Abstract
Background
Reports on emergency surgery performed soon after a COVID-19 infection that are not controlled for premorbid risk-factors show increased 30-day mortality and pulmonary complications. This contributed to a virtual cessation of elective surgery during the pandemic surge. To inform evidence-based guidance on the decisions for surgery during the recovery phase of the pandemic, we compare 30-day outcomes in patients testing positive for COVID-19 before their operation, to contemporary propensity-matched COVID-19 negative patients undergoing the same procedures.
Methods
This prospective multicentre study included all patients undergoing surgery at 170 Veterans Health Administration (VA) hospitals across the United States. COVID-19 positive patients were propensity matched to COVID-19 negative patients on demographic and procedural factors. We compared 30-day outcomes between COVID-19 positive and negative patients, and the effect of time from testing positive to the date of procedure (≤10 days, 11–30 days and >30 days) on outcomes.
Results
Between March 1 and August 15, 2020, 449 COVID-19 positive and 51,238 negative patients met inclusion criteria. Propensity matching yielded 432 COVID-19 positive and 1256 negative patients among whom half underwent elective surgery. Infected patients had longer hospital stays (median seven days), higher rates of pneumonia (20.6%), ventilator requirement (7.6%), acute respiratory distress syndrome (ARDS, 17.1%), septic shock (13.7%), and ischemic stroke (5.8%), while mortality, reoperations and readmissions were not significantly different. Higher odds for ventilation and stroke persisted even when surgery was delayed 11–30 days, and for pneumonia, ARDS, and septic shock >30 days after a positive test.
Discussion
30-day pulmonary, septic, and ischaemic complications are increased in COVID-19 positive, compared to propensity score matched negative patients. Odds for several complications persist despite a delay beyond ten days after testing positive. Individualized risk-stratification by pulmonary and atherosclerotic comorbidities should be considered when making decisions for delaying surgery in infected patients.
Keywords: COVID-19, Risk factors, Respiratory distress syndrome, Adult, Postoperative complications, Surgical procedures, Operative
Introduction
Coronavirus Disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was declared a pandemic by the World Health Organization on March 11, 2020. Reports indicate high rates of mortality and pulmonary complications after surgical procedures performed on COVID-19 positive patients.1, 2, 3 A report of 1128 surgical procedures (74% emergency procedures) conducted in 24 countries, during a COVID surge demonstrated 30-day mortality in 23.8%, postoperative pneumonia in 40.4%, and need for unexpected mechanical ventilation in 14.4%.4 These data are similar to other studies on periprocedural risks for COVID-19 positive patients.1, 2, 3 Given the extraordinary circumstances surrounding COVID-19, including decreased healthcare capacity,5 delay in seeking or obtaining care,6 and unique factors selecting for surgery, a contemporary control group of COVID-19 negative patients undergoing similar procedures is necessary to make inferences about the risk attributable to the infection.
The Veterans Health Administration (VA), one of the largest healthcare systems in the United States (US), provides care to over nine million individuals.7 Starting March 17, 2020, the VA deferred elective surgery nationwide to prevent the spread of COVID-19, preserve emergency surgery capabilities, and avoid adverse outcomes in non-emergency surgery. This deferral followed guidelines issued by the Centers for Medicare and Medicaid Services8 and the American College of Surgeons.9 As COVID-19 cases decreased across the country, VA facilities selectively restarted elective procedures. The current approach involves delaying elective surgery for at least ten days after a positive test in asymptomatic patients or ten days after the resolution of symptoms in symptomatic patients.10 Emergency surgery is performed without delay.
There are limited data describing the contribution of a COVID-19 infection to postoperative outcomes or the optimal delay in surgery after infection. With a national database containing electronic medical records (EMR) from serving nine million veterans across the US, the VA provides an opportunity to study the impact of SARS-CoV-2 on surgical patients. To help establish evidence-based guidance on the risk of surgery during the COVID-19 pandemic, we compared COVID-19 positive to contemporary COVID-19 negative patients undergoing a broad range of surgeries, after a propensity match that included identical procedure and case urgency.
Methods
Study design
We conducted a nationwide, multicenter, prospective study comparing COVID-19 positive and negative patients undergoing identical surgical procedures with identical urgency classifications. The COVID-19 patients were propensity score matched to patients who did not have COVID-19. Study participants were obtained from all 170 VA hospitals and 1255 VA health care centers across the US. The protocol was reviewed and approved by the University of Maryland Institutional Review Board and the Baltimore VA Medical Center Research and Development Committee.
Participants
We identified patients who underwent a surgical procedure between March 1 and August 15, 2020 and collected data on demographic, medical, and surgical characteristics. Our exposure was a positive SARS-CoV-2 quantitative RT-PCR test. Laboratory testing for SARS-CoV-2 infection was uniform across VA facilities.
All included patients had a test for SARS-CoV-2 prior to or on the date of surgery. Patients who were positive by PCR before or on the date of surgery were classified COVID-19 positive. Patients who were tested and never found to be positive at any time during the study period were classified COVID-19 negative. Patients who were not tested were excluded. If multiple procedures were performed during the study period in a COVID-19 positive patient, the procedure performed nearest to the date of the first positive test was defined as the index procedure. Similarly, in the COVID-19 negative group, the first procedure was considered the index procedure.
Data collection
Study data were obtained from the Department of Veterans Affairs Informatics and Computing Infrastructure (VINCI) database which stores data from the VA’s EMR (Computerized Patient Record System, CPRS).11
Data on age, sex, race and ethnicity, body mass index (BMI), and American Society of Anesthesiologists (ASA) physical status classification were obtained.12 The Charlson Comorbidity Index (CCI) was computed from a composite of 17 comorbidities using International Classification of Diseases-10 (ICD-10) codes for the 2 years prior to the date of surgery.13 The Current Procedural Terminology (CPT) code, case urgency (elective, urgent, or emergency), and anesthesia used for each procedure were obtained. Anesthesia was classified as general or other (a composite of sedation, spinal, epidural and local).
Outcomes
Patient outcomes up to 30-days after the index procedure were recorded. We studied four primary outcome measures: mortality, readmission, reoperation, and hospital length of stay (LOS). Secondary outcome measures (related to pulmonary complications) included postoperative pneumonia, need for mechanical ventilation, and acute respiratory distress syndrome (ARDS). Secondary outcomes (related to inflammatory, thrombotic, and ischemic syndromes) including septic shock, myocardial infarction (MI), ischemic stroke, and acute pulmonary embolism (PE). ICD-10 diagnosis codes were used to identify the outcome measures; ICD-10 and CPT codes were used to identify mechanical ventilation.
Statistical analysis
Summary statistics comparing COVID-19 positive and negative patients are presented as frequencies and percentages; means and standard deviations (SD); or medians and interquartile ranges (IQR). Comparisons of the two groups were performed using Pearson’s Chi2 test and Fisher’s Exact test for categorical data. Student’s t-test was used for normally distributed continuous data, and the Mann-Whitney-U test was used for non-normally distributed continuous data. Outcomes were first compared between unmatched COVID-19 positive and negative patients.
COVID-19 positive patients were propensity score matched to COVID-19 negative patients (propensity to be COVID-19 positive, matching ratio 3:1, control to case). The match started with an exact match on all digits of the CPT code for the index procedure and an exact match on case urgency (elective, urgent or emergency). This was followed by a nearest neighbor match on age, sex, race, ethnicity, BMI, smoking status, CCI, ASA class, type of anesthesia, and history of cancer. For each outcome measure, we performed two analyses. The first compared outcomes in the matched COVID-19 positive and negative patients. The second analysis used a simple logistic regression with a 4-level independent variable for time from positive test to surgery: 1) COVID-19 negative, 2) ≤10 days from positive test to surgery, 3) 11–30 days, and 4) >30 days. The rationale for these strata are as follows: 1) According to CDC guidelines, the isolation period after onset of COVID-19 symptoms is ten days.14 2) It has been reported that 98% of patients with COVID-19 exposure develop symptoms within 11.5 days of infection.15 3) Little is known about the risk for adverse events up to and greater than one month after testing positive. Odds ratios (OR) and 95% confidence intervals (CI) for clinical outcome measures were calculated for each time strata relative to COVID-19 negative patients. A two-sided α of ≤0.05 was considered statistically significant. Statistical analyses were performed using R version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria).
Role of the funding source
The VA and the funding agencies had no role in the study design, analysis, interpretation, or manuscript.
Results
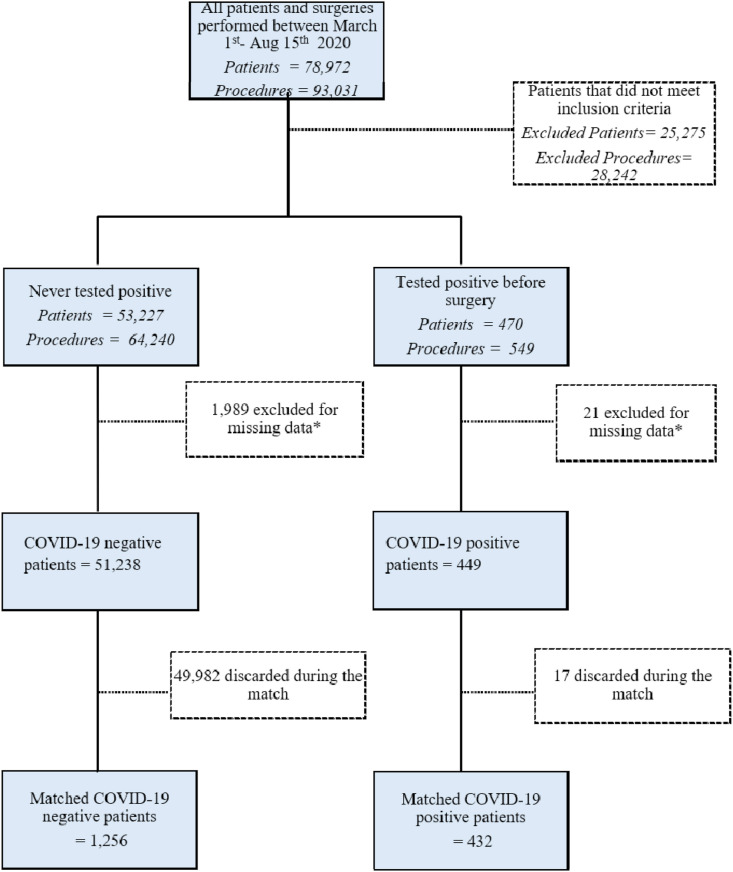
At the time of analysis (September 25, 2020), 30-day follow-up was complete on all patients undergoing surgery between March 1 and August 15, 2020. 93,031 surgical procedures were performed on 78,972 patients, with 53,697 patients meeting inclusion criteria. A total of 470 patients tested positive for SARS-CoV-2 infection, and 53,227 never tested positive before or on the date of their procedure. After excluding patients with missing data, we had 449 patients in the COVID-19 positive group and 51,238 patients in the negative group. Matching on propensity for being in the COVID-19 positive group resulted in a final cohort of 432 COVID-19 positive and 1256 negative patients (Fig. 1 ).
Fig. 1.
Flow diagram of patients included in the study.
In the unmatched cohort, the COVID-19 positive group included more black patients (43% vs 19%; p < 0.001), greater comorbidity burden (3.6 vs 3.1; p < 0.001), more patients with ASA class four or five (25.2% vs 12.7%; P < 0.001), and more urgent or emergency procedures (55.3% vs 36.5%, p < 0.001) compared to the COVID-19 negative group (Supplemental Table 1). In unadjusted 30-day outcomes, COVID-19 positive patients had higher mortality rates (3.3% vs. 0.8%, p < 0.001) and longer median LOS (7 vs 3 days, p < 0.001) but no differences in readmission (8.9% vs. 7.1%, p = 0.17) or reoperation (8.7% vs. 6.9%, p = 0.16). COVID-19 positive patients had higher rates of pneumonia (21.4% vs. 3.3%, p < 0.001), postoperative mechanical ventilation (7.6% vs. 1.9%, p < 0.001), ARDS (16.9% vs. 2.8%, p < 0.001), septic shock (14.3% vs. 3.4%, p < 0.001), MI (4.7% vs. 1.5%, p < 0.001), ischemic stroke (6.0% vs. 1.9%, p < 0.001) and PE (3.8% vs. 1.8%, p = 0.01).
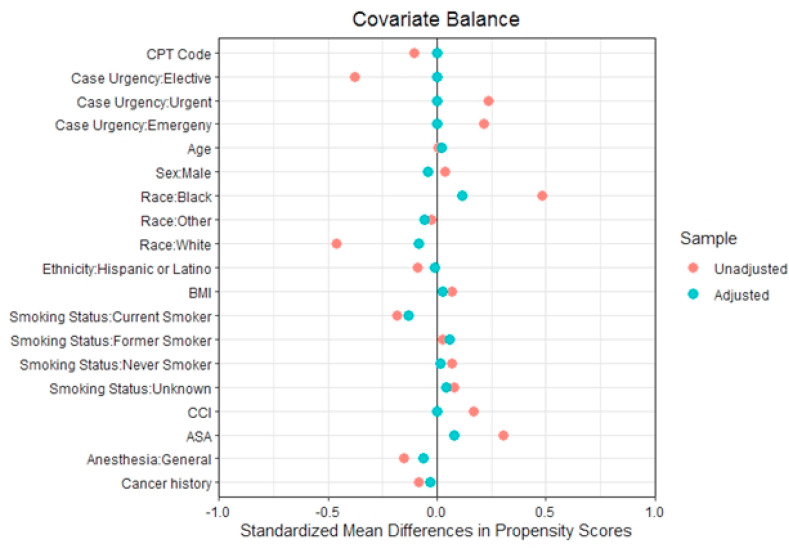
After propensity score matching, the standardized mean differences of the propensity scores comparing COVID-19 positive and negative patients were zero for their full 5-digit CPT codes, levels of procedural urgency, ethnicity, and comorbidity burden (CCI), and close to zero for all other variables included in the matching algorithm (Fig. 2 ). Demographic, clinical, and procedural characteristics including levels of urgency were similar in the matched cohort (Table 1 ).
Fig. 2.
Covariate balance for all the variables included in the propensity score match after achieving an exact match on the full Clinical Procedural Terminology (CPT) code and on the level of case urgency∗.
Table 1.
Description of patients by COVID-19 status after propensity score matching.
| COVID-19 Negative |
COVID-19 Positive |
p | |
|---|---|---|---|
| N = 1256 | N = 432 | ||
| DEMOGRAPHICS | |||
| Age, years (SD) | 64.8 (12.3) | 65.1 (12.7) | 0.69 |
| Sex, male | 1171 (93.2) | 398 (92.1) | 0.51 |
| Race | 0.14 | ||
| Black | 478 (38.1) | 187 (43.3) | |
| White | 96 (7.6) | 27 (6.2) | |
| Other | 682 (54.3) | 218 (50.5) | |
| Hispanic | 1160 (92.4) | 398 (92.1) | 0.96 |
| BMI, kg/m2(SD) | 29.8 (6.9) | 30.0 (6.9) | 0.71 |
| CLINICAL RISK FACTORS | |||
| Smoking Status | 0.18 | ||
| Current | 237 (18.9) | 62 (14.4) | |
| Former | 549 (43.7) | 201 (46.5) | |
| Never | 406 (32.3) | 143 (33.1) | |
| Unknown | 64 (5.1) | 26 (6.0) | |
| Comorbidity index, CCI (SD) | 3.6 (3.4) | 3.6 (3.3) | 0.91 |
| Heart failure | 16 (1.3) | 10 (2.3) | 0.20 |
| COPD | 305 (24.3) | 101 (23.4) | 0.75 |
| Cirrhosis | 49 (3.9) | 18 (4.2) | 0.92 |
| HIV | 16 (1.3) | 10 (2.3) | 0.20 |
| Dialysis dependent renal failure | 119 (9.5) | 33 (7.6) | 0.30 |
| PROCEDURAL RISK FACTORS | |||
| ASA Class | 0.06 | ||
| 1 | 3 (0.2) | 3 (0.7) | |
| 2 | 195 (15.5) | 58 (13.4) | |
| 3 | 778 (61.9) | 260 (60.2) | |
| 4 | 279 (22.2) | 108 (25.0) | |
| 5 | 1 (0.1) | 3 (0.7) | |
| General anesthesia | 737 (58.7) | 269 (62.3) | 0.21 |
| Organ system of surgery | 1.00 | ||
| Musculoskeletal | 263 (20.9) | 89 (20.6) | |
| Urological | 250 (19.9) | 85 (19.7) | |
| Gastrointestinal | 194 (15.4) | 68 (15.7) | |
| Cardiovascular | 163 (13.0) | 57 (13.2) | |
| Ophthalmological | 111 (8.8) | 37 (8.6) | |
| Respiratory | 105 (8.4) | 35 (8.1) | |
| Integumentary | 74 (5.9) | 27 (6.2) | |
| Neurological | 69 (5.5) | 25 (5.8) | |
| Endocrine | 9 (0.7) | 3 (0.7) | |
| Gynecological | 6 (0.5) | 2 (0.5) | |
| Lymphatic | 6 (0.5) | 2 (0.5) | |
| Auditory | 3 (0.2) | 1 (0.2) | |
| Mediastinum/Diaphragm | 3 (0.2) | 1 (0.2) | |
| Urgency level | 0.92 | ||
| Elective | 580 (46.2) | 196 (45.4) | |
| Urgent | 544 (43.3) | 188 (43.5) | |
| Emergency | 132 (10.5) | 48 (11.1) | |
Data represents frequency (%) unless indicated. Averages presented as means. SD, standard deviation; BMI, body mass index; CCI, Charlson comorbidity index; COPD, chronic obstructive pulmonary disease; HIV, human immunodeficiency virus; ASA, American Society of Anesthesiologists.
After matching, COVID-19 positive patients demonstrated longer median LOS (7 vs 5 days, p < 0.001). Infected patients had higher 30-day rates of pneumonia (20.6% vs. 6.0%, p < 0.001), postoperative mechanical ventilation (7.6% vs. 4.1%, p = 0.01) and ARDS (17.1% vs 6.8%, p < 0.001). Mortality, reoperations, and readmissions were not different between the groups (Table 2 ). Among COVID-19 positive patients, 24.3% (105/432) had at least one pulmonary complication and 12.4% (13/105) of those died. Among COVID negative patients, 10.7% (134/1256) had at least one pulmonary complication and 14.9% (20/134) of those died. Infected patients had higher rates of septic shock (13.7% vs. 6.8%. p < 0.001) and ischemic stroke (5.8% vs. 2.9%, p = 0.01). Rates of PE were similar and rates of MI were nominally higher among COVID-19 positive patients but did not reach statistical significance (4.6% vs. 2.7%, p = 0.07).
Table 2.
30-day postoperative outcomes COVID-19 status after propensity score matching.
| COVID-19 Negative |
COVID-19 Positive |
p-value | |
|---|---|---|---|
| N = 1256 | N = 432 | ||
| Mortality | 28 (2.2) | 15 (3.5) | 0.22 |
| Reoperation | 131 (10.4) | 36 (8.3) | 0.24 |
| Readmission | 113 (9.0) | 33 (7.6) | 0.44 |
| Length of stay, days∗ | 5 [2, 10] | 7 [2, 23] | <0.001 |
| Pneumonia | 75 (6.0) | 89 (20.6) | <0.001 |
| Postoperative mechanical ventilation | 51 (4.1) | 33 (7.6) | 0.01 |
| ARDS | 86 (6.8) | 74 (17.1) | <0.001 |
| Septic shock | 86 (6.8) | 59 (13.7) | <0.001 |
| Pulmonary embolism | 27 (2.1) | 15 (3.5) | 0.18 |
| Myocardial infarction | 34 (2.7) | 20 (4.6) | 0.07 |
| Ischemic stroke | 37 (2.9) | 25 (5.8) | 0.01 |
The data is expressed as number (percentage) unless specified otherwise. ∗Median [interquartile range]. ARDS, Acute respiratory distress syndrome.
The median time interval between the COVID-19 positive test and index procedure 42 days (IQR 21, 73). COVID-19 negative patients had a negative test 2 days prior to their procedure (IQR 1, 3). SARS-CoV-2 infection was diagnosed within ten days prior to surgery in 70 patients (16.2%), between 11 and 30 days prior in 96 patients (22.2%), and between 30 and 150 days prior in 266 patients (61.6%; Supplemental Fig. 1). The greatest odds of pneumonia and septic shock occurred in procedures within ten days of a positive test; however, higher odds of pneumonia, ARDS, and septic shock persisted even when a procedure was performed 11–30 days or more than 30 days after a positive test (Table 3 ). Odds for mechanical ventilation and ischemic stroke were elevated up to 30 days after a positive test. Frequency and percentage of postoperative complications by time interval from test to surgery is presented in Supplemental Table 2.
Table 3.
Odds ratios (95% confidence intervals) for 30-day postoperative outcomes by time interval from positive COVID-19 test to procedure after propensity score matching.
| COVID-19 Negative (n = 1256) | COVID-19 Positive Interval between testing and date of procedure |
|||
|---|---|---|---|---|
| ≤10 days (n = 70) | 11–30 days (n = 96) | >30 days (n = 266) | ||
| Pneumonia | Reference | 7.7 (4.4–13.3) | 6.2 (3.7–10.1) | 2.7 (1.8–4.1) |
| Postoperative mechanical ventilation | Reference | 3.1 (1.3–6.4) | 3.1 (1.5–5.9) | 1.3 (0.7–2.3) |
| ARDS | Reference | 4.0 (2.2–7.2) | 4.5 (2.7–7.5) | 2.0 (1.3–3.0) |
| Septic shock | Reference | 3.4 (1.8–6.2) | 2.7 (1.5–4.8) | 1.7 (1.1–2.6) |
| Myocardial infarction | Reference | 2.2 (0.6–5.7) | 1.6 (0.5–4.0) | 1.7 (0.8–3.2) |
| Ischaemic stroke | Reference | 1.0 (0.2–3.3) | 3.4 (1.5–7.0) | 1.8 (0.9–3.4) |
ARDS: Acute respiratory distress syndrome.
Discussion
Postprocedural pulmonary and septic complications are higher in patients with preprocedural SARS-CoV-2 infection compared to similar patients without infection. The rates for these complications are lower than earlier reports based largely on uncontrolled studies with large proportions of high-risk emergency procedures performed early (within ten days) after COVID-19 diagnosis. The propensity matched controls in this study allowed for a better estimation of the true impact of COVID-19 on outcomes. Periprocedural ischemic stroke occurs more frequently in COVID-19 positive compared to negative patients, and MI rates trend this direction. This finding argues for careful cardiac and cerebrovascular risk-stratification of infected patients being considered for surgery. The risk for pulmonary complications and septic shock persists even in patients undergoing procedures delayed more than 30 days after positive COVID-19 testing. The odds for stroke are elevated up to 30 days after a positive test. These findings have important implications for clinical decision making since persistent COVID-related risks must be weighed against the risk for prolonged delays in offering surgery.
Early in the pandemic, high periprocedural pulmonary complications, systemic sepsis and mortality resulted in a virtual cessation of elective surgery across the world. Available outcomes data present challenges, as they derive primarily from emergency procedures performed soon after a COVID-19 infection and lack adjustment for premorbid risk-factors known to contribute to complications. In 9 COVID-19 positive patients undergoing emergency surgery for hip fractures, the mortality was 56%.16 COVID-19 positive transplant recipients also demonstrated high rates of postoperative infections and mortality.3 Procedures performed early after a COVID-19 infection also result in high complication rates. In 34 patients undergoing an operation within 2.5 days of being diagnosed with the infection, 100% developed pneumonia, 32.4% developed ARDS, 33.3% required mechanical ventilation, 29.4% had septic shock, and 20.5% died.1 Finally, patient risk factors also influence COVID-19-related complications. In one report, overall mortality in 72,000 infected patients was 2.3%, while the mortality rose to 8.0% in 70 to 79-year-olds and to 14.8% in those aged ≥80 years.17 The odds for patients with severe compared to non-severe infection were elevated 2- to 4-fold in patients with hypertension, diabetes, respiratory system disease, and cardiovascular disease.18 Similar to prior studies, our unadjusted cohort demonstrated higher proportions of urgent/emergency procedures and complications in COVID-19 positive patients.
There are limited studies on the risk of postprocedural adverse events attributable to COVID-19 after adjusting for important covariates. Available reports have three main limitations: 1) They compare COVID-19 positive patients to pre-pandemic patients2 , 19, 20, 21, 22; 2) they do not include elective surgery2 , 16 , 21 , 23; or 3) they do not adjust for comorbidity burden.2 , 16 , 22 , 23 Propensity score-matching offers an opportunity to compare outcomes between COVID-19 positive and negative patients with similar demographic features and comorbidities undergoing similar operations (emergency or elective). Given sample size limitations, it is difficult to achieve this analysis using data from a single center. One group reported a propensity score matched analysis but was limited to 25 COVID-19 patients undergoing emergency surgery within 2.12 ± 0.93 days of testing positive.19 The VINCI database provides the breadth and volume of data needed to match individuals on the specific procedure, level of urgency, demographics, and risk factors. After propensity score-matching, we found that COVID-19 positive patients demonstrated elevated rates of post-procedural pneumonia, ARDS, postoperative mechanical ventilation, and septic shock compared to uninfected controls. These translated into a prolonged LOS. The observed rates for complications were lower than in previous reports and likely reflect the use of appropriate controls, our COVID-19 positive case mix (54.6% urgent/emergency procedures, 62.3% with general anesthesia), and lead time from test to surgery (16.2% of COVID-19 positive patients had surgery within ten days of testing). Infected patients had similar rates of mortality, reoperation, readmission, and acute PE compared to uninfected patients. These findings may reflect increased collective knowledge driving improved anticipation, prevention, detection, and management of COVID-related complications.
It is reassuring that a delay in surgery by ten days after a positive test for COVID-19 reduces postprocedural complications. However, not all complications returned to non-COVID-19 levels. The odds for pneumonia, ARDS and septic shock were high when a procedure was performed within ten days of a positive test and remained elevated even when procedures were delayed by ≥ 30 days. The odds of postoperative mechanical ventilation were elevated up to 30 days after a positive test. Scattered reports have suggested the possibility of persistent pulmonary risks when procedures are performed 2–4 weeks after a test is positive.20 , 24 Our findings argue for consideration of delaying elective surgery for 30 or more days, particularly in patients with pre-existing pulmonary risk factors such as smoking, chronic obstructive pulmonary disease and severe cardiac disease, which have all been associated with COVID-19 pneumonia.
Periprocedural ischemic stroke was elevated in COVID-19 patients and the risk persisted up to 30 days post testing, while MI was nominally higher, raising several concerns. The risk for myocardial and brain ischemia must be weighed against the risk of delaying an operation. A high index of suspicion must be maintained since the diagnosis may be confounded by procedure-related pain or narcotic use. A low threshold for coronary or cerebrovascular imaging may be warranted, particularly in high-risk patients with severe atherosclerotic comorbidities, as these patients are at increased risk of myocardial or cerebrovascular ischemia during influenza or other viral infections.25 , 26 Pre-existing atherosclerosis worsens clinical outcomes in COVID-19 positive patients.18 Like other coronaviruses, SARS-CoV-2 may induce systemic inflammation, localized destabilization of atherosclerotic plaques, direct inflammation of the myocardium, and a hypercoagulable state, all of which can lead to end-organ ischemia.25 , 27 The exact mechanism for our findings requires further investigation. Potential preventative measures such as deferring elective surgery must be considered, and short term periprocedural dual antiplatelet or aggressive statin prophylaxis in active or severe infections, particularly in patients with pre-existing atherosclerosis, will need to be evaluated.
Limitations
Propensity-matching cannot account for all potential differences, and unknown yet important factors may not have been controlled. The study describes the surgical experience at VA facilities as the pandemic was evolving. Testing for COVID-19 was not instituted consistently until later phases; therefore, a large proportion of patients was excluded from the analysis due to unknown COVID-19 status. The VA population is largely male, older, and has more comorbidities than the general population, which must be considered when generalizing these findings. The study included a wide range of procedures from all organ systems. The issue of surgical procedure-specific outcomes for different levels of urgency was beyond the scope of this study. However, the VINCI database is accumulating more cases, and as sample sizes increase, further studies will investigate this important issue. This early in the pandemic, the study could only report 30-day outcomes. COVID-19 may have long-term, postprocedural effects that will not be identified until future studies provide follow-up beyond 30 days. Lastly, we did not assess institutional protocols to mitigate COVID-19 transmission, but future studies will need to investigate the relative merit of different strategies to guide restrictions on surgical services during surge periods.
Conclusions
We did not find higher rates of postprocedural mortality, reoperation, or readmission in patients with prior COVID-19 infection, potentially due to increased elective cases and improved care for COVID-19 in our cohort. COVID-19 positive patients did have higher risks of pulmonary, septic, and ischemic complications, which persisted for 30 days or longer after a positive test. These findings underscore the importance of preoperative screening for SARS-CoV-2 infection and consideration for deferring elective surgery up to or beyond 30 days, particularly in those with pulmonary or atherosclerotic risk factors. Strategies for targeted prophylaxis, reduced thresholds for testing, and aggressive treatments for these complications need to be evaluated.
Contributors
The co-authors contributed to study conception, protocol development, data collection, data interpretation, and critical revision of the manuscript. BKL is the guarantor.
Data sharing
Data sharing requests will be considered upon written request to the corresponding author. Deidentified participant data or other prespecified data will be available subject to a written proposal and a signed data sharing agreement with the Veterans Affairs Informatics and Computing Infrastructure.
Funding sources
This study was funded by Veterans Affairs awards HSRD C19-20-407, RRD RX000995 and CSRD CX001621, and NIH awards NS080168, NS097876 and AG000513 (BKL); National Institutes of Health awards AG028747, DK072488, and Baltimore VA Medical Center GRECC (JDS); National Institutes of Health T32 AG00262 (NKP).
The views expressed are those of the authors and not necessarily those of the Veterans Affairs or the National Institutes of Health.
Declaration of competing interest
We declare no competing interests.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amjsurg.2020.12.024.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Lei S., Jiang F., Su W., et al. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. EClinicalMedicine. 2020;21 doi: 10.1016/j.eclinm.2020.100331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Egol K.A., Konda S.R., Bird M.L., et al. Increased mortality and major complications in hip fracture care during the COVID-19 pandemic: a New York city perspective. J Orthop Trauma. 2020;34(8):395–402. doi: 10.1097/BOT.0000000000001845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michaels M.G., La Hoz R.M., Danziger-Isakov L., et al. Coronavirus disease 2019: implications of emerging infections for transplantation. Am J Transplant. 2020;20(7):1768–1772. doi: 10.1111/ajt.15832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nepogodiev D., Bhangu A., Glasbey J.C., et al. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet. 2020;396:27–38. doi: 10.1016/S0140-6736(20)31182-X. 10243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rocco B., Sighinolfi M.C., Sandri M., et al. The dramatic COVID-19 outbreak in Italy is responsible of a huge drop in urological surgical activity: a multicenter observational study. BJU Int. 2020 doi: 10.1111/bju.15149. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong Laura E., Hawkins Jessica E., Langness Simone, Murrell Karen L., Iris Patricia, Sammann Amanda. Where are all the patients? Addressing Covid-19 fear to encourage sick patients to seek emergency care. NEJM Catal. 2020 https://catalyst.nejm.org/doi/abs/10.1056/CAT.20.0193 Published online. [Google Scholar]
- 7.Veterans Affairs Health Administration U.S. Department of veterans Affairs. 2020. https://www.va.gov/health/ Published.
- 8.Centers for Medicare and Medicaid Services Non-emergent, elective medical services, and treatment recommendations. 2020. https://www.cms.gov/Files/Document/31820-Cms-Adult-Elective- Surgery-and-Procedures- Recommendations.Pdf Published.
- 9.American College of Surgeons COVID-19: guidance for triage of non-emergent surgical procedures. https://www.facs.org/covid-19/clinical-guidance/triagehttps://www.facs.org/about-acs/covid-19/information-for-surgeons/triagehttps://www.facs.org/COVID-19/clinical-guidance/triage %5C%25 0Ainternal-pdf://0.0.6.158/triage.html
- 10.Centres for Disease Control and Prevention Interim guidance for rapid antigen testing for SARS-CoV-2 CDC. 2020. https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antigen-tests-guidelines.html Published.
- 11.U.S. Department of Veterans Affairs VA Informatics and computing infrastructure (VINCI) homepage. 2018. https://www.hsrd.research.va.gov/for_researchers/vinci/
- 12.Mayhew D., Mendonca V., Murthy B.V.S. A review of ASA physical status – historical perspectives and modern developments. Anaesthesia. 2019;74(3):373–379. doi: 10.1111/anae.14569. [DOI] [PubMed] [Google Scholar]
- 13.Quan H., Sundararajan V., Halfon P., et al. Coding_Algorithms_for_Defining_Comorbidities_in.10. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 14.Wölfel R., Corman V.M., Guggemos W., et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 15.Lauer S.A., Grantz K.H., Bi Q., et al. The incubation period of coronavirus disease 2019 (CoVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172(9):577–582. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.LeBrun D.G., Konnaris M.A., Ghahramani G.C., et al. Hip fracture outcomes during the COVID-19 pandemic: early results from New York. J Orthop Trauma. 2020;34(8):403–410. doi: 10.1097/BOT.0000000000001849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA - J Am Med Assoc. 2020 doi: 10.1001/jama.2020.2648. Published online. [DOI] [PubMed] [Google Scholar]
- 18.Yang J., Zheng Y., Gou X., et al. Prevalence of comorbidities and its effects in coronavirus disease 2019 patients: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barkhordari K., Khajavi M.R., Bagheri J., et al. Early respiratory outcomes following cardiac surgery in patients with COVID-19. J Card Surg. 2020 doi: 10.1111/jocs.14915. Published online August 13, jocs.14915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glasbey J.C., Nepogodiev D., Omar O., et al. Delaying surgery for patients with a previous SARS-CoV-2 infection. Br J Surg. 2020 doi: 10.1002/bjs.12050. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Konda S.R., Ranson R.A., Solasz S.J., et al. Modification of a validated risk stratification tool to characterize geriatric hip fracture outcomes and optimize care in a post-COVID-19 World. J Orthop Trauma. 2020 doi: 10.1097/BOT.0000000000001895. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghermandi R., Pipola V., Terzi S., et al. The impact of SARS-CoV-2 pandemic on oncologic and degenerative spine surgery department activity: the experience of rizzoli orthopaedic institute under COVID-19 lockdown. Eur Rev Med Pharmacol Sci. 2020 doi: 10.26355/eurrev_202007_21926. Published online. [DOI] [PubMed] [Google Scholar]
- 23.Kayani B., Onochie E., Patil V., et al. The effects of COVID-19 on perioperative morbidity and mortality in patients with hip fractures: a multicentre cohort study. Bone Joint Lett J. 2020;102(9):1–10. doi: 10.1302/0301-620X.102B9.BJJ-2020-1127.R1. [DOI] [PubMed] [Google Scholar]
- 24.Stevenson J.D., Evans S., Morris G., et al. Mortality of high-risk orthopaedic oncology patients during the COVID-19 pandemic: a prospective cohort study. J Surg Oncol. 2020 doi: 10.1002/jso.26127. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system. JAMA Cardiol. 2020;5(7):831. doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 26.Lee K.R., Bae J.H., Hwang I.C., Kim K.K., Suh H.S., Ko K.D. Effect of influenza vaccination on risk of stroke: a systematic review and meta-analysis. Neuroepidemiology. 2017;48(3-4):103–110. doi: 10.1159/000478017. [DOI] [PubMed] [Google Scholar]
- 27.Merkler A.E., Parikh N.S., Mir S., et al. Risk of ischemic stroke in patients with coronavirus disease 2019 (COVID-19) vs patients with influenza. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.2730. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.