Abstract
Background
Healthcare workers (HCWs) have been disproportionately affected by coronavirus disease 2019 (COVID-19), which may be driven, in part, by nosocomial exposure. If HCW exposure is predominantly nosocomial, HCWs in paediatric facilities, where few patients are admitted with COVID-19, may lack antibodies to severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) and be at increased risk during the current resurgence.
Aim
To compare the seroprevalence of SARS-CoV-2 amongst HCWs in paediatric facilities in seven European countries and South Africa (N=8).
Methods
All categories of paediatric HCWs were invited to participate in the study, irrespective of previous symptoms. A single blood sample was taken and data about previous symptoms were documented. Serum was shipped to a central laboratory in London where SARS-CoV-2 immunoglobulin G was measured.
Findings
In total, 4114 HCWs were recruited between 1st May and mid-July 2020. The range of seroprevalence was 0–16.93%. The highest seroprevalence was found in London (16.93%), followed by Cape Town, South Africa (10.36%). There were no positive HCWs in the Austrian, Estonian and Latvian cohorts; 2/300 [0.66%, 95% confidence interval (CI) 0.18–2.4] HCWs tested positive in Lithuania; 1/124 (0.81%, 95% CI 0.14–4.3) HCWs tested positive in Romania; and 1/76 (1.3%, 95% CI 0.23–7.0) HCWs tested positive in Greece.
Conclusion
Overall seroprevalence amongst paediatric HCWs is similar to their national populations and linked to the national COVID-19 burden. Staff working in paediatric facilities in low-burden countries have very low seroprevalence rates and thus are likely to be susceptible to COVID-19. Their susceptibility to infection may affect their ability to provide care in the face of increasing cases of COVID-19, and this highlights the need for appropriate preventative strategies in paediatric healthcare settings.
Keywords: COVID-19, SARS-CoV-2, Seroprevalence, Healthcare workers, Hospital workers
Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) was first recognized in December 2019 and rapidly spread worldwide, with the World Health Organization (WHO) declaring a pandemic situation on 11th March 2020. Soon after the identification and genetic sequencing of the virus, diagnostic tests became available for the detection of live virus in human secretions, followed rapidly by tests designed to measure serum antibodies to SARS-CoV-2 antigens. Antibodies to SARS-CoV-2 are known to start increasing within 5 days of exposure [1], and immunoglobulin G (IgG) can be detected in the serum for many months following exposure. Sero-epidemiology, the presence of antibody in a representative community sample, can shed light on overall population exposure and, when correlates of protection are better understood, may help to predict both individual and community susceptibility to infection.
Healthcare workers (HCWs) have had to work throughout the pandemic and the incidence of coronavirus disease 2019 (COVID-19) among HCWs has been shown, in some studies, to be higher than in the general population [2,3]. Recently, WHO has indicated that while HCWs represent <3% of the population in the large majority of countries and <2% in almost all low- and middle-income countries, approximately 14% of cases of COVID-19 reported to WHO are among HCWs. In some countries, the proportion is as high as 35% (https://www.who.int/news-room/detail/17-09-2020-keep-health-workers-safe-to-keep-patients-safe-who).
Seroprevalence has been measured in HCWs to help understand the transmission potential of SARS-CoV-2 in the context of nosocomial exposure, and also for insight into the true burden of underlying COVID-19 infection. In many settings, it has been shown to be significantly higher in HCWs than in the relevant general population [[4], [5], [6], [7]]. As relatively few children globally have been admitted to hospital with COVID-19 [8], staff working in paediatric facilities have been unlikely to face a significant risk of exposure from their patients [9]. Only two studies of seroprevalence in paediatric HCWs have been published to date. In Barcelona, Spain, seroprevalence of 4% was discovered in HCWs [10], similar to the rate in a population-based random sample from the general population in Barcelona (5.4%) analysed at approximately the same time (March/April 2020). In Argentina, the seroprevalence rate in paediatricians from a single children's hospital 3 months into the pandemic was 0.9% [11]. While SARS-CoV-2 has been studied intensely in high-prevalence countries that bore the brunt of the initial pandemic, nothing is known about paediatric HCW serostatus in other countries. Understanding levels of seroprevalence in such staff could provide insight into the general prevalence of SARS-CoV-2 in cities or countries where mass testing for the presence of virus in swabs or community-based sero-surveys have not been widely implemented or are suboptimal, and thereby uncover rates of asymptomatic infection that have led to seroconversion. Additionally, such information can help healthcare facilities plan for the current surge in SARS-CoV-2 infection and, where relevant, target vaccine delivery.
There is currently little standardization of assays designed to measure antibodies to SARS-CoV-2, resulting in assays of varying sensitivity and specificity being used [12] and consequent difficulty in the comparison of seroprevalence rates between studies and/or countries. As such, this study was designed to compare rates of SARS-CoV-2 antibody positivity in HCWs working in paediatric facilities in seven different European countries and South Africa. By centralizing all of the testing in a single laboratory in London, UK, methodological laboratory issues that may influence comparisons were eliminated. In order to contextualize potential differences in seroprevalence rates between the countries participating in this study, publicly available data were accessed regarding the dates of the initial cases and subsequent epidemiology of COVID-19 in each participating country, the mobility of citizens in each country relative to national restrictions on movement, and government responses during the pandemic.
Methods
This study was undertaken in HCWs in paediatric facilities in seven European sites and one South African site. The study was initiated at Great Ormond Street Hospital, London where local hospital staff were invited to enrol in a prospective longitudinal cohort study of SARS-CoV-2 serology (COSTARS, IRAS 282713, ClinicalTrials.gov Identifier: NCT04380896). Collaborators based in paediatric healthcare facilities were invited to join a multi-centre study with a similar design. Ethical approval was obtained locally by the lead investigators at each site. A material transfer agreement which defined the aims of the study and governed the transfer of data and serum was signed between each centre and the principal investigator's laboratory at Great Ormond Street Institute of Child Health, University College London.
Staff of all categories in each healthcare setting were invited to join the study, irrespective of symptoms or whether they suspected they had previously had COVID-19. The number of staff recruited was a convenience sample matched to the capacity for laboratory testing. Staff who consented to join the study provided a single 2-mL blood sample and completed a questionnaire focused on documenting the symptoms of COVID-19 since the onset of the pandemic, prior known exposure, and the outcome if a viral swab for SARS-CoV-2 RNA polymerase chain reaction (PCR) had been obtained.
Serology
Serum was prepared and aliquoted, given a unique identifier locally, and stored frozen until batch shipping to the WHO International Reference Laboratory for Pneumococcal Serology at University College London, where samples were analysed for the presence of IgG to SARS-CoV-2 nucleocapsid protein (Epitope Diagnostics Inc, San Diego, CA, USA), as described previously [13]. All positive or equivocal samples were re-assayed in a multiplexed assay measuring IgG to SARS-CoV-2 nucleocapsid protein, receptor binding domain of S1 and trimeric spike antigen (MSD SARS-Coronavirus Plate 1, Rockville, MD, USA) to confirm positivity, and to establish whether equivocal samples were positive or negative. The MSD assay has undergone extensive evaluation in the London laboratory [14].
Data sources
National case counts at the time of sampling were taken from data collated from WHO, US Centers for Disease Control and Prevention, and other sources, and available on the public website bing.com (https://www.bing.com/covid). Numbers of cases were converted to rates of COVID-19 per 100,000 population using population estimates published by Eurostat (https://ec.europa.eu/eurostat/data/database). Mobility data for each individual country in the period between the onset of the first case and cohort recruitment were taken from publicly available Google Mobility data available at https://www.google.com/COVID-19/mobility/. Government responses to the pandemic were accessed at the publicly available website hosted by the Blavatnik School of Government at the University of Oxford (https://www.bsg.ox.ac.uk/research/research-projects/coronavirus-government-response-tracker). The Oxford COVID-19 Government Response Tracker systematically collects information on several different common policy responses that governments have taken to respond to the pandemic on 17 indicators, such as school closures and travel restrictions.
Results
Cohort characteristics
In total, 4114 HCWs were recruited between 1st May and mid-July 2020 from the nine sites, although the cohort size varied significantly between the centres (Table I ). For Estonia, Latvia, Lithuania and Romania, >90% of the staff included in the study were female, and females dominated all of the cohorts reflecting the gender make-up of staff in the paediatric health setting. The mean age of participants in each cohort was similar and ranged from 38 to 50 years, although cohorts had a wide age range (19–78 years). The time taken to recruit the entire cohort varied between sites. Some sites recruited staff over days, while others continued recruitment over months. The proportion of HCWs in each cohort reporting symptoms compatible with COVID-19 prior to recruitment varied significantly: <1% (Greece and Romania), 9–18% (Austria, Estonia and Lithuania), 22% (Latvia) and 30–45% (South Africa and UK).
Table I.
Overall demographics and results for the eight cohorts studied
| Country | N of samples | % female | Age in years (mean, range) | Date of first nationally recorded case of COVID-19 | Sample collection date range | N with clinical symptoms | N with positive PCR results | Proportion with positive PCR results | Proportion of cohort with symptoms | If symptomatic, time between symptom onset and blood test | N seropositive | seroprevalence rate (95% CI) | National COVID-19 rate/100,000 population at time of sampling |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Austria | 196 | 84.2 | 38, 22–65 | 25/02/2020 | 17–24/07/20 | 33 | 0 | 0 | 16.8 | 116 days | 0 | 0 (0–1.92) | 225.8 |
| Estonia | 130 | 96.2 | 50, 19–71 | 27/02/2020 | 10–12/06/20 | 23 | 0 | 0 | 17.7 | 96 days | 0 | 0 (0–2.87) | 148.2 |
| Greece | 77 | 77.6 | 45, 18–67 | 26/02/2020 | 19/06–16/07/20 | 0 | 0 | 0 | 0.0 | 0 | 1 | 1.3 (0.23–7.0) | 34.5 |
| Latvia | 177 | 92.7 | 42, 20–73 | 03/03/2020 | 19/05–20/06/20 | 39 | 0 | 0 | 22.0 | 82 days | 0 | 0 (0–2.14) | 54.7 |
| Lithuania | 300 | 93.0 | 49, 22–70 | 28/02/2020 | 01–12/06/20 | 28 | 2 | 0.67 | 9.3 | 93 days | 2 | 0.66 (0.18–2.4) | 59.1 |
| Romania | 124 | 94.4 | 43, 21–65 | 26/02/2020 | 14/05/20–27/05/20 | 1 | 1 | 0.81 | 0.8 | Not available | 1 | 0.81 (0.14–4.3) | 87.6 |
| South Africa | 222 | 78.8 | 41, 19–67 | 05/03/2020 | 10/06–17/08/20 | 69 | 17 | 7.66 | 31.1 | 46 days | 23 | 10.36 (7–15.07) | 974.4 |
| UK | 1754 | 65.5 | 38, 19–69 | 15/02/2020 | 01/05–31/05/2020 | 772 | 15 | 0.86 | 44.0 | 64 days | 269 | 15.34 (13.73–17.1) | 390.1 |
| UK | 1134 | 72.9 | 37, 19–78 | 15/02/2020 | 01/06–30/06/2020 | 869 | 15 | 1.32 | 76.6 | 89 days | 192 | 16.93 (14.86–19.22) | 459.1 |
PCR, polymerase chain reaction; COVID-19, coronavirus disease 2019; CI, confidence interval.
The majority of cohort recruitment took place between May and June 2020 with the exception of Greece, where recruitment started in mid-June and extended into July 2020; Austria, where recruitment was only undertaken in July 2020; and South Africa, where recruitment extended from mid-June until mid-August 2020 which coincided with the peak of the pandemic (https://www.nicd.ac.za/diseases-a-z-index/covid-19/surveillance-reports/). London recruited throughout May and June 2020, and the two periods were separated out for analytical purposes.
Despite 16–22% of staff in Austria, Estonia and Latvia reporting symptoms prior to study recruitment, none had a positive viral swab for SARS-CoV-2 RNA recorded. In Austria, all staff participating in the study were swabbed regularly (two weekly) as part of local health measures. The proportion of the cohort with a positive PCR result in Lithuania, Romania and the UK was <1%, and all those with positive PCR results were also antibody-positive. The positive PCR rate in South Africa was higher at 7.66%.
Serology
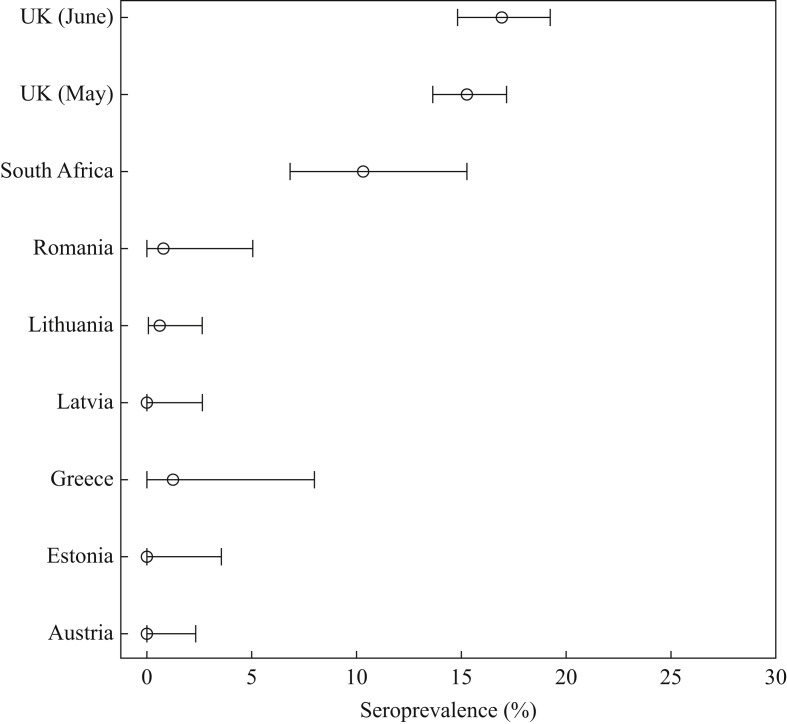
Seroprevalence rates for three of the four countries with no positive PCR results were zero, although one of 76 workers was IgG-positive in Greece (Figure 1 ). Similarly low seroprevalence rates were found in Romania [one of 224 HCWs tested IgG-positive (0.8%)] and Lithuania [two of 300 HCWs tested IgG-positive (0.66%)]. Seroprevalence in Cape Town HCWs was 10.4%, and 15.4% and 16.93% of the London cohort were IgG-positive for the May and June cohorts, respectively. When comparing seroprevalence rates for the individual cohorts with the rates of COVID-19 cases/100,000 population in each country at the time of sampling, some anomalies were noted (Table I). For those countries with <100 cases per 100,000 population (Greece, Latvia, Lithuania and Romania), seroprevalence rates were low (0–1.3%). However, Estonia and Austria, with rates of 148.23 and 225.76/100,000 population, respectively, had no seropositive HCWs in their cohorts despite 17% of their cohorts reporting symptoms compatible with COVID-19 (although no PCR-positive results). The UK and South Africa had high rates of COVID-19 at the time of recruitment, and this was reflected in high seroprevalence rates.
Figure 1.
Seroprevalence estimates for severe acute respiratory syndrome coronavirus-2 antinucleocapsid immunoglobulin G in healthcare worker cohorts in eight countries.
The time reported between symptoms compatible with COVID-19 and blood sampling was similar for those cohorts with significant numbers of symptomatic staff. The number of days ranged from 89 (UK, June cohort) to 116 days (Austria), and this is unlikely to be responsible for the differences noted between countries. South Africa had the shortest mean time between symptoms and blood sampling (46 days), yet had a seroprevalence rate lower than the UK. Furthermore, the two UK cohorts had different times between symptoms and sampling (64 days for the May cohort and 89 days for the June cohort), but seroprevalence rates were higher for the cohort with a longer gap, suggesting that waning antibody is unlikely to be relevant over a period of 60–90 days.
Mobility and government response
Google Mobility data, looking at changes in non-residential activities including visits to retail outlets, parks, driving and use of public transport for the relevant areas of each country in this analysis, revealed some differences between the countries but none appeared to correlate with seroprevalence (Table II ). The smallest change for the eight countries was Estonia with a -11% change, followed by Latvia and Lithuania with -19 and -21% change, respectively; however, these three countries had seroprevalence rates <1%. For the other six countries, there was a greater reduction in mobility during the initial phases of the pandemic, with changes ranging from -31% (Austria) to -42% (Romania). The countries with the highest seroprevalence rates showed changes in mobility of -40% (South Africa) and -33% (UK).
Table II.
Google Mobility and the Oxford COVID-19 Government Response Tracker for the eight participating countries
| Country | Average Google mobility reduction in non-residential activity (%) | Oxford COVID-19 Government Response Tracker score (%) |
|---|---|---|
| Austria | -31 | 62 |
| Estonia | -11 | 50 |
| Greece | -37 | 63 |
| Latvia | -19 | 70 |
| Lithuania | -21 | 60 |
| Romania | -42 | 50 |
| South Africa | -40 | 90 |
| UK | -33 | 75 |
The Oxford COVID-19 Government Response Tracker score was compared for each country at 100 days following the first case, which was approximately the first or second week of June 2020 for the eight countries in this study (Table II). For the countries with low seroprevalence rates (and generally lower case rates), the scores ranged from 50% to 70%. The country with the highest score was South Africa (90%), which reflects the severe lockdown imposed early on in the pandemic. Despite the high score, the seroprevalence rate in the South African cohort was the second highest in this study, suggesting that the score may accurately reflect national responses to the pandemic, but the response does not necessarily predict the spread of SARS-CoV-2 in a population.
Discussion
HCWs have continued to work during the pandemic to maintain health services, and have therefore been at increased risk of exposure to SARS-CoV-2. As COVID-19 may be asymptomatic, additional methods are required to estimate the true burden of disease and, by implication, the number of staff that might be protected as a result of recovering from infection. Detection of virus in throat/nasal swabs and serological tests of specific antibody have both been used in studies of clinical facing front-line HCWs to try and quantify the burden of disease in this group. However, estimates of seroprevalence amongst HCWs have varied widely; for example, within Europe, Germany [15], Greece [16], Croatia [17] and Austria [18] have demonstrated low seroprevalence rates for HCWs (1%, 1.07%, 2% and 3.2%, respectively), while Belgium (6.4%) [19], Spain (9.3%) [6] and the UK [4,20] have had higher rates in some studies. However, interpretation of differences in seroprevalence rates between countries is complicated by the fact that rates have been shown to vary widely between studies in the same country. For example, five independent studies conducted within hospitals that are part of the UK National Health Service have reported seroprevalence estimates ranging from 10.6% [21] or 10.7% [22] at study entry to an overall rate of 24.4% [4] or 31.64% [7], or, in one study, 25% at entry with a subsequent overall rate of 45% [20]. In the USA, in a convenience sample of front-line HCWs who worked with patients with COVID-19 at 13 geographically diverse US academic medical centres, seroprevalence rates were found to range by hospital from 0.8% to 31.2% (median 3.6%). Higher rates were found in those hospitals situated in areas with high community cumulative incidence of COVID-19 [5].
Apart from differences between countries or hospitals, other factors have also been shown to influence seropositivity rates, including gender [5], ethnicity [4,5] and category of hospital work (e.g. housekeepers higher than intensive care unit staff) [4]. In contrast, in Belgium, a study found no relationship between direct clinical care of patients with COVID-19 and seroprevalence rates, but instead found that rates correlated with reported household contacts [23].
Comparison of seroprevalence rates between studies is complicated by the varying performance of assays used to measure SARS-CoV-2 serum responses. There is currently no standardization of assays and, as shown within the same seroprevalence study, the performance of seven different assays may differ widely [19].
To reliably undertake transnational comparison, this study centralized testing in a single laboratory. Staff in all participating centres were recruited irrespective of a history of clinical symptoms that could be construed as COVID-19, and thus should be considered as an unbiased sample of HCWs. All worked in paediatric facilities, although some were embedded in larger adult facilities. No attempt was made to stratify between clinical facing and non-clinical staff, as HCWs in paediatric facilities are generally not in high-risk environments as children represent only a small number (6%) of total COVID-19 admissions [24], and young children may be less likely to transmit virus than older individuals [25]. All cohorts in this study were dominated by females, so gender differences in seroprevalence rates should not account for the differences noted in the cohorts, and the mean ages and age ranges overlapped significantly and were thus comparable. The recruitment period (both time taken in days, and months in which recruitment took place) differed between the cohorts, although the majority of recruitment took place between May and July 2020 either during or after the initial peak of disease in most countries. The UK site enrolled two cohorts, one in May and one in June 2020 – both periods were associated with similar numbers of new cases in London, and relatively small differences in seroprevalence rates were seen between the two cohorts. This suggests that month of sampling is unlikely to have had a major effect on the results of this study as most of the sampling in the eight participating centres occurred at a similar time in relation to the peak of disease. The time between symptom onset and blood testing was comparable for most cohorts, with the shortest mean time being 46 days (South African cohort). As most assays detect antibody reliably at least 15 days after exposure [26], most positive cases would have been captured in the study cohorts, although it is unclear if some staff may have lost IgG and thus sero-reverted. If this were the case, it would have affected the seven cohorts with longer time periods between symptom onset and testing equally. As in other studies [23], there was discrepancy between the proportion of staff reporting symptoms compatible with COVID-19 and the seroprevalence rate. This was most pronounced for the UK cohort in June 2020 where 76.6% of the cohort reported compatible symptoms yet seroprevalence was ‘only’ 16.93%.
Interestingly, the seroprevalence rates for several of the HCW cohorts were similar to population-based estimates for the same city or region. Seroprevalence for the London cohort of HCWs (15.4–17%) was very similar to that measured for London-based blood donors analysed by Public Health England in May and June 2020 (15–16%) [27]. Seroprevalence for the Greek HCW cohort was 1.3% (based on a single case), which was similar to that found in a community-based study of seroprevalence in residual sera in Athens (0.93%) [28]. The absence of any IgG-positive HCWs in Austria similarly reflects the very low national seroprevalence rate of 0.15% (http://www.statistik.at/web_en/statistics/PeopleSociety/health/123052.html). The absence of any IgG-positive HCWs in the Estonian cohort reflects the very low seroprevalence rate in Tartu (1.6%) [29], and the low seroprevalence rate in the Lithuanian cohort (0.66%) was below the rate of 1.31% seen in a population-based survey in Vilnius (https://sam.lrv.lt/lt/naujienos/pristatyti-pirminiai-populiacijos-tyrimo-rezultatai). Similarly, blood donor testing in Latvia revealed a seroprevalence rate of 0.4% (personal communication). The authors found one study of paediatric HCWs conducted in Spain, where the seroprevalence rate among the HCWs was similar to that in the general population [10], and one study of physicians in a children's hospital in Argentina with a seroprevalence rate of 0.9% [11]. Together, these data suggest that HCWs in paediatric settings are no more likely than the general population to be seropositive for SARS-CoV-2, and household contacts may be an important source of infection [23].
This study has a number of limitations. The cohort sizes differed significantly between centres, although all but one centre enrolled >120 subjects. No attempt was made to adjust for sensitivity of the EDI assay, as this study was comparative and the key outcome was comparison of rates between the participating countries. The true seropositive rate might be slightly higher as assay sensitivity was assessed as 92.4% with published sensitivity as low as 80% [30]. However, all equivocal samples that were borderline in the EDI assay were re-assayed in the MSD assay which has very high sensitivity [14], increasing the overall sensitivity for detecting positive cases in these cohorts. As the cohorts separated into six countries with relatively low seropositivity rates and two countries with relatively high seropositivity rates, this limited the opportunity to correlate findings with factors measured by Google Mobility data or the response of individual governments to the pandemic.
In conclusion, this study shows that HCWs in paediatric facilities have seroprevalence rates that are similar to their local general population, which are closely related to the overall burden of COVID-19 in the areas where the hospitals are based. While this may be interpreted as the success of personal protection in paediatric healthcare facilities, it is more likely to be the absence of nosocomial exposure or lack of transmission from infected children to adults, and thus a risk of exposure to SARS-CoV-2 that is similar to that of the general public. The relatively small proportions of HCWs with antibodies in six of the eight participating centres suggest that, as the current second wave of infections increases, staff working in paediatric facilities remain susceptible to infection. A recent outbreak amongst staff in a paediatric intensive care unit in Germany illustrates the serious consequences of infection for health services [31]. Measures to monitor and protect paediatric staff both at work and at home should be instituted and adhered to in order to avoid shortages of critical staff in the health service, and seronegative staff should be targeted for vaccination where available.
Acknowledgements
The authors wish to thank the staff at all participating centres for their willingness to volunteer for this study. The authors also thank Riga Children's Hospital Foundation's for support with Latvian sample transportation costs.
Conflict of interest statement
None declared.
Funding
DG receives support from the NIHR Great Ormond Street Biomedical Research Centre. HZ is supported by the South African Medical Research Council.
References
- 1.To K.K., Tsang O.T., Leung W.S., Tam A.R., Wu T.C., Lung D.C. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20:565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nguyen L.H., Drew D.A., Graham M.S., Joshi A.D., Guo C.G., Ma W. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Health. 2020;5:e475–e483. doi: 10.1016/S2468-2667(20)30164-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rudberg A.S., Havervall S., Manberg A., Jernbom Falk A., Aguilera K., Ng H. SARS-CoV-2 exposure, symptoms and seroprevalence in healthcare workers in Sweden. Nat Commun. 2020;11:5064. doi: 10.1038/s41467-020-18848-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shields A., Faustini S.E., Perez-Toledo M., Jossi S., Aldera E., Allen J.D. SARS-CoV-2 seroprevalence and asymptomatic viral carriage in healthcare workers: a cross-sectional study. Thorax. 2020;75:1089–1094. doi: 10.1136/thoraxjnl-2020-215414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Self W.H., Tenforde M.W., Stubblefield W.B., Feldstein L.R., Steingrub J.S., Shapiro N.I. Seroprevalence of SARS-CoV-2 among frontline health care personnel in a multistate hospital network – 13 academic medical centers, April–June 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1221–1226. doi: 10.15585/mmwr.mm6935e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia-Basteiro A.L., Moncunill G., Tortajada M., Vidal M., Guinovart C., Jimenez A. Seroprevalence of antibodies against SARS-CoV-2 among health care workers in a large Spanish reference hospital. Nat Commun. 2020;11:3500. doi: 10.1038/s41467-020-17318-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grant J.J., Wilmore S.M.S., McCann N.S., Donnelly O., Lai R.W.L., Kinsella M.J. Seroprevalence of SARS-CoV-2 antibodies in healthcare workers at a London NHS trust. Infect Control Hosp Epidemiol. 2020:1–3. doi: 10.1017/ice.2020.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gotzinger F., Santiago-Garcia B., Noguera-Julian A., Lanaspa M., Lancella L., Calo Carducci F.I. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. 2020;4:653–661. doi: 10.1016/S2352-4642(20)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amendola A., Tanzi E., Folgori L., Barcellini L., Bianchi S., Gori M. Low seroprevalence of SARS-CoV-2 infection among healthcare workers of the largest children hospital in Milan during the pandemic wave. Infect Control Hosp Epidemiol. 2020;41:1468–1469. doi: 10.1017/ice.2020.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dacosta-Urbieta A.R.-C.I., Pardo-Seco J., Redondo-Collazo L., Salas A., Gómez-Rial J., Martinón-Torres F. Seroprevalence of SARS-CoV-2 among pediatric healthcare workers in Spain. Front Pediatr. 2020;8:547. doi: 10.3389/fped.2020.00547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Insua C., Stedile G., Figueroa V., Hernandez C., Svartz A., Ferrero F. Seroprevalence of SARS-CoV-2 antibodies among physicians from a children's hospital. Arch Argent Pediatr. 2020;118:381–385. doi: 10.5546/aap.2020.eng.381. [DOI] [PubMed] [Google Scholar]
- 12.Flower B., Brown J.C., Simmons B., Moshe M., Frise R., Penn R. Clinical and laboratory evaluation of SARS-CoV-2 lateral flow assays for use in a national COVID-19 seroprevalence survey. Thorax. 2020;75:1082–1108. doi: 10.1136/thoraxjnl-2020-215732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kruttgen A., Cornelissen C.G., Dreher M., Hornef M., Imohl M., Kleines M. Comparison of four new commercial serologic assays for determination of SARS-CoV-2 IgG. J Clin Virol. 2020;128:104394. doi: 10.1016/j.jcv.2020.104394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson M., Wagstaffe H.R., Gilmour K.C., Mai A.L., Lewis J., Hunt A. Evaluation of a novel multiplexed assay for determining IgG levels and functional activity to SARS-CoV-2. J Clin Virol. 2020;130:104572. doi: 10.1016/j.jcv.2020.104572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Behrens G.M.N., Cossmann A., Stankov M.V., Witte T., Ernst D., Happle C. Perceived versus proven SARS-CoV-2-specific immune responses in health-care professionals. Infection. 2020;48:631–634. doi: 10.1007/s15010-020-01461-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Psichogiou M.K.A., Pavlpoulou I.D., Basoulis D., Petsios K., Roussos A., Patrikaki M. Antibodies against SARS-CoV-2 among health care workers in a country with low burden of COVID-19. medRxiv. 2020;23 doi: 10.1371/journal.pone.0243025. 20137620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vilibic-Cavlek T., Stevanovic V., Tabain I., Betica-Radic L., Sabadi D., Peric L. Severe acute respiratory syndrome coronavirus 2 seroprevalence among personnel in the healthcare facilities of Croatia, 2020. Rev Soc Bras Med Trop. 2020;53 doi: 10.1590/0037-8682-0458-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuereder T., Berghoff A.S., Heller G., Haslacher H., Perkmann T., Strassl R. SARS-CoV-2 seroprevalence in oncology healthcare professionals and patients with cancer at a tertiary care centre during the COVID-19 pandemic. ESMO Open. 2020;5:e000889. doi: 10.1136/esmoopen-2020-000889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moscola J., Sembajwe G., Jarrett M., Farber B., Chang T., McGinn T. Prevalence of SARS-CoV-2 antibodies in health care personnel in the New York City area. JAMA. 2020;324:893–895. doi: 10.1001/jama.2020.14765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Houlihan C.F., Vora N., Byrne T., Lewer D., Kelly G., Heaney J. Pandemic peak SARS-CoV-2 infection and seroconversion rates in London frontline health-care workers. Lancet. 2020;396 doi: 10.1016/S0140-6736(20)31484-7. e6–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pallett S.J.C., Rayment M., Patel A., Fitzgerald-Smith S.A.M., Denny S.J., Charani E. Point-of-care serological assays for delayed SARS-CoV-2 case identification among health-care workers in the UK: a prospective multicentre cohort study. Lancet Respir Med. 2020;8:885–894. doi: 10.1016/S2213-2600(20)30315-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eyre D.W., Lumley S.F., O'Donnell D., Campbell M., Sims E., Lawson E. Differential occupational risks to healthcare workers from SARS-CoV-2 observed during a prospective observational study. Elife. 2020;9:e60675. doi: 10.7554/eLife.60675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steensels D., Oris E., Coninx L., Nuyens D., Delforge M.L., Vermeersch P. Hospital-wide SARS-CoV-2 antibody screening in 3056 staff in a tertiary center in Belgium. JAMA. 2020;324:195–197. doi: 10.1001/jama.2020.11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo H., Liu S., Wang Y., Phillips-Howard P.A., Ju S., Yang Y. Age differences in clinical features and outcomes in patients with COVID-19, Jiangsu, China: a retrospective, multicentre cohort study. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-039887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park Y.J., Choe Y.J., Park O., Park S.Y., Kim Y.M., Kim J. Contact tracing during coronavirus disease outbreak, South Korea, 2020. Emerg Infect Dis. 2020;26:2465–2468. doi: 10.3201/eid2610.201315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deeks J.J., Dinnes J., Takwoingi Y., Davenport C., Spijker R., Taylor-Phillips S. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst Rev. 2020;6:CD013652. doi: 10.1002/14651858.CD013652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Public Health England . PHE; London: 2020. Weekly coronavirus disease 2019 (COVID-19) surveillance report. Summary of COVID-19 surveillance systems.https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/923668/Weekly_COVID19_Surveillance_Report_week_40.pdf . Available at: [last accessed January 2021] [Google Scholar]
- 28.Tsitsilonis O.E., Paraskevis D., Lianidou E., Pierros V., Akalestos A., Kastritis E. Seroprevalence of antibodies against SARS-CoV-2 among the personnel and students of the National and Kapodistrian University of Athens, Greece: a preliminary report. Life (Basel) 2020;10:214. doi: 10.3390/life10090214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jõgi P.I.D., Soots M., Lättekivi F., Naaber P., Toompere K., Vaas H. Seroprevalence of SARS-CoV-2 IgG antibodies in two regions of Estonia (KoroSero-EST-1) medRxiv. 2020;21 20216820. [Google Scholar]
- 30.Patel E., Bloch E.M., Clarke W., Hsieh Y.H., Boon D., Eby Y.J. Comparative performance of five commercially available serologic assays to detect antibodies to SARS-CoV-2 and identify individuals with high neutralizing titers. medRxiv. 2020;31 doi: 10.1128/JCM.02257-20. 2018478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knoll R.L., Klopp J., Bonewitz G., Grondahl B., Hilbert K., Kohnen W. Containment of a large SARS-CoV-2 outbreak among healthcare workers in a pediatric intensive care unit. Pediatr Infect Dis J. 2020;39:e336–e339. doi: 10.1097/INF.0000000000002866. [DOI] [PubMed] [Google Scholar]