Abstract
Introduction
Among patients with coronavirus disease 2019 (COVID-19), the factors that affect anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibody production remain unclear. This study aimed to identify such factors among patients convalescing from COVID-19.
Methods
This study comprised patients who had been diagnosed with COVID-19 between January 1 and June 30, 2020 and gave consent for anti-SARS-CoV-2 spike protein antibody measurement using enzyme-linked immunosorbent assay during their acute and/or convalescent phases. Factors related to elevated antibody titers and the relationship between the days from disease onset and the development of antibody titers were assessed.
Results
A total of 84 participants enrolled in the study. Nineteen participants had antibody titers measured during the convalescent phase only, and 65 participants had antibody titers measured during the acute and convalescent phases. The antibody titers peaked in weeks 5 and 6. The stepwise multivariate log-normal analysis revealed that male sex (P = 0.04), diabetes mellitus (P = 0.03), and high C-reactive protein levels during the disease course (P < 0.001) were associated with elevated IgG antibodies. Glucocorticoid use was not associated with antibody titers.
Conclusion
The study found that high values of maximum CRP levels during the acute phase, male sex, and diabetes mellitus were associated with elevated antibody titers. Antibody titers tended to be highest in the first 5 or 6 weeks after the onset of symptoms.
Keywords: COVID-19, SARS-CoV-2, Convalescent, Anti-SARS-CoV-2 spike protein antibody
1. Introduction
The global spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection following an outbreak in Wuhan, China, at the end of 2019 has had a significant effect on people's health and lives [1]. As of August 6, 2020, approximately 18.6 million people have been infected worldwide, of which 3.8% have died [2].
Many aspects of coronavirus disease 2019 (COVID-19) antibody production remain unknown. In patients with COVID-19, seroconversion of specific anti-SARS-CoV-2 IgM and IgG antibodies has been observed as early as the fourth day after symptom onset [3]. Long et al. [4] reported that patients with COVID had elevated anti-SARS-CoV-2 IgM and IgG antibodies within 19 days. However, precise information is limited concerning the persistence of antibody and factors that influence antibody levels.
In addition, there are no known effective drugs against COVID-19, other than remdesivir, which has been reported to shorten the course of the disease [5,6]. Under these circumstances, transfusion of convalescent plasma (CPT) donated by patients who have recovered from the infection is a promising treatment option. For life-threatening viruses such as severe acute respiratory syndrome coronavirus (SARS-CoV), Middle East respiratory syndrome coronavirus (MERS-CoV) and Ebola virus, CPT has been used based on the premise that neutralizing antibodies (nAbs) in the donor plasma, attenuate viral production in the recipients [7]. Although several CPT clinical trials have been conducted in COVID-19 patients and have had favorable clinical outcomes [[8], [9], [10], [11], [12], [13], [14]], most studies were conducted on small numbers of participants; thus, they could not draw definite conclusions regarding CPT efficacy. To prove CPT efficacy, controlled trials on large numbers of patients are essential, necessitating establishing a large donor cohort of COVID-19 patients with high neutralizing antibody activity.
SARS-CoV-2, a β-coronavirus with approximately 80% genetic sequence identity with SARS-CoV, has 4 structural viral proteins of nucleocapsid, envelope, membrane and spike proteins, and 16 non-structural proteins [15]. Among these molecules, the spike protein is a promising target for nAbs, to which several neutralizing monoclonal antibodies to SARS-CoV and MERS-CoV have been generated [15]. The spike protein is a large molecule with approximately 1250 amino acids composed of S1 and S2 fragments: S1 has an amino-terminal domain and receptor-binding domain (RBD) attaches to host cells via a cellular receptor, angiotensin-converting enzyme-2. In contrast, S2 contains a carboxy-terminal domain required to fuse the virus to the cellular membrane [15]. In recently reported CPT clinical trials, convalescent plasmas were screened by an enzyme-linked immunosorbent assay (ELISA) using RBD as an antigen [8,9]. However, it has been reported that nAbs in SARS-CoV and MERS-CoV patients also targets the S2 or S1/S2 proteolytic cleavage site [15], implying that it is feasible to screen COVID-19 convalescent patients by using a full-length spike protein instead of RBD alone. Additionally, if we can find clinical information and laboratory data closely linked to the high anti-spike antibody titers, it would be advantageous to select candidate donors efficiently. This study aimed to determine factors that are likely to influence adequate antibody production in patients convalescing after COVID-19.
2. Materials and methods
2.1. Study design and participants
Patients diagnosed with COVID-19 between January 1 and June 30, 2020, in Japan, and who gave consent for antibody titer measurement during their convalescent phases were enrolled in the study. A diagnosis of COVID-19 was defined as a positive SARS-CoV-2 polymerase chain reaction test on a nasopharyngeal or sputum sample. The date of onset and the date of blood collection were recorded, and the number of days since the onset was calculated and recorded for each sample tested for antibody titers. In addition to the measurement of antibody titers, data on the date of onset, age, sex, height, weight, medical history, oral medications, illness severity, oxygen administered during hospitalization, presence of pneumonia, use of respiratory support, steroid administration, maximum temperature, maximum levels of C-reactive protein (CRP), lactate dehydrogenase (LDH), fibrinogen, and D-dimer during acute phase of COVID-19 were extracted from the participant medical records.
2.2. Detection of anti-SARS-CoV-2 spike protein antibody using an ELISA
Plasmid DNA encoding a full-length SARS-CoV-2 spike protein was provided by Drs. N.M.A. Okba and B.L. Haagmans (Department of Viroscience, Erasmus Medical Center, The Netherlands) [16]. A recombinant spike protein was expressed using an Expi293 Expression System (Thermo Fisher Scientific, Waltham, MA). Briefly, Expi293 cells were maintained in an Expi293 expression medium at 37 °C in 5% CO2 at a vibration of 125 rpm. The plasmid DNA was transfected with ExpiFectamine 293 Reagent (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer's protocol. The cells were harvested after 3 days and suspended in lysis buffer (20 mM Tris-HCl [pH 8.0], 500 mM NaCl, 1 mM EDTA, 10 mM β-mercaptoethanol, 0.1% NP-40, and 5% glycerol), then sonicated and centrifuged for 30 min at 10,000 rpm. The supernatant was incubated with Strep-Tactin Superflow Agarose beads (Merk Millipore, Burlington, MA) for 3 h at 4 °C. After washing in the lysis buffer, protein was eluted using a Strep-Tactin Elution Buffer with Desthiobiotin (IBA Life Sciences, Göttingen, Germany). The purified protein was coated onto a MaxiSoap 96-well ELISA plate (Thermo Fisher Scientific) at 2.5 μg/mL and then incubated with a 1/800 diluted serum/plasma sample obtained from patients with COVID-19. The captured anti-spike antibodies were detected with an anti-human IgG conjugated with horseradish peroxidase (GeneTex, Irvine, CA, USA) and 3,3′,5,5′-tetramethylbenzidine substrate solution (Nacalai Tesque, Kyoto, Japan). Then, absorbance at 450 nm wave-length (AbOD450) was measured using a microplate reader (Bio-Rad, Irvine, CA, USA). Samples of individuals who were free of SARS-CoV-2 infection were used as a negative control. In contrast, samples of infected patients with high levels of anti-spike antibodies were used as positive control. Each sample was assayed in triplicate, and all measurement values were normalized in terms of positive control values.
2.3. Statistical analysis
Quantitative variables were described as medians and interquartile ranges. Categorical variables were presented as frequencies and percentages. The stepwise multivariate log-normal analysis was used to determine the significant predictors of high antibody titers. In the multivariate model, we included factors that showed a univariate association of P < 0.1 and factors that were considered clinically important. When there was a correlation among the variables, the study investigators selected the most relevant factor contributing to increased antibody titers. All analyses were conducted using R (Version 3.5.1., R Core Team, Vienna, Austria, 2018).
Ethical approval
The study was approved by the Ethics Commission of the National Center for Global Health and Medicine, Japan (NCGM-G-003536-00 and NCGM-G-003472-03). Written informed consent was obtained from all patients.
3. Results
A total of 84 participants were enrolled in this study. Nineteen participants had antibody titers measured during the convalescent phase only, and 65 patients had antibody titers measured during the acute and convalescent phases. In the 65 patients with two antibody measurements, all of whom had higher antibody titers during the convalescent phase, we included the titers during the convalescent phase in our analysis.
Table 1 shows the basic characteristics of the 84 participants. Significant differences were found between antibody titer levels and age (P = 0.006), sex (P = 0.06), hypertension (P = 0.07), diabetes mellitus (DM) (P = 0.02), hyperlipidemia (P = 0.009), smoking (P = 0.02), chronic obstructive pulmonary disease (P = 0.04), inhalation of oxygen during hospitalization (P = 0.02), ventilatory management (P = 0.06), glucocorticoid use (P = 0.03), maximum temperature (P = 0.003), maximum CRP level (P < 0.001), LDH level (P < 0.001), and fibrinogen level (P = 0.01) during the course of the disease (Table 2 ). The stepwise multivariate log-normal analysis revealed that male sex (P = 0.04), DM (P = 0.03), and high values of maximum CRP levels during the acute phase (P < 0.001) were associated with elevated IgG antibodies (Table 2). Glucocorticoid use was not associated with the antibody titers.
Table 1.
Participant characteristics and clinical information related to the hospital stay for COVID-19.
| Characteristic |
Total (N = 84) |
|---|---|
| Baseline and demographic data | |
| Age (years), median (IQR) | 50 (38–65) |
| Male, n (%) | 59 (70.2) |
| Height (cm), median (IQR) | 167.8 (162.2–173.9) |
| Weight (kg), median (IQR) | 67.1 (59–75.5) |
| BMI, median (IQR) | 24 (21.3–26.4) |
| Medical history and smoking status | |
| Hypertension, n (%) | 27 (32.1) |
| DM, n (%) | 18 (21.4) |
| Hyperlipidemia, n (%) | 20 (23.8) |
| Smoker, n (%) | 32 (38.1) |
| COPD, n (%) | 5 (6.0) |
| Myocardial Infarction, n (%) | 1 (1.2) |
| Arrhythmia, n (%) | 7 (8.3) |
| Medications | |
| ACEI/ARB, n (%) | 14 (16.7) |
| Beta-blocker, n (%) | 8 (9.5) |
| During hospital stay | |
| Oxygen inhalation, n (%) | 35 (41.7) |
| Presence of pneumonia, n (%) | 71 (84.5) |
| Mechanical ventilation, n (%) | 15 (17.9) |
| Glucocorticoid use, n (%) | 24 (28.6) |
| Peak body temperature, median (IQR) | 38.5 (37.6–39) |
| Maximum CRP, Median (IQR) | 6.8 (1.7–16.3) |
| Maximum LDH, Median (IQR) | 304 (241–467.8) |
| Maximum fibrinogen, median (IQR) | 548 (381–654) |
| Maximum D-dimer, median (IQR) | 0.8 (0.5–2.3) |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; DM, diabetes mellitus; IQR, interquartile range; LDH, lactate dehydrogenase.
Table 2.
Univariate and stepwise multivariate log-normal analysis of antibody titers related to various potentially associated factors.
| Variables |
Estimate |
Standard error |
P |
|---|---|---|---|
| Univariate analysis | |||
| Age | 0.0149 | 0.0053 | .006 |
| Sex | −0.5430 | 0.2808 | .06 |
| Height | 0.0138 | 0.0119 | .25 |
| Weight | 0.0064 | 0.0062 | .31 |
| BMI | 0.0126 | 0.0224 | .58 |
| Hypertension | 0.3490 | 0.1898 | .07 |
| DM | 0.4468 | 0.1908 | .02 |
| Hyperlipidemia | 0.4933 | 0.1849 | .009 |
| Smoking | 0.4483 | 0.1891 | .02 |
| COPD | 0.5423 | 0.2612 | .04 |
| Myocardial Infarction | −1.0400 | 2.5189 | .68 |
| Arrhythmia | −0.0116 | 0.3585 | .97 |
| ACEI/ARB | 0.0973 | 0.2471 | .70 |
| Beta-blocker use | 0.3840 | 0.2478 | .13 |
| Oxygen inhalation | 0.4530 | 0.1954 | .02 |
| Presence of pneumonia | 1.1872 | 0.8770 | .18 |
| Intubation | 0.3851 | 0.2034 | .06 |
| Glucocorticoid use | 0.4222 | 0.1879 | .03 |
| Peak body temperature | 0.2775 | 0.0916 | .003 |
| Maximum CRP | 0.0362 | 0.0065 | <.001 |
| Maximum LDH | 0.0010 | 0.0002 | <.001 |
| Fibrinogen | 0.0018 | 0.0007 | .01 |
| D-dimer | 0.0011 | 0.0030 | .72 |
| Stepwise multivariate log-normal analysis | |||
| (Intercept) | −0.3377 | 0.1461 | .02 |
| Sex | −0.597 | 0.2899 | .04 |
| DM | 0.3521 | 0.1588 | .03 |
| Maximum CRP | 0.0316 | 0.0062 | <.001 |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; DM, diabetes mellitus; IQR, interquartile range; LDH, lactate dehydrogenase; n, number.
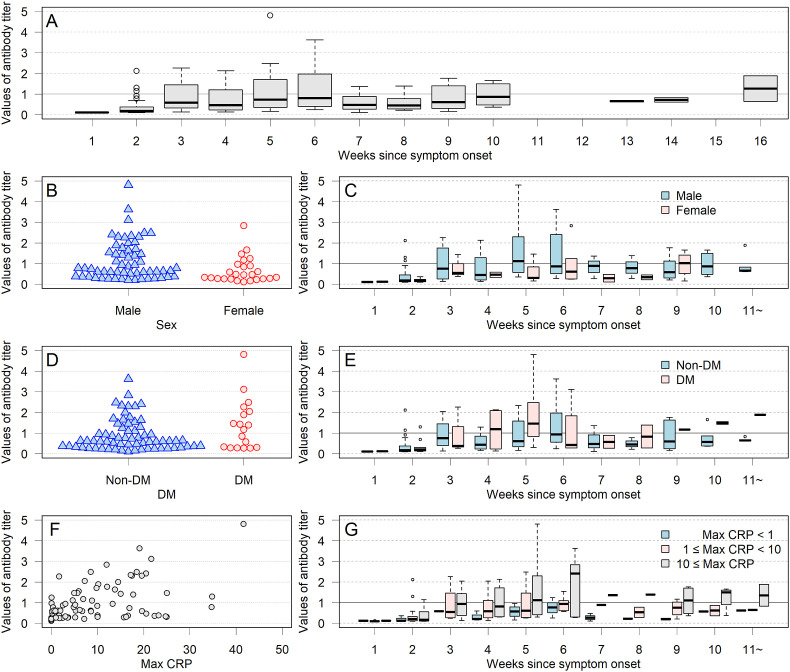
The antibody titers according to time since disease onset are shown in Fig. 1 . The antibody titers peaked between weeks 5 and 6. Fig. 1 also shows the distribution of antibody titers according to sex, the presence or absence of DM, and CRP levels.
Fig. 1.
Changes in IgG antibody titer levels in the 84 participants, according to sex, diabetes mellitus (DM) status, and C-reactive protein (CRP) levels during COVID-19. Antibody titers were highest in the first 5 or 6 weeks after the onset of the disease and then decreased gradually. Antibody titers tended to be higher in males, participants with DM, and those with high values of maximum CRP levels during the acute phase.
4. Discussion
We first carried out an ELISA with a full-length S protein and detected that 65 out of 84 participants (77.3%) were positive for the antibody. Then, by comparative studies with clinical information, we successfully extracted 4 clinical indices, sex, diabetes, no intubation but the highest CRP level, which are correlated with the high anti-spike antibody titers. Our data are partially consistent with a preprint report that high nAbs are correlated with high CRP levels on admission [17]. Our study found that male sex, DM, and high values of maximum CRP levels during the acute phase were associated with higher antibody titers, with CRP levels showing a strong correlation.
Most patients with COVID-19 appear to produce IgM, IgG, and neutralizing antibodies within 14 days [18], however, not all patients convalescing after COVID-19 produce similar levels of antibody titers [19]. Long et al. [20] found that many patients had lower IgG antibody titers during the convalescent phase than during the acute phase and that 40% of asymptomatic infections and 12.9% of symptomatic infections were seronegative in the early convalescent phase. In our study, the highest antibody titer levels were found in the fifth, sixth, and seventh week after onset. In terms of collecting convalescent plasma from patients, it appears that the highest levels of antibody titers can be obtained from patients at 5–6 weeks from the disease onset.
Our analysis did not test for differences in antibody titers with severity of disease. In our study in the same cohort in which the relationship between disease severity and antibody titers was examined, antibody titers tended to be higher in moderate and severe disease than in mild disease. However, both moderate and severe disease titers tended to decline after 60 days from onset [20]. However, Long et al. [21] reported lower IgG levels in asymptomatically infected individuals than in symptomatic infected individuals, and Robbiani et al. [19] reported higher anti-RBD antibodies in hospitalized patients than in outpatients, suggesting an association with disease severity. Similarly, Zhao et al. [22] reported that the severity of the disease correlated with the degree of antibody elevation. On the other hand, some reports suggest that elevated antibody titers do not correlate with the severity of the disease [23]. In our study, DM was shown to result in high antibody titers, but DM has also been identified as a risk factor for severe COVID-19 [24]. The relationship between the risk of severe disease and antibody production may be complex and challenging to understand.
In our study, higher CRP levels were more associated with higher antibody titers than disease severity. Measurement of serum CRP levels is in widespread clinical use as a sensitive marker of inflammation. In response to cytokines such as IL-6 and IL-1β, the hepatic expression of CRP increases [25]. IL-6 is reported to be elevated in patients with COVID-19, ranging from 1 in 4 to approximately half of such patients [26,27]. Investigators have reported that patients with severe COVID-19 were more likely to have elevated IL-6 than non-critical cases. In addition to IL-6, CRP itself has been reported to correlate with disease severity [24,28]. Although disease severity and CRP may be confounders of each other; we believe that CRP is more likely to be the factor associated with elevated antibody titers.
In our study, higher antibody titers were found in men than in women. Zeng et al. [29] have reported on the differences in the production of SARS-CoV-2 IgG antibodies occurring between males and females. They found no difference in mild and moderate disease, whereas, in severe cases, females were found to have higher antibody titers than males. In contrast, as with our study, Robbiani et al. [19] reported higher anti-RBD IgG in men than in women. Sex differences in relation to COVID-19 have been a focus of study, with more deaths reported among males than females in China, Italy, and the United States [[3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32]]. Androgens may play a role in the difference in the severity of the disease between males and females [33]. The androgen receptor regulates transcription of the transmembrane protease, serine 2 (TMPRSS2), required for SARS-CoV-2 infectivity. There have been reports of more severe cases in patients with androgenetic alopecia [34,35]. The difference in the risk of severe disease and levels of antibody titers between males and females may be related to the role of androgens and expression levels of TMPRSS2.
This study had some limitations. First, serum samples during the convalescent period were collected more than 21 days after the onset of illness. The number of days from disease onset was not the same for all participants, and some cases may have been measured on days when antibody titers were not at their highest level. Therefore, it is possible to have underestimated the number of cases that would have otherwise achieved higher antibody titers. Second, we measured the IgG antibody titers of spike proteins. These would be expected to correlate generally with neutralizing antibodies [16], but may not be a perfect match. Third, we found higher antibody titers in diabetic patients, but we did not test for differences in antibody titers according to the severity of diabetes. This point needs to be verified in another study.
In conclusion, we examined factors that could lead to elevated antibody titers and found that high values of maximum CRP levels during the acute phase, male sex, and DM were associated with elevated antibody titers. Antibody titers tended to be highest in the first 5 or 6 weeks after the onset of symptoms. Our results may contribute to future discussions of herd immunity to COVID-19 and the collection of convalescent plasma.
Funding
This work was supported by the Health, Labor and Welfare Policy Research Grants, Research on Emerging and Re-emerging Infectious Diseases and Immunization (grant number 20HA1006), and Japan Agency for Medical Research and Development (grant number JP19fk0108163, JP20fk0108160).
Declaration of competing interest
The authors declare that they have no conflicts of interest.
Acknowledgments
We express our sincere thanks to Drs. N.M.A. Okba and B.L. Haagmans (Department of Viroscience, Erasmus Medical Center, The Netherlands) for providing us with plasmid DNA that encoded a full-length SARS-CoV-2 spike protein.
References
- 1.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;26(382):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . 2020. Coronavirus disease (COVID-19) Situation Report –199.https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200806-covid-19-sitrep-199.pdf?sfvrsn=6b9d262d_2 accessed 7 August 2020. [Google Scholar]
- 3.Xiang F., Wang X., He X., Peng Z., Yang B., Zhang J., et al. Antibody detection and dynamic characteristics in patients with COVID-19. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa461. ciaa461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Long Q.X., Liu B.Z., Deng H.J., Wu G.C., Deng K., Chen Y.K., et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26:845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 5.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., et al. Remdesivir for the treatment of COVID-19 — preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2007764. [DOI] [PubMed] [Google Scholar]
- 6.Ito K., Ohmagari N., Mikami A., Sugiura W. Major ongoing clinical trials for COVID-19 treatment and studies currently being conducted or scheduled in Japan. Global Health Med. 2020;2:96–101. doi: 10.35772/ghm.2020.01034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barone P., DeSimone R.A. Convalescent plasma to treat coronavirus disease 2019 (COVID-19): considerations for clinical trial design. Transfusion. 2020;60:1123–1127. doi: 10.1111/trf.15843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J., et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323:1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duan K., Liu B., Li C., Zhang H., Yu T., Qu J., et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci Unit States Am. 2020;117:9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li L., Zhang W., Hu Y., Tong X., Zheng S., Yang J., et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 2020 doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salazar E., Perez K.K., Ashraf M., Chen J., Castillo B., Christensen P.A., et al. Treatment of COVID-19 patients with convalescent plasma in Houston, Texas. Preprint. medRxiv. 2020:2020. doi: 10.1101/2020.05.08.20095471. [DOI] [Google Scholar]
- 12.Zhang B., Liu S., Tan T., Huang W., Dong Y., Chen L., et al. Treatment with convalescent plasma for critically ill patients with severe acute respiratory syndrome coronavirus 2 infection. Chest. 2020;158 doi: 10.1016/j.chest.2020.03.039. e9–e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ye M., Fu D., Ren Y., Wang F., Wang D., Zhang F., et al. Treatment with convalescent plasma for COVID-19 patients in Wuhan, China. J Med Virol. 2020 doi: 10.1002/jmv.25882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeng Q.L., Yu Z.J., Gou J.J., Li G.M., Ma S.H., Zhang G.F., et al. Effect of convalescent plasma therapy on viral shedding and survival in COVID-19 patients. J Infect Dis. 2020;222:38–43. doi: 10.1093/infdis/jiaa228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang S., Hillyer C., Du L. Neutralizing antibodies against SARS-CoV-2 and other human coronaviruses. Trends Immunol. 2020;41:355–359. doi: 10.1016/j.it.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okba N.M.A., Müller M.A., Li W., Wang C., GeurtsvanKessel C.H., Corman V.M., et al. Severe acute respiratory syndrome coronavirus 2-specific antibody responses in coronavirus disease patients. Emerg Infect Dis. 2020;26:1478–1488. doi: 10.3201/eid2607.200841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu F., Wang A., Liu M., Wang Q., Chen J., Xia S., et al. Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications. MedRxiv. 2020:20047365. doi: 10.1101/2020.03.30.20047365. [DOI] [Google Scholar]
- 18.Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A., et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 19.Robbiani D.F., Gaebler C., Muecksch F., Lorenzi J.C.C., Wang Z., Cho A., et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. 2020;584:437–442. doi: 10.1038/s41586-020-2456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kutsuna S., Asai Y., Matsunaga A. Loss of anti–SARS-CoV-2 antibodies in mild covid-19. N Engl J Med. 2020;383 doi: 10.1056/NEJMc2027051. [DOI] [PubMed] [Google Scholar]
- 21.Long Q.X., Tang X.J., Shi Q.L., Li Q., Deng H.J., Yuan J., et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26:1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 22.Zhao J., Yuan Q., Wang H., Liu W., Liao X., Su Y., et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa344. ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.To K.K., Tsang O.T., Leung W.S., Tam A.R., Wu T.C., Lung D.C., et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20:565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rhodes B., Fürnrohr B.G., Vyse T.J. C-reactive protein in rheumatology: biology and genetics. Nat Rev Rheumatol. 2011;7:282–289. doi: 10.1038/nrrheum.2011.37. [DOI] [PubMed] [Google Scholar]
- 26.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun D., Li H., Lu X.X., Xiao H., Ren J., Zhang F.R., et al. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center's observational study. World J Pediatr. 2020:1–9. doi: 10.1007/s12519-020-00354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Z., Yang B., Li Q., Wen L., Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. 2020;71:769–777. doi: 10.1093/cid/ciaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeng F., Dai C., Cai P., Wang J., Xu L., Li J., et al. A comparison study of SARS-CoV-2 IgG antibody between male and female COVID-19 patients: a possible reason underlying different outcome between sex. J Med Virol. 2020 doi: 10.1002/jmv.25989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323:1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 32.Chen T., Wu D., Chen H., Yan W., Yang D., Chen G., et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wambier C.G., Goren A., Vaño-Galván S., Ramos P.M., Ossimetha A., Nau G., et al. Androgen sensitivity gateway to COVID-19 disease severity. Drug Dev Res. 2020 doi: 10.1002/ddr.21688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goren A., Vaño-Galván S., Wambier C.G., McCoy J., Gomez-Zubiaur A., Moreno-Arrones O.M., et al. A preliminary observation: male pattern hair loss among hospitalized COVID-19 patients in Spain — a potential clue to the role of androgens in COVID-19 severity. J Cosmet Dermatol. 2020;19:1545–1547. doi: 10.1111/jocd.13443. [DOI] [PubMed] [Google Scholar]
- 35.Wambier C.G., Vaño-Galván S., McCoy J., Gomez-Zubiaur A., Herrera S., Hermosa-Gelbard Á., et al. Androgenetic alopecia present in the majority of hospitalized COVID-19 patients - the "Gabrin sign". J Am Acad Dermatol. 2020;21(83):680–682. doi: 10.1016/j.jaad.2020.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]