Abstract
Objectives
The objective of this study was to investigate the differences between the results of two serology assays for detection of COVID-19 among medical staff, who are at higher risks of infection.
Methods
The immunochromatography (ICG) rapid test kit and the chemiluminescence immunoassay (CLIA) quantitative antibody test were performed. The differences in IgM and IgG antibody prevalence in different serological assays were descriptively analyzed.
Results
A total of 637 participants were included in this research. Two staff were IgM positive in the CLIA quantitative antibody test (cutoff value: 10 AU/ml) of 51 staff who were IgM positive in the rapid test kit. Six staff were IgG positive in the CLIA quantitative antibody test of 56 staff who were IgG positive in the rapid test kit.
The proportion of antibody positive staff differed greatly between the rapid test kit and the CLIA quantitative antibody test.
Conclusions
There was a vast difference in the proportions of IgG and IgM antibody positive staff in the rapid test kit and the CLIA quantitative antibody test results. The results from the only rapid test kit might have to be interpreted with caution. Further studies to evaluate antibody testing accuracy are required to promote the understanding of each assay's characteristics and determine their purposes in each community.
Keywords: COVID-19, Serological assay, Antibody prevalence
1. Introduction
The COVID-19 pandemic is an ongoing global pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). To end this pandemic, various serologic assays including the chemiluminescence immunoassay (CLIA) and immunochromatography (ICG) assay have been developed. In particular, antibody tests are useful for evaluating the extent of the disease in the population, infection control, assessing the effects of a new vaccination, and as a marker of advancing in severity of COVID-19 [1], [2], [3]. Further, it was reported that higher levels of serological assay readings have been seen in those with symptoms and those with severe diseases, while asymptomatic infections demonstrate a variable response [4]. Thus, improving the accuracy of antibody tests and increasing its usage in communities has become a vital public health issue in recent times.
Meanwhile, how antibody tests can be effectively utilized is under discussion mainly because sensitivity, specificity, or threshold vary between each assay and different products of the same assay [1], [5], [6], [7], [8], [9]. Since the results differ depending on the method and the test used, it is necessary to gather information on the differences in results for different antibody tests for future use. Nevertheless, most antibody test surveys within communities have been evaluated using a single assay or a single production. Furthermore, a few studies have been conducted to compare the results of different antibody tests from communities and when done on a large-scale basis [10]; however, the number of such studies is limited.
Thus, the objective of this study was to investigate the differences between the results of the rapid COVID-19 test kit and the CLIA quantitative antibody test among medical staff, who are at higher risks of infection. To this end, this study set out to evaluate the concordance between a lab-based assay and a point-of-care rapid test kit assay in an asymptomatic but high-risk population.
2. Method
Seireikai group is a private health care group located in the central part of Fukushima Prefecture, Japan. It runs Hirata Central Hospital, which has 142 beds for inpatients and is located in Hirata Village, approximately 190 km north of Tokyo.
The immunochromatography rapid test kit and the CLIA quantitative antibody test were performed on 680 hospital staff to identify COVID-19 infection statuses; of these, we set aside the 637 participants who worked as Seireikai group staff and agreed to participate in this study. The blood sample for each test was obtained between 8 May and 28 May 2020 in Fukushima Prefecture, where approximately 1850 thousand residents live and 81 COVID-19 cases has been reported as of 28 May 2020 [11].
The 2019-nCoV IgG/IgM kit made by Vazyme Biotech Co., LTD (YHLO Biotech, Shenzhen, China) was used for the rapid test. The testing method process was followed by the official testing method adequately [12]. The serum was used for the examination. Two independent laboratory technicians certified the line judgment. The CLIA quantitative antibody test was performed using a high throughput assay apparatus, called iFlash 3000, and with assay reagents, iFlash-SARS-CoV-2 IgM/IgG (YHLO Biotech, Shenzhen, China). The testing method process was as per official guidelines. (Refer to the official instruction manual for iFlash Immunoassay Analyzer for SARS-CoV-2 IgG and IgM). The cutoff of the CLIA quantitative antibody test was 10 AU/ml. S antigen, which may induce the production of neutralizing antibodies, as well as N antigen were targets for the antibody test. Moreover, the samples for the CLIA quantitative antibody test and rapid test were obtained at the same time (see Table 1 ).
Table 1.
Participants characteristics.
| Number | % | |
|---|---|---|
| Gender | ||
| Female | 483 | 75.82 |
| Male | 154 | 24.18 |
| Age | ||
| 18–44 | 324 | 50.86 |
| 45–64 | 265 | 41.6 |
| 65–78 | 48 | 7.54 |
| Occupation | ||
| Doctor | 15 | 2.35 |
| Nurse | 112 | 17.58 |
| Caregiver | 275 | 43.17 |
| Other medical staff | 66 | 10.36 |
| Office worker | 51 | 8.01 |
| Other non-medical staff | 118 | 18.52 |
| Working place | ||
| Hospital | 160 | 25.12 |
| Clinic | 53 | 8.32 |
| Long term care health facility | 371 | 58.24 |
| Nursery school | 22 | 3.45 |
| Other | 31 | 4.87 |
| IgM in Immunochromatography kit test | ||
| Positive | 51 | 8.01 |
| Negative | 586 | 91.99 |
| IgG in Immunochromatography kit test | ||
| Positive | 56 | 8.79 |
| Negative | 581 | 91.21 |
| IgM in CLIA quantitative test | ||
| Positive | 2 | 0.31 |
| Negative | 635 | 99.69 |
| IgG in CLIA quantitative test | ||
| Positive | 6 | 0.94 |
| Negative | 631 | 99.06 |
CLIA = chemiluminescence immunoassay.
The quality check test was performed every day before measuring the CLIA samples. The expected value and the confidential range of the calibration reagent for each lot were determined by the company, and the tests for participants were performed after confirming that the values were within the decided range. The data for the value of the calibration reagent on each day are shown in the supplemental table 1. The CLIA quantitative antibody tests of the participants were performed on three different days. Further, reverse transcription polymerase chain reaction (RT-PCR) was employed using the LightCycler® 480 Instrument II [13].
The differences in IgM and IgG antibody prevalence in different serological assays were descriptively analyzed using STATA IC15 (Lightstone, Texas USA, version15). This study was approved by the ethics committee of Hirata Central Hospital (Ethics Committee ID: 2020-0427-1), and all participants individually provided written informed consent.
3. Results
A total of 637 participants were included in this research. The median age [25th, 75th centiles] of participants was 44 [34, 56], and the proportion of males was 24.18% (n = 154).
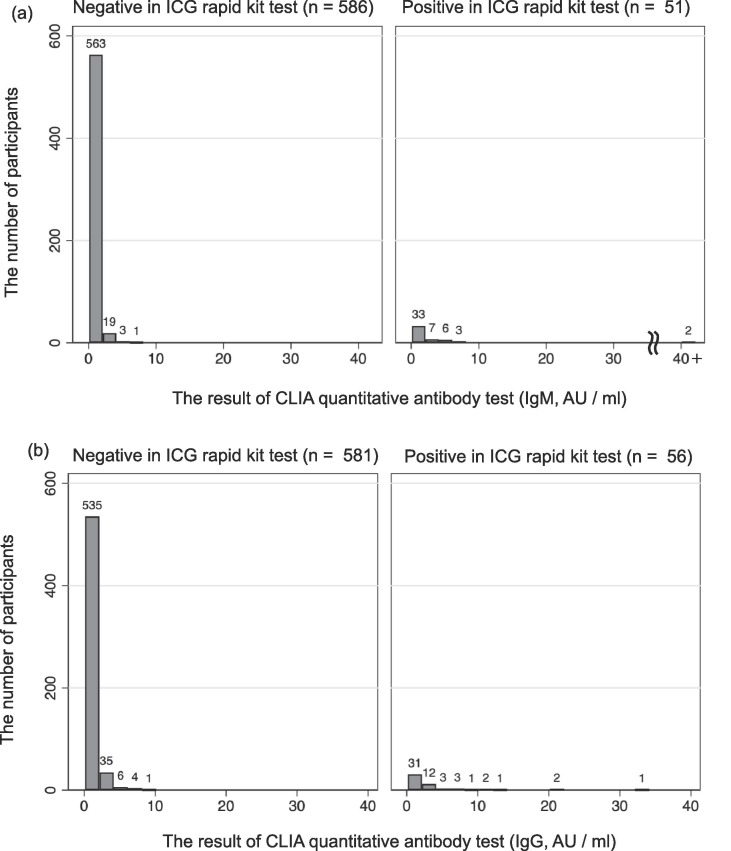
Fig. 1(a) shows the results of the IgM test. Two staff members were positive in the CLIA quantitative antibody test (cutoff value: 10 AU/ml) of the 51 staff members who were IgM positive in the rapid test kit. The proportions of IgM positives in the rapid test kit for each range of the CLIA quantitative antibody test results were 4.3% (0–0.5 AU/ml), 2.6% (0.5–1.0 AU/ml), 13.9% (1.0–1.5 AU/ml), 18.5% (1.5–2.0 AU/ml), 0.0% (2.0–2.5 AU/ml), 40.9% (2.5–5.0 AU/ml), 70.0% (5.0–7.5 AU/ml), and 100.00% (over10 AU/ml).
Fig. 1.
The results of the chemiluminescence immunoassay quantitative antibody test (AU/ml). (a) IgM, (b) IgG. The cut off value of this chemiluminescence immunoassay quantitative antibody test is 10AU/ml. ICG = immunochromatography, CLIA = Chemiluminescence immunoassay.
Fig. 1(b) shows the results of the IgG test. Six staff members were positive in the CLIA quantitative antibody test (cutoff value: 10 AU/ml) of the 56 staff members who were IgG positive in the rapid test kit. The proportions of IgG positive in the rapid test kit for each range of the quantitative antibody test results were 2.7% (0–0.5 AU/ml), 5.6% (0.5–1.0 AU/ml), 7.8% (1.0–1.5 AU/ml), 9.6% (1.5–2.0 AU/ml), 14.3% (2.0–2.5 AU/ml), 31.4% (2.5–5.0 AU/ml), 50.0% (5.0–7.5 AU/ml), 33.33% (7.5–10 AU/ml), and 100.0% (over10 AU/ml).
The IgG antibody prevalence ratio among health care workers in the rural area of Fukushima Prefecture was 0.9% based on the CLIA quantitative antibody test. A RT-PCR assay was performed for 51 participants who were IgM-positive with the rapid test kit, and the results were all negative. However, the findings of IgM positives in the rapid test and RT-PCR negative individuals does not mean that there were false positive IgM bands in the rapid kit tests in each case because the term from infection to appearing positive results is different for each assay [14]. Moreover, all the staff members did not have any subjective symptoms related to COVID-19 during the survey period. Additionally, none were confirmed to have COVID-19 in the following two months after the survey. This means that these tests were conducted on asymptomatic populations.
4. Discussion
The proportion of antibody positive staff differed greatly between the rapid test kit and the CLIA quantitative antibody test (8.8% in the rapid test kit and 0.9% in the CLIA quantitative antibody test for IgG; 8.0% and 0.3% for IgM). Thus, the results from the rapid test kit might have to be interpreted with caution.
This study suggested that detailed data on the sensitivity of positive control among the subclinical infection population and the specificity of negative control among the absolutely non-infected population are required for each test. Moreover, the 2019-nCoV IgG/IgM detection kit by Vazyme Biotech Co., Ltd. used in this study reported a sensitivity of 92% and a specificity of 97%; however, the positive control was set within the population that had developed severe symptoms of COVID-19 following hospitalization. For an epidemiological study of COVID-19 to determine the proportion of subclinical infections, it is necessary to consistently evaluate the sensitivity of subclinical infections with the serum of people who have not had any symptoms with positive RT-PCR results as a positive control, as well as specificity with the serum before COVID-19 as a negative control.
Whenever rapid test kits are used in hospitals and communities, it is crucial to decide in advance as to how participants who have tested IgM positive are to be handled. In the present study, RT-PCR tests were performed on all IgM-positive participants for risk management of a possible outbreak of COVID-19 among hospital staff. However, the evaluation of IgM using rapid test kits for diagnosis has proved difficult in previous studies [1], [5], [15]. When the 2019-nCoV IgG/IgM detection kit by Vazyme Biotech Co., Ltd, is used in hospitals or communities, it might be a plausible option for the rapid test kit results to be focused only on the IgG rather than IgG and IgM. Additionally, to avoid confusion, these assays need to be independently evaluated prior to use, and must be used for a minimum population size against PT-PCR positive results within a suitable time frame post the RT-PCR results.
Nevertheless, antibody tests have had positive effects; for instance, in this study, staff awareness of COVID-19 increased post testing. This is because the staff was widely notified about the purpose of this antibody test to protect the safety of patients and to raise their awareness about the exact situations in hospitals. It is thus vital to inform the community on the purpose of antibody testing, so that the tests have a positive effect, rather than cause anxiety relating to test results and confusion.
This study has some limitations. First, when interpreting these findings, the possibility that the cross reactions with other coronaviruses might have caused the higher seroprevalence in the rapid test should be considered. Second, the confirmatory virus neutralization test was not performed in the present study. Third, the differences in the target antigen might affect the differences in the results from each test. Fourth, while a clear correlation was not found between quantitative antibody test values and rapid test line density, the present study did not have enough quality record of band intensity to assess.
5. Conclusion
There was a vast difference in the proportions of IgG and IgM antibody positive staff in the rapid test kit and the CLIA quantitative antibody test results. Further studies to evaluate antibody testing accuracy are required to promote the understanding of each assay's characteristics and determine their purposes in each community. Additionally, an epidemiological survey is required to further the discussion on the effective usage of antibody assays and infection control.
Funding sources
This research did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Author contributions
All the authors made a substantial contribution to this research. YK and TM contributed to writing the paper. All members contributed to the study design, data collection, and coordination with local stakeholders.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank Mr. Fumihiko Sagawa, Mr. Masahiko Nihei and all the staff at the Seireikai group for providing critical data for this research.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.intimp.2020.107360.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Whitman J.D., Hiatt J., Mowery C.T., Shy B.R., Yu R., Yamamoto T.N., et al. Test performance evaluation of SARS-CoV-2 serological assays. MedRxiv. 2020 doi: 10.1101/2020.04.25.20074856. [DOI] [Google Scholar]
- 2.Daverio M., Amigoni A., Cavicchiolo M.E. Testing for novel coronavirus antibodies: a necessary adjunct. J. Infect. Dis. 2020;222(3):517–518. doi: 10.1093/infdis/jiaa283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plebani M., Padoan A., Negrini D., Carpinteri B., Sciacovelli L. Diagnostic performances and thresholds: The key to harmonization in serological SARS-CoV-2 assays? Clin. Chim. Acta. 2020;509:1–7. doi: 10.1016/j.cca.2020.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang B., Zhou X., Zhu C., Song Y., Feng F., Qiu Y., et al. Immune phenotyping based on the neutrophil-to-lymphocyte ratio and IgG level predicts disease severity and outcome for patients with COVID-19. Front. Mol. Biosci. 2020;7:157. doi: 10.3389/fmolb.2020.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Elslande J., Houben E., Depypere M., Brackenier A., Desmet S., André E., et al. Diagnostic performance of seven rapid IgG/IgM antibody tests and the Euroimmun IgA/IgG ELISA in COVID-19 patients. Clin. Microbiol. Infect. 2020;26(8):1082–1087. doi: 10.1016/j.cmi.2020.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montesinos I., Gruson D., Kabamba B., Dahma H., Wijngaert S.V., Reza S., et al. Evaluation of two automated and three rapid lateral flow immunoassays for the detection of anti-SARS-CoV-2 antibodies. J. Clin. Virol. 2020;128:104413. doi: 10.1016/j.jcv.2020.104413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rashid Z.Z., Othman S.N., Samat M.N.A., Ali U.K., Wong K.K. Diagnostic performance of COVID-19 serology assays. Malaysian J. Pathol. 2020;42(1):13–21. [PubMed] [Google Scholar]
- 8.Castro R., Luz P.M., Wakimoto M.D., Veloso V.G., Grinsztejn B., Perazzo H. COVID-19: a meta-analysis of diagnostic test accuracy of commercial assays registered in Brazil. Braz. J. Infect. Dis. 2020;24(2):180–187. doi: 10.1016/j.bjid.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plebani M., Padoan A., Sciacovelli L., Basso D. Towards the rational utilization of SARS-CoV-2 serological tests in clinical practice. Clin. Chem. Lab Med. 2020 doi: 10.1515/cclm-2020-0880. [DOI] [PubMed] [Google Scholar]
- 10.Flower B., Brown J.C., Simmons B., Moshe M., Frise R., Penn R., et al. Clinical and laboratory evaluation of SARS-CoV-2 lateral flow assays for use in a national COVID-19 seroprevalence survey. Thorax. 2020;75:1082–1088. doi: 10.1136/thoraxjnl-2020-215732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ministry of Health Labour and Welfare, About Coronavirus Disease 2019 (COVID-19). https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/newpage_00032.html, 2020 (accessed 12 August 2020).
- 12.Vazyme Medical Technology Co., 2019-nCoV IgG/IgM Detection Kit. https://www.medsrl.com.ar/wp-content/uploads/2020/08/Vazyme.pdf, 2020 (accessed 5 December 2020).
- 13.Roche Molecular Systems, Inc., LightCycler® 480 Instrument II. https://lifescience.roche.com/global_en/products/lightcycler14301-480-instrument-ii.html#documents, 2018 (accessed 5 December 2020).
- 14.Zhao J., Yuan Q., Wang H., Liu W., Liao X., Su Y., et al. Antibody responses to SARS-CoV-2 in patients with novel coronavirus disease 2019. Clin. Infect. Dis. 2020;71(16):2027–2034. doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imai K., Tabata S., Ikeda M., Noguchi S., Kitagawa Y., Matuokaet M., et al. Clinical evaluation of an immunochromatographic IgM/IgG antibody assay and chest computed tomography for the diagnosis of COVID-19. J. Clin. Virol. 2020;128:104393. doi: 10.1016/j.jcv.2020.104393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.