Abstract
Immunotherapy is the newest approach to combat cancer. It can be achieved using several strategies, among which is the dendritic cell (DC) vaccine therapy. Several clinical trials are ongoing using DC vaccine therapy either as a sole agent or in combination with other interventions to tackle different types of cancer. Immunotherapy can offer a potential treatment to coronavirus disease 2019 (COVID-19) the worst pandemic facing this generation, a disease with deleterious effects on the health and economic systems worldwide. We hypothesize that DC vaccine therapy may provide a potential treatment strategy to help combat COVID-19. Cancer patients are at the top of the vulnerable population owing to their immune-compromised status. In this review, we discuss DC vaccine therapy in the light of the body’s immunity, cancer, and newly emerging infections such as COVID-19 in hopes of better-customized treatment options for patients with multiple comorbidities.
Keywords: Dendritic cell vaccine, Cancer, Immunotherapy, Combination therapy, Advantages, Disadvantages, COVID-19
Background
Cancer immunotherapy is one of the new approaches that is currently studied in an attempt to uncover new opportunities for better outcomes for cancer patients [1], [2]. It is a newly added pillar of oncotherapy in addition to the conventional therapies, i.e., chemotherapy, surgery, and radiotherapy. Traditional treatments mainly rely on either the tumor site or the abnormal cell tendency to divide. In contrast, immunotherapy uses the inherent aptitude of the body’s immune system to discriminate between healthy self-tissues and pathologic/ foreign ones. This concept was introduced by William Coley (founder of cellular immunity) using viable streptococcus bacteria, and later, Paul Ehrlich (founder of humoral immunity) using antibodies [3], [4], [5]. The main benefits of cancer immunotherapy compared to other treatment strategies are the capacity of possible long-lasting immunity and the ability of the immune system to adapt to the tumor’s changing conditions [6].
Cellular immunotherapy can be achieved using various approaches including; Adoptive T Cell therapy (ACT), Dendritic Cell (DC) vaccine therapy, and Natural Killer (NK) cell therapy, in which great efforts are being exerted to enhance the effectiveness of these technologies for the purpose of rendering them clinically reasonable remedies [3], [7], [8], [9], [10].
The current COVID-19 epidemic outbreak that started in December 2019 [11] caused by the SARS-CoV-2 virus leads to higher demands of intensive care facilities and exhaustion of the medical facilities. This urges the swift need to find novel technologies for vaccine development that can both fight the active disease and prevent future infections. It is also crucial to implement a system that could help the rapid development of vaccines for future emerging infectious outbreaks [12].
In the current review, we aim to concisely discuss DC vaccine therapy to better understand the interactions between the body’s immunity and conditions such as cancer and other emerging infections as SARS-CoV-2 to pave the way for the emergence of better-designed immunotherapies, especially for the vulnerable population of immune-compromised cancer patients.
Immunity and cancer
It is crucial to understand the mechanisms by which tumor cells escape immunity and the interactions occurring between cancer and the immune system, which involves both the recognition and eradication of transformed cancer cells. Surprisingly, the immune system could also enhance tumor progression by favoring the fittest cancer cells’ survival in an immune-competent host or by altering the tumor microenvironment in a way that favors tumor growth. This is called cancer immunoediting, where the immune system can act both as a tumor stimulatory and as a tumor suppressor [4], [13].
Accumulated pieces of evidence from preclinical and clinical studies illustrate the anticancer role of immunoediting in terms of 3 consecutive phases; elimination, equilibrium, and evasion. This cancer immunoediting suppressive mechanism is effective in cases of failure of intrinsic tumor suppressor mechanisms. In the elimination step, the immune system conquers the tumors cells ahead of clinical detection. Many immune cells and molecules from both adaptive and innate immune systems cooperate to perform this task. If successful, the cascade is halted, and the host is protected. However, if some cancer cells escape detection, the second phase of equilibrium comes into play to prevent the creation of an inflammatory milieu that favors tumor growth. This is achieved by the attempt to keep the cancer cells dormant by the action of the adaptive immunity, specifically lymphocytic T cells, interleukin-12 (IL-12), and interferon-γ (IFN-γ). It is worth noting that the tumor immunogenicity editing takes place in this phase, and thus it could be the end of cancer cells.
Nevertheless, as a result of the continuous immune selection pressure on tumor cells, some genetically unstable ones may evolve, leading to the third escape phase. In this phase, cancer cells fall under one of three categories. The first is unrecognized tumor cells, by the adaptive immune system owing to their antigen loss. The second is resistant tumor cells that resist the elimination mechanisms. And third, tumor cells with an immune-suppressive microenvironment. At this point, tumor cell growth could no longer be controlled by the immune system, leading to a clinically detectable tumor [13].
Inflammation plays a vital role in cancer progression [14]. Tumor-related chronic inflammation affects both local and systemic immunological reactions and encourages the emergence of immune-suppressive microenvironment and tumor development. On the other hand, acute inflammation stimulates both dendritic cells and effector T cells functions and subsequently promotes the antitumor activity [5].
Plasmacytoid dendritic cells (pDCs) are a rare type of innate immune cells that are involved in body responses to tumors and viruses [15]. Concerning cancer, pDCs were shown to be involved in both pro- and antitumor responses [15]. pDCs contribute to the immunosuppression associated with cancer chiefly via the secretion of inducible co-stimulatory ligand (ICOS-L) that activates pro-tumoral CD4+ T cells which enhances intra-tumoral infiltrating pDCs in different types of cancer [16], [17], [18]. While pDCs’ anti-tumoral action is mainly by stimulation of the immune response against cancer, their antitumor functions can be summarized as follows; transporting the tumor-associated antigens (TAAs) to lymph nodes, cross-priming of CD8+ T cells, controlling the tumor microenvironment, and intratumor infiltration and secretion of cytokines that modulate tumor-associated immunosuppression [15].
Immunity and viral infections
Some live viruses like the influenza virus can infect all cell types except pDCs that are responsible for virus phagocytosis and the subsequent secretion of type 1 interferon. On the other hand, killed virus-based vaccines; the pDCs instead engulf the whole dead virus to produce type 1 interferon. Type 1 interferon production comes as a result of the activation of the TLR7/MyD88-dependent pathway that plays a crucial role in the adaptive immunity reaction against viruses [19]. It worths mentioning that the TLR7/MyD88-dependent pathway activation and type 1 interferon production are also crucial in combating cancer by modulating the Hippo signaling pathway, chiefly one of its main components; the large tumor suppressor 1/2 (LATS1/2) which plays important role in many types of cancers [20], [21]. The RNA particles in the vaccine composition are pivotal to its effectiveness, especially in the non-infected cohort. At the same time, its absence may still have some shielding effect in the previously infected population [19].
Indeed, pDCs augment antiviral immunity through crosstalk between the adaptive and innate immune reactions [15]. In this antiviral context, antigen-specific interactions occur between DCs and T cells, which evoke the adaptive cellular immunity response. Activated CD8+ T cells stimulate the recruitment of the XCR1 chemokine receptor-expressing DCs (XCR1+ DCs), which reside at the lymph nodes. Thus, enhancing the cooperation between the pDCs and XCR1+ DCs, which in turn boost the maturation and antigen cross-presentation of XCR1+DCs. This validates the model stating that antigen-activated CD8+ T cells modulate their microenvironment via recruiting more DCs at the antigen recognition site [22].
Dendritic cell-based vaccine therapy and its different implementation strategies in cancer
Effective cancer vaccines could serve as preventive agents against cancers caused by infectious diseases, e.g., human papillomavirus or hepatitis B virus, or as onco-therapeutic agents. The latter approach relies on the fact that the host body may have CD3+ T cells recognizing specific TAAs. Accordingly, vaccination can intensify the strength of this existing reaction against TAAs, or produce a De Novo one. DCs are known for their high efficiency as antigen-presenting cells (APC) on both major histocompatibility complex molecules class I (MHC I) and II (MHC II) to CD8+ and CD4+ T cells respectively [6]. Furthermore, DCs migrate between lymphoid and non-lymphoid tissues and modulate cytokine and chemokine gradients and hence regulate inflammation and homing of lymphocytes [15]. Hundreds of contemporary trials are testing and evaluating DCs alone, or in combination with other regimens to combat cancer. Examples of some of these trials are shown in Table 1 .
Table 1.
Examples of some completed clinical trials using DC vaccination in different types of cancer [71].
| NCT number | Tumor type | Intervention | Clinical Trial Phases | Population |
|---|---|---|---|---|
| NCT02018458 | Breast Cancer | DC vaccine + Preoperative chemotherapy | Phase 1 and 2 | 8 enrolled females Age: 18 – 80 years |
| NCT01042535 | Breast Cancer | Adenovirus p53 transduced dendritic cell (DC) vaccine + 1-methyl-dtryptophan | Phase I/II | 44 enrolled from all sex Age:18 years and older adults |
| NCT00082641 | Breast Cancer | Autologous dendritic cell adenovirus p53 vaccine | Phase I/II | 24 enrolled females Age: 19 – 120 years |
| NCT01876212 | Metastatic melanoma | DC vaccine + Dasatinib | Phase II | 15 enrolled from all sex Age: 18 years and older adults |
| NCT00289341 | Prostate cancer | Autologous DCs Pulsed With Apoptotic Tumor Cells (DC/LNCaP) | Phase I/II | 24 enrolled males Age: 18 Years and older |
| NCT00345293 | Prostate cancer | Autologous DCs Pulsed With Apoptotic Tumor Cells (DC/PC3) | Phase I/II | 13 enrolled males Age: 18 Years and older |
| NCT00085436 | Kidney cancer | • Aldesleukin, •Autologous tumor cell vaccine •Recombinant IFN-α | Phase II | 18 enrolled from all sex Age: 18 years and older adults |
| NCT00103116 | Lung cancer | Autologous dendritic cell cancer vaccine | Phase II | 32 enrolled from all sex Age: 18 – 80 years |
| NCT00617409 | Lung cancer | Paclitaxel + Ad.p53-DC vaccines + All –trans Retinoic Acid (ATRA) | Phase I/II | 24 enrolled females Age: 18 – 80 years |
First-generation DC vaccine failure in randomized trials was due to the incomplete comprehension of DCs role and the ability of cancer to create an immune-suppressive environment leading to tolerance, a condition not applicable in vaccines against infectious diseases-caused cancers. Thus, overcoming this tolerance is a challenge faced by DC vaccines. To solve this problem, DCs are loaded with large amounts of antigens for further activation and expansion with the help of immune-stimulatory molecules [6], [23].
Sipuleucel-T (Provenge Dendreon Corporation) is the first DC vaccine therapy that was approved in April 2010 by the US Food and Drug Administration (FDA) for the treatment of metastatic prostate cancer. It is a DC-based vaccine based on the co-culturing of APCs enriched blood collected using leukapheresis, with prostatic acid phosphatase (PAP) fused to granulocyte-macrophage colony-stimulating factor (GM-CSF) for 36 to 44 h. This product succeeded in increasing the average survival by around four months. However, clinical trials did not show a significant tumor size reduction, nor halt tumor progression [1], [6]. Using standard response evaluation criteria in solid tumors (RECIST) criteria, only a single case had some improved outcomes. A decrease of prostate-specific antigen levels to half in 2.6% of the patients receiving Sipuleucel-T compared to 1.3% receiving the placebo, which was still viewed as a significant effect by FDA owing to the limited availability of treatment choices [23].
A possible perspective that could explain the low-efficiency outcome based on the RECIST criteria is the unsuitability of these criteria to measure the outcomes in vaccine cancer immunotherapy compared to conventional cancer therapy. This is because cancer immunotherapy functions by inducing inflammation at the tumor microenvironment rather than direct cytotoxicity. And the RECIST follow up criteria are built on appraising the cytotoxic effects. So in some scenarios of cancer immunotherapy, when the tumor sizes are stable, failing the RECIST criteria leads to discontinuation of the immunotherapy treatment protocol preventing the documentation of possible positive responses. Thus, a new version of RECIST was proposed to comprehend if vaccination could be a valid alternative for conventional cancer therapies [24].
Current DC vaccination approaches use one of 3 strategies; (i) conventional vaccination, (ii) in vivo DC targeting, and (iii) DC vaccination. Conventional vaccination involves the use of antigens in the form of proteins or long peptides plus an adjuvant that aid in the maturation of DC. This approach lacks accurate targeting, and accordingly, many attempts were implemented to enhance it, using, for instance, recombinant vectors with high affinity to DC or novel adjuvants that favorably seek DC.
In vivo DC targeting strategy involves injecting the host with anti-DC antibodies (example: anti-C type lectin that targets the C-type lectin expressed on the surface of DC) joined with antigens. Although several tumor mechanisms induce tolerance to this approach, nevertheless, strong immunity is triggered by this mechanism upon the delivery of an appropriate maturation stimulus, e.g., the Toll-like Receptors (TLR) ligand- antigen conjugates. This strategy is a promising future approach after solving its limitations, as the DCs and their receptors may be altered in some patients.
Finally, the DC vaccination strategy comprises the adoptive transfer of ex vivo generated DCs. DCs are either separated from peripheral blood mononuclear cells (PBMC) from the host blood or CD34+ precursors that are mobilized from the bone marrow, and are co-cultured with GM-CSF and other factors like IL-4 in case of monocytes, and Flt3 ligand and TNF-α in case of CD34+ cells, to induce differentiation into immature DCs. These DCs are then loaded with TAA (in the form of peptides, proteins, or tumor cells) and activated with pro-inflammatory cytokines ex vivo then injected back into the host [1], [25], [26].
Many of the DC vaccines showed promising ex vivo effects, although modest efficacy was seen clinically, especially with late stages of cancer. DCs used in clinical trials are administrated via different routes and prepared using different methodologies that affect their efficiency. Although first generation DC vaccine therapies showed encouraging results early in phase I and II of clinical trials, it failed in phase III to produce a clinical response [26].
Currently, many preclinical studies are running on the development of next-generation DC vaccines to enhance their effectiveness by boosting the immunogenicity using different maturation cocktails to enhance the effector T lymphocytes function [27].
Strategies adopted to increase the efficacy of DC-based cancer vaccines
Understanding the obstacles that currently exist with cancer vaccines can open the door to their advancement. We summarize six different categories that need to be addressed for DC vaccine enhancement.
-
1.
The first category goal is the ultimate TAA, which should have significantly different expression levels in cancer cells versus the normal ones in a statistically significant number of aimed patients. Also, it should be an important protein for the cancer cell viability, or else the tumor cells can down-regulate the antigen expression and subsequently escape the immune system. Another consideration is the fact that this TAA should either be associated with a specific type of tumor or a universal one or both also whether it is a surface antigen or not [28].
-
2.
The second category focuses on the epitopes used in vaccination, whether they are multiple epitopes or a single one. Using multiple epitopes strategy is preferred over the single one to overcome the tumor immune escape strategy that we previously mentioned. Indeed, the more versatile and abundant the tumor antigens involved at the same time, the more expected positive outcomes of the vaccination and less probability of tumor escape [29]. Other crucial aspects at this stage of planning the vaccine are, the selection of more immunogenic epitope structures, the necessity of the activation of both kinds of tumor-specific T cells (CD8+ and CD4+) for more guaranteed elimination of tumor, and to make the vaccine efficient for a wide range of patients by optimizing multi-peptide vaccines that can potentially bind to diverse HLA molecules [30].
-
3.
The third category is the involvement of multiple pathways in the tumor eradication process, and the inclusion of active innate immunity. Innate immunity upon the sensation of erroneous signals by its pattern recognition receptors (PRRs) produces effector signals to activate various pathways that are inevitable for the adaptive immune system activation; thus, the adjuvant selection is fundamental. Multiple adjuvants with complementary roles are generally more preferred to single adjuvant use [19], [28]. For instance, the multi-peptide and dual adjuvant GX301 vaccine that is substantially immunogenic in renal and prostate cancer patients. It has four telomerase peptides and two adjuvants; Montanide ISA-51 and Imiquimod [31]. The 2 adjuvants have complementary actions, Montanide ISA-51 shields the vaccine peptides from being digested with the tissue proteases and enhance their uptake by APCs. Besides, it stimulates the innate immune system to produce IFN-γ, enhancing the HLA molecules expression by cancer cells. Imiquimod is a strong activator of the TLR-7 and TLR-8, which are responsible for the potent activation of DCs [32], [33].
-
4.
The fourth category deals with the inhibition of the pro-tumor regulatory machinery in the tumor microenvironment or the activation of antitumor machinery in an attempt to boost cancer vaccine action. Among the pro-tumor regulatory systems inhibited are the tumor-associated macrophages (M2), myeloid-derived suppressor cells, the regulatory CD8+/ CD28- & CD4+ T cells, type 2 CD4+ T cells, type 2 NK T cells, B cells, mast cells, and IL-10 inhibitory cytokine. While the antitumor processes activated are M1 macrophages, CD8+ T cells, type 1 CD4+ T cells, NK cells, type 1 NK T cells, and immune killer dendritic cells [14], [34].
-
5.
Nanomedicine presents the fifth potential strategy. Nanoparticle formulations promote the uptake of tumor-specific antigens and adjuvant by DCs and boost the activation of DCs. Hence, the nanoparticles platform could be exploited to augment the antitumor immune responses of DC-based cancer vaccines [35].
-
6.
The final category involves the tailoring of finest schedules and routes of administration for the therapeutic vaccine protocols and the possibility of co-administration of other therapeutic interventions like cytokines or chemotherapy [28].
To put it briefly, tailoring a cancer vaccine should combine all of the above aspects with the feasibility both financially and technically to be able to treat more patients globally.
Virus-based cancer vaccines
An alternative system to evoke an immune response for a TAA is its insertion into a viral backbone whose proteins are responsible for a strong immune response. This is a double-sided weapon, where its advantages are their high immunogenicity and that it could be used for any patient without prior individual tailoring, so-called “off-the-shelf” nature. On the other hand, the main disadvantage is the complexity of the viral backbone directing the immune response mainly to the viral particle itself rather than the antigen of interest. This could be partially solved by the repetition of dosing, which could neutralize the immune response to the antigen of interest [36].
Therefore, the National Cancer Institute (NCI) attempted to overcome these obstacles by using a couple of inventions targeting the vector immunogenicity. The first was incorporating a triad of co-stimulatory factors, namely, ICAM −1 (CD54), LFA-3 (human CD58), and B7-1 (CD80). These proteins interact with different molecules needed for T-cell activation (CD11a/CD18 complex, LFA-2 (CD2), and CD28 and CTLA-4, respectively). Second, by using heterologous prime-boost under specific conditions that was proven to have synergistic positive results. This strategy was attempted in prostate cancer [37], [38].
Advantages versus disadvantages of DC-based cancer vaccines
Dendritic cell cancer vaccine strategy proved efficient in many preclinical and clinical trials studying various solid [39] and hematological [40] cancers. However, as discussed earlier several issues should be addressed before becoming mainstream in cancer therapy. Current clinical trials involving different types of tumors (as in Table 1) are currently exploring the significance of the DC vaccine as a monotherapy or in combination with other interventions in different types of tumors as metastatic melanoma [41], breast cancer [42], and prostate cancer [36].
This therapeutic intervention is ordinarily well-tolerated without frequent severe side effects. Common side effects include local reactions (rash, erythema, pruritus, or pain) at the injection site since it is mostly administered intradermally. Occasionally, flu-like symptoms (fever, malaise, myalgia, or arthralgia) could be experienced, but all are of mild grade. In case it is injected intra-nodal, which is rare, there is a probability of nodal rupture and, consequently, failure of therapy. A specific issue associated with immunotherapy, in general, is the provocation of autoimmunity; however, this is rare in cancer vaccine immunotherapy compared to cytokines and monoclonal antibodies immunotherapeutic interventions [43].
Combination therapies strategies to boost the DC-based cancer vaccines
Chemotherapy
Chemotherapeutic drugs primary target is to kill cancer cells and to decrease the tumor burden. However, studies recently reported some off-target immunological consequences that develop according to the chemotherapeutic agent. For instance, tumor immunogenic cell death (ICD) occurs through stimulation of the antitumor immunity by increasing the damage-associated molecular patterns (DAMPs) production, which in turn recruits the endogenous DCs in the tumor and stimulate their antigen uptake, maturation, and activation. Moreover, other chemotherapeutic agents exhaust suppressive immune cells like T-regulatory lymphocytes. The timing and sequence of introducing the chemotherapeutics in the treatment schedule, together with the DC cancer vaccine, dictates the purpose of its use. For example, if the goal is reducing the tumor size, then chemotherapy should be started before DC vaccine therapy. While if the aim is to deplete the suppressive immune cells, then chemotherapy should be co-administrated or started shortly before DC-based therapy [41], [44].
Different chemotherapeutic agents are used in preclinical and clinical trials to assess their potential as a treatment option for different cancer types using high and low doses. Among them is cyclophosphamide, an alkylating agent that stimulates ICD, the antitumor activities, and depletes the suppressive immune cells. It was studied in combination with DC-based therapy in several preclinical and clinical settings with different dosage schedules for the treatment of mesothelioma, melanoma, pancreatic cancer, colon cancer, and several others [45], [46], [47], [48]. Temozolomide is another alkylating agent with a dose-dependent action. At high doses, temozolomide promotes lympho-ablation while at low doses affects mainly the regulatory T-cells. An advantage of temozolomide is its ability to cross the blood-brain barrier; it is used to treat glioblastoma and melanoma, which frequently metastasize to the brain[41], [49].
Radiotherapy
Radiotherapy immune-modulatory effects promote the ICD and the antitumor immunity cascade of DC activation and migration to the lymph nodes. In the lymph nodes, DC initiates the systemic antitumor immune response via the antigen cross-presentation (from dying tumor cells and DAMPs) to naïve T lymphocytes. These activated T cells then create a systemic antitumor immunity when it departs from the lymph nodes and attacks metastatic tumor lesions that were not previously irradiated. Besides, other radiotherapy antitumor activity mechanisms include the increased expression of FAS, MHC I, and NKG2D ligands and production of CXCL16 by tumor cells [50], [51].
Immune checkpoint inhibitors
Immune checkpoint inhibitors (anti-PD-1/ PD-L1/ CTLA-4) function by enhancing the antitumor immunity via suppressing the co-inhibitory molecules over-presented by both cancer and immune cells [52]. Moreover, their co-administration with DC vaccine therapy augments its therapeutic effect. This could be explained by the fact that the PD-1/ PD-L1/ CTLA-4 inhibitory molecules’ expressions negatively affect the DC vaccine therapy through either changing the DCs themselves or the naïve T lymphocytes that are activated by DCs [41]. The PD-1/PD-L1 axis blocks the effector T lymphocytes and NK cells action and promotes T lymphocytes exhaustion, thus adversely affects the tumor-infiltrating lymphocytes (TILs) to the tumor microenvironment.
Additionally, PD-L1 using the STAT3/caspase 7 dependent pathway directly blocks the interferon-γ mediated cytotoxicity [53], [54]. Therefore, blocking the PD-1/PD-L1 axis will have a synergistic therapeutic action when co-administrated with DC vaccine therapy, thus affecting the tumor microenvironment, decreasing IL-10, increasing interferon-γ, reducing regulatory T lymphocytes, and enhancing cytotoxic T lymphocytes (CTLs) function [55]. In preclinical mouse models, DC vaccine therapy, in combination with anti-PD-1, decreased the tumor volumes of melanoma and increased survival in glioblastoma compared to monotherapy. Anti-PD-L1 antibodies demonstrated similar effects on breast cancer [56], [57], [58].
Anti-CTLA-4 antibodies (tremelimumab & ipilimumab) block the inhibitory actions of CTLA-4. CTLA-4 prevents CD28 on T cells to bind to the CD80/CD86 on APCs, thus inhibiting the naïve T-cell activation [59], [60]. In a retrospective study of advanced melanoma patients whose case advanced after DC vaccine therapy, the addition of ipilimumab promoted tumor-specific cytotoxic T lymphocytic action, although it did not increase the overall survival [61]. On the other hand, in late-stage melanoma patients, the response rate was 38% of the patients receiving the combination therapy of ipilimumab and DC therapy. DCs were electroporated with CD40, CD70, TLR-4 encoding mRNA and a melanoma-associated antigen (MAGE-A3, MAGE-C2, tyrosinase, or gp100) joined to MHCII [62].
Dendritic cell possible role in SARS-CoV-2 infection
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of COVID-19, is one of the Corona viridae family of viruses that are famous for their surface crown-shaped glycoproteins, from where it acquired its name. The minimal amount of structure proteins comprised by the virion particle is 4: spike (S) protein, envelope (E) protein, membrane (M) protein, and nucleocapsid (N) protein [63]. Some of the family species are only confined to replication in mammals like the alpha and beta coronaviruses while others can replicate in both, but mainly in avian than mammals, as the gamma and delta coronaviruses [64]. Some beta corona strains that replicate in humans are known to cause the common cold. Still, others are more aggressive in their attacks as the SARS-CoV-1, the Middle Eastern Respiratory Syndrome coronavirus (MERS-CoV), and most among them the SARS-CoV-2.
The genomic content of SARS-CoV-2 is a single-stranded, positive-sense RNA encoding many open reading frames, among which are the important ones responsible for the S-protein. The S protein is responsible for the virus attachment to the host cells’ surface angiotensin-converting enzyme-2 (ACE2) receptor and subsequent engulfment by the endosomes. Other encoded proteins are the replicase enzyme and other accessory and structural proteins, although till now, not all accessory proteins functions are clearly defined (reviewed in Amanat and Krammer, 2020).
The entry of coronavirus into host cells achieved by the trans-membrane spike (S) glycoprotein on the virus surface determines the scope of infected host cells. Therefore, it is a target for emerging vaccines and antibodies against coronavirus. S is made up of S1 and S2 functional subunits, S1 controls the virus binding to the ACE2 receptor, and S2 mediates the fusion between the virus and cell membranes [63]. Respiratory DCs are among the cells infected by SARS-CoV-2 together with the lung type 2 alveolar cells and other endothelial cells. This could partially clarify the vigorous immunopathology found upon infection with the SARS-CoV-2 virus [65].
A C-type lectin expressed on the surface of DC found in peripheral mucosa called Dendritic Cell-Specific Intercellular adhesion molecule-Grabbing Nonintegrin (DC-SIGN) was found to play an important role in the attachment of many viruses to host cells as in the case of the human immunodeficiency virus (HIV) through binding to the viral E protein. In the HIV context, there are contradicting reports as to whether the DC themselves are infected by HIV, or they don’t serve as a viral entry receptor but help in trans infection of target cells by transporting the virus to the secondary lymphoid tissue rich in CD4+ T helper cells. These CD4+ T helper cells express both CD4+ and chemokine CCR5 receptors that together form an important complex for the viral entry. DCs are highly potent APCs, the immature DC catch the viral antigen from the distal tissues and transports them to the inactive T-cells in lymph nodes. These immature DCs also express CD4+ and CCR5 receptors, albeit in lower amounts. During the DCs transportation process, the immature DC changes their profile of expressed molecules, among which are chemokine receptors, and also become incapable of capturing more antigens. They are responsible for generating the adaptive immune system’s primary reaction against viruses and for the strong infection of the targeted T cells, which consequently leads to the systemic viral distribution in the host [66].
DC-SIGNR (CD209L), also called L-SIGN, is a homolog of DC-SIGN (CD209) protein and promotes many viral infections [67], [68], [69]. DC-SIGN and DC-SIGNR expressing cells promote infection with SARS-CoV-S particles. It was shown that although DC-SIGN does not function as a receptor for SARS-CoV infection, it is important for the trans infection of targeted T-cells [70]. Antibodies against these DC-SIGN inhibit some of the DC infections and accordingly could serve as a promising target for designing novel therapies.
Promising SARS-CoV-2 vaccines platforms
Several approaches are under investigation in an attempt to develop an effective and safe vaccine that can be used on a large scale both to confront the current COVID-19 epidemic and to overcome the possibility of the virus reinfection. Many technologies are implemented after the extensive study of the coronavirus structure to locate the most interesting targets for vaccine development, which was found to be the spike protein (S-protein) [12].
The target of all vaccines that are under development is to initiate the immune response when exposing the body to a non-virulent form of the virus antigens, thus destroying the virus upon entrance into the host. Clinical trials currently taking place can be classified under one of the following platforms: i) Weakened or inactivated viral vaccines like polio and measles vaccines (e.g. Sinovac Biotech in Beijing and Codagenix in Farmingdale, New York). ii) Nucleic acid-based vaccines, where only a part of the RNA (mostly the part encoding the S protein) is used to evoke an immune reaction. However, to date, this technology did not produce any licensed product, iii) Viral vector vaccines, where a viral backbone is used to generate the antigen (for instance, coronavirus proteins) inside the body. These used virus backbones either can replicate in host cells, such as the Ebola new certified vaccine, which generates a safe yet robust immune reaction. The disadvantage is the possibility of getting neutralized by the immune system. The second type is the viral vectors that lost their ability to self-replicate (e.g., Johnson & Johnson are operating on them). iv) Protein-based vaccines which involve the injection of the viral coat or viral-mimicking proteins (specifically the S-proteins of the viral coat or its receptor-binding domain) into the host [12], [71], [72], [73].
Raised questions and concluding remarks
The use of the DC-vaccine therapy approach in cancer patients has proved advantageous in treating cancer via its antitumor immunity mechanisms, which can be further augmented when co-administrated with other interventions or adjuvants. More preclinical and clinical trials should explore different strategies and combinations that will lead to better outcomes.
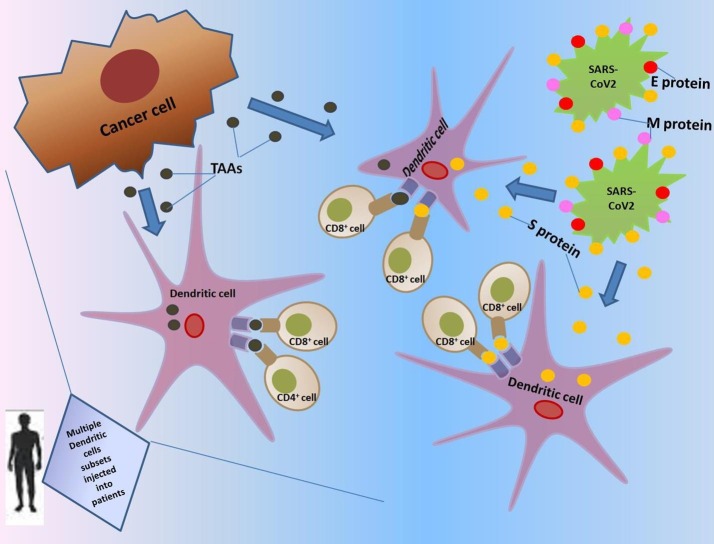
The whole world is currently suffering from the harmful effects of the COVID-19 epidemic. Cancer patients are more vulnerable to such infection and its adverse effects owing to their profound deteriorated health status and low immunity. Theories evolving from clinical and preclinical observations could be of great benefit in overcoming the unprecedented challenge. With our current understanding of the virus, cancer, and DC nature, a question that could be addressed is the possibility of targeting both cancer and COVID-19 virus by one weapon, like for instance tailoring a DC vaccine therapy which could both block viral infection and serve as an immune-boosting approach owing to its efficient antigen-presenting capability, thus sensitizing the immune system to act against the tumor and the virus simultaneously (Fig. 1 ). The viral entry and life cycle steps, together with the immune system regulation and different cancers natures, could serve as potential targets for drug therapy. It could be speculated that an ideal potent vaccine would comprise multiple DC subsets to benefit from their complementary actions.
Fig. 1.
Graphical abstract showing the hypothesized DC vaccine therapy approach against COVID-19 in cancer patients.
This could also be of an added benefit to the elderly population, which are mostly affected by the adverse effects of COVID-19, yet have a weakened immune system, which dictates multiple dosing and higher needs for antigen-exposure. The timing of the co-administration of combinations in the therapeutic schedule is an important factor that should be considered owing to the crucial effect it has on the efficiency of the therapies.
Limitations
The current review article has several limitations. First, the DC vaccine immunotherapy in combating cancer is still in its infancy phase, and lots of preclinical and clinical trials should be done to optimize its usage. Furthermore, the incomplete comprehension of the SARS-CoV-2 virus nature and the presence of several murine isoforms of the viral target DC-SIGN molecules make it more challenging. Moreover, there is an immense amount of theories and potential treatments to COVID-19 evolving every day upon verifying new evidence. Nevertheless, most of the data are obtained from adult patients recruited in small clinical trials or observational studies and cannot be extended to pediatric patients.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Pérez R., Campillo-Davo D., Van Tendeloo V.F.I., Benítez-Ribas D. Cellular immunotherapy: a clinical state-of-the-art of a new paradigm for cancer treatment. Clin Transl Oncol. 2020 doi: 10.1007/s12094-020-02344-4. [DOI] [PubMed] [Google Scholar]
- 2.Abdel-Aziz A.K., Pallavicini I., Ceccacci E., Meroni G., Saadeldin M.K., Varasi M. Tuning mTORC1 activity dictates the response to LSD1 inhibition of acute myeloid leukemia. Haematologica. 2020;105:2105–2117. doi: 10.3324/haematol.2019.224501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perica K., Varela J.C., Oelke M., Schneck J. Adoptive T Cell Immunotherapy For Cancer. Rambam Maimonides Med J. 2015;6 doi: 10.5041/rmmj.10179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ribatti D. The concept of immune surveillance against tumors. The first theories. Oncotarget. 2017;8:7175–7180. doi: 10.18632/oncotarget.12739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang Y. Cancer immunotherapy : harnessing the immune system to battle cancer Find the latest version : Cancer immunotherapy : harnessing the immune system to battle cancer. J Clin Invest. 2015;125:3335–3337. doi: 10.1172/JCI83871.It. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farkona S., Diamandis E.P., Blasutig I.M. Cancer immunotherapy: The beginning of the end of cancer? BMC Med. 2016;14:1–18. doi: 10.1186/s12916-016-0623-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bashyam H. Ralph Steinman: Dendritic cells bring home the Lasker. J Exp Med. 2007;204:2245–2248. doi: 10.1084/jem.20071995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenberg S.A., Restifo N.P. Adoptive cell transfer as personalized immunotherapy for human cancer. Science (80 -) 2015;348:62–68. doi: 10.1126/science.aaa4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eshhar Z., Waks T., Gross G., Schindler D.G. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the γ or ζ subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci USA. 1993;90:720–724. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimasaki N., Jain A., Campana D. NK cells for cancer immunotherapy. Nat Rev Drug Discov. 2020;19:200–218. doi: 10.1038/s41573-019-0052-1. [DOI] [PubMed] [Google Scholar]
- 11.Go Y.Y., Kim Y., Cheon S., Nam S., Ku B., Kim M. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amanat F., Krammer F. SARS-CoV-2 Vaccines: Status Report. Immunity. 2020 doi: 10.1016/j.immuni.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schreiber Robert D., Old Lloyd J.S.M.J. Cancer immunoediting: Integrating the role of immunity in cancer suppression and promotion. Sci Signal. 2011;331:78. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 14.Ostrand-Rosenberg S. Immune surveillance: a balance between protumor and antitumor immunity. Curr Opin Genet Dev. 2008;18:11–18. doi: 10.1016/j.gde.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perez C.R., De Palma M. Engineering dendritic cell vaccines to improve cancer immunotherapy. Nat Commun. 2019;10:1–10. doi: 10.1038/s41467-019-13368-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faget J., Sisirak V., Blay J.Y., Caux C., Bendriss-Vermare N., Ménétrier-Caux C. ICOS is associated with poor prognosis in breast cancer as it promotes the amplification of immunosuppressive CD4+ T cells by plasmacytoid dendritic cells. Oncoimmunology. 2013;2:1–4. doi: 10.4161/onci.23185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conrad C., Gregorio J., Wang Y.H., Ito T., Meller S., Hanabuchi S. Plasmacytoid dendritic cells promote immunosuppression in ovarian cancer via ICOS costimulation of Foxp3+ T-regulatory cells. Cancer Res. 2012;72:5240–5249. doi: 10.1158/0008-5472.CAN-12-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aspord C., Leccia M.T., Charles J., Plumas J. Plasmacytoid dendritic cells support melanoma progression by promoting Th2 and regulatory immunity through OX40L and ICOSL. Cancer Immunol Res. 2013;1:402–415. doi: 10.1158/2326-6066.CIR-13-0114-T. [DOI] [PubMed] [Google Scholar]
- 19.Akira S. Innate immunity and adjuvants. Philos Trans R Soc B Biol Sci. 2011;366:2748–2755. doi: 10.1098/rstb.2011.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang W., Zhou Y., To K.F. The large tumor suppressor family: Friend or foe. J Thorac Dis. 2017;9:1748–1751. doi: 10.21037/jtd.2017.06.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saadeldin M.K., Shawer H., Mostafa A., Kassem N.M., Amleh A., Siam R. New genetic variants of LATS1 detected in urinary bladder and colon cancer. Front Genet. 2015;5:1–11. doi: 10.3389/fgene.2014.00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brewitz A., Eickhoff S., Dähling S., Quast T., Bedoui S., Kroczek R.A. CD8+ T Cells Orchestrate pDC-XCR1+ Dendritic Cell Spatial and Functional Cooperativity to Optimize Priming. Immunity. 2017;46:205–219. doi: 10.1016/j.immuni.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mellman I., Coukos G., Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolchok J.D., Hoos A., O’Day S., Weber J.S., Hamid O., Lebbé C. Guidelines for the evaluation of immune therapy activity in solid tumors: Immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 25.Schuler G. Dendritic cells in cancer immunotherapy. 2010:2123–2130. doi: 10.1002/eji.201040630. [DOI] [PubMed] [Google Scholar]
- 26.Sabado R.L., Bhardwaj N. Dendritic cell immunotherapy. 2013:31–45. doi: 10.1111/nyas.12125. [DOI] [PubMed] [Google Scholar]
- 27.Nina S.M.B. Re-emergence of Dendritic Cell Vaccines for Cancer Treatment. Physiol Behav. 2018;176:139–148. doi: 10.1016/j.physbeh.2017.03.040. [DOI] [Google Scholar]
- 28.Fenoglio D., Traverso P., Parodi A., Kalli F., Zanetti M., Filaci G. Generation of more effective cancer vaccines. Hum Vaccines Immunother. 2013;9:2543–2547. doi: 10.4161/hv.26147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phuphanich S., Wheeler C.J., Rudnick J.D., Mazer M., Wang H., Nuño M.A. Phase i trial of a multi-epitope-pulsed dendritic cell vaccine for patients with newly diagnosed glioblastoma. Cancer Immunol Immunother. 2013;62:125–135. doi: 10.1007/s00262-012-1319-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamada A., Sasada T., Noguchi M., Itoh K. Next-generation peptide vaccines for advanced cancer. Cancer Sci. 2013;104:15–21. doi: 10.1111/cas.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fenoglio D., Traverso P., Parodi A., Tomasello L., Negrini S., Kalli F. A multi-peptide, dual-adjuvant telomerase vaccine (GX301) is highly immunogenic in patients with prostate and renal cancer. Cancer Immunol Immunother. 2013;62:1041–1052. doi: 10.1007/s00262-013-1415-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aucouturier J., Dupuis L., Deville S., Ascarateil S., Ganne V. Montanide ISA 720 and 51: A new generation of water in oil emulsions as adjuvants for human vaccines. Expert Rev Vaccines. 2002;1:111–118. doi: 10.1586/14760584.1.1.111. [DOI] [PubMed] [Google Scholar]
- 33.Schön M.P., Schön M. Imiquimod: Mode of action. Br J Dermatol. 2007;157:8–13. doi: 10.1111/j.1365-2133.2007.08265.x. [DOI] [PubMed] [Google Scholar]
- 34.Filaci G., Fenoglio D., Fravega M., Ansaldo G., Borgonovo G., Traverso P. CD8 + CD28 − T Regulatory Lymphocytes Inhibiting T Cell Proliferative and Cytotoxic Functions Infiltrate Human Cancers. J Immunol. 2007;179:4323–4334. doi: 10.4049/jimmunol.179.7.4323. [DOI] [PubMed] [Google Scholar]
- 35.Hashemi V., Farhadi S., Ghasemi Chaleshtari M., Seashore-Ludlow B., Masjedi A., Hojjat-Farsangi M. Nanomedicine for improvement of dendritic cell-based cancer immunotherapy. Int Immunopharmacol. 2020;83 doi: 10.1016/j.intimp.2020.106446. [DOI] [PubMed] [Google Scholar]
- 36.Drake C.G. Update on prostate cancer vaccines. Cancer J. 2011;17:294–299. doi: 10.1097/PPO.0b013e3182325e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hodge J.W., Sabzevari H., Yafal A.G., Gritz L., Lorenz M.G.O., Schlom J. A triad of costimulatory molecules synergize to amplify T-cell activation. Cancer Res. 1999;59:5800–5807. [PubMed] [Google Scholar]
- 38.Lebeau A.M., Kostova M., Craik C.S., Denmeade S.R. Prostate-specific antigen: An overlooked candidate for the targeted treatment and selective imaging of prostate cancer. Biol Chem. 2010;391:333–343. doi: 10.1515/BC.2010.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song N., Guo H., Ren J., Hao S., Wang X. Synergistic anti-tumor effects of dasatinib and dendritic cell vaccine on metastatic breast cancer in a mouse model. Oncol Lett. 2018;15:6831–6838. doi: 10.3892/ol.2018.8188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Acker H.H., Versteven M., Lichtenegger F.S., Roex G., Campillo-Davo D., Lion E. Dendritic Cell-Based Immunotherapy of Acute Myeloid Leukemia. J Clin Med. 2019;8:579. doi: 10.3390/jcm8050579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Gulijk M., Dammeijer F., Aerts J.G.J.V., Vroman H. Combination Strategies to Optimize Efficacy of Dendritic Cell-Based Immunotherapy. Front Immunol. 2018;9:2759. doi: 10.3389/fimmu.2018.02759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ayoub N.M., Al-Shami K.M., Yaghan R.J. Immunotherapy for HER2-positive breast cancer: Recent advances and combination therapeutic approaches. Breast Cancer Targets Ther. 2019;11:53–69. doi: 10.2147/BCTT.S175360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anguille S., Smits E.L., Lion E., Van Tendeloo V.F., Berneman Z.N. Clinical use of dendritic cells for cancer therapy. Lancet Oncol. 2014;15:257–267. doi: 10.1016/S1470-2045(13)70585-0. [DOI] [PubMed] [Google Scholar]
- 44.Galluzzi L., Buqué A., Kepp O., Zitvogel L., Kroemer G. Immunological Effects of Conventional Chemotherapy and Targeted Anticancer Agents. Cancer Cell. 2015;28:690–714. doi: 10.1016/j.ccell.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 45.Veltman J.D., Lambers M.E.H., Van Nimwegen M., De Jong S., Hendriks R.W., Hoogsteden H.C. Low-dose cyclophosphamide synergizes with dendritic cell-based immunotherapy in antitumor activitys. J Biomed Biotechnol. 2010;2010. doi: 10.1155/2010/798467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu J.Y., Wu Y., Zhang X.S., Yang J.L., Li H.L., Mao Y.Q. Single administration of low dose cyclophosphamide augments the antitumor effect of dendritic cell vaccine. Cancer Immunol Immunother. 2007;56:1597–1604. doi: 10.1007/s00262-007-0305-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Borch T.H., Engell-Noerregaard L., Zeeberg Iversen T., Ellebaek E., Met Ö., Hansen M. mRNA-transfected dendritic cell vaccine in combination with metronomic cyclophosphamide as treatment for patients with advanced malignant melanoma. Oncoimmunology. 2016;5. doi: 10.1080/2162402X.2016.1207842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cornelissen R., Hegmans J.P.J.J., Maat A.P.W.M., Kaijen-Lambers M.E.H., Bezemer K., Hendriks R.W. Extended tumor control after dendritic cell vaccination with low-dose cyclophosphamide as adjuvant treatment in patients with malignant pleural mesothelioma. Am J Respir Crit Care Med. 2016;193:1023–1031. doi: 10.1164/rccm.201508-1573OC. [DOI] [PubMed] [Google Scholar]
- 49.Dréan A, Goldwirt L, Verreault M, Canney M, Schmitt C, Guehennec J, et al. Blood-brain barrier, cytotoxic chemotherapies and glioblastoma. vol. 16. 2016. https://doi.org/10.1080/14737175.2016.1202761. [DOI] [PubMed]
- 50.Teitz-Tennenbaum S., Li Q., Okuyama R., Davis M.A., Sun R., Whitfield J. Mechanisms involved in radiation enhancement of intratumoral dendritic cell therapy. J Immunother. 2008;31:345–358. doi: 10.1097/CJI.0b013e318163628c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brix N., Tiefenthaller A., Anders H., Belka C., Lauber K. Abscopal, immunological effects of radiotherapy: Narrowing the gap between clinical and preclinical experiences. Immunol Rev. 2017;280:249–279. doi: 10.1111/imr.12573. [DOI] [PubMed] [Google Scholar]
- 52.Abdel-Aziz A.K., Saadeldin M.K., D’Amico P., Orecchioni S., Bertolini F., Curigliano G. Preclinical models of breast cancer: Two-way shuttles for immune checkpoint inhibitors from and to patient bedside. Eur J Cancer. 2019;122:22–41. doi: 10.1016/j.ejca.2019.08.013. [DOI] [PubMed] [Google Scholar]
- 53.Baumeister S.H., Freeman G.J., Dranoff G., Sharpe A.H. Coinhibitory Pathways in Immunotherapy for Cancer. Annu Rev Immunol. 2016;34:539–573. doi: 10.1146/annurev-immunol-032414-112049. [DOI] [PubMed] [Google Scholar]
- 54.Gato-Cañas M., Zuazo M., Arasanz H., Ibañez-Vea M., Lorenzo L., Fernandez-Hinojal G. PDL1 Signals through Conserved Sequence Motifs to Overcome Interferon-Mediated Cytotoxicity. Cell Rep. 2017;20:1818–1829. doi: 10.1016/j.celrep.2017.07.075. [DOI] [PubMed] [Google Scholar]
- 55.Rosenblatt J., Glotzbecker B., Mills H., Vasir B., Tzachanis D., Levine J.D. PD-1 blockade by CT-011, anti-PD-1 antibody, enhances ex vivo t-cell responses to autologous dendritic cell/myeloma fusion vaccine. J Immunother. 2011;34:409–418. doi: 10.1097/CJI.0b013e31821ca6ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nagaoka K., Hosoi A., Iino T., Morishita Y., Matsushita H., Kakimi K. Dendritic cell vaccine induces antigen-specific CD8+ T cells that are metabolically distinct from those of peptide vaccine and is well-combined with PD-1 checkpoint blockade. Oncoimmunology. 2018;7:1–14. doi: 10.1080/2162402X.2017.1395124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Antonios J.P., Soto H., Everson R.G., Orpilla J., Moughon D., Shin N. PD-1 blockade enhances the vaccination-induced immune response in glioma. JCI Insight. 2019;1:1–13. doi: 10.1172/jci.insight.87059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ge Y., Xi H., Ju S., Zhang X. Blockade of PD-1/PD-L1 immune checkpoint during DC vaccination induces potent protective immunity against breast cancer in hu-SCID mice. Cancer Lett. 2013;336:253–259. doi: 10.1016/j.canlet.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 59.Chambers C.A., Kuhns M.S., Egen J.G., Allison J.P. CTLA -4-M EDIATED I NHIBITION IN R EGULATION OF T C ELL R ESPONSES : Mechanisms and Manipulation in Tumor Immunotherapy. Annu Rev Immunol. 2001;19:565–594. doi: 10.1146/annurev.immunol.19.1.565. [DOI] [PubMed] [Google Scholar]
- 60.Blank C.U.E.A. Therapeutic use of anti-CTLA-4 antibodies. Int Immunol. 2017;27:3–10. doi: 10.1093/intimm/dxu076. [DOI] [PubMed] [Google Scholar]
- 61.Boudewijns S., Koornstra R.H.T., Westdorp H., Schreibelt G., van den Eertwegh A.J.M., Geukes Foppen M.H. Ipilimumab administered to metastatic melanoma patients who progressed after dendritic cell vaccination. Oncoimmunology. 2016;5 doi: 10.1080/2162402X.2016.1201625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wilgenhof S., Corthals J., Heirman C., Van Baren N., Lucas S., Kvistborg P. Phase II study of autologous monocyte-derived mRNA electroporated dendritic cells (TriMixDC-MEL) plus ipilimumab in patientswith pretreated advanced melanoma. J Clin Oncol. 2016;34:1330–1338. doi: 10.1200/JCO.2015.63.4121. [DOI] [PubMed] [Google Scholar]
- 63.Tortorici M.A.V.D. Structural insights into coronavirus entry. Adv Virus Res. 2019;105:93–116. doi: 10.1016/bs.aivir.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cui J., Li F., Shi Z.L. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brufsky A., Lotze M.T. DC/L-SIGNs of Hope in the COVID-19 Pandemic. J Med Virol. 2020 doi: 10.1002/jmv.25980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Geijtenbeek T.B.H., Kwon D.S., Torensma R., Van Vliet S.J., Van Duijnhoven G.C.F., Middel J. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–597. doi: 10.1016/S0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 67.Gardner J.P., Durso R.J., Arrigale R.R., Donovan G.P., Maddon P.J., Dragic T. L-SIGN (CD 209L) is a liver-specific capture receptor for hepatitis C virus. Proc Natl Acad Sci U S A. 2003;100:4498–4503. doi: 10.1073/pnas.0831128100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Halary F., Amara A., Lortat-Jacob H., Messerle M., Delaunay T., Houlès C. Human Cytomegalovirus binding to DC-SIGN is required for dendritic cell infection and target cell trans-infection. Immunity. 2002;17:653–664. doi: 10.1016/S1074-7613(02)00447-8. [DOI] [PubMed] [Google Scholar]
- 69.Tassaneetrithep B., Burgess T.H., Granelli-Piperno A., Trumpfheller C., Finke J., Sun W. DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells. J Exp Med. 2003;197:823–829. doi: 10.1084/jem.20021840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marzi A., Gramberg T., Simmons G., Möller P., Rennekamp A.J., Krumbiegel M. DC-SIGN and DC-SIGNR Interact with the Glycoprotein of Marburg Virus and the S Protein of Severe Acute Respiratory Syndrome Coronavirus. J Virol. 2004;78:12090–12095. doi: 10.1128/jvi.78.21.12090-12095.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shang J., Ye G., Shi K., Wan Y., Luo C., Aihara H. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020:1–8. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Callaway Ewen S.N. The race for coronavirus vaccines. Nature. 2020;580:576–577. doi: 10.1038/d41586-020-01221-y. [DOI] [PubMed] [Google Scholar]
- 73.Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic Treatments for Coronavirus Disease (COVID-19): A Review. JAMA - J Am Med Assoc. 2019;2020:2019. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]