Lucia O., Cincinelli G., Cimaz R., Caporali R. COVID-19 infection and rheumatoid arthritis: faraway, so close! Autoimmun. Rev. 2020;19:102523. doi: 10.1016/j.autrev.2020.102523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conticini E., Bargagli E., Bardelli M., Rana G.D., Baldi C., Cameli P., Gentileschi S., Bennett D., Falsetti P., Lanzarone N., Bellisai F., Barreca C., D'Alessandro R., Cantarini L., Frediani B. COVID-19 pneumonia in a large cohort of patients treated with biological and targeted synthetic antirheumatic drugs. Ann. Rheum. Dis. 2020 doi: 10.1136/annrheumdis-2020-217681. [DOI] [PubMed] [Google Scholar]
- 29.Aries P., Iking-Konert C. No increased rate of SARS-CoV-2 infection for patients with inflammatory rheumatic diseases compared with the general population in the city of Hamburg (Germany) Ann. Rheum. Dis. 2020 doi: 10.1136/annrheumdis-2020-218400. [DOI] [PubMed] [Google Scholar]
- 30.Colmenero J., Rodríguez-Perálvarez M., Salcedo M., Arias-Milla A., Muñoz-Serrano A., Graus J., Nuño J., Gastaca M., Bustamante-Schneider J., Cachero A., Lladó L., Caballero A., Fernández-Yunquera A., Loinaz C., Fernández I., Fondevila C., Navasa M., Iñarrairaegui M., Castells L., Pascual S., Ramírez P., Vinaixa C., González-Dieguez M.L., González-Grande R., Hierro L., Nogueras F., Otero A., Álamo J.M., Blanco-Fernández G., Fábrega E., García-Pajares F., Montero J.L., Tomé S., De la Rosa G., Pons J.A. Epidemiological pattern, incidence and outcomes of COVID-19 in liver transplant patients. J. Hepatol. 2020 doi: 10.1016/j.jhep.2020.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meltzer D.O., Best T.J., Zhang H., Vokes T., Arora V., Solway J. Association of Vitamin D status and other clinical characteristics with COVID-19 test results. JAMA Netw. Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.19722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Entrenas Castillo M., Entrenas Costa L.M., Vaquero Barrios J.M., Alcalá Díaz J.F., López Miranda J., Bouillon R., Quesada Gomez J.M. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: a pilot randomized clinical study. J. Steroid Biochem. Mol. Biol. 2020;203:105751. doi: 10.1016/j.jsbmb.2020.105751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia-Vidal C., Sanjuan G., Moreno-García E., Puerta-Alcalde P., Garcia-Pouton N., Chumbita M., Fernandez-Pittol M., Pitart C., Inciarte A., Bodro M., Morata L., Ambrosioni J., Grafia I., Meira F., Macaya I., Cardozo C., Casals C., Tellez A., Castro P., Marco F., García F., Mensa J., Martínez J.A., Soriano A. COVID19-researchers group, Incidence of co-infections and superinfections in hospitalised patients with COVID-19: a retrospective cohort study. Clin. Microbiol. Infect. 2020 doi: 10.1016/j.cmi.2020.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allocca M., Fiorino G., Zallot C., Furfaro F., Gilardi D., Radice S., Danese S., Peyrin-Biroulet L. Incidence and patterns of COVID-19 among inflammatory bowel disease patients from the nancy and milan cohorts. Clin. Gastroenterol. Hepatol. 2020;18:2134–2135. doi: 10.1016/j.cgh.2020.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodríguez-Lago I., Ramírez de la Piscina P., Elorza A., Merino O., Ortiz de Zárate J., Cabriada J.L. Characteristics and prognosis of patients with inflammatory bowel disease during the SARS-CoV-2 pandemic in the Basque Country (Spain) Gastroenterology. 2020;159:781–783. doi: 10.1053/j.gastro.2020.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simon D., Tascilar K., Krönke G., Kleyer A., Zaiss M.M., Heppt F., Meder C., Atreya R., Klenske E., Dietrich P., Abdullah A., Kliem T., Corte G., Morf H., Leppkes M., Kremer A.E., Ramming A., Pachowsky M., Schuch F., Ronneberger M., Kleinert S., Maier C., Hueber A.J., Manger K., Manger B., Berking C., Tenbusch M., Überla K., Sticherling M., Neurath M.F., Schett G. Patients with immune-mediated inflammatory diseases receiving cytokine inhibitors have low prevalence of SARS-CoV-2 seroconversion. Nat. Commun. 2020;11:3774. doi: 10.1038/s41467-020-17703-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Veenstra J., Buechler C.R., Robinson G., Chapman S., Adelman M., Tisack A., Dimitrion P., Todter E., Kohen L., Lim H.W. Antecedent immunosuppressive therapy for immune-mediated inflammatory diseases in the setting of a COVID-19 outbreak. J. Am. Acad. Dermatol. 2020:19–21. doi: 10.1016/j.jaad.2020.07.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hasseli R., Mueller-Ladner U., Schmeiser T., Hoyer B.F., Krause A., Lorenz H.M., Regierer A.C., Richter J.G., Strangfeld A., Voll R.E., Pfeil A., Schulze-Koops H., Specker C. National registry for patients with inflammatory rheumatic diseases (IRD) infected with SARS-CoV-2 in Germany (ReCoVery): a valuable mean to gain rapid and reliable knowledge of the clinical course of SARS-CoV-2 infections in patients with IRD. RMD Open. 2020;6:1–9. doi: 10.1136/rmdopen-2020-001332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haberman R.H., Castillo R., Chen A., Yan D., Ramirez D., Sekar V., Lesser R., Solomon G., Niemann A.L., Blank R.B., Izmirly P., Webster D.E., Ogdie A., Troxel A.B., Adhikari S., Scher J.U., Haberman R.H., Castillo R., Chen A., Yan D., Ramirez D., Sekar V., Lesser R., Solomon G., Niemann A.L., Blank R.B., Izmirly P., Webster D.E., Ogdie A., Troxel A.B., Adhikari S., Scher J.U. COVID‐19 in patients with inflammatory arthritis: a prospective study on the effects of comorbidities and DMARDs on clinical outcomes. Arthritis Rheum. 2020 doi: 10.1002/art.41456. 0–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.RECOVERY Collaborative Group. Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., Staplin N., Brightling C., Ustianowski A., Elmahi E., Prudon B., Green C., Felton T., Chadwick D., Rege K., Fegan C., Chappell L.C., Faust S.N., Jaki T., Jeffery K., Montgomery A., Rowan K., Juszczak E., Baillie J.K., Haynes R., Landray M.J. Dexamethasone in hospitalized patients with covid-19 - preliminary report. N. Engl. J. Med. 2020:1–11. doi: 10.1056/NEJMoa2021436. [DOI] [Google Scholar]
- 41.Furst D.E. The risk of infections with biologic therapies for rheumatoid arthritis. Semin. Arthritis Rheum. 2010;39:327–346. doi: 10.1016/j.semarthrit.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 42.Mehta B., Pedro S., Ozen G., Kalil A., Wolfe F., Mikuls T., Michaud K. Serious infection risk in rheumatoid arthritis compared with non-inflammatory rheumatic and musculoskeletal diseases: a US national cohort study. RMD Open. 2019;5 doi: 10.1136/rmdopen-2019-000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Akiyama S., Hamdeh S., Micic D., Sakuraba A. Prevalence and clinical outcomes of COVID-19 in patients with autoimmune diseases: a systematic review and meta-analysis. Ann. Rheum. Dis. 2020 doi: 10.1136/annrheumdis-2020-218946. annrheumdis-2020-218946. [DOI] [PubMed] [Google Scholar]
- 44.de Salud Carlos Instituto, III, Ministerios de Ciencia e Innovación, de España Gobierno. Encuesta de seroprevalencia ENECOVID. https://www.isciii.es/Noticias/Noticias/Paginas/Noticias/PrimerosDatosEstudioENECOVID19.aspx (n.d.)
- 45.Pablos J.L., Abasolo L., Alvaro-Gracia J.M., Blanco F.J., Blanco R., Castrejón I., Fernandez-Fernandez D., Fernandez-Gutierrez B., Galindo-Izquierdo M., Gonzalez-Gay M.A., Manrique-Arija S., Mena Vázquez N., Mera Varela A., Retuerto M., Seijas-Lopez A. RIER investigators group, Prevalence of hospital PCR-confirmed COVID-19 cases in patients with chronic inflammatory and autoimmune rheumatic diseases. Ann. Rheum. Dis. 2020;79:1170–1173. doi: 10.1136/annrheumdis-2020-217763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Michelena X., Borrell H., López-Corbeto M., López-Lasanta M., Moreno E., Pascual-Pastor M., Erra A., Serrat M., Espartal E., Antón S., Añez G.A., Caparrós-Ruiz R., Pluma A., Trallero-Araguás E., Barceló-Bru M., Almirall M., De Agustín J.J., Lladós J., Julià A., Marsal S. Incidence of COVID-19 in a cohort of adult and paediatric patients with rheumatic diseases treated with targeted biologic and synthetic disease-modifying anti-rheumatic drugs. Semin. Arthritis Rheum. 2020;50:564–570. doi: 10.1016/j.semarthrit.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rodríguez Y., Novelli L., Rojas M., De Santis M., Acosta-Ampudia Y., Monsalve D.M., Ramírez-Santana C., Costanzo A., Ridgway W.M., Ansari A.A., Gershwin M.E., Selmi C., Anaya J.-M. Autoinflammatory and autoimmune conditions at the crossroad of COVID-19. J. Autoimmun. 2020:102506. doi: 10.1016/j.jaut.2020.102506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gianfrancesco M.A., Hyrich K.L., Gossec L., Strangfeld A., Carmona L., Mateus E.F., Sufka P., Grainger R., Wallace Z., Bhana S., Sirotich E., Liew J., Hausmann J.S., Costello W., Robinson P., Machado P.M., Yazdany J. COVID-19 global Rheumatology alliance steering committee, rheumatic disease and COVID-19: initial data from the COVID-19 global Rheumatology alliance provider registries. Lancet Rheumatol. 2020;2:e250–e253. doi: 10.1016/S2665-9913(20)30095-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carfì A., Bernabei R., Landi F. Gemelli against COVID-19 post-acute care study group, persistent symptoms in patients after acute COVID-19. J. Am. Med. Assoc. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.