Abstract
Introduction and objectives
Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2. Atrial fibrillation (AF) is common in acute situations, where it is associated with more complications and higher mortality.
Methods
Analysis of the international HOPE registry (NCT04334291). The objective was to assess the prognostic information of AF in COVID-19 patients. A multivariate analysis and propensity score matching were performed to assess the relationship between AF and mortality. We also evaluated the impact on mortality and embolic events of the CHA2DS2-VASc score in these patients.
Results
Among 6217 patients enrolled in the HOPE registry, 250 had AF (4.5%). AF patients had a higher prevalence of cardiovascular risk factors and comorbidities. After propensity score matching, these differences were attenuated. Despite this, patients with AF had a higher incidence of in-hospital complications such as heart failure (19.3% vs 11.6%, P = .021) and respiratory insufficiency (75.9% vs 62.3%, P = .002), as well as a higher 60-day mortality rate (43.4% vs 30.9%, P = .005). On multivariate analysis, AF was independently associated with higher 60-day mortality (hazard ratio, 1.234; 95%CI, 1.003-1.519). CHA2DS2-VASc score acceptably predicts 60-day mortality in COVID-19 patients (area ROC, 0.748; 95%CI, 0.733-0.764), but not its embolic risk (area ROC, 0.411; 95%CI, 0.147-0.675).
Conclusions
AF in COVID-19 patients is associated with a higher number of complications and 60-day mortality. The CHA2DS2-VASc score may be a good risk marker in COVID patients but does not predict their embolic risk.
Keywords: COVID-19, SARS-CoV-2, Mortality, Registry, Prognosis, Atrial fibrillation, CHA2DS2-VASc, Bleeding
Abbreviations: AF, atrial fibrillation; PSM, propensity score matching
Abstract
Introducción y objetivos
La enfermedad por coronavirus de 2019 (COVID-19) está causada por el segundo coronavirus del síndrome respiratorio agudo y grave. La fibrilación auricular (FA) es común en situaciones agudas, en las que conlleva más complicaciones y mortalidad.
Métodos
Análisis del Registro internacional HOPE (NCT04334291); el objetivo es evaluar la información pronóstica de FA en pacientes con COVID-19. Se realizó un análisis multivariable y un emparejamiento por puntuación de propensión para evaluar la relación entre FA y mortalidad. Además, se evaluó en estos pacientes el impacto en la mortalidad y los eventos embólicos de la puntuación CHA2DS2-VASc.
Resultados
Entre los 6.217 pacientes inscritos en el registro HOPE, 250 tenían FA (4,5%). Los pacientes con FA tenían una mayor prevalencia de factores de riesgo cardiovascular y comorbilidades. Después del emparejamiento por puntuación de propensión, estas diferencias se atenuaron. A pesar de ello, los pacientes con FA tuvieron una mayor incidencia de complicaciones hospitalarias como insuficiencia cardiaca (el 19,3 frente al 11,6%; p = 0,021) e insuficiencia respiratoria (el 75,9 frente al 62,3%; p = 0,002), así como una mayor tasa de mortalidad a los 60 días (el 43,4 frente al 30,9%; p = 0,005). En el análisis multivariado, la FA se asoció de manera independiente con una mayor mortalidad a los 60 días (hazard ratio = 1,234; IC95%, 1,003-1,519). La puntuación CHA2DS2-VASC predice de manera aceptable la mortalidad a los 60 días de los pacientes con COVID-19 (área ROC = 0,748; IC95%, 0,733-0,764), pero no su riesgo embólico (área ROC = 0,411; IC95%, 0,147-0,675).
Conclusiones
La FA en pacientes con COVID-19 se asocia con más complicaciones y mayor mortalidad a los 60 días. La puntuación CHA2DS2-VASc puede ser un buen marcador de riesgo en pacientes con COVID-19, pero no predice su riesgo embólico.
Palabras clave: COVID-19, SARS-CoV-2, Mortalidad, Registro, Pronóstico, Fibrilación auricular, CHA2DS2-VASc, Hemorragia
INTRODUCTION
In January, 2020, a novel virus, known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was identified as the sole causative agent for a cluster of pneumonia cases initially detected in Wuhan City, Hubei province, China.1 SARS-CoV-2, which causes the disease now named coronavirus disease 2019 (COVID-19), has spread from China to the rest of the world.2, 3
Currently the percentage of asymptomatic infected carriers is unknown, but several studies indicate that it could be very high.4 In symptomatic patients, the clinical spectrum of SARS-CoV-2 infection appears to be wide, encompassing mild upper respiratory tract illness, and severe viral pneumonia with respiratory failure and even death.5 Most fatal cases occurred in patients with advanced age or underlying medical comorbidities such us cardiovascular disease, hypertension, diabetes mellitus, chronic lung disease, and chronic kidney disease.6, 7
Atrial fibrillation (AF) is the most frequent arrythmia worldwide, and its prevalence is higher in patients with cardiovascular risk factors and other comorbidities.8 This arrythmia is common in the context of acute situations such as myocardial infarction, cardiac surgery or infections, where it is linked with a higher risk of complications and mortality.9 However, there is no work specifically addressing the impact of AF on the prognosis of COVID-19. Here, we present details of an international registry of patients discharged from a hospital with laboratory-confirmed or high suspicion SARS-CoV-2 infection and definite clinical outcomes. We aimed to describe the clinical features and prognosis of COVID-19 patients with AF and to evaluate the impact of this arrythmia on the short-term prognosis of the disease. Additionally, we also aimed to investigate the impact of the CHA2DS2-VASc score and anticoagulation treatment during admission for the prognosis in this population. The CHA2DS2-VASc score is a simple stroke risk stratification schema, based on a risk factor approach (congestive heart failure/left ventricular dysfunction, hypertension, age, diabetes mellitus, stroke, vascular disease, and sex), and offers good predictive value for embolic events in patients with AF.8, 10
METHODS
Study design and population
This is a subanalysis of the Health Outcome Predictive Evaluation (HOPE) COVID-19 registry, with an overall study sample of 6217 patients with a definitive diagnosis or high suspicion of SARS-CoV-2 infection.11 In brief, the HOPE registry is a retrospective cohort registry (ie, a “real-world” all comers type, with voluntary participation and with no financial compensation. All patients discharged (deceased or alive) after hospital admissions for COVID-19 were suitable for the study. There were no exclusion criteria, except for patients’ explicit refusal to participate. From March 23, 2020 to June 1, 2020 all patients fulfilling the inclusion criteria from 24 centers in Spain were assessed in the present study. Clinical and demographic data were collected at inclusion and during hospitalization. The study was performed according to the ethical principles of the Declaration of Helsinki and Good Clinical Practice Guidelines and was approved by the local Ethics Research Committee of the Hospital Clínico San Carlos (Madrid, Spain) (20/241-E). Written informed consent was waived because of the characteristics of the anonymized registry and the severity of the situation. However, at least verbal authorization from the patient (or familiar or caregiver when unavailable) was required. A list of participating hospitals, investigators, collaborators and the protocol are available in the appendix of the supplementary data.
Definitions and study outcomes
Enrolled patients were divided into 2 groups according to AF history. Study definitions are available elsewhere on the registry's website.12 The primary endpoint was all-cause mortality at 60-days in either cohort. We evaluated whether the use of the CHA2DS2-VASc risk score was useful to assess the risk of death or embolism in patients with COVID-19. The CHA2DS2-VASc score was not recorded in the original dataset, but was obtained retrospectively for this research study. Enrolled patients were divided into 3 groups according to their CHA2DS2-VASc score (group 1: CHA2DS2-VASc = 0 in men and ≤ 1 in women; group 2: CHA2DS2-VASc = 1 in men or 2 in women; and group 3: CHA2DS2-VASc > 1 in men or > 2 in women). Other clinically relevant events were recorded as secondary endpoints: invasive mechanical ventilation, noninvasive mechanical ventilation, respiratory insufficiency, heart failure, renal failure, sepsis, systemic inflammatory response syndrome, clinically relevant bleeding, and embolic events. Events were classified following local researchers’ criteria according to the definitions of the HOPE COVID-19 registry. The vital status at 60 days of the patients discharged alive was confirmed by telephone interview.
Statistical analysis
Data are presented as mean ± standard deviation for continuous variables with a normal distribution, median (interquartile range [IQR]) for continuous variables with a nonnormal distribution, and as frequency (%) for categorical variables. The student t test and the Mann-Whitney U test were used to compare continuous variables with normal and nonnormal distributions, when needed. The chi-square test or Fisher exact test was used to compare categorical variables. Univariate analysis was performed for qualitative variables and reported as odds ratios with 95% confidence interval (95%CI). Given the multiplicity of variables, only factors associated with all-cause mortality with P < .01 on univariate analysis (age, sex, hypertension, dyslipidemia, diabetes mellitus, smoke, chronic kidney failure, ischemic heart disease, heart failure, lung disease, cerebrovascular disease, cancer, renin-angiotensin-aldosterone system inhibitor treatment, AF, aspirin treatment, anticoagulation treatment, saturation O2 < 92% on admission, D-dimer elevation, C-reactive protein elevation, lactate dehydrogenase elevation) were entered into the Cox multivariate regression analysis to define independent risk factors for the main outcome in the matched population. The assumption of proportionality of risks was verified by analyzing Schoenfeld residuals. The C-index and Gronesby Borgan test were calculated to determine discrimination and calibration, respectively. Kaplan-Meier curves were used to estimate survival function and compare subgroups with the log-rank test. Possible collinearity and interactions were evaluated with the introduction of multiplicative terms calculating the tolerance and the variance inflation factor. Propensity score matching (PSM) was estimated with AF as the dependent variable and the main clinical profiles at admission (PSM 1:1; 0.01 tolerance, without replacement, nearest neighbor) to obtain balanced pairs. The MatchIt package (Ho, Imai, King, & Stuart, 2007) was used. Variables included in the PSM were age, dyslipidemia, smoking, cerebrovascular disease, lung disease and were exactly added (sex, hypertension, diabetes, chronic kidney failure, and any type of cancer). Quality adjustment generated by the PSM model is shown in the figure 1 of the supplementary data. The area under the receiver operating characteristic curve (ROC curve) was used to measure how well the models discriminated the CHA2DS2-VASc score for 60-day all-cause mortality and risk of in-hospital embolic event. All tests were 2-sided. The statistical analysis was performed with the IBM SPSS 24.0 software package, STATA software, version 15 and R Core Team (2020).
RESULTS
Of the 6217 patients consecutively enrolled in the HOPE COVID-19 registry, 250 had history of AF (4.2%) and 1687 patients were men (60.3%). After matching for the main baseline confounding factors, 233 patients with AF and 233 without AF were selected for the definitive analysis.
Baseline characteristics
The percentage of patients testing positive patients for SARS-CoV-2 infection by nasopharyngeal PCR was 89%. The baseline characteristics of COVID-19 patients are shown in table 1 . Mean age was 66 ± 17 years, 57.6% of patient were male and the median interval from disease onset to admission was 6 [IQR 5] days. Of the total reported patients, 3020 (50.3%) had hypertension, 2237 (37.5%) dyslipidemia, 1237 (19.9%) diabetes mellitus, 1243 (30.5%) previous pulmonary disease, 265 (4.2%) heart failure, and 416 (7.1%) chronic kidney failure.
Table 1.
Features of COVID-19 patients and comparative analysis according to atrial fibrillation
| Before PSM |
After PSM |
|||||||
|---|---|---|---|---|---|---|---|---|
| Overall N = 6217 |
AF n = 250 |
No AF n = 5967 |
P | Overall n = 466 |
AF n = 233 |
No AF n = 233 |
P | |
|
Demographic | ||||||||
| Male, % |
3579 (57.6) |
171 (68.4) |
3408 (57.1) |
< .001 |
268 (57.5) |
134 (57.5) |
134 (57.5) |
1 |
| Age, y |
65.7 ± 16.8 |
79.9 ± 9.9 |
65.1 ± 16.7 |
< .001 |
79.4 ± 10.7 |
79.7 ± 9.7 |
79.1 ± 11.5 |
.538 |
| BMI, kg/m2 |
28.8 ± 5.2 |
28.8 ± 4.4 |
28.8 ± 5.2 |
.229 |
28.5 ± 4.2 |
28.5 ± 4.2 |
28.6 ± 4.4 |
.858 |
|
Comorbidities | ||||||||
| Hypertension |
3020 (50.3) |
203 (81.2) |
2817 (49.0) |
< .001 |
378 (81.1) |
189 (81.1) |
189 (81.1) |
1 |
| Diabetes mellitus |
1237 (19.9) |
77 (30.8) |
1160 (19.4) |
< .001 |
138 (29.6) |
69 (29.6) |
69 (29.6) |
1 |
| Hypercholesterolemia |
2237 (37.5) |
140 (56.7) |
2097 (36.7) |
< .001 |
262 (56.2) |
132 (56.7) |
130 (55.8) |
.852 |
| Smoker |
271 (5.1) |
10 (4.1) |
261 (5.1) |
.478 |
23 (4.9) |
10 (4.3) |
13 (6.3) |
.354 |
| Lung disease |
1243 (30.5) |
87 (47.3) |
1156 (29.7) |
< .001 |
159 (34.1) |
81 (34.8) |
78 (33.5) |
.769 |
| Chronic kidney disease |
416 (7.1) |
370 (88.9) |
46 (18.8) |
< .001 |
82 (17.6) |
41 (17.6) |
41 (17.6) |
1 |
| Obesity |
1174 (24.5) |
59 (27.7) |
1115 (24.3) |
.263 |
103 (27.2) |
56 (28.1) |
47 (26.1) |
.657 |
| Heart failure |
265 (4.2) |
15 (6.0) |
250 (4.2) |
.234 |
176 (37.8) |
110 (47.2) |
66 (28.3) |
< .001 |
| Ischemic heart disease |
396 (6.4) |
9 (3.6) |
387 (6.5) |
.067 |
35 (7.5) |
9 (3.9) |
26 (11.2) |
.003 |
| Cardiomyopathy |
127 (2.0) |
6 (2.4) |
121 (2.0) |
.684 |
19 (4.1) |
6 (2.6) |
13 (5.6) |
.101 |
| Cerebrovascular disease |
479 (8.1) |
33 (13.4) |
446 (7.9) |
.002 |
68 (14.6) |
31 (13.3) |
37 (15.9) |
.431 |
| Any cancer |
861 (14.6) |
51 (20.7) |
810 (14.3) |
.005 |
94 (20.2) |
47 /20.2) |
47 (20.2) |
1 |
|
Concomitant treatment | ||||||||
| Beta-blockers |
933 (15.7) |
123 (49.6) |
810 (14.2) |
< .001 |
179 (38.6) |
120 (51.7) |
59 (25.4) |
< .001 |
| ACEi/ARBs |
2214 (37.1) |
2090 (36.6) |
124 (49.8) |
< .001 |
261 (56.3) |
118 (50.9) |
143 (61.6) |
.019 |
| Antiplatelet therapy |
901 (15.2) |
23 (9.2) |
878 (15.4) |
.008 |
85 (18.4) |
22 (9.5) |
63 (27.3) |
< .001 |
| Oral anticoagulation therapy |
651 (10.9) |
214 (85.6) |
437 (7.6) |
< .001 |
230 (49.3) |
198 (85.0) |
32 (13.8) |
< .001 |
| Vitamin K antagonists |
535 (82.2) |
145 (67.8) |
390 (89.2) |
163 (70.9) |
137 (69.2) |
26 (81.3) |
||
| Direct-acting oral anticoagulants |
116 (17.8) |
69 (32.2) |
47 (10.8) |
67 (29.1) |
61 (30.8) |
6 (18.7) |
||
|
Laboratory parameters | ||||||||
| Creatinine, mg/dL |
1.1 ± 1.7 |
1.5 ± 1.3 |
1.1 ± 1.6 |
.035 |
1.4 ± 1.2 |
1.3 ± 1.0 |
1.5 ± 1.4 |
.071 |
| Hemoglobin, g/dL |
13.5 ± 1.9 |
12.7 ± 2.4 |
13.6 ± 1.9 |
< .001 |
12.9 ± 2.3 |
13.1 ± 2.2 |
12.8 ± 2.4 |
.146 |
| Platelet count, x 109/L |
213 ± 95 |
192 ± 90 |
213 ± 85 |
.513 |
203 ± 95 |
231 ± 99 |
192 ± 89 |
.017 |
| Lymphocytes, g/dL |
1229 ± 1715 |
1276 ± 3279 |
1228 ± 161 |
.015 |
1318 ± 3063 |
1330 ± 2726 |
1305 ± 3377 |
.930 |
| Elevated D-dimer |
3633 (69.7) |
151 (71.2) |
3482 (69.6) |
.619 |
292 (75.1) |
140 (71.4) |
152 (78.8) |
.095 |
| Elevated procalcitonin |
832 (13.4) |
41 (30.1) |
791 (21.6) |
.018 |
87 (30.5) |
40 (31.3) |
47 (29.9) |
.811 |
| Elevated C-reactive protein |
5249 (90.0) |
217 (90.0) |
5032 (90.0) |
.990 |
408 (90.5) |
202 (90.2) |
206 (90.7) |
.837 |
| Elevated troponins |
382 (14.1) |
28 (31.5) |
354 (13.5) |
< .001 |
50 (25.8) |
26 (31.3) |
24 (21.6) |
.126 |
| Elevated transaminases |
2304 (41.9) |
73 (31.1) |
2231 (42.4) |
.001 |
150 (34.6) |
69 (31.5) |
81 (37.9) |
.165 |
| Elevated ferritin |
2018 (64.0) |
70 (65.4) |
1948 (63.9) |
.753 |
140 (63.1) |
67 (67.0) |
73 (59.8) |
.271 |
| Elevated LDH | 4155 (77.0) | 175 (77.4) | 3980 (77.0) | .879 | 327 (78.2) | 164 (77.7) | 163 (78.7) | .801 |
ACEi, angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; BMI, body mass index; LDH, lactate dehydrogenase; PSM, propensity score matching.
The data are presented as No. (%) or mean ± standard deviation.
Patients were categorized in 2 groups according to history of AF. When we compared these groups, we observed that patients with AF were older and had a greater number of comorbidities. Furthermore, this group had more frequently received prior treatment with antiplatelets, anticoagulants, and renin-angiotensin-aldosterone system inhibitors. These differences were controlled after statistical matching (table 1).
Treatment and outcomes during admission
Management is depicted in table 2 . The specific COVID-19 drugs most frequently used were hydroxychloroquine (86.1%), followed by antibiotics (77.9%) and lopinavir/ritonavir (54.4%). Corticoids were prescribed in approximately 31% of the patients. For respiratory support, prone positioning was used in 7%, and noninvasive mechanical ventilation in 10%. An invasive mechanical ventilation approach was required in more than 6%. When we compared these groups according to AF, antiviral drugs and tocilizumab were more frequently used in non-AF patients; in contrast, corticoids and antibiotics were more frequently used in AF patients. After PSM these differences were attenuated (table 2).
Table 2.
Adverse events during hospitalization in patients with COVID-19 and comparative analysis according to atrial fibrillation
| Before PSM | After PSM | |||||||
|---|---|---|---|---|---|---|---|---|
| Overall N = 6217 |
AF n = 250 |
No AF n = 5967 |
P | Overall N = 466 |
AF n = 233 |
No AF n = 233 |
P | |
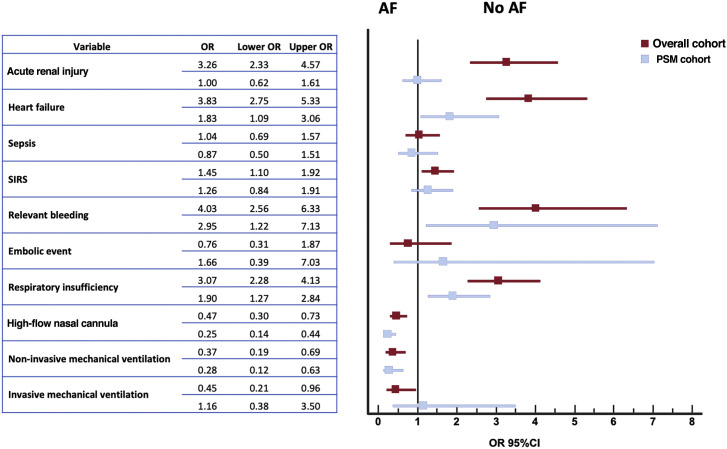
| Acute renal injury | 1047 (17.8) | 95 (38.6) | 952 (16.8) | < .001 | 156 (33.5) | 87 (37.3) | 69 (29.6) | .077 |
| Heart failure | 406 (6.9) | 49 (20.0) | 357 (6.3) | < .001 | 72 (15.5) | 45 (19.3) | 27 (11.6) | .021 |
| Sepsis | 1351 (23.2) | 27 (10.9) | 589 (10.5) | .842 | 57 (12.4) | 27 (11.6) | 30 (13.2) | .608 |
| Systemic inflammatory response syndrome | 1351 (23.2) | 74 (30.1) | 1277 (22.9) | .009 | 126 (27.5) | 69 (29.7) | 57 (25.1) | .266 |
| Relevant bleeding | 169 (2.9) | 24 (9.8) | 145 (2.6) | < .001 | 27 (5.9) | 20 (8.7) | 7 (3.1) | .012 |
| Embolic event | 154 (2.6) | 5 (2.0) | 149 (2.7) | .544 | 8 (1.7) | 5 (2.2) | 3 (1.3) | .487 |
| Respiratory insufficiency | 3100 (52.3) | 190 (76.3) | 2910 (51.2) | < .001 | 320 (69.1) | 176 (75.9) | 144 (62.3) | .002 |
| High flow nasal cannula | 994 (17.1) | 22 (9.1) | 972 (17.4) | .001 | 76 (16.8) | 18 (7.9) | 58 (25.7) | < .001 |
| Noninvasive mechanical ventilation | 589 (10.0) | 10 (4.0) | 579 (10.3) | .001 | 34 (7.4) | 8 (3.4) | 26 (11.4) | .001 |
| Invasive mechanical ventilation | 350 (6.0) | 7 (2.8) | 343 (6.1) | .033 | 13 (2.8) | 7 (3.0) | 6 (2.6) | .797 |
| Use of corticoids | 1819 (31.2) | 106 (42.6) | 1713 (30.7) | < .001 | 166 (36.5) | 98 (42.2) | 68 (30.5) | .009 |
| Use of hydroxychloroquine | 5094 (86.1) | 207 (83.1) | 4887 (86.2) | .166 | 374 (81.1) | 191 (82.3) | 183 (79.9) | .508 |
| Use of antiviral drugs | 3204 (54.4) | 97 (39.3) | 3107 (55.1) | < .001 | 190 (41.8) | 91 (39.6) | 99 (44.0) | .038 |
| Use of interferon or similar | 659 (11.4) | 21 (8.5) | 638 (11.5) | .142 | 46 (10.2) | 19 (8.2) | 27 (12.2) | .165 |
| Use of tocilizumab or similar | 540 (9.3) | 8 (3.3) | 532 (9.5) | .001 | 19 (4.2) | 8 (3.5) | 11 (4.9) | .452 |
| Use of antibiotics | 4323 (77.9) | 199 (84.3) | 4124 (77.6) | .015 | 345 (79.7) | 184 (83.3) | 161 (75.9) | .059 |
| Anticoagulation | < .001 | < .001 | ||||||
| No | 990 (20.2) | 41 (17.3) | 949 (20.4) | 116 (25.3) | 38 (16.7) | 78 (33.9) | ||
| Prophylactic dose | 3068 (62.7) | 61 (25.7) | 3007 (64.6) | 179 (39.1) | 58 (25.4) | 121 (52.6) | ||
| Complete dose | 837 (17.1) | 135 (57.0) | 702 (15.0) | 163 (35.6) | 132 (57.9) | 31 (13.5) | ||
AF, atrial fibrillation; PSM, propensity score matching.
The data are presented as No. (%).
In-hospital events are shown in figure 1 . The most common was bilateral pneumonia (75%) with concomitant respiratory insufficiency in 52.3% of all patients. In the overall COVID-19 population, acute renal injury, sepsis and systemic inflammatory response syndrome were reported in roughly 20% of the patients. Systemic inflammatory response syndrome, heart failure and respiratory failure were more frequent in the AF group. In parallel, we observed a higher incidence of hemorrhagic complications in the AF group (9.8% vs 2.6%; OR, 4.03; 95%CI, 2.56-6.33), but with comparable outcomes in embolic events between the 2 groups. Despite the statistical matching, the AF group continued to show a higher incidence of all these complications (table 2).
Figure 1.
Adverse events during hospitalization in patients with COVID-19 and comparative analysis according to atrial fibrillation. 95%CI, 95% confidence interval; AF, atrial fibrillation; OR, odds ratio; PSM, propensity score matching; SIRS, systemic inflammatory response syndrome.
In-hospital anticoagulation management
During hospitalization, close to 80% of all patients received some type of anticoagulation therapy, with 62.7% of them receiving a prophylactic dose, while 17.1% received the full anticoagulant dose. In particular, in the AF group, only 135 (57%) patients received anticoagulation at an appropriate dose [102 (75.6%) of them using intravenous/subcutaneous anticoagulation with heparin/enoxaparin, 18 (13.3%) with acenocumarol and 15 (11.1%) with a direct-acting anticoagulant], 61 (25.7%) received a prophylactic dose, and 41 (17.3%) did not receive anticoagulant treatment. We did not observe differences in age (79.4 vs 81.5; P = .248) or in the CHA2DS2-VASc score (3.8 vs 3.8, P = .277) between patients who had received some dose of anticoagulant treatment and those who had not. Despite this low percentage of patients treated with appropriate doses of anticoagulant treatment, the incidence of relevant bleeding complications during admission was higher in the AF group (9.8% vs 2.6%; OR, 4.03; 95%CI, 2.56-6.33). When we compared the entire cohort of patients, we observed that those who received full anticoagulant doses had a higher risk of bleeding than those who received only a prophylactic dose or did not receive any (OR full dose vs prophylactic dose 4.17; 95%CI, 2.90-6.00; and OR full dose vs any dose 3.32; 95%CI, 2.05-5.35). However, these differences were not observed when we analyzed only the group of patients with AF (OR full dose vs prophylactic dose 1.42, 95%CI, 0.49-4.11; and OR full dose vs any dose 1.57, 95%CI, 0.43-5.72). When we analyzed embolic events, we observed no differences between the groups based on the type of anticoagulant (2 [6.1%] in nonvitamin K antagonist oral anticoagulant group vs 4 [6.6%] in vitamin K antagonist group vs 33 [5.1%] in unfractionated heparin group vs 115 [3.9%] in low-molecular-weight-heparin group; P = .298) and dose received (40 [4.7%] in the full-dose anticoagulation group vs 99 [3.2%] prophylaxis group vs 15 [1.5%] group without anticoagulation; P = .231).
Prognostic impact of atrial fibrillation on COVID-19
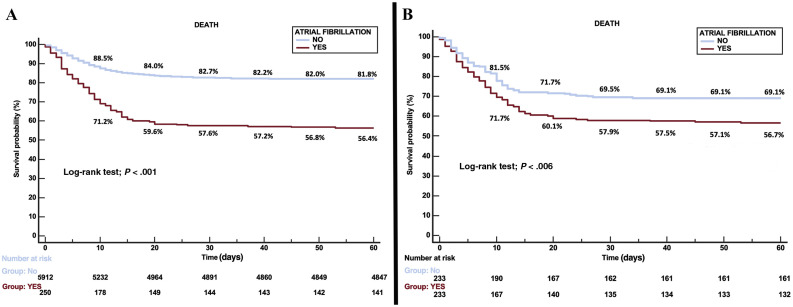
Univariable analysis of 60-day all-cause mortality from COVID-19 showed a linear relation between AF development and mortality (mortality in patients with AF 43.6% vs mortality in patients without AF 18%; OR, 3.51; 95%CI, 2.71-4.55). In the multivariate analysis (C-Index and 95% Jackknife CI, 0.750 (0.71-0.788), Groennesby and Borgan test P = .782) (table 3 ), the presence of AF was independently associated with 60-day all-cause mortality in these patients (HR, 1.234; 95%CI, 1.003-1.519) together with other variables such as age, lactate dehydrogenase elevated on admission, saturation on admission < 92%, and chronic kidney disease. In addition, we observed high mortality in patients who were on anticoagulant treatment before admission (41.9% vs 16.9%; OR 3.55; 95%CI, 2.99-4.28), specifically, 43.9% within the AF group. The 60-day Kaplan-Meier survival analysis after PSM confirmed the higher mortality among AF patients (figure 2 ).
Table 3.
Multivariate Cox regression analysis for evaluating the risk of 60-day all-cause mortality
| HR (95%CI) | P | |
|---|---|---|
| Age (per year) | 1.04 (1.02-1.06) | < .001 |
| Saturation on admission < 92% | 3.84 (2.65-5.58) | < .001 |
| Elevated LDH on admission | 1.65 (1.06 -1.58) | .027 |
| Chronic kidney disease | 1.78 (1.29-2.58) | .002 |
| Atrial fibrillation | 1.57 (1.12-2.20) | .009 |
95%CI, 95% confidence interval; HR, hazard ratio; LDH, lactate dehydrogenase.
Adjustment variables included in the full model: age, sex, hypertension, dyslipidemia, diabetes mellitus, smoking, chronic kidney failure, ischemic heart disease, heart failure, lung disease, cerebrovascular disease, cancer, renin-angiotensin-aldosterone system inhibitors treatment, atrial fibrillation, aspirin treatment, anticoagulation treatment, saturation O2 < 92% on admission, D-dimer elevation, C-reactive protein elevation, lactate dehydrogenase elevation.
Figure 2.
Kaplan-Meier survival landmark analysis according to atrial fibrillation history. A: before propensity score matching. B: after propensity score matching.
A total of 1185 patients died during the 60-day follow-up. The main causes of mortality in our registry were respiratory failure (59.2%), combined cause (19.9%), infectious etiology (6.2%), and systemic inflammatory response (5.0%). Cardiovascular cause was the main cause of death in 1.4% of the patients. In addition, a total of 154 embolic events were recorded during hospital admission, with no differences between the groups (table 2).
CHA2DS2-VASc score and mortality risk assessment
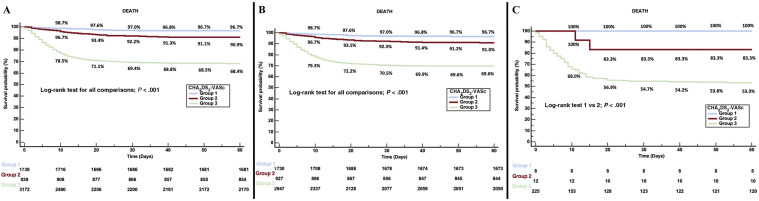
Kaplan-Meier survival landmark analysis according to the CHA2DS2-VASc score is shown in figure 3 . CHA2DS2-VASc score had a modest ability to predict 60-day all-cause mortality in the entire cohort (area ROC, 0.748; 95%CI, 0.733-0.764); however, it had a poor performance when the group of patients with AF was specifically evaluated (area ROC, 0.618; 95%CI, 0.546-0.689). Furthermore, CHA2DS2-VASc score was also unable to predict the incidence of embolism during admission in the overall cohort (area ROC, 0.519; 95%CI, 0.471-0.568) and AF group (area ROC, 0.411; 95%CI, 0.147-0.675).
Figure 3.
Kaplan-Meier survival landmark analysis according to the CHA2DS2-VASc score. Group 1: CHA2DS2-VASc = 0 in men and ≤ 1 in women; Group 2: CHA2DS2-VASc = 1 in men or 2 in women; and Group 3: CHA2DS2-VASc > 1 in men or > 2 in women. A: entire cohort. B: patients without AF. C: patients with AF.
DISCUSSION
COVID-19 patients with underlying cardiovascular disease have an increased risk of morbidity and mortality from complicated myocardial injury, myocarditis, congestive heart failure, thromboembolism, and arrhythmias.13 Interestingly, AF is the most common arrhythmia seen in tertiary care and critically ill patients.14 In fact, cardiac arrhythmias are among the most common comorbidities in COVID-19 patients and have been identified in almost all fatal cases.15 However, to date there is no evidence on whether AF contributes somehow to COVID-19 prognosis and we therefore report the first work specifically addressing the influence and prognostic role of AF on COVID-19. The main findings are: a) 4% of patients with COVID-19 had a prior history of AF before hospitalization; b) patients with COVID-19 and AF had higher 60-day all-cause mortality; c) AF is an independent predictor of mortality; d) patients with a high CHA2DS2-VASc score had higher 60-day all-cause mortality; e) CHA2DS2-VASc score is not useful for predicting the incidence of embolic events during SARS-CoV-2 infection; f) full-dose anticoagulant therapy may increase bleeding complications.
AF is the most common arrhythmia worldwide, and it is known that its prevalence is higher among the elderly and patients with conditions such as hypertension, diabetes mellitus, chronic kidney disease, and heart disease.16 Our data confirm a higher burden of cardiovascular risk factors in this group of patients, but in the multivariate analysis, the presence of AF was independently associated with COVID-19 prognosis. Therefore, the following question arises: is AF in COVID-19 a simple bystander or a marker of increased risk? Several theories have been postulated to try to explain why patients with AF may be at higher risk from SARS-CoV-2 infection, but they are probably based on both inflammatory status and the mechanisms of cellular entry of the virus.17 It has been previously demonstrated that high AF burden is associated with higher activity levels of angiotensin-converting enzyme 2, the peptide through which the virus binds to human cells.18 Up-regulation of angiotensin-converting enzyme 2 can potentially increase susceptibility to COVID-19.18 Interestingly, angiotensin-converting enzyme 2 levels also correlate with structural and functional remodeling of the left atrium, which are in turn substrates for a greater susceptibility to AF.19 In addition, one of the key pathways of COVID-19 is represented by the abnormal inflammatory response of the host. Importantly, systemic inflammation precedes and predicts AF in the community. From this perspective, AF may reflect the existence of an increased inflammatory substrate that favors worse outcomes, amplified when coupled with COVID-19. The presence of AF itself is a poor prognostic factor in multiple clinical contexts; likewise, new onset AF worsens the prognosis of patients admitted for serious diseases.20 In the context of infections, this worse prognosis is accentuated and is prolonged during the mid-term follow-up.21 In addition, it is known that AF increases mortality both in patients with and without previous cardiovascular disease.22, 23.
It is important to note that most patients with AF require anticoagulation to prevent the risk of embolism, but in some cases, this treatment is related to hemorrhagic complications. Both complications can be accentuated in patients with SARS-CoV-2 infection. COVID-19 frequently induces hypercoagulability with inflammation driving increased levels of procoagulant clotting factors and disruption of the normal homeostasis of vascular endothelial cells, which results in microangiopathy, local thrombus formation, and a systemic coagulation defect leading to large vessel thrombosis and hence major thromboembolic complications.24 Whether anticoagulation alone is sufficient to prevent these thrombotic events, especially those driven by endothelial dysfunction, is unknown, although it is recommended that all admitted patients should receive prophylaxis for deep vein thrombosis.25 However, the prevalence of drug-drug interactions from anticoagulation was reported to be as high as 26.3% in the AF population. Such interactions increased the risk of bleeding up to 7-fold and it is expected to be higher in COVID-19 patients. Although the guiding principles for anticoagulation in COVID-19 patients with AF are the same as in patients without SARS-CoV-2 infection, little is known about potential complications with COVID-19. Our data show that patients receiving full dose anticoagulation have a higher incidence of bleeding complications. In contrast, they do not have a higher incidence of embolic complications. In addition, the CHA2DS2-VASc score is not capable of predicting in-hospital embolic risk in this population. Therefore, its use to assess the need for in-hospital anticoagulation should not be justified by this scale alone and should be individualized; it could be considered that in patients with AF who are admitted because of COVID-19, only treatment with prophylactic anticoagulation regimens might be considered during admission.
Limitations
The design of this study entails some constraints. Some incident events in the participating centers may not have been diagnosed and/or reported. The calculation of the incidence of the events is not precise since recruitment was performed in participating centers without other sampling procedures other than the broad inclusion criteria (hospital discharge). Regarding the management applied, at all times it was decided by the attending medical team, as well as in the comparison group.
Other considerations to take into account are that we did not have information on the type of AF (paroxysmal/permanent) so we cannot know if this classification influenced the prognosis or management of these patients; although we performed a PSM, some variables such as heart failure and ischemic heart disease were not balanced; although we attempted to adjust for many confounders, other unmeasured or unknown confounders might have played a role; we tried to report all the treatments used during admission, but the protocols differed between the centers, which may influence the results. In addition, we were not able to record possible in-hospital AF events, because the health situation experienced in some hospitals included in the registry did not allow electrocardiographic monitoring or daily electrocardiograms to be performed in many patients. The variable “previous anticoagulation” was not included in the matching process; therefore, we cannot rule out that this variable could have modified our results. Despite this, we did include this variable in the multivariate analysis and it was not predictive of a worse prognosis. Furthermore, our registry only included in-hospital complications (except for mortality) and, therefore, we cannot exclude the possibility that some of the patients may have had an embolic or hemorrhagic event at discharge. The precise impact of AF on COVID-19 warrant further investigation.
CONCLUSIONS
Our findings show that AF in patients with COVID-19 is associated with a high 60-day all-cause mortality rate. CHA2DS2-VASc score may be a good risk marker in COVID patients, but it does not predict their embolic risk. More studies are needed to confirm these findings.
FUNDING
Unconditioned grant (Fundación Interhospitalaria para la Investigación Cardiovascular [FIC] Madrid, Spain). This nonprofit institution had no role in the study design; in the collection, analysis, interpretation of data; in the writing of the report; nor in the decision to submit the paper for publication.
CONFLICTS OF INTEREST
The authors declare that there is no conflict of interest.
WHAT IS KNOWN ABOUT THE TOPIC?
-
–
COVID-19 ranges from asymptomatic to critical forms, and several prognostic factors have been described, however, there is no work addressing the specific impact of atrial fibrillation.
WHAT DOES THIS STUDY ADD?
-
–
AF in COVID-19 patients is associated with a higher number of complications and 60-day mortality. The CHA2DS2-VASc score may be a good risk marker in COVID patients, but it does not predict their embolic risk. Clinicians should systematically assess CHA2DS2-VASc in patients with COVID-19 and atrial fibrillation at the time of hospital admission in order to optimize risk stratification and improve resource allocation. However, its use to assess the need for in-hospital anticoagulation should not be justified by this scale alone and should be individualized.
Acknowledgements
Cardiovascular Excellence SL, for their essential support in the database and HOPE webpage. All HOPE researchers.
APPENDIX. SUPPLEMENTARY DATA
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2020.12.009
Appendix B. SUPPLEMENTARY MATERIAL
References
- 1.Word Health Organization. Novel coronavirus – China. 2020. Available at: http://www.who.int/csr/don/12-january-2020-novel-coronavirus-china/en/. Accessed 14 Dec 2020.
- 2.Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). COVID-19 Dashboard. 2020. Available at: https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6. Accessed 12 Dec 2020
- 3.Chan J.F., Yuan S., Kok K.H. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pollán M., Pérez-Gómez B., Pastor-Barriuso R. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020:396535–396544. doi: 10.1016/S0140-6736(20)31483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petrilli C.M., Jones S.A., Yang J. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uribarri A., Núñez Gil I.J., Aparisi A. Impact of renal function on admission in COVID-19 patients: an analysis of the international HOPE COVID-19 (Health Outcome Predictive Evaluation for COVID 19) Registry. J Nephrol. 2020;33:737–745. doi: 10.1007/s40620-020-00790-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hindricks G., Potpara T., Dagres N., 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2020 doi: 10.1093/eurheartj/ehaa612. [DOI] [Google Scholar]
- 9.Steinberg B.A., Zhao Y., He X. Management of postoperative atrial fibrillation and subsequent outcomes in contemporary patients undergoing cardiac surgery: insights from the Society of Thoracic Surgeons CAPS-Care Atrial Fibrillation Registry. Clin Cardiol. 2014;37:7–13. doi: 10.1002/clc.22230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lip G.Y.H., Nieuwlaat R., Pisters R., Lane D.A., Crijns H.J.G.M. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 11.ClinicalTrials.gov. International COVID19 Clinical Evaluation Registry, (HOPE COVID 19) Available at: https://clinicaltrials.gov/ct2/show/NCT04334291. Accessed 12 Dec 2020.
- 12.HOPE registry website. Available at: https://hopeprojectmd.com. Accessed 14 Dec 2020.
- 13.Driggin E., Madhavan M.V., Bikdeli B. Cardiovascular Considerations for Patients, Health Care Workers, and Health Systems During the COVID-19 Pandemic. J Am Coll Cardiol. 2020;75:2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bosch N.A., Cimini J., Walkey A.J. Atrial Fibrillation in the ICU. Chest. 2018;154:1424–1434. doi: 10.1016/j.chest.2018.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanchis-Gomar F., Perez-Quilis C., Lavie C.J. Should atrial fibrillation be considered a cardiovascular risk factor for a worse prognosis in COVID-19 patients? Eur Heart J. 2020;41:3092–3093. doi: 10.1093/eurheartj/ehaa509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chugh S.S., Havmoeller R., Narayanan K. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inciardi R.M., Adamo M., Lupi L., Metra M. Atrial fibrillation in the COVID-19 era: simple bystander or marker of increased risk? Eur Heart J. 2020;41:3094. doi: 10.1093/eurheartj/ehaa576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tomasoni D., Italia L., Adamo M. COVID-19 and heart failure: from infection to inflammation and angiotensin II stimulation. Searching for evidence from a new disease. Eur J Heart Fail. 2020;22:957–966. doi: 10.1002/ejhf.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walters T.E., Kalman J.M., Patel S.K., Mearns M., Velkoska E., Burrell L.M. Angiotensin converting enzyme 2 activity and human atrial fibrillation: increased plasma angiotensin converting enzyme 2 activity is associated with atrial fibrillation and more advanced left atrial structural remodelling. Europace. 2017;19:1280–1287. doi: 10.1093/europace/euw246. [DOI] [PubMed] [Google Scholar]
- 20.Moss T.J., Calland J.F., Enfield K.B. New-Onset Atrial Fibrillation in the Critically Ill. Crit Care Med. 2017;45:790–797. doi: 10.1097/CCM.0000000000002325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gundlund A., Olesen J.B., Butt J.H. One-year outcomes in atrial fibrillation presenting during infections: a nationwide registry-based study. Eur Heart J. 2020;41:1112–1119. doi: 10.1093/eurheartj/ehz873. [DOI] [PubMed] [Google Scholar]
- 22.Benjamin E.J., Wolf P.A., D’Agostino R.B., Silbershatz H., Kannel W.B., Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 23.Pedersen O.D., Bagger H., Køber L., Torp-Pedersen C., The occurrence and prognostic significance of atrial fibrillation/-flutter following acute myocardial infarction. TRACE Study group. TRAndolapril Cardiac Evalution Eur Heart J. 1999;20:748–754. doi: 10.1053/euhj.1998.1352. [DOI] [PubMed] [Google Scholar]
- 24.Iba T., Levy J.H., Levi M., Connors J.M., Thachil J. Coagulopathy of Coronavirus Disease 2019. Crit Care Med. 2020;48:1358–1364. doi: 10.1097/CCM.0000000000004458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.