Abstract
Marine-derived sulfated polysaccharides possess various antiviral activities against a broad range of enveloped and non-enveloped viruses. It has become the potential source of antiviral drugs for pharmaceutical development. In this review, we will discuss the different types of sulfated polysaccharides and their structural classification. Some of the major sulfated polysaccharides with potent antiviral activity, including carrageenan, agar, ulvan, fucoidan, and alginates, are considered in this review. The mechanism of these sulfated polysaccharides in inhibiting the different stages of the viral infection process inside the host cell is also demonstrated. It involves blocking the initial entry of the virus or inhibiting its transcription and translation by modulating the immune response of the host cell. In addition, we explore the potential of sulfated polysaccharides as antiviral agents in preventing recent Corona Virus Disease-2019 (COVID-19).
Keywords: Sulfated galactans, Virucidal activity, Respiratory syndrome, Immune response, Structural classification, Drug development
Graphical abstract
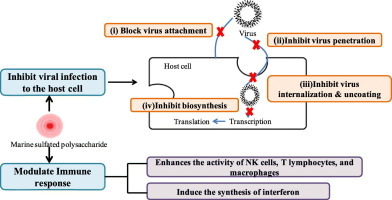
1. Introduction
In recent years, there is a sudden outburst of some viral diseases that have caused severe harm to human health (Wang et al., 2020). In the discovery and development of drugs, natural products play a vital role during the last decades. Various natural products, mostly from plant sources (root, bark, flowers, or essential oils) and algae-derived compounds are considered effective alternative against multiple diseases (Dutta et al., 2019; Kushwaha et al., 2020; Singh et al., 2019). These natural products contain structurally diverse active substances with a wide range of biological activities (Goswami et al., 2020; Kushwaha et al., 2015). The use of algae to prevent or treat numerous diseases has been exploited for many years and is still used in healthcare in many countries (Kushwaha et al., 2017; Xian et al., 2020). Seaweeds have been recognized as rich and valuable sources of bioactive compounds because of their various biological activities (Goswami et al., 2019a; Khalid et al., 2018). Marine sulfated polysaccharides are considered as a potential source of biologically active compounds for drug development (Bind et al., 2019; Hans et al., 2019). These compounds have been reported to have varieties of pharmacological activities such as antitumor, antiviral, antioxidant, antimicrobial, anticoagulant, and immune-inflammatory effects (Bind et al., 2018; Fedorov et al., 2013; Goswami et al., 2019b).
Sulfated polysaccharides (SPs) are natural complex polymers found majorly in the cell walls of marine algae. Some of the essential SPs include carrageenan and agar from red macroalgae, ulvan from green macroalgae, and fucoidan and laminarian from brown macroalgae (Wijesekara et al., 2011). The sulfated polysaccharides of seaweeds have been shown to exhibit antiviral activity against a broad spectrum of viruses. It has been reported to inhibit antiviral activity against Herpes Simplex virus (HSV) (Gomaa and Elshoubaky, 2016), Human immunodeficiency virus type-1 (HIV-1) (Besednova et al., 2019), chikungunya virus (Cirne-Santos et al., 2019), and many other enveloped and non-enveloped viruses. Also, the antiviral activity of SPs against the currently on-going pandemic coronavirus disease 2019 (COVID-19) is reported (Chen et al., 2020). COVID-19 is a severe acute respiratory syndrome that may cause illness in animals or humans. This virus primarily spreads between people during close contact, often via small droplets from the nose or mouth, which are expelled by coughing, sneezing, or talking. According to worldometer, as of 20th November 2020, over 57,019,580 identified cases of COVID 19 worldwide in 218 countries and territories (Worldometer, 2020). There is no specific medicine to prevent or treat this disease. Marine sulfated polysaccharide exerts virucidal effect by intervening in different stages of viral infection. Thus, it raises the possibility for the advancement of antiviral agents in therapeutics (Wang et al., 2012). These natural nontoxic and effective antiviral drugs are currently investigated to analyze their potential role in the prevention of contagious diseases in humans.
In this mini-review, the main focus is on the neoteric development in the field of antiviral activity of sulfated polysaccharides isolated from the marine biomasses, their molecular significances, and their mechanism of action in preventing and regulating immune responses of the host cell. Also, the role of sulfated polysaccharides in preventing COVID-19 is briefly discussed. In the last decade, the research and developments on the structural or molecular characteristics and biological potency of sulfated polysaccharides have been done to a more considerable extent. From this pile of research findings, a glimpse of information is tried to be illustrated in this review paper.
2. Worldwide production of marine sulfated polysaccharides
The global production of marine macroalgae is approximately 33 million tonnes in 2017, out of which 32 million tonnes were harvested from the culture sector (Ferdouse et al., 2018). The leading macroalgae producing countries are China, Norway, Indonesia, France, Ireland, and India (FAO, 2019). The universal seaweed market size was valued at $4097.93 million in 2017 and is estimated to reach $9075.65 million by 2024, which will lead to an increase in annual growth rate to 12.0% in 2024 (Allied market research, 2018). In 2012, the highest production of some of the common red seaweeds was 6.1 million tonnes of Eucheuma species (worth 1.2 billion USD), 0.8 million tonnes of Gracilaria species (worth 34 million USD), 2.1 million tonnes of Kappaphycus species (371 million USD), and 0.7 million tonnes of Porphyra species (worth 13 billion USD) (Bjerregaard et al., 2016). Some of the brown seaweed production is 5.7 million tonnes of Saccarina species worth 330 million USD and 2.1 million tonnes of Undaria pinnatifida worth 0.9 billion USD. Green seaweed production is 2 million tonnes of Ulva species, 1.2 million tonnes of Caulerpa species (Ferdouse et al., 2018).
Red macroalgae are commercially more important than green and brown macroalgae as it covers three-fourth of the global value. There is an increase in demand for red seaweeds in the manufacturing of hydrocolloids such as agar and carrageenan. These hydrocolloids have various applications in food, pharmaceutical, and biotechnological industries. Worldwide production of agar in 2014 was about 10,600 tonnes, with a wholesale value of some $191 million. World carrageenan production exceeded 60,000 tonnes in 2014, with a value of over US$626 million. In 2014 production of alginates was 30,000 tonnes with a value of about US$339 million (Rhein-Knudsen et al., 2015). Many other macroalgae derived products like biomass, protein or organic chemicals have growing needs globally. Large-scale seaweed production offers research and development to expand the range of algae-derived products to upgrade the industrial market.
3. Marine-derived sulfated polysaccharides
3.1. Carrageenan and agar from red macroalgae
Sulfated galactans are the main polysaccharide component of red algae, which have a linear backbone of alternating 3-linked β-d-galactopyranose and 4-linked α-galactopyranose. There exist two major instances of the sulfated galactans isolated from the red algae, where agarans possess the 4-linked α-galactose moiety with levorotatory (l-) configuration and the other one termed as carrageenans consist of the similar linkage with dextrorotatory (d-) configuration (Al-Alawi et al., 2011). Thus carrageenans consist of linear chains of alternating β-d-galactopyranose units (G-units) with a linkage between the 1 and 3 positions of the monomeric units and α-galactopyranose units (D-units) with a linkage between 1 and 4 positions or 3, 6-α-galactopyranose (Anhydrous) units (AnGal units) (Ferreira et al., 2012). The most relevant carrageenans which are commercially produced are kappa (κ) [Fig. 1a], iota (ι) [Fig. 1b] and lambda (λ) [Fig. 1c], which differ according to the number and position of sulfate ester groups (S) and the occurrence of 3, 6-anhydro-d-galactopyranose units. κ-carrageenan has one (25–30%), ι-carrageenan has two (28–30%), and λ-carrageenan has three (32–39%) of sulfate ester groups (Muthukumar et al., 2020). The composition of carrageenan varies among species such as Kappaphycus alvarezii is a significant source of κ-carrageenan (Rudke et al., 2020), Eucheuma denticulatum consists of ι-carrageenan (Jönsson et al., 2020) and Gigartina skottsbergii and Chondrus crispus comprises of λ-carrageenan (Zhu et al., 2018). Biological precursor carrageenan named μ (mu) and ν (nu) is subjected to hot alkaline treatment, which leads to the cyclization of 3,6 anhydro rings. This pretreatment converts mu and nu into commercial κ- and ι- carrageenan, respectively (Ortiz-Tena et al., 2017). During carrageenan preparation, galactose and sulfate can be either substituted by xylose, glucose, uronic acid, methyl ethers, or pyruvate groups (Yu et al., 2010).
Fig. 1.
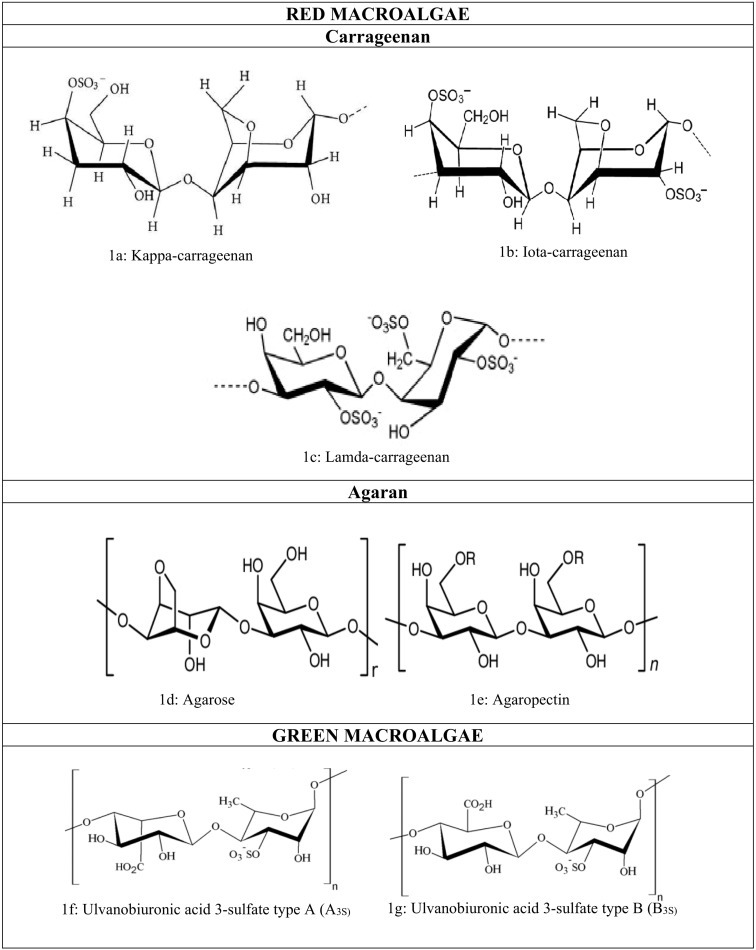
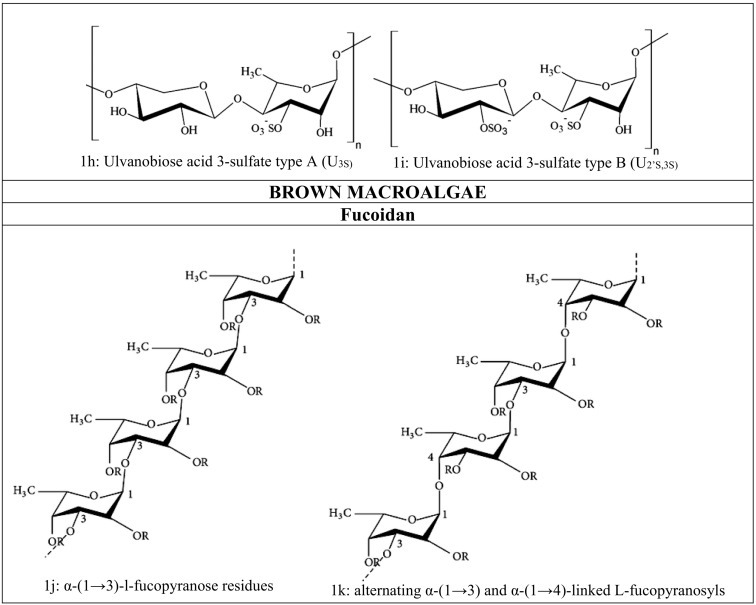
(a–k): Chemical structure of Marine derived-sulfated polysaccharides.
Agar from red macroalgae is composed of two polysaccharides, agarose [Fig. 1d] and agaropectin [Fig. 1e]. It is mainly found in Gelidium and Gracilaria species. It is built of repeating alternating chains of 1 → 3-β-d-galactopyranose unit and 1 → 4-α-l-galactopyranose or 3 → 6-α-l-galactopyranose (Anhydrous) unit (Usov, 2011). The β-d-galactopyranose unit can be substituted either by sulfate esters, methoxy groups, or pyruvic acid acetals groups (Lee et al., 2017a). For example, structural analysis of sulfated agar in Polysiphonia nigrescens is mainly replaced with sulfate on C6, with minor proportions of methyl ether and β-d-xylose (Bouhlal et al., 2011). Similarly, the sulfate group is present at the C2 position of agar isolated from Acanthophora spicifera with minor substitutions of pyruvylated, sulfated, and sulfated/pyruvylated disaccharide alditols (Gonçalves et al., 2002).
3.2. Ulvan from green macroalgae
Ulvan is one of the most complex sulfated polysaccharides representing about 9–36% of the algae dry weight and found in Ulva, Gayralia, and Monostroma species. Ulvan mainly consists of l-rhamnose constituting 5.0–92.2 M%, d- glucuronic acid comprising 2.6–52.0 M%, d-xylose constituting 0.0–38.0 M%, l-iduronic acid, which constitutes 0.6–15.3 M%, and sulfate in different proportions (Kim, 2015). The ulvan backbone mostly consists of α- and β-(1 → 4)- linked sugars with characteristic repeating disaccharide units. Aldobiuronic acids, also known as ulvanobiuronic acid (types A and B) [Fig. 1f, g], and aldobioses, known as ulvanobioses (type U) are two major disaccharide repeating units [Fig. 1h, i] (Kidgell et al., 2019). One of the most common disaccharide units, Ulvanobiuronic acid type A3S consists of β-d-glucuronic acid (1,4)-linked to α-L rhamnose 3-sulfate, while in type B3S α-l-iduronic acid is (1 → 4)-linked to α-l-rhamnose 3-sulfate. Ulvanobiose U3S consists of β-d-xylose 2-sulfate (1 → 4)-linked to α-l-rhamnose 3-sulfate and type U2 ' S,3S consist of β-d-xylose (1 → 4)-linked to α-l-rhamnose 3-sulfate (Figueira et al., 2020). Some of the marine algae synthesized these sulfated polysaccharides. Such as Monostroma nitidum composed of rhamnan sulfate polysaccharides (Lee et al., 2010). Gayralia oxysperma is consisting of heterorhamnan sulfate polysaccharides units (Li et al., 2012). Enteromorpha compressa composed of sulfated heteroglycuronan units, which contains rhamnose with terminal linkages between 1 and 4 position or 1 to (2,4) positions, xylopyranose unit with the linkage between 1 and 4 positions, and (1,4)- glucuronic acid units linked terminally (Ray, 2006).
3.3. Fucoidan from brown macroalgae
Fucoidan is a heterogeneous sulfated polysaccharide that constitutes 25–30% of the dry algal weight. It is consists of a backbone of α-(1 → 3)-l-fucopyranose residues [Fig. 1j] or alternating α-(1 → 3) and α-(1 → 4)-linked l-fucopyranosyls [Fig. 1k]. These residues are either substituted with sulfate, acetate, or glycosyl units such as glucuronic acid. It also contains minimal quantities of monosaccharides like d-xylose, d-galactose, d-mannose, and uronic acids (Ale et al., 2011). Fucoidan from Fucus evanescens and Ascophyllum nodosum contain alternative units of α-(1 → 3)-linked and α-(1 → 4)-linked l-fucopyranose. In Fucus evanescens, the sulfate group is substituted on C-2 and C-4 fucopyranose residues, and Ascophyllum nodosum consists of sulfate at the C-2 position of the 3-linked fucose and C-2- and C-3 positions of the 4-linked fucose (Yuguchi et al., 2016).
4. The antiviral mechanism of sulfated polysaccharide
Marine sulfated polysaccharides exhibit unique structures that exert antiviral effects. It obstructs different phases of the viral life cycle by directly inactivating virions before infection or by inhibiting its replication inside the host cell. Thus, seaweeds rich in polysaccharides largely contribute to the discovery and development of antiviral drugs. The primary stages in the life cycle of the virus are attachment, penetration, uncoating, biosynthesis, viral assembly, and release, which differ with species [Fig. 2 ].
Fig. 2.
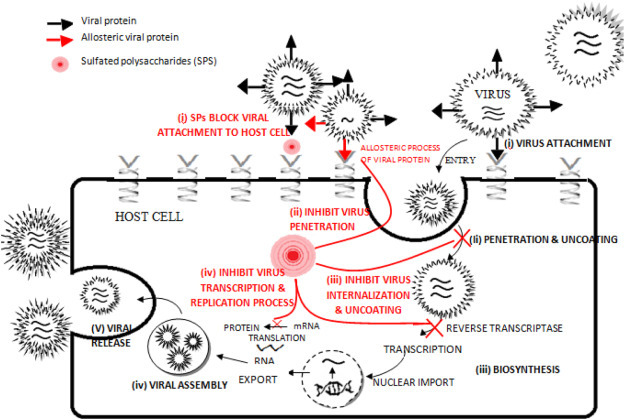
Stages in life cycle of virus (Black) - (i) virus attachment, (ii) virus penetration and uncoating (iii) biosynthesis (iv) viral assembly (v) viral release.
Mechanism of antiviral actions of Sulfated polysaccharides (SPs) (Red)- (i) inhibit virus attachment (ii) inhibit virus penetration (iii) inhibit virus internalization and uncoating (iv) Inhibit virus transcription and replication process. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4.1. Inhibition of virus attachment
The initial contact occurs by the ionic interaction between positively charged external glycoproteins that are present on the enveloped virus surface and the negatively charged constituents of the host cell surface. A high density of negative charge is present on the cell surface due to the existence of sulfate residues that interact with the positively charged domain of viral glycoprotein that disrupts the initial virus-cell contact (Sepúlveda-Crespo et al., 2017). Carrageenan might prevent virus infection into the host cell by directly inhibiting its binding to the cell surface.
4.2. Inhibition of virus penetration
After binding of the virus onto the host cell, the subsequent virus invasion process is followed by irreversible adsorption via electrostatic interaction between the host cell and virus receptors. To cease this penetration operation, some of the sulfated marine polysaccharides interact with virus receptors, which block its interaction with the host cell surface or directly interact with virions to prevent virus infection. Several studies have shown that negative charges residing over the sulfate group of carrageenan interact with the virus by covering up the positive charge of the viral receptors (Wang et al., 2012).
4.3. Inhibit interiorization and uncoating of the virus
The virus is penetrated inside the host cell by invagination of the outer membrane to form a vacuole. Then it is transported through the intracellular fluid, i.e., cytoplasm, and delivered to endosomes and other intracellular organelles. After endocytosis, the virus interacts with the cell membrane or creates an intracellular compartment enclosing virus, thus changing the structure of the viral capsid. When a virus interacts with receptor protein around the endosome, specific signals are produced, which uncoats and discharges the virions (Mercer et al., 2010). The certain sulfated marine polysaccharides are reported to interfere with the virus internalization by interacting with virus membrane proteins. They bind with carbohydrate groups linked to the polypeptide chains of the virus to inhibit its penetration. Also, sulfated polysaccharides bind at the allosteric site of the viral capsid, which prevents the uncoating of the virus inside the host cell.
4.4. Inhibition of virus transcription and translation process
After internalization and uncoating, the virus is replicated inside the host cell. The replication of the virus involves the synthesis of viral messenger RNA, viral protein synthesis, and then viral genome replication mediated by regulatory protein expression. Many marine polysaccharides can inhibit the virus transcription and replication process after entering into the host cells by interfering with the replication enzymes such as reverse transcriptase or by preventing the formation of proteins from messenger RNA in the host cell (Queiroz et al., 2008).
5. Antiviral activity of marine-derived sulfated polysaccharides
Studies conducted over 50 years ago showed the effects of polysaccharides from marine algae in the inhibition of influenza B and mumps virus. These findings have introduced algae-derived polysaccharides as a potent source of antiviral agents. The scientific research on antiviral compounds and various biological activities of marine sulfated polysaccharides towards several deadly viruses has eventually increased in the past decade. Since then, a vast number of studies have been published on the antiviral property of various sulfated polysaccharides. They prevent a wide range of contagion diseases such as human immunodeficiency virus (HIV), hepatitis-C virus (HCV), dengue virus (DENV), herpesvirus (HSV-1 and HSV-2), human papillomavirus (HPV), and many respiratory tract viruses. Antiviral activity of some of the sulfated polysaccharides and their mode of action are discussed below [Table 1 ].
Table 1.
Antiviral activity of some sulfated polysaccharides from marine algae.
| Organism | Compounds | Activity | References |
|---|---|---|---|
| Red macroalgae | |||
| Acanthophora spicifera | Agaran | aHSV-1, HSV-2, inhibit initial attachment of virus to the cells | Bedoux et al., 2017 |
| Meristiella gelidium | Iota/kappa/nu-hybrid carrageenan | HSV-2, bDENV-2, no cytotoxicity on Vero cells | De Sf-Tischer et al., 2006 |
| Acanthophora spicifera | Carrageenan | HSV-1, cRVFV, inhibit virus replication | Gomaa and Elshoubaky, 2016 |
| Sigma–Aldrich | Kappa-carrageenan | Enterovirus 71 prevent viral replication before and after adsorption | Chiu et al., 2012b |
| Purchased from FMC Biopolymers | Iota-carrageenan | dHRV, prevent binding and replication of virions | Grassauer et al., 2008 |
| Gymnogongrus griffithsiae, Cryptonemia crenulata | Sulfated galactans | HSV-1, HSV-2, inhibit virus adsorption | Talarico et al., 2004 |
| Gracilaria corticata | Sulfated galactan | HSV-1, HSV-2, inhibition of virus attachment | Mazumder et al., 2002 |
| Purchased from FMC Biopolymers | Iota-carrageenan | Pandemic eH1NI/2009 reduced viral replication | Leibbrandt et al., 2010 |
| Gymnogongrus torulosus | dl-galactan | HSV-2, DENV-2, inhibit virus adsorption | Pujol et al., 2002 |
| Sebdenia polydactyla | Sulfated xylomannans | HSV-1 | Ghosh et al., 2009 |
| Lithothamnion muelleri | Sulfated xylogalactans | HSV-1, HSV-2, inhibit viral adsorption and penetration into the cells | Malagoli et al., 2014 |
| Scinaia hatei | Sulfated xylomannan | HSV-1, HSV-2, inhibit virus binding | Mandal et al., 2008 |
| Sphaerococcus coronopifolius, Boergeseniella thuyoides | Water-soluble sulfated polysaccharides | HSV-1, fHIV-1, inhibit adsorption and replication of the virus | Bouhlal et al., 2011 |
| Green macroalgae | |||
| Enteromorpha compressa | Ulvan | HSV, inhibit adsorption and replication of the virus | Lopes et al., 2017 |
| Ulva intestinalis | Ulvan | Measles virus, reduction of syncytia formation and low cytotoxicity | Morán-Santibañez et al., 2016 |
| Ulva armoricana | Enzyme assisted-Ulvan | HSV-1, no cytotoxicity on Vero cells | Hardouin et al., 2016 |
| Monostroma latissimum | Rhamnan sulfate | HSV-1, HIV-1, gHCMV, virus adsorption and replication | Wang et al., 2018 |
| Brown macroalgae | |||
| Adenocystis utricularis | Fucoidan (galactofucan) | HSV-1, HSV-2, no cytotoxicity | Ponce et al., 2003 |
| Dictyota dichotoma | Fucoidan (galactofucan) | HSV-1, reduction in plaque formation | Rabanal et al., 2014 |
| Cladosiphon okamuranus | Fucoidan (glucuronic acid, sulfated fucose) | DENV-2, direct binding | Hidari et al., 2008 |
| Adenocystis utricularis | Fucoidan | HIV-1, block entry of the virus | Trinchero et al., 2009 |
| Cystoseira indica | Sulfated fucans | HSV-1, HSV-2, inhibit virus adsorption | Mandal et al., 2007 |
| Sargassum mcclurei | Fucoidan | HIV-1, block entry of the virus | Thuy et al., 2015 |
| Caulerpa brachypus | Xylan-fucoidan | HSV-1, inhibit attachment, penetration, and later stage replication | Lee et al., 2004 |
| Fucus vesiculosus | Fucoidan | hBVDV, inhibit binding of the virus | Güven et al., 2020 |
| Laminaria japonica | Fucoidan | iH5N1, inhibit entry of the virus | Makarenkova et al., 2010 |
| Undaria pinnatifida | Galactofucan | HSV-1, HSV-2, HCMV, Inhibit virus entry, and host-virus binding | Hemmingson et al., 2006 |
| Sargassum trichophyllum | Fucoidan | HSV-2, inhibit virus adsorption, penetration and replication | Lee et al., 2011 |
Herpes simplex virus.
Dengue virus.
Rift valley fever virus.
Human rhinovirus.
Influenza A virus.
Human immunodeficiency virus.
Human cytomegalovirus.
Bovine viral diarrhea virus.
Avian influenza virus.
5.1. The antiviral potential of sulfated polysaccharides carrageenan from red macroalgae
The presence of 3,6-anhydrogalactopyranose and allocation of sulfate groups on carrageenan show different inhibitory effects on different viruses (Jiao et al., 2011). Buck et al. (2006) reported that iota-carrageenan is a potent infection inhibitor for a specific sexually transmitted human papillomavirus (HPV) by preventing the binding of HPV virions to cells. It has shown a thousand-fold more susceptibility, with a 50% inhibitory concentration value (IC50) at low concentration. They have also reported that contraceptives lubricated with carrageenans could effectively block HPV infection transmission through sexual contact. Besides, carrageenans incorporated within infant formulas have shown restricted vertical transmission of HPV from mother to baby.
It is reported that λ-carrageenan and hybrid μ/ι carrageenan isolated from Gigartina skottsbergii can inhibit the activity of type1 and type-2 herpes simplex virus (HSV). It interferes between cell surface heparin sulfate and HSV glycoprotein interlinkage at an early stage of infection. Carrageenan and its oligosaccharide derivatives bind with the glycoprotein present on the virus surface. This linkage leads to the denaturation and inactivation of HSV glycoprotein. Thus it inhibits the viral adsorption and replication inside the host cell (Carlucci et al., 2002).
Linear chains of galactopyranosyl residues of λ- and ι-carrageenan are found to be potent inhibitors of dengue virus type-2 (DENV-2) and type-3 (DENV-3) in monkey kidney cells and human hepatic cells. Results have shown that the dengue virus can be inhibited by carrageenan either at the early stage of virus adsorption or after the encapsulation of the capsid of virus-containing nucleic acid into the cytoplasm (Talarico and Damonte, 2007). Yamada and his co-workers demonstrated the anti-HIV activity of carrageenan. Carrageenan was modified by depolymerization and sulfation to increase its activity. Depolymerized and sulfated κ and ι carrageenan show the highest anti-HIV activity (Yamada et al., 2000). Also, low molecular weight κ-carrageenan obtained by microwave-aided acid hydrolysis was acetylated and sulfated. The obtained derivative of carrageenan displayed the highest activity against the influenza virus (H1N1) in mice (Tang et al., 2013). Wang and his co-workers have reported that carrageenan could inhibit mRNA and protein expression of the influenza A virus. Their result shows that after the virus is internalized inside the host cell, carrageenan blocks the viral replication cycle. It inhibited the formation of nucleic acids and proteins, which cease the release of the virus outside the host cell (Wang et al., 2011).
Therefore, the antiviral activity of carrageenan has distinct inhibitory actions on different viruses because of their molecular mass and level of sulfation, which comprises of sulfate content and the position of sulfation.
5.2. The antiviral potential of sulfated polysaccharide ulvan from green macroalgae
Chiu and his colleagues have tested the antiviral effects of ulvan from Ulva lactuca on the Japanese encephalitis virus (JEV). It is a mosquito-borne flavivirus that causes acute encephalitis in humans. They reported that ulvan could inhibit JEV infection in Vero cells by blocking virus adsorption and making the virus unable to enter cells (Chiu et al., 2012a).
Aguilar-Briseño and his teammates have isolated Ulvan from Ulva clathrata and fucoidan from Cladosiphon okamuranus. They evaluated the antiviral activity and mode of action of isolated Ulvan, fucoidan, and their mixture on Newcastle disease virus (NDV). Their antiviral activity was assessed by syncytia reduction assay. They stated that syncytia formation could only be inhibited if ulvan and its mixture were added before protein cleavage as once protein is cleaved via trypsin digestion; ulvan will lose the ability to inhibit syncytia formation. Their result shows that ulvan was able to inhibit 67% of syncytia formation, fucoidan inhibits 47%, and their mixture inhibits 59% of syncytia formation (Aguilar-Briseño et al., 2015).
5.3. The antiviral potential of sulfated polysaccharides fucoidan from brown macroalgae
Queiroz and his associates have examined the potential of fucoidan to inhibit HIV reverse transcriptase (RT) enzyme before integrating the virus into the host cell. RT enzyme is encoded by a retrovirus that catalyzes the viral RNA (ribonucleic acid) genome into DNA (deoxyribonucleic acid). They have also studied the role of sulfate and carboxyl groups present on the fucoidan to inhibit HIV. They have assessed that fucoidan has a prominent effect on reverse transcriptase in vitro, even at low concentration values. Their results have also shown that fucoidan polysaccharide, modified by carboxy-reduction and desulfation, has reduced the inhibitory activity for HIV RT four times compared to unmodified ones. Therefore, they have suggested that chemical structures of molecules such as degree of sulfation, disaccharides linkages, polymeric backbone, and the degree of polymer chemical reactions could play an important function in affecting antiviral properties of fucoidan polysaccharides (Queiroz et al., 2008).
Dinesh et al. (2016) reported the anti-HIV-1 property of fucoidan extracted from Sargassum swartzii. They observed that it exhibit inhibitory activity against HIV-1 p24 antigen (95.6 ± 1.1%) and reverse transcriptase (78.9 ± 1.43%) at a concentration of 25 μg/ml. Yuguchi et al. (2016) reported that fucoidan obtained from certain brown seaweeds displayed similar anti-HIV activity by blocking the early steps of HIV entry into target cells. They have suggested that sulfate content and its position in the fucoidan backbone is not associated with antiviral activity. Hayashi and his colleagues have reported that fucoidan from Undaria pinnatifida can prevent the replication of influenza A virus inside the host cell by blocking the transcription process. They have also said that fucoidan helps in stimulating both innate and adaptive immune functions by regulating B- lymphocytes (bone marrow cells) and T-(thymus cells) lymphocytes in virus-infected mice (Hayashi et al., 2013).
6. Modulation of host antiviral immune responses with seaweed polysaccharides
The viral infection can induce the antiviral immune responses of the host cell. Type I interferon system (IFN), host natural killer (NK) cells, and phagocytic cells contribute significantly against viral infection. IFN interacts with receptors on the cell surface that generates the antiviral proteins and enhances the activity of NK cells, T lymphocytes, and macrophages (Sozzani et al., 2010). Several seaweed polysaccharides help indirectly in the inhibition of viral infection and speed up the process of virus removal by activating the NK cells and stimulating antiviral immune factors.
The λ-carrageenan polysaccharide has been shown to induce the synthesis of interferon, which contributes to the biological effects on the immune response. Microwave degradation of λ-carrageenan from Chondrus ocellatus has been reported to inhibit the growth of tumors by improving the activity of interferons and enhancing lymphocyte multiplication (Liu et al., 2019). Fucoidan has various immune-modulating effects, such as stimulating the production of NK cells, development of dendritic cells, and the function of cytotoxic lymphocytes. Moreover, it enhances the Th1-type immune response by producing antibodies against determinants of specific antigen and generates memory T cells against the particular virus (Zhang et al., 2015). Carrageenan from Kappaphycus striatum modulates the immune response by enhancing the activity of macrophages and NK cells and increases the expression of interleukin-2 (IL-2) and tumor necrosis factor-alpha (TNF-α) (Yuan et al., 2006). The sulfated polysaccharides from Enteromorpha prolifera can stimulate macrophages cells. It can also induce the production of various cytokines such as interferon-gamma (IFN-γ) and interleukin-2 (IL-2), thus upregulating the Th-1 response (Kim et al., 2011). Polysaccharides from brown and green macroalgae are also reported to improve the antiviral immune response of the host cell. Alginate polysaccharide 911 can block the absorption of the virus and inhibit its replication by modulating the immune response of T and B lymphocytes (Xianliang et al., 2000).
In conclusion, marine sulfated polysaccharides can impede the virus adsorption and its replication and, thus, activate the immune response of the host directly by accelerating the process of virus clearance.
7. Potential antiviral application of marine polysaccharide in combating COVID-19
In late December 2019, an outbreak of novel coronavirus diseases (COVID-19) in Wuhan, China has spread quickly nationwide. According to the World Health Organization (WHO), it is a pandemic disease caused by a unique beta-coronavirus, one of the four genera of coronavirus (alpha, beta, delta, and gamma). At present, it is formally known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Gorbalenya et al., 2020). It is transmitted through the air (cough or sneeze), physical contact, contaminated objects, or mass gathering. Infected people experience a dry cough, high fever, sore throat, and face difficulty in breathing.
The COVID-19 is a novel enveloped beta-coronavirus which mainly caused infections in the pulmonary and digestive tract (Zhu et al., 2020). The outer membrane of the virus is made up of spike proteins that protrude out of the cell surface. The inside virus contains capsid protein along with its genome and single-stranded RNA. It is reported that glycoproteins on the surface of coronavirus bind to the receptors of host cells. This attachment leads to the entrance of the virus inside the cell. Spike proteins on the virus surface bind with blood vessel cells of the heart and kidney and with epithelial cells of lungs and intestine via angiotensin-converting enzyme (Xu et al., 2020).
There are currently no vaccine or effective antiviral treatments for COVID-19 in general, although hundreds of clinical trials are underway. Recently, marine sulfated polysaccharides have gain attention because of their antiviral effects [Table 2 ]. It has been proved that the antiviral properties of carrageenan have shown promising inhibitory effects on many types of viruses. It can effectively disrupt the interaction between virus and host cell receptors, thus block the internalization of virus particles. Koenighofer and co-workers have developed carrageenan-based nasal spray, which was effective in a patient with a common cold infected by a human coronavirus OC43 (beta) and human coronavirus 229E (alpha). Its application has shown a 2.5-fold reduction in relapses of symptoms and increased viral clearance compared to placebo-treated patients during clinical trials (Koenighofer et al., 2014). To treat throat infection caused by a human coronavirus OC43, Morokutti-Kurz and his colleagues have prepared lozenges comprising 10 mg iota-carrageenan as active pharmaceutical constituents. Their results show that glycoproteins on the coronavirus surface were denatured during the residence time of lozenge inside the mouth. Carrageenan based lozenges were responsible for the morphological changes of glycoproteins due to the low pH maintained inside the mouth. Denaturation of glycoproteins leads to inhibit the virucidal actions of coronavirus (Morokutti-Kurz et al., 2017). Graf and his partners have formed a nasal spray formulation comprising 0.05% of xylometazoline hydrochloride and 0.12% of iota-carrageenan. This formulation has been reported to relieve nasal congestion in the upper respiratory tract and simultaneously provides antiviral protection of respiratory mucosa (Graf et al., 2018).
Table 2.
Some of the marine sulfated polysaccharides reported to combat COVID-19.
| Compound | Efficacy | Effect/mechanism | Reference |
|---|---|---|---|
| Iota-carrageenan based nasal spray | Concentration as low as 6 μg/ml | Inhibit SARS-CoV-2 in vitro | Bansal et al., 2020 |
| Carrageenan based nasal spray | – | 2.5-Fold reduction in relapses of symptoms | Koenighofer et al., 2014 |
| Fucoidan and carrageenan | At 3.90-500 μg/ml concentration | Prevent SARS-CoV-2 entry into the cell by binding to the S-glycoprotein | Song et al., 2020 |
| Iota-carrageenan based lozenges | At a concentration of 10 mg | Denaturation of coronavirus glycoprotein | Morokutti-Kurz et al., 2017 |
| Fucoidan Kappa carrageenan Iota- carrageenan | Concentration of 0.01–10% by weight | Prevent or treat respiratory tract infections caused by the virus | Grassauer and Prieschl-Grassauer, 2019 |
| Fucoidan | At approx. 83 nM concentration | SP bind to spike protein of SARS-CoV-2 in vitro, preventing its binding to the host cell | Kwon et al., 2020 |
| Iota- carrageenan based nasal spray | At the concentration of 0.12% | Relieve nasal congestion in the upper respiratory tract | Graf et al., 2018 |
| Iota- carrageenan | Total 1 mg dose daily | 2.40 fold increase in recovery rate from coronavirus infection | Hemilä and Chalker, 2020 |
| Lamda-carrageenan | At 0.3–1.4 μg/ml concentration | Target viral attachment to cell surface receptors | Jang et al., 2020 |
Grassauer and Prieschl-Grassauer have investigated the effect of carrageenan on the inhibition of coronavirus mediated cell death in cat kidney cells. Iota-carrageenan shows significant inhibition at low levels (4 μg/ml), but kappa- and lambda-carrageenan are not sufficient. They reported that pretreatment of the host cell with the highest experimental carrageenan concentration of 400 μg/ml shows only 35% inhibition. This result indicates that pretreatment is not significant enough to protect cells from coronavirus infection. Therefore, they suggested that antiviral active agent carrageenan should be present at the time of infection, i.e., when virus and host cell interaction is about to take place. Thus, carrageenan facilitates protection against coronavirus. It also suggests that carrageenan can be coated or impregnated to a solid surface of hygiene or sanitary item such as gloves, tissue paper, cotton swab, and facial masks (Grassauer and Prieschl-Grassauer, 2019).
8. Benefits of marine SPs over other natural compounds
Several potential vaccines are being studied for COVID 19, but no specific effective treatment is developed. Although many natural compounds other than sulfated polysaccharides from marine algae also shows promising results on COVID 19 patients. Various herbal traditional medicines, plant extracts containing flavonoids and phenolic compounds, and essential oils from medicinal plants are reported to have antiviral bioactivities for COVID 19. Shree et al. (2020) have reported that phytochemicals from medicinal plants such as Ashwagandha (Withania somnifera), Giloy (Tinospora cordifolia), and Tulsi (Ocimum sanctum) showed potential inhibitor against SARS-CoV-2 main protease. Studies show that essential oil from Eucalyptus and Neem (Azadirachta indica) helps in the symptomatic treatment of COVID 19 (Roy and Bhattacharyya, 2020). Wahedi et al. (2020) have reported that phenolic compounds from plants such as Stilbene can disrupt the affinity binding of COVID spike protein on the human ACE2 receptor. Jo et al. (2020) has reported that the antiviral activity of some of the flavonoids can efficiently block the enzymatic activity of coronavirus 3C-like protease. Other plant secondary metabolites such as alkaloids, terpenoids, and polyphenols were also reported to prevent the binding of the virus to the host cell (Jahan and Onay, 2020).
Although potential anti-SARS-CoV-2 activity is exhibited by both algae-based and plant-based compounds, they both have their own merits and demerits. Marine macroalgae have been explored by many researchers as an excellent opportunity to become an inexhaustible source of biologically active compounds for the discovery of novel and useful therapeutic drugs. Algae-based and plant-based compounds are safe, biocompatible, and biodegradable, but the production cost of algae-based SPs is less than plant-based natural compounds due to their abundance in the ocean (Ruocco et al., 2016). Marine SPs are water-soluble and can easily be extracted using an aqueous extraction method, unlike plant-based compounds extracted using harmful organic compounds. Physicochemical and mechanical properties of SPs can easily be modified, which increases its use in pharmaceutical industries (Lee et al., 2017b). No clear health risk has been identified in the literature; however, initiatives are required to study the chemical composition, biological potency, bioavailability, toxicity, and associated mechanisms of sulfated polysaccharides in pharmaceutical sectors.
9. Conclusion
The sulfated polysaccharides are considered as a promising antiviral drug in the future. The diverse structure of SPs has an essential role in boosting the host antiviral response by interfering with virus attachment, adsorption, and its replication process. There are several antiviral studies of marine polysaccharides have been performed in vitro or on specific animal models. Across 40 compounds are commercially available in the market, and many more are being implemented at the preclinical or clinical stages of human trials. More initiatives are required to study the chemical composition, biological potency, and associated mechanisms of SPs in pharmaceutical sectors.
CRediT authorship contribution statement
Nidhi Hans: Conceptualization, Methodology, Validation, Investigation, Writing an original draft, Visualization. Anushree Malik: Conceptualization, Resources, Writing- review & editing, Supervision, Project administration, funding acquisition. Satyanarayan Naik: Conceptualization, Resources, Writing- review & editing, Supervision, Project administration, funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgment
The authors sincerely acknowledge the Centre for Rural Development and Technology, Indian Insititute of Technology Delhi, New Delhi, India, for financial and technical support to carry out this research work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Aguilar-Briseño J.A., Cruz-Suarez L.E., Sassi J.F., Ricque-Marie D., Zapata-Benavides P., Mendoza-Gamboa E., Rodríguez-Padilla C., Trejo-Avila L.M. Sulphated polysaccharides from Ulva clathrata and Cladosiphon okamuranus seaweeds both inhibit viral attachment/entry and cell-cell fusion, in NDV infection. Mar. Drugs. 2015;13:697–712. doi: 10.3390/md13020697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Alawi A.A., Al-Marhubi I.M., Al-Belushi M.S.M., Soussi B. Characterization of carrageenan extracted from Hypnea bryoides in Oman. Mar. Biotechnol. 2011;13:893–899. doi: 10.1007/s10126-010-9350-7. [DOI] [PubMed] [Google Scholar]
- Ale M.T., Mikkelsen J.D., Meyer A.S. Review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar. Drugs. 2011;9:2106–2130. doi: 10.3390/md9102106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allied market research, 2018. Seaweed Market by Product (Red, Brown, and Green) and Application (Human Food, Hydrocolloids, Fertilizers, Animal Feed Additives, and Others) - Global Opportunity Analysis and Industry Forecast. (Accessed 14 April 2020).
- Bansal S., Jonsson C.B., Taylor S.L., Figueroa J.M., Dugour A.V., Palacios C., Vega J.C. Iota-carrageenan and xylitol inhibit SARS-CoV-2 in cell culture. BioRxiv. 2020 doi: 10.1101/2020.08.19.225854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedoux G., Caamal-Fuentes E., Boulho R., Marty C., Bourgougnon N., Freile-Pelegrín Y., Robledo D. Antiviral and cytotoxic activities of polysaccharides extracted from four tropical seaweed species. Nat. Prod. Commun. 2017;12(6) doi: 10.1177/1934578X1701200602. 1934578X1701200602. [DOI] [Google Scholar]
- Besednova N.N., Zvyagintseva T.N., Kuznetsova T.A., Makarenkova I.D., Smolina T.P., Fedyanina L.N., Kryzhanovsky S.P., Zaporozhets T.S. Marine algae metabolites as promising therapeutics for the prevention and treatment of HIV/AIDS. Metabolites. 2019;9(5):87. doi: 10.3390/metabo9050087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bind A., Goswami L., Prakash V. Comparative analysis of floating and submerged macrophytes for heavy metal (copper, chromium, arsenic and lead) removal: sorbent preparation, characterization, regeneration and cost estimation. Geol. Ecol. Landsc. 2018;2(2):61–72. doi: 10.1080/24749508.2018.1452460. [DOI] [Google Scholar]
- Bind A., Kushwaha A., Devi G., Goswami S., Sen B., Prakash V. Biosorption valorization of floating and submerged macrophytes for heavy-metal removal in a multi-component system. Appl Water Sci. 2019;9(4):95. doi: 10.1007/s13201-019-0976-y. [DOI] [Google Scholar]
- Bjerregaard R., Valderrama D., Radulovich R., Diana J., Capron M., Mckinnie C.A., Cedric M., Hopkins K., Yarish C., Goudey C., Forster J. World Bank Group; Washington, DC: 2016. Seaweed Aquaculture for Food Security, Income Generation and Environmental Health in Tropical Developing Countries. [Google Scholar]
- Bouhlal R., Haslin C., Chermann J.C., Colliec-Jouault S., Sinquin C., Simon G., Cerantola S., Riadi H., Bourgougnon N. Antiviral activities of sulfated polysaccharides isolated from Sphaerococcus coronopifolius (Rhodophytha, Gigartinales) and Boergeseniella thuyoides (Rhodophyta, Ceramiales) Mar. Drugs. 2011;9(7):1187–1209. doi: 10.3390/md9071187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck C.B., Thompson C.D., Roberts J.N., Müller M., Lowy D.R., Schiller J.T. Carrageenan is a potent inhibitor of papillomavirus infection. PLoS Pathog. 2006;2(7) doi: 10.1371/journal.ppat.0020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlucci M.J., Scolaro L.A., Damonte E.B. Herpes simplex virus type 1 variants arising after selection with an antiviral carrageenan: lack of correlation between drug susceptibility and syn phenotype. J. Med. Virol. 2002;68:92–98. doi: 10.1002/jmv.10174. [DOI] [PubMed] [Google Scholar]
- Chen X., Han W., Wang G., Zhao X. Application prospect of polysaccharides in the development of anti-novel coronavirus drugs and vaccines. Int. J. Biol. Macromol. 2020 doi: 10.1016/j.ijbiomac.2020.07.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu Y.H., Chan Y.L., Li T.L., Wu C.J. Inhibition of Japanese encephalitis virus infection by the sulfated polysaccharide extracts from Ulva lactuca. Mar. Biotechnol. 2012;14:468–478. doi: 10.1007/s10126-011-9428-x. [DOI] [PubMed] [Google Scholar]
- Chiu Y.H., Chan Y.L., Tsai L.W., Li T.L., Wu C.J. Prevention of human enterovirus 71 infection by kappa carrageenan. Antivir. Res. 2012;95:128–134. doi: 10.1016/j.antiviral.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Cirne-Santos C.C., Barros C.D.S., Nogueira C.C.R., Azevedo R.C., Yamomoto K.A., Meira G.L.S., Vasconcelos Z.F.M.D., Ratcliffe N.A., Teixeira V.L., Schmidt-Chanasit J., Ferreira D.F., Paixão I.C.N.D.P. Inhibition by marine algae of chikungunya virus isolated from patients in a recent disease outbreak in Rio de Janeiro. Front. Microbiol. 2019;10:2426. doi: 10.3389/fmicb.2019.02426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sf-Tischer P.C., Talarico L.B., Noseda M.D., Guimarães S.M.P.B., Damonte E.B., Duarte M.E.R. Chemical structure and antiviral activity of carrageenans from Meristiella gelidium against herpes simplex and dengue virus. Carbohydr. Polym. 2006;63:459–465. doi: 10.1016/j.carbpol.2005.09.020. [DOI] [Google Scholar]
- Dinesh S., Menon T., Hanna L.E., Suresh V., Sathuvan M., Manikannan M. In vitro anti-HIV-1 activity of fucoidan from Sargassum swartzii. Int. J. Biol. Macromol. 2016;82:83–88. doi: 10.1016/j.ijbiomac.2015.09.078. [DOI] [PubMed] [Google Scholar]
- Dutta M., Kushwaha A., Kalita S., Devi G., Bhuyan M. Assessment of bioaccumulation and detoxification of cadmium in soil-plant-insect food chain. Bioresour. Technol. Rep. 2019;7:100242. doi: 10.1007/978-981-15-6564-9_11. [DOI] [Google Scholar]
- FAO., 2019. FAO yearbook. Fishery and Aquaculture Statistics 2017/FAO annuaire.
- Fedorov S.N., Ermakova S.P., Zvyagintseva T.N., Stonik V.A. Anticancer and cancer preventive properties of marine polysaccharides: some results and prospects. Mar. Drugs. 2013;11:4876–4901. doi: 10.3390/md11124876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdouse, F., Holdt, S. L., Smith, R., Murúa, P., & Yang, Z., 2018. The global status of seaweed production, trade and utilization. Globefish Research Programme. 124, I.
- Ferreira L.G., Noseda M.D., Gonçalves A.G., Ducatti D.R., Fujii M.T., Duarte M.E. Chemical structure of the complex pyruvylated and sulfated agaran from the red seaweed Palisada flagellifera (Ceramiales, Rhodophyta) Carbohydr. Res. 2012;347:83–94. doi: 10.1016/j.carres.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Figueira T.A., da Silva A.J.R., Enrich-Prast A., Yoneshigue-Valentin Y., de Oliveira V.P. Structural characterization of ulvan polysaccharide from cultivated and collected Ulva fasciata (Chlorophyta) Adv. Biosci. Biotechnol. 2020;11(5):206–216. doi: 10.4236/abb.2020.115016. [DOI] [Google Scholar]
- Ghosh T., Pujol C.A., Damonte E.B., Sinha S., Ray B. Sulfated xylomannans from the red seaweed Sebdenia polydactyla: structural features, chemical modification and antiviral activity. Antivir. Chem. Chemother. 2009;19:235–242. doi: 10.1177/095632020901900603. [DOI] [PubMed] [Google Scholar]
- Gomaa H.H., Elshoubaky G.A. Antiviral activity of sulfated polysaccharides carrageenan from some marine seaweeds. Int. J. Curr. Pharm. Rev. Res. 2016;7:34–42. [Google Scholar]
- Gonçalves A.G., Ducatti D.R., Duarte M.E.R., Noseda M.D. Sulfated and pyruvylated disaccharide alditols obtained from a red seaweed galactan: ESIMS and NMR approaches. Carbohydr. Res. 2002;337:2443–2453. doi: 10.1016/S0008-6215(02)00318-X. [DOI] [PubMed] [Google Scholar]
- Gorbalenya A.E., Baker S.C., Baric R., Groot R.J.D., Drosten C., Gulyaeva A.A., Haagmans B.L., Lauber C., Leontovich A.M., Neuman B.W., Penzar D., Perlman S., Poon L., Samborskiy D., Sidorov I.A., Solá G.I., Ziebuhr J. Severe acute respiratory syndrome-related coronavirus: the species and its viruses–a statement of the Coronavirus Study Group. BioRxiv. 2020 doi: 10.1101/2020.02.07.937862. [DOI] [Google Scholar]
- Goswami L., Kumar R.V., Manikandan N.A., Pakshirajan K., Pugazhenthi G. Anthracene biodegradation by Oleaginous Rhodococcus opacus for biodiesel production and its characterization. Polycycl. Aromat. Compd. 2019 doi: 10.1080/10406638.2017.1302971. [DOI] [Google Scholar]
- Goswami L., Kumar R.V., Pakshirajan K., Pugazhenthi G. A novel integrated biodegradation—microfiltration system for sustainable wastewater treatment and energy recovery. J. Hazard. Mater. 2019;365:707–715. doi: 10.1016/j.jhazmat.2018.11.029. [DOI] [PubMed] [Google Scholar]
- Goswami L., Pakshirajan K., Pugazhenthi G. Biological treatment of biomass gasification wastewater using hydrocarbonoclastic bacterium Rhodococcus opacus in an up-flow packed bed bioreactor with a novel waste-derived nano-biochar based bio-support material. J. Clean. Prod. 2020;256:120253. doi: 10.1016/j.jclepro.2020.120253. [DOI] [Google Scholar]
- Graf C., Bernkop-Schnürch A., Egyed A., Koller C., Prieschl-Grassauer E., Morokutti-Kurz M. Development of a nasal spray containing xylometazoline hydrochloride and iota-carrageenan for the symptomatic relief of nasal congestion caused by rhinitis and sinusitis. Int. J. Gen. Med. 2018;11:275. doi: 10.2147/IJGM.S167123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassauer, A., & Prieschl-Grassauer, E., 2019. U.S. Patent No. 10,342,820. Washington, DC: U.S. Patent and Trademark Office.
- Grassauer A., Weinmuellner R., Meier C., Pretsch A., Prieschl-Grassauer E., Unger H. Iota-Carrageenan is a potent inhibitor of rhinovirus infection. Virol. J. 2008;5:107. doi: 10.1186/1743-422X-5-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güven K.C., Coban B., Özdemir O. Pharmacology of marine macroalgae. Encycl. Mar. Biotechnol. 2020;1:585–615. doi: 10.1002/9781119143802.ch20. [DOI] [Google Scholar]
- Hans N., Malik A., Naik S.N. In: Handbook of Algal Technologies and Phytochemicals: Volume I Food, Health and Nutraceutical Applications. Ravishankar G.A., Ambati R.R., editors. CRC Press; 2019. Platform molecules from algae by using supercritical CO2 and subcritical water extraction. [DOI] [Google Scholar]
- Hardouin K., Bedoux G., Burlot A.S., Donnay-Moreno C., Bergé J.P., Nyvall-Collén P., Bourgougnon N. Enzyme-assisted extraction (EAE) for the production of antiviral and antioxidant extracts from the green seaweed Ulva armoricana (Ulvales, Ulvophyceae) Algal Res. 2016;16:233–239. doi: 10.1016/j.algal.2016.03.013. [DOI] [Google Scholar]
- Hayashi K., Lee J.B., Nakano T., Hayashi T. Anti-influenza A virus characteristics of a fucoidan from sporophyll of Undaria pinnatifida in mice with normal and compromised immunity. Microbes Infect. 2013;15:302–309. doi: 10.1016/j.micinf.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Hemilä H., Chalker E. 2020. Carrageenan Nasal Spray May Double the Rate of Recovery From Coronavirus and Influenza Virus Infections: Re-analysis of Randomized Trial Data. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmingson J.A., Falshaw R., Furneaux R.H., Thompson K. Structure and antiviral activity of the galactofucan sulfates extracted from Undariapinnatifida (Phaeophyta) J. Appl. Phycol. 2006;18:185. doi: 10.1007/s10811-006-9096-9. [DOI] [Google Scholar]
- Hidari K.I., Takahashi N., Arihara M., Nagaoka M., Morita K., Suzuki T. Structure and anti-dengue virus activity of sulfated polysaccharide from a marine alga. Biochem. Biophys. Res. Commun. 2008;376:91–95. doi: 10.1016/j.bbrc.2008.08.100. [DOI] [PubMed] [Google Scholar]
- Jahan I., Onay A. Potentials of plant-based substance to inhabit and probable cure for the COVID-19. Turk. J. Biol. 2020;44(3):228. doi: 10.3906/biy-2005-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang Y.J., Shin H., Lee M.K., Kwon O.S., Shin J.S., Kim Y., Kim M. Antiviral activity of lambda-carrageenan against influenza viruses in mice and severe acute respiratory syndrome coronavirus 2 in vitro. BioRxiv. 2020 doi: 10.1101/2020.08.23.255364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao G., Yu G., Zhang J., Ewart H.S. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar. Drugs. 2011;9:196–223. doi: 10.3390/md9020196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo S., Kim S., Shin D.H., Kim M.S. Inhibition of SARS-CoV 3CL protease by flavonoids. J. Enzyme Inhib. Med. Chem. 2020;35(1):145–151. doi: 10.1080/14756366.2019.1690480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jönsson M., Allahgholi L., Sardari R.R., Hreggviðsson G.O., Nordberg Karlsson E. Extraction and modification of macroalgal polysaccharides for current and next-generation applications. Molecules. 2020;25(4):930. doi: 10.3390/molecules25040930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalid S., Abbas M., Saeed F., Bader-Ul-Ain H., Suleria H.A.R. Therapeutic potential of seaweed bioactive compounds. Seaweed Biomater. 2018;1:7–26. doi: 10.5772/intechopen.74060. [DOI] [Google Scholar]
- Kidgell J.T., Magnusson M., de Nys R., Glasson C.R. Ulvan: a systematic review of extraction, composition and function. Algal Res. 2019;39:101422. doi: 10.1016/j.algal.2019.101422. [DOI] [Google Scholar]
- Kim J.K., Cho M.L., Karnjanapratum S., Shin I.S., You S.G. In vitro and in vivo immunomodulatory activity of sulfated polysaccharides from Enteromorpha prolifera. Int. J. Biol. Macromol. 2011;49:1051–1058. doi: 10.1016/j.ijbiomac.2011.08.032. [DOI] [PubMed] [Google Scholar]
- Kim S.K., editor. Springer Handbook of Marine Biotechnology. Springer; 2015. [DOI] [Google Scholar]
- Koenighofer M., Lion T., Bodenteich A., Prieschl-Grassauer E., Grassauer A., Unger H., Mueller C.A., Fazekas T. Carrageenan nasal spray in virus confirmed common cold: individual patient data analysis of two randomized controlled trials. Multidiscip. Respir. Med. 2014;9:57. doi: 10.1186/2049-6958-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushwaha A., Rani R., Kumar S., Gautam A. Heavy metal detoxification and tolerance mechanisms in plants: implications for phytoremediation. Environ. Rev. 2015;24(1):39–51. doi: 10.1139/er-2015-0010. [DOI] [Google Scholar]
- Kushwaha A., Rani R., Kumar S. In: Environmental Science and Engineering, 8. Kumar P., Gurjar B.R., Govil J.N., editors. Studium Press LLC; U.S.A: 2017. Mechanism of soil-metal-microbe interactions and their implication on microbial bioremediation and phytoremediation; p. 462. [Google Scholar]
- Kushwaha A., Rani R., Patra J.K. Adsorption kinetics and molecular interactions of lead [Pb (II)] with natural clay and humic acid. Int. J. Environ. Sci. Technol. 2020;17(3):1325–1336. doi: 10.1007/s13762-019-02411-6. [DOI] [Google Scholar]
- Kwon P.S., Oh H., Kwon S.J., Jin W., Zhang F., Fraser K., Hong J.J., Linhardt R.J., Dordick J.S. Sulfated polysaccharides effectively inhibit SARS-CoV-2 in vitro. Cell Discov. 2020;6(1):1–4. doi: 10.1038/s41421-020-00192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.B., Hayashi K., Maeda M., Hayashi T. Antiherpetic activities of sulfated polysaccharides from green algae. Planta Med. 2004;70:813–817. doi: 10.1055/s-2004-827228. [DOI] [PubMed] [Google Scholar]
- Lee J.B., Koizumi S., Hayashi K., Hayashi T. Structure of rhamnan sulfate from the green alga Monostroma nitidum and its anti-herpetic effect. Carbohydr. Polym. 2010;81:572–577. doi: 10.1016/j.carbpol.2010.03.014. [DOI] [Google Scholar]
- Lee J.B., Takeshita A., Hayashi K., Hayashi T. Structures and antiviral activities of polysaccharides from Sargassum trichophyllum. Carbohydr. Polym. 2011;86:995–999. doi: 10.1016/j.carbpol.2011.05.059. [DOI] [Google Scholar]
- Lee W.K., Lim Y.Y., Leow A.T.C., Namasivayam P., Abdullah J.O., Ho C.L. Biosynthesis of agar in red seaweeds: a review. Carbohydr. Polym. 2017;164:23–30. doi: 10.1016/j.carbpol.2017.01.078. [DOI] [PubMed] [Google Scholar]
- Lee Y.E., Kim H., Seo C., Park T., Lee K.B., Yoo S.Y., Hong S.C., Kim J.T., Lee J. Marine polysaccharides: therapeutic efficacy and biomedical applications. Arch. Pharm. Res. 2017;40(9):1006–1020. doi: 10.1007/s12272-017-0958-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibbrandt A., Meier C., König-Schuster M., Weinmüllner R., Kalthoff D., Pflugfelder B., Graf P., Frank-Gehrke B., Beer M., Fazekas T., Unger H., Prieschl-Grassauer E., Grassauer A. Iota-carrageenan is a potent inhibitor of influenza a virus infection. PLoS One. 2010;5(12) doi: 10.1371/journal.pone.0014320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Mao W., Hou Y., Gao Y., Qi X., Zhao C., Chen Y., Chen Y., Li N., Wang C. Preparation, structure and anticoagulant activity of a low molecular weight fraction produced by mild acid hydrolysis of sulfated rhamnan from Monostroma latissimum. Bioresour. Technol. 2012;114:414–418. doi: 10.1016/j.biortech.2012.03.025. [DOI] [PubMed] [Google Scholar]
- Liu Z., Gao T., Yang Y., Meng F., Zhan F., Jiang Q., Sun X. Anti-cancer activity of porphyran and carrageenan from red seaweeds. Molecules. 2019;24(23):4286. doi: 10.3390/molecules24234286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes N., Ray S., Espada S.F., Bomfim W.A., Ray B., Faccin-Galhardi L.C., Linhares R.E.C., Nozawa C. Green seaweed Enteromorpha compressa (Chlorophyta, Ulvaceae) derived sulphated polysaccharides inhibit herpes simplex virus. Int. J. Biol. Macromol. 2017;102:605–612. doi: 10.1016/j.ijbiomac.2017.04.043. [DOI] [PubMed] [Google Scholar]
- Makarenkova I.D., Deriabin P.G., L’vov D.K., Zviagintseva T.N., Besednova N.N. Antiviral activity of sulfated polysaccharide from the brown algae Laminaria japonica against avian influenza A (H5N1) virus infection in the cultured cells. Vopr. Virusol. 2010;55:41–45. [PubMed] [Google Scholar]
- Malagoli B.G., Cardozo F.T., Gomes J.H.S., Ferraz V.P., Simões C.M., Braga F.C. Chemical characterization and antiherpes activity of sulfated polysaccharides from Lithothamnion muelleri. Int. J. Biol. Macromol. 2014;66:332–337. doi: 10.1016/j.ijbiomac.2014.02.053. [DOI] [PubMed] [Google Scholar]
- Mandal P., Mateu C.G., Chattopadhyay K., Pujol C.A., Damonte E.B., Ray B. Structural features and antiviral activity of sulphated fucans from the brown seaweed Cystoseira indica. Antivir. Chem. Chemother. 2007;18:153–162. doi: 10.1177/095632020701800305. [DOI] [PubMed] [Google Scholar]
- Mandal P., Pujol C.A., Carlucci M.J., Chattopadhyay K., Damonte E.B., Ray B. Anti-herpetic activity of a sulfated xylomannan from Scinaia hatei. Phytochemistry. 2008;69:2193–2199. doi: 10.1016/j.phytochem.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Mazumder S., Ghosal P.K., Pujol C.A., Carlucci M.J., Damonte E.B., Ray B. Isolation, chemical investigation and antiviral activity of polysaccharides from Gracilaria corticata (Gracilariaceae, Rhodophyta) Int. J. Biol. Macromol. 2002;31:87–95. doi: 10.1016/S0141-8130(02)00070-3. [DOI] [PubMed] [Google Scholar]
- Mercer J., Schelhaas M., Helenius A. Virus entry by endocytosis. Annu. Rev. Biochem. 2010;79:803–833. doi: 10.1146/annurev-biochem-060208-104626. [DOI] [PubMed] [Google Scholar]
- Morán-Santibañez K., Cruz-Suárez L.E., Ricque-Marie D., Robledo D., Freile-Pelegrín Y., Peña-Hernández M.A., Rodríguez-Padilla C., Trejo-Avila L.M. Synergistic effects of sulfated polysaccharides from Mexican seaweeds against measles virus. Biomed. Res. Int. 2016:2016. doi: 10.1155/2016/8502123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morokutti-Kurz M., Graf C., Prieschl-Grassauer E. Amylmetacresol/2, 4-dichlorobenzyl alcohol, hexylresorcinol, or carrageenan lozenges as active treatments for sore throat. Int. J. Gen. Med. 2017;10:53. doi: 10.2147/IJGM.S120665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumar J., Chidambaram R., Sukumaran S. Sulfated polysaccharides and its commercial applications in food industries—a review. J. Food Sci. Technol. 2020:1–14. doi: 10.1007/s13197-020-04837-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Tena J.G., Schieder D., Sieber V. In: Tropical Seaweed Farming Trends, Problems and Opportunities. Hurtado A., Critchley A., Neish I., editors. vol. 9. Springer; Cham: 2017. Carrageenan and more: biorefinery approaches with special reference to the processing of Kappaphycus; pp. 155–164.http://doi-org-443.webvpn.fjmu.edu.cn/10.1007/978-3-319-63498-2_10 (Developments in Applied Phycology). [Google Scholar]
- Ponce N.M., Pujol C.A., Damonte E.B., Flores M.L., Stortz C.A. Fucoidans from the brown seaweed Adenocystis utricularis: extraction methods, antiviral activity and structural studies. Carbohydr. Res. 2003;338:153–165. doi: 10.1016/S0008-6215(02)00403-2. [DOI] [PubMed] [Google Scholar]
- Pujol C.A., Estevez J.M., Carlucci M.J., Ciancia M., Cerezo A.S., Damonte E.B. Novel DL-galactan hybrids from the red seaweed Gymnogongrus torulosus are potent inhibitors of herpes simplex virus and dengue virus. Antivir. Chem. Chemother. 2002;13:83–89. doi: 10.1177/095632020201300202. [DOI] [PubMed] [Google Scholar]
- Queiroz K.C.S., Medeiros V.P., Queiroz L.S., Abreu L.R.D., Rocha H.A.O., Ferreira C.V., Jucá M.B., Aoyama H., Leite E.L. Inhibition of reverse transcriptase activity of HIV by polysaccharides of brown algae. Biomed. Pharmacother. 2008;62:303–307. doi: 10.1016/j.biopha.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Rabanal M., Ponce N.M., Navarro D.A., Gómez R.M., Stortz C.A. The system of fucoidans from the brown seaweed Dictyota dichotoma: chemical analysis and antiviral activity. Carbohydr. Polym. 2014;101:804–811. doi: 10.1016/j.carbpol.2013.10.019. [DOI] [PubMed] [Google Scholar]
- Ray B. Polysaccharides from Enteromorpha compressa: isolation, purification and structural features. Carbohydr. Polym. 2006;66:408–416. doi: 10.1016/j.carbpol.2006.03.027. [DOI] [Google Scholar]
- Rhein-Knudsen N., Ale M.T., Meyer A.S. Seaweed hydrocolloid production: an update on enzyme assisted extraction and modification technologies. Mar. Drugs. 2015;13:3340–3359. doi: 10.3390/md13063340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S., Bhattacharyya P. Possible role of traditional medicinal plant Neem (Azadirachta indica) for the management of COVID-19 infection. Int. J. Res. Pharm. Sci. 2020;11:122–125. doi: 10.26452/ijrps.v11iSPL1.2256. [DOI] [Google Scholar]
- Rudke A.R., de Andrade C.J., Ferreira S.R.S. Kappaphycus alvarezii macroalgae: an unexplored and valuable biomass for green biorefinery conversion. Trends Food Sci. Technol. 2020;103:214–224. doi: 10.1016/j.tifs.2020.07.018. [DOI] [Google Scholar]
- Ruocco N., Costantini S., Guariniello S., Costantini M. Polysaccharides from the marine environment with pharmacological, cosmeceutical and nutraceutical potential. Molecules. 2016;21(5):551. doi: 10.3390/molecules21050551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepúlveda-Crespo D., Ceña-Díez R., Jiménez J.L., Ángeles Muñoz-Fernández M. Mechanistic studies of viral entry: an overview of dendrimer-based microbicides as entry inhibitors against both HIV and HSV-2 overlapped infections. Med. Res. Rev. 2017;37(1):149–179. doi: 10.1002/med.21405. [DOI] [PubMed] [Google Scholar]
- Shree P., Mishra P., Selvaraj C., Singh S.K., Chaube R., Garg N., Tripathi Y.B. Targeting COVID-19 (SARS-CoV-2) main protease through active phytochemicals of ayurvedic medicinal plants–Withania somnifera (Ashwagandha), Tinospora cordifolia (Giloy) and Ocimum sanctum (Tulsi)–a molecular docking study. J. Biomol. Struct. Dyn. 2020;1-14 doi: 10.1080/07391102.2020.1810778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P.K., Kushwaha A., Hans N., Gautam A., Rani R. Evaluation of the cytotoxicity and interaction of lead with lead resistant bacterium Acinetobacter junii Pb1. Braz. J. Microbiol. 2019;50(1):223–230. doi: 10.1007/s42770-019-00041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S., Peng H., Wang Q., Liu Z., Dong X., Wen C., Ai C., Zhang Y., Wang Z., Zhu B. Inhibitory activities of marine sulfated polysaccharides against SARS-CoV-2. Food Funct. 2020;11(9):7415–7420. doi: 10.1039/D0FO02017F. [DOI] [PubMed] [Google Scholar]
- Sozzani S., Bosisio D., Scarsi M., Tincani A. Type I interferons in systemic autoimmunity. J. Autoimmun. 2010;43:196–203. doi: 10.3109/08916930903510872. [DOI] [PubMed] [Google Scholar]
- Talarico L.B., Damonte E.B. Interference in dengue virus adsorption and uncoating by carrageenans. Virology. 2007;363:473–485. doi: 10.1016/j.virol.2007.01.043. [DOI] [PubMed] [Google Scholar]
- Talarico L.B., Zibetti R.G., Faria P.C., Scolaro L.A., Duarte M.E., Noseda M.D., Pujol C.A., Damonte E.B. Anti-herpes simplex virus activity of sulfated galactans from the red seaweeds Gymnogongrus griffithsiae and Cryptonemia crenulata. Int. J. Biol. Macromol. 2004;34:63–71. doi: 10.1016/j.ijbiomac.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Tang F., Chen F., Li F. Preparation and potential in vivo anti-influenza virus activity of low molecular-weight κ-carrageenans and their derivatives. J. Appl. Polym. Sci. 2013;127:2110–2115. doi: 10.1002/app.37502. [DOI] [Google Scholar]
- Thuy T.T.T., Ly B.M., Van T.T.T., Quang N.V., Tu H.C., Zheng Y., Seguin-Devaux C., Mi B., Ai U. Anti-HIV activity of fucoidans from three brown seaweed species. Carbohydr. Polym. 2015;115:122–128. doi: 10.1016/j.carbpol.2014.08.068. [DOI] [PubMed] [Google Scholar]
- Trinchero J., Ponce N.M., Córdoba O.L., Flores M.L., Pampuro S., Stortz C.A., Salomón H., Turk G. Antiretroviral activity of fucoidans extracted from the brown seaweed Adenocystis utricularis. Phytother. Res. 2009;23:707–712. doi: 10.1002/ptr.2723. [DOI] [PubMed] [Google Scholar]
- Usov A.I. In: Advances in Carbohydrate Chemistry and Biochemistry. Horton D., editor. Academic Press; 2011. Polysaccharides of the red algae; pp. 115–217. [DOI] [PubMed] [Google Scholar]
- Wahedi H.M., Ahmad S., Abbasi S.W. Stilbene-based natural compounds as promising drug candidates against COVID-19. J. Biomol. Struct. Dyn. 2020;1-10 doi: 10.1080/07391102.2020.1762743. [DOI] [PubMed] [Google Scholar]
- Wang S., Wang W., Hao C., Yunjia Y., Qin L., He M., Mao W. Antiviral activity against enterovirus 71 of sulfated rhamnan isolated from the green alga Monostroma latissimum. Carbohydr. Polym. 2018;200:43–53. doi: 10.1016/j.carbpol.2018.07.067. [DOI] [PubMed] [Google Scholar]
- Wang W., Zhang P., Hao C., Zhang X.E., Cui Z.Q., Guan H.S. In vitro inhibitory effect of carrageenan oligosaccharide on influenza A H1N1 virus. Antivir. Res. 2011;92:237–246. doi: 10.1016/j.antiviral.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Wang W., Wang S.X., Guan H.S. The antiviral activities and mechanisms of marine polysaccharides: an overview. Mar. Drugs. 2012;10:2795–2816. doi: 10.3390/md10122795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Di Y., Ye J., Wei W. Study on the public psychological states and its related factors during the outbreak of coronavirus disease 2019 (COVID-19) in some regions of China. Psychol. Health Med. 2020:1–10. doi: 10.1080/13548506.2020.1746817. [DOI] [PubMed] [Google Scholar]
- Wijesekara I., Pangestuti R., Kim S.K. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr. Polym. 2011;84:14–21. doi: 10.1016/j.carbpol.2010.10.062. [DOI] [Google Scholar]
- Worldometer [Internet] COVID-19 Coronavirus Pandemic., 2020. Available from: https://www.worldometers.info/coronavirus/ (Accessed 20 November 2020).
- Xian Y., Zhang J., Bian Z., Zhou H., Zhang Z., Lin Z., Xu H. Bioactive natural compounds against human coronaviruses: a review and perspective. Acta Pharm. Sin. B. 2020;10:1163–1174. doi: 10.1016/j.apsb.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xianliang X., Hua D., Meiyu G., Pingfang L., Yingxia L., Huashi G. Studies of the anti-AIDS effects of marine polysaccharide drug 911 and its related mechanisms of action. Chin. J. Mar. Drugs. 2000;19(6):4–8. [Google Scholar]
- Xu X., Chen P., Wang J., Feng J., Zhou H., Li X., Zhong W., Hao P. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci. China Life Sci. 2020;63:457–460. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T., Ogamo A., Saito T., Uchiyama H., Nakagawa Y. Preparation of O-acylated low-molecular-weight carrageenans with potent anti-HIV activity and low anticoagulant effect. Carbohydr. Polym. 2000;41:115–120. doi: 10.1016/S0144-8617(99)00083-1. [DOI] [Google Scholar]
- Yu G., Hu Y., Yang B., Zhao X., Wang P., Ji G., Wu J., Guan H. Extraction, isolation and structural characterization of polysaccharides from a red alga Gloiopeltis furcata. J. Ocean Univ. China. 2010;9:193–197. doi: 10.1007/s11802-010-0193-7. [DOI] [Google Scholar]
- Yuan H., Song J., Li X., Li N., Dai J. Immunomodulation and antitumor activity of κ-carrageenan oligosaccharides. Cancer Lett. 2006;243:228–234. doi: 10.1016/j.canlet.2005.11.032. [DOI] [PubMed] [Google Scholar]
- Yuguchi Y., Bui L.M., Takebe S., Suzuki S., Nakajima N., Kitamura S., Thanh T.T.T. Primary structure, conformation in aqueous solution, and intestinal immunomodulating activity of fucoidan from two brown seaweed species Sargassum crassifolium and Padina australis. Carbohydr. Polym. 2016;147:69–78. doi: 10.1016/j.carbpol.2016.03.101. [DOI] [PubMed] [Google Scholar]
- Zhang W., Oda T., Yu Q., Jin J.O. Fucoidan from Macrocystis pyrifera has powerful immune-modulatory effects compared to three other fucoidans. Mar. Drugs. 2015;13:1084–1104. doi: 10.3390/md13031084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu B., Ni F., Sun Y., Zhu X., Yin H., Yao Z., Du Y. Insight into carrageenases: major review of sources, category, property, purification method, structure, and applications. Crit. Rev. Biotechnol. 2018;38(8):1261–1276. doi: 10.1080/07388551.2018.1472550. [DOI] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]


