Abstract
The coronavirus disease-2019 (COVID-19) pandemic has profoundly changed clinical care and research, including the conduct of clinical trials, and the clinical research ecosystem will need to adapt to this transformed environment. The Heart Failure Academic Research Consortium is a partnership between the Heart Failure Collaboratory and the Academic Research Consortium, composed of academic investigators from the United States and Europe, patients, the U.S. Food and Drug Administration, the National Institutes of Health, and industry members. A series of meetings were convened to address the challenges caused by the COVID-19 pandemic, review options for maintaining or altering best practices, and establish key recommendations for the conduct and analysis of clinical trials for cardiovascular disease and heart failure. This paper summarizes the discussions and expert consensus recommendations.
Key Words: clinical trial, COVID-19, endpoint ascertainment, heart failure
Abbreviations and Acronyms: AE, adverse event; COVID-19, coronavirus disease-2019; FDA, Food and Drug Administration; HF, heart failure; HF-ARC, Heart Failure Collaboratory–Academic Research Consortium; HFC, HF Collaboratory; NYHA, New York Heart Association; PGA, patient global assessment; PRO, patient-reported outcome; SAP, statistical analysis plans
Central Illustration
Highlights
-
•
The COVID-19 pandemic has profoundly affected patient care and the conduct of clinical trials.
-
•
HF-ARC scientific expert panel developed recommendations for the conduct and analysis of heart failure trials during the pandemic.
-
•
The HF-ARC consensus recommendations support ongoing clinical trials and strengthen the clinical trial ecosystem, which should have sustained benefits in the future.
The coronavirus disease-2019 (COVID-19) pandemic has dramatically altered health care delivery and clinical research worldwide. Clinical trial activity apart from COVID-19 studies has plummeted. In April 2020, approximately 90% of clinical trial sites closed to enrollment worldwide, with a 95% decline in cardiovascular trial activity (1,2). The rapid initiation of over 1,000 COVID-19 clinical trials contrasts with the decline in non–COVID-19 research.
In response to these issues, regulators, sponsors, and professional organizations including the U.S. Food and Drug Administration (FDA), National Institutes of Health (NIH), the European Medicines Agency, and the European Society of Cardiology have released guidance on the performance of clinical trials in the era of COVID-19 (3, 4, 5, 6). These statements promote the principles of safety for patients and research team members while striving to preserve trial integrity (7). Strategies to limit in-person data collection and maintain physical distancing are suggested. In response, trial telehealth and remote monitoring protocol amendments have surged (8). Nevertheless, although new regulatory and governmental guidance documents may facilitate remote and electronic data collection, specific methodologies and standardized practices have not been established (6,7).
The HF Collaboratory (HFC) is a multistakeholder organization that seeks to improve evidence generation for new therapies, implementation of safe and effective treatments, and clinical trial efficiency; HFC includes patients, clinicians, clinical investigators, the FDA, NIH, industry, and payers (9). The Heart Failure Academic Research Consortium (HF-ARC) working group was convened through a partnership between the HFC and ARC, with representatives from the United States and Europe, to develop a scientific expert statement of standardized methods for heart failure (HF) clinical trials in response to the COVID-19 pandemic.
General Principles
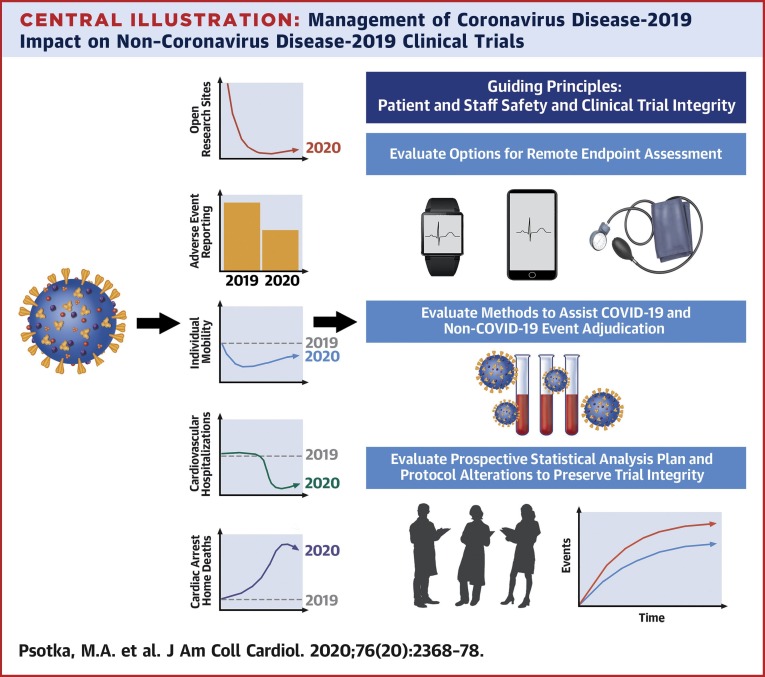
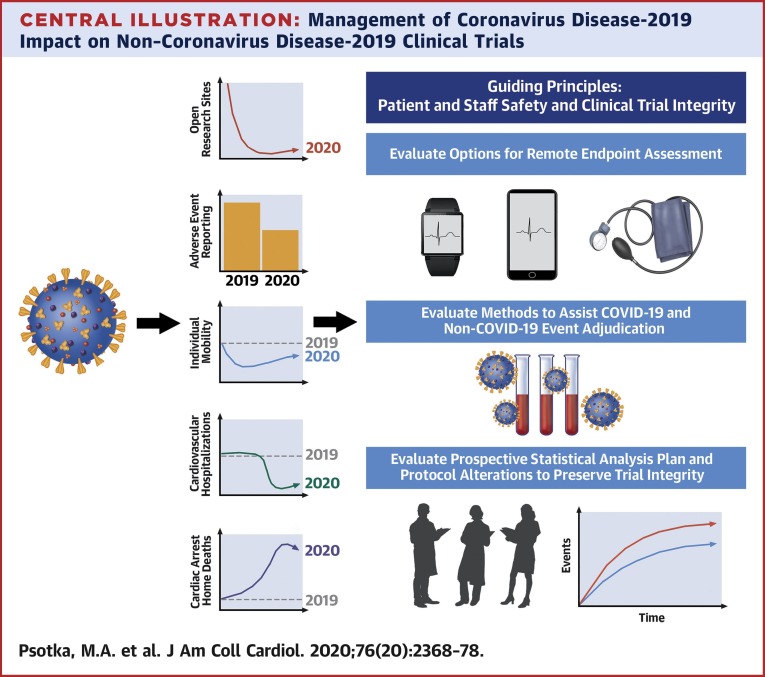
The methods for adjudicating consensus are described in the Supplemental Appendix, Supplemental Figure 1, and Supplemental Table 1. These recommendations focus largely on data collection and analysis (3, 4, 5,7). Sponsors and regulators must together decide which protocol procedures can be safely and accurately completed and which data are critical, and seek to maximize evidence acquisition while embracing flexibility, including protocol amendments and deviations when appropriate. When necessary, collection of imperfect data is preferred to noncollection to minimize difficulties with interpretability and statistical assessment arising from missing data. Case report forms may require specific fields for COVID-19–focused elements to facilitate understanding of the pandemic’s impact, including on enrollment, investigational product delivery, follow-up completeness, safety assessments, event rates, and to assist clinical event committee adjudications (an example can be found in the Supplemental Appendix). Regional and country-specific COVID-19 infection rates may also aid interpretation of study results. To assure integrity of the clinical trial, permanent changes to study protocols, analysis plans, and informed consents should be documented prospectively, receive Institutional Review Board approval, and be communicated to patients (Central Illustration ).
Central Illustration.
Management of Coronavirus Disease-2019 Impact on Non–Coronavirus Disease-2019 Clinical Trials
Coronavirus disease-2019 (COVID-19) has globally affected clinical trial performance, patient behavior, and clinical care. Most clinical trial sites ceased site-based enrollments and procedures; adverse event reporting declined for enrolled patients. Patient mobility declined in response to governmental orders and fear of infection, and patient-care changes include widespread reductions in cardiovascular hospitalizations and increased numbers of at-home cardiac arrests, which may be due to COVID-19 or cardiovascular conditions. We evaluate methods to promote valid remote endpoint ascertainment, adjudication of COVID-19 and non–COVID-19 events, and suggest considerations for careful statistical analysis plan and protocol alterations that may be made in consultation with regulators.
Ideally these activities will accelerate use of innovative methodologies and digital tools by the clinical trial ecosystem, acknowledging that individual studies and research sites will react variably to local disease burdens, resources, and institutional capabilities (9). Additionally, although these suggestions aim to reinvigorate clinical research during and following the current pandemic, lasting transformation and global stakeholder cooperation will be needed to prepare for future events, including protocols for virtual screening, electronic consenting, central institutional review boards, remote enrollment, randomization, follow-up visits, site monitoring, endpoint ascertainment, and sample collection, as well as statistical analysis plans to handle mid-trial changes.
Data and Trial Integrity
Typically, contract research organizations, sponsors, and study staff engage in frequent queries of site data to help prevent missingness, retain patients, and identify protocol deviations. However, sponsors and contract research organizations have increasingly implemented remote monitoring during the pandemic to collect data and ensure integrity with adherence to Good Clinical Practice guidelines (10). Typically, standardized direct counseling from trained research staff encourages patients to adhere to trial protocols, but remote pill counts may monitor medication adherence and improve accountability at lower cost than in-person visits (11). The stress and mental health sequelae of the pandemic may require increased attention to patient well-being and counseling by trial personnel to ensure completion of protocolled activities. Central statistical monitoring may provide an alternative method to in-person site visits (12). This technique searches for sites or regions with data inconsistencies and may better identify sites for audit. The integrity and timely delivery of investigational pharmaceutical products to patients and investigators has been affected by the pandemic, and must also be proactively managed by sponsors and regulators.
Endpoint Collection
Digital and remote methods of endpoint ascertainment have been suggested when possible (3, 4, 5,7). Virtualization merits individual consideration for each clinical trial, as the ability to acquire and analyze these data depend on the patient population, investigational agent studied, and endpoints. Early and direct communication with regulators is paramount to agree on changes to trial design or analysis. Although endpoints collectable only in a clinical setting, such as cardiopulmonary exercise testing, may have to be modified or delayed, others may be collected by alternative or remote methods on time. Decision-making is influenced by trial characteristics and whether the outcomes lie within the primary endpoints. As detailed later in the text, alternative methods of assessment often lack sufficient evidence of reliability compared with established methodologies. Studies under development should investigate at baseline the within-patient agreement between in-clinic and remotely obtained measurements to prepare for unforeseen future challenges to study execution.
Patient-reported outcomes and quality-of-life assessments
Patient-reported outcome (PRO) instruments have been extensively evaluated, including for remote administration (13). The HF-specific Kansas City Cardiomyopathy Questionnaire (KCCQ) and Minnesota Living With Heart Failure Questionnaire (MLHFQ) are both designated Medical Device Development Tools by the FDA Center for Devices and Radiological Health, and may be translated to digital or telephone formats from the original self-administered paper (14,15). Anchoring these PROs with patient global assessments (PGA) may assist with establishing thresholds for meaningful change (16). Nevertheless, collection of these endpoints using remote methodologies during the pandemic, compared to prior, may increase within-subject variability through environmental effects on patient responses, coaching by assessors and family or caregivers, and heightened susceptibility to missing data. It also remains unclear whether data from interviewer-based assessments can be pooled with self-administered data. Thus, although implementation of remote PROs appears feasible, the preferred collection method will depend on specifics of the incorporating study and PRO instrument (Table 1 ).
Table 1.
Advantages and Drawbacks of Remote Collection Strategies for PRO and Dyspnea Assessment
| Strategy | Advantages | Drawbacks |
|---|---|---|
| Mail, paper |
|
|
| Mail, paper, with telephone script |
|
|
| Telephone-based interview, paper or digital |
|
|
| Internet-based, digital |
|
|
| Mobile phone-based, digital |
|
|
IRB = institutional review board; PRO = patient-reported outcomes.
Few clinical trials independently assess dyspnea, but it remains the most common multifactorial patient-reported symptom in HF (17). Dyspnea is often measured using Likert or visual analog scales. The Likert is commonly assessed in paper format, but may be done by telephone; the visual analog scale requires a visual reference either on paper or a digital monitor. Although the psychometric properties of these methods have not been carefully evaluated, interviewer and staff feedback are known to affect patient responses, and selection of the appropriate administration strategy is situationally dependent (Table 1).
General health status PROs may be useful for patients with HF, separately or in combination with HF-specific instruments (16). PGA captures the global impact of disease and is one of the most widely used PRO instruments in clinical trials (18). PGA does not require baseline assessment as it incorporates comparison with a prior time point when asking whether and how the patient’s overall status has changed, though patients may interpret baseline differently than investigators and recall may be imperfect. Like dyspnea, PGA is commonly measured on a 7-point Likert scale ranging from markedly better to markedly worse, and can be assessed remotely and digitally (19). The Short Form-36 (SF-36) and EQ-5D questionnaires are other general PRO instruments that have been used with paper collection, telephone interview, and over the Internet. Among these, EQ-5D may be preferred to SF-36 due to greater validation.
COVID-19 may impair PRO interpretation. Infection may worsen dyspnea and health-related quality of life, perhaps persistently; social distancing and home lockdown may limit patient activities that provoke breathlessness. The economic impact and concerns for infection may affect mental health and quality of life. Accordingly, PRO data during COVID-19 may require statistical adjustment for temporality and regionality, and contextualization of the pandemic’s psychological burden, which may be concurrently captured with disease-agnostic PROs.
-
•
When a PRO is assessed, the mode of administration should be recorded and similar scripts, instruments, and devices should be used before, during, and after the pandemic, modifying only as necessary to maximize data collection while maintaining procedural integrity.
-
•
PGA, EQ-5D, KCCQ, and MLHFQ are validated PROs well-suited for HF trials employing remote study visits, including those performed during the COVID-19 pandemic.
-
•
COVID-19 could influence PRO responses in complex ways. Results should be interpreted in the context of regional timing and severity of the pandemic, patient symptoms, and COVID-19 infection status, which may be assessed by serological status.
Functional and exercise measures
New York Heart Association (NYHA) functional classification is widely used to assess functional status for patients with HF (20). NYHA functional class can be collected remotely by patient interview but may be affected by environmental factors similarly to PROs. Objective measurement of maximal or submaximal exercise capacity requires equipment, and trials with short-term hemodynamic or functional endpoints may be temporarily suspended then restarted when the pandemic is better controlled. Although cardiopulmonary exercise testing and peak Vo 2 measurement may not be possible remotely, there are established strategies to collect accelerometer-based activity for timed walking tests such as the 6-min walk test (21). Validation studies have been published for 2 home 6-min walk test smartphone applications, however, the remote capture of these data elements has limitations due to measurement error, sometimes poor device reliability, lack of performance testing, and frequent missing data (21, 22, 23, 24). In addition, patients may need reminders to wear nonimplantable devices to record their activity, the technology may have psychological consequences that alter behavior, it is unclear whether data from different modalities can be pooled, and mask-wearing may affect performance (25). Activity data from implantable devices may also be used, but many of the technologies required to capture these data may be differentially available in the population. Finally, lifestyle changes with social distancing may substantially reduce overall physical activity and exertional tolerance, independent of disease-related changes.
-
•
Some exertional and functional testing measures have digital remote options, but changes in assessment method and variability associated with remote data collection may add statistical noise.
-
•
To facilitate analysis, it is best to record the mode of test administration, modifying only as necessary to maximize data collection while maintaining procedural integrity.
-
•
NYHA functional class may be remotely assessed but the means of collection should be recorded and its value to clinical trials should continue to be re-examined.
Imaging
Although remote use of many cardiac imaging modalities is infeasible, the growth of miniaturized and portable echocardiographic platforms could lessen the technological barriers to image acquisition (26). Nonetheless, echocardiography has been validated only with trained users, and patient-acquired images are anticipated to be of variable quality with missing data points. Although technician home visits may be the option of highest quality, and greatest expenditures, because of suboptimal positioning and privacy concerns, they face data collection and accuracy limitations. Thus, image acquisition may be best delayed until it can be safely performed in the health care setting.
-
•
Multiple portable ultrasound-based data acquisition platforms are in use for clinical purposes; however, remote echocardiographic assessment is only validated with trained users.
-
•
Technician home visits may be possible for small studies, but require training and quality control by a centralized core laboratory to ensure data reliability.
Biomarkers
Biomarkers including troponin and natriuretic peptides are commonly used for clinical trial inclusion criteria, endpoints, event adjudication, safety, and mechanistic evaluation or monitoring (27). COVID-19 complicates biomarker use because infection is associated with increased circulating levels, and they are risk markers for severe COVID-19 disease and worse outcomes (28,29). Although in many cases, biomarker changes appear to be due to noncardiac critical illness, the COVID-19 coronavirus appears to have tropism for the myocardium and vasculature (30). Thus, COVID-19 complicates adjudication of myocardial injury events and worsening HF events, because the symptomatology and biomarkers may be consistent with either COVID-19 or primary cardiovascular conditions (27,31). Additionally, biomarker measurement and serum chemistries may not be feasible due to site closures. Once accurate antibody assays become available, stored biorepositories can provide a more complete evaluation of known and unknown COVID-19 infections in trial populations.
-
•
Home-based biomarker and serum chemistry testing may be leveraged as an option for patients without obvious COVID-19; technical and interpretation challenges for biomarkers remain, and shared risk assessment and decision-making with patients should determine which methodology for safety laboratory assessment, if any, can be achieved.
-
•
Biosample collection for all clinical trial enrollees may assist with adjudication and interpretation of COVID-19–associated events.
Hospitalization and mortality endpoints
Assessment of traditional clinical trial outcomes, including emergency department visits, hospitalizations, and mortality, may be confounded by signs, symptoms, and biomarkers of COVID-19 that overlap with HF, increased overall death rates due to COVID-19, direct myocardial injury with COVID-19, and altered patient and clinician behavior from societal changes and governmental recommendations (28,30). Patients with cardiovascular comorbidities and HF appear to be at greater risk of more severe COVID-19 disease in addition to an increased risk of cardiovascular adverse events (32). Although overall COVID-19 hospitalization and mortality rates remain low and vary by geographic location, COVID-19 could contribute to the outcome in any individual case, complicating event adjudication and data interpretation.
Patient behavior has changed markedly in response to fears of infection and to social distancing measures enacted by local authorities, leading to decreased medical care and altered nonfatal event rates. In Italy and the United States, the COVID-19 pandemic was temporally associated with a 40% decrease in presentation for acute coronary syndromes (33,34). Worldwide, HF hospitalization rates have dropped up to 50% with regional COVID-19 spread and governmental activity restrictions (35) (Supplemental References). Patients may forego care to avoid contact with the medical system. Alternatively, diminished rates of myocardial infarction and HF hospitalization may be due to misdiagnosis, fewer true events associated with increased sedentary behavior, or outpatient treatment of worsening HF. The consequences of the complex interplay of individual and societal factors on cardiovascular health and event rates remain unclear in some cases (Table 2 ). Nevertheless, decreased events may lengthen the follow-up required to demonstrate efficacy, and given difficulties ascertaining cause of death, hamper interpretation of clinical trial results if fatal event rates increase.
Table 2.
Competing Influences of COVID-19 on CV Event Rates
| Effects on Events | Type of Event |
|
|---|---|---|
| Nonfatal CV Events | Fatal CV Events | |
| Reduction in event rates |
|
|
|
||
| Increase in event rates |
|
|
|
||
| Unclear effect on event rates |
|
|
| Increased missing events or events of unknown cause |
|
|
COPD = chronic obstructive pulmonary disease; COVID-19 = coronavirus disease-2019; CV = cardiovascular; HF = heart failure.
The effect of COVID-19 on patient behavior may also lead to altered rates and misclassification of fatal events. In Lombardy, Italy, during the first 40 days of the outbreak, there was an almost 60% increase in out-of-hospital cardiac arrests compared with the same time period the previous year (36). These deaths rose contemporaneously with regional infections, but of the excess, only 75% were suspected or diagnosed with COVID-19, suggesting a potential concomitant increase in non–COVID-19 mortality, possibly from avoidance of appropriate medical care. However, deaths due to COVID-19 or other noncardiovascular etiologies are unlikely to be treatable with investigational HF therapies and may dilute a treatment effect. In addition, with limited data and documentation available for adjudication, clinical events committees may be unable to determine the cause of death. This may be particularly pertinent to out-of-hospital sudden death or events of unknown etiology, which are frequently ascribed to cardiovascular causes, but may be best pre-specified as noncardiovascular or analyzed as part of sensitivity analyses. Local COVID-19 infection prevalence and mortality patterns may be needed to allow proper adjudication of events, acknowledging that patient deaths are often due to a combination of concurrent illnesses, and that consistency in adjudication is more crucial than complete accuracy.
-
•
Changes in cardiovascular event rates in response to COVID-19, and the adjudication and analysis of these events, will be complex (Table 2).
-
•
For nonfatal and fatal events, information on COVID-19 status at the time aids in adjudication and analysis: specifically, whether the patient was definitely positive for COVID-19, definitely negative, unknown but suspected positive, or unknown and suspected negative.
-
•
Prospective and retrospective collection of COVID-19 symptoms, infection status, and events, which may include nucleic acid and serological testing, should be performed to aid analysis and interpretation of the data (see case report form in the Supplemental Appendix).
-
•
Clinical events committees should adjudicate whether COVID-19 was uninvolved, contributory, or the primary cause of cardiovascular and noncardiovascular events.
Adverse events
Behavioral and societal forces decreasing are also likely to limit ascertainment of adverse events (AEs). Typically, AEs are collected by clinical chart review and patient report; thus, decreased contact and chart review by monitors can reduce ascertainment. Telephone or virtual visits that include AE checklists may capture some of this information, but rely on access and may be more difficult for older and sicker patients (37). Traditional mail may be more accessible but at greater cost and subject to increased risk of missing data.
Nevertheless, there is general consensus that clinical trials collect excessive AE information and the pandemic presents an opportunity to reduce that burden in consultation with regulators (38). The FDA has suggested collecting more extensive AE data on a limited sample of enrolled patients, or a more focused symptom catalog on all subjects, to reduce the recording of AEs unlikely to contribute new knowledge about the risks of the intervention (38).
-
•
Patient reporting of AEs can be accomplished remotely by virtual visit or telephone and may be streamlined with questionnaires or focused event checklists.
-
•
In consultation with regulators, selective safety data collection may be acceptable when the intervention’s safety profile is well-characterized, for instance, on a subset of the enrolled population.
Socioeconomic Disparities
Ample evidence shows that socioeconomic factors affect the risk of exposure, transmission, and development of severe COVID-19, with patients of traditionally disadvantaged backgrounds experiencing higher rates of disease exposure, contraction, and critical illness. In New York, areas of the city with greater poverty and composed of more ethnic and racial minorities had increased rates of hospitalization and death from COVID-19 than wealthier and more homogenous regions after adjustment for population density (39). Furthermore, racial and ethnic minorities had disproportionately increased rates of death (40). In China, Italy, and the United States, the pandemic has particularly affected older patients, with more hospitalizations and higher rates of critical illness and mortality (41). In the Campania region of Italy, the drop in presentation of patients for acute coronary syndromes was greater for elderly than for younger patients, and more pronounced for women than men (33). These disparities must be considered in the context of clinical trial execution, because COVID-19 exacerbates longstanding inequalities in clinical trial participation for women, the elderly, and minority populations; additional sponsor resources may be needed to manage these differences (9). On one hand, remote patient assessment may improve inclusion of under-represented populations, particularly those in rural areas with transportation or access barriers. However, for those without telephone, Internet, or mailing addresses, the disparity may hamper participation. The differential effect on outcomes may also merit consideration of prospectively identified sensitivity analyses among socioeconomic and demographic groups to understand the impact on overall treatment effect within a clinical trial.
Statistical Considerations
COVID-19 challenges the design, execution, and analysis of clinical trials. Although statistical analysis plans (SAP) were written following extensive negotiations between statisticians, sponsors, investigators, and regulators, the pandemic may require prospective SAP alterations to allow the collected data to best answer the clinical questions under study. SAP revision is complicated and consequential, and should be done and documented prospectively in concert with regulators to help ensure integrity of the study and analysis. Although sponsors, regulators, and investigators recognize that trials in early stages of implementation face the greatest potential adverse impact of the pandemic, appropriate adaptation and targeted flexibility could allow these trials to succeed. Statistical considerations may influence what data to collect, given variable susceptibility to statistically informative missing data and bias, and statistical adjustment may allow data collected by altered methods to be used. Furthermore, projection of statistical power on the basis of the number of collected events, alterations in event rates, and enrollee counts may inform sponsor and scientific decisions surrounding initiating or pausing enrollment, as well as potentially stopping the clinical trial earlier than originally intended. We recommend considering the following issues:
-
•
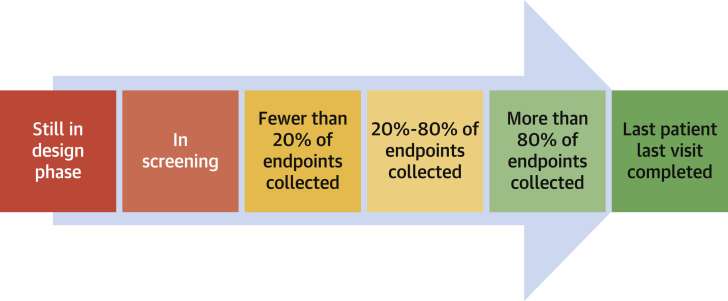
For a time-to-event endpoint, the decision regarding data censoring in response to the pandemic needs to balance loss of statistical power with confounding of inference caused by including pandemic-affected data. For a trial that has collected approximately 80% of its endpoints before COVID-19, there may only be a small decrement in statistical power, and censoring may be favored; sponsors may consider stopping the trial immediately and analyzing the data to avoid the analytic and statistical complications of the pandemic (Figure 1 ). Alternatively, creation of a modified composite endpoint including only reliably ascertained components may be used.
-
•
If approximately 20% to 80% of endpoints have been collected, endpoints may either need to be modified or stratified on the basis of patient-specific impact of the COVID-19 pandemic and the feasibility of endpoint ascertainment. A new SAP would specifically describe the analytic approach for data missing because of COVID-19, which may affect the primary outcome analysis or subsequent sensitivity analyses.
-
•
For a trial that has enrolled few patients or for which few endpoints have been collected, sponsors may consider either substantially revising the SAP, or designing a new trial to begin after the COVID-19 pandemic has sufficiently waned, to account for changes to the clinical trial ecosystem.
-
•
Statistically accounting for the timeline and intensity with which COVID-19 affected local or regional clinical care and patient behavior could help adjust for the comprehensive changes in health care utilization and efficacy associated with the COVID-19 pandemic, including increased overall mortality rates, decreased health care utilization, excess mortality due to COVID-19, and potentially increased HF mortality rates. The timeline and intensity of COVID-19 will vary by country and region. Ongoing assessments of COVID-19 impact will continue to refine these plans.
-
•
Methods for missing data analyses for continuous outcomes include multiple imputation, imputation of a surrogate, opposite arm mean imputation, and linear mixed and tipping point models. Each of these rely on distinct statistical assumptions that are best prospectively identified and justified in the SAP. The reasons data are missing cannot be verified from the nonmissing data, thus distinct approaches should be used as sensitivity analyses to confirm treatment effects are unaltered by different missingness postulations. No single solution will apply to all studies affected by COVID-19. Stakeholders including regulators will likely use multiple coordinated assessments to understand the data, and noninferiority trials may be more challenging than superiority trials to evaluate in the presence of missing data.
-
•
Studies under development should prepare for unexpected catastrophic events similar to this pandemic by outlining in their SAP how to handle such natural disasters.
Figure 1.
Trial Timeline Statistical Assessment
The progression of a clinical trial from design to completion, color coded by proximity to database lock and analysis (dark green = ready for analysis; dark red = inappropriate for analysis). This concept can help assess initiation and stopping decisions. For instance, given difficulties with recruitment and enrollment, trials with fewer than approximately 20% of endpoints collected in the dark red period may consider pausing initiation or recruitment in consultation with regulators, until COVID-19 has waned. Alternatively, trials with >80% of endpoints collected in green, may recalculate their power and consider stopping the trial to avoid the consequences of COVID-19 on endpoint ascertainment, adjudication, and statistical analysis plan (SAP) adjustment; with at least 80% of events collected, there may be only a modest 10% decrement in statistical power. Trials with approximately 20% to 80% of endpoints collected need to consider how best to adapt to the issues covered in this paper, including remote endpoint collection, methods to minimize missing data, and SAP modifications.
Conclusions
The COVID-19 pandemic has profoundly changed clinical care and research including the performance and analysis of clinical trials. We have summarized expert consensus recommendations for clinical trials; these principles should also be incorporated into contingency planning for future public health crises and systemic disruptions, including potential resurgence of COVID-19, to allow more seamless continuation of clinical research. Although some clinical research programs may unfortunately be substantially adversely affected by COVID-19, we hope our suggestions will help salvage ongoing clinical investigation and strengthen the clinical trial environment for the future.
Author Relationship With Industry
Dr. Psotka has received consulting fees from Amgen, Cytokinetics, and Windtree; and has received grant support from the U.S. Food and Drug Administration. Dr. Abraham has received consulting fees from Abbott, Boehringer Ingelheim, CVRx, Edwards Lifesciences, and Respicardia; has received salary support from V-Wave; and has received research support from the U.S. National Institutes of Health/National Heart, Lung, and Blood Institute. Dr. Filippatos has received consulting income from Boehringer Ingelheim, Medtronic, Novartis, Servier, and Vifor; and has received lecture fees from Merck. Dr. Lindenfeld has received consulting fees from Novartis, Abbott, ResMed, V-Wave, CVRx, and Cytokinetics. Dr. Ahmad has received consulting fees from Amgen, Cytokinetics, Relypsa, and Novartis. Dr. Bhatt has received consulting fees from Sanofi Pasteur; and participates in clinical endpoint committees for the U.S. National Institutes of Health. Dr. Cleland has received consulting fees from Abbott, Amgen, Bayer, Medtronic, Novartis, Pharmacosmos, Vifor, Bristol Myers Squibb, and Servier; and has received research grants from Amgen, Bayer, Novartis, Pharmacosmos, Vifor, Bristol Myers Squibb, and Servier. Dr. Felker has received research grants from the National Heart, Lung, and Blood Institute, American Heart Association, Amgen, Merck, Cytokinetics, and Roche Diagnostics; and has received consulting fees from Novartis, Amgen, Bristol Myers Squibb, Cytokinetics, Medtronic, Cardionomic, Relypsa, V-Wave, Myokardia, Innolife, EBR Systems, Arena, Abbott, Sphingotec, Roche Diagnostics, Alnylam, LivaNova, Windtree Therapeutics, Rocket Pharma, and scPharmaceuticals. Dr. Januzzi is a trustee of the American College of Cardiology; has received grant support from Applied Therapeutics, Novartis Pharmaceuticals, and Abbott Diagnostics; has received consulting income from Abbott, Janssen, Novartis, Pfizer, Merck, and Roche Diagnostics; and participates in clinical endpoint committees/data safety monitoring boards for Abbott, AbbVie, Amgen, Boehringer Ingelheim, Janssen, and Takeda. Dr. Kitzman has received consulting fees from AstraZeneca, Bayer, Novartis, Merck, Pfizer, Boehringer Ingelheim, AbbVie, DCRI, and Corvia; has received grant support from AstraZeneca, Bayer, and Novartis; and has stock in Gilead. Dr. Lewis has received consulting fees from Novartis; and has received institutional research grant support from Amgen, Novartis, and Sanofi. Dr. McMurray has received financial support from Bayer, Cardiorentis, Amgen, Oxford University, Theracos, Abbvie, DalCor, Pfizer, Merck, Novartis, GlaxoSmithKline, Bristol Myers Squibb, Vifor-Fresenius, Kidney Research UK, and AstraZeneca. Dr. Mentz has received consulting fees from Abbott, Amgen, AstraZeneca, Bayer, Boston Scientific, Janssen, Luitpold, Medtronic, Merck, Novartis, and Sanofi; and has received research grants from Akros, Amgen, AstraZeneca, Bayer, Bristol Myers Squibb, GlaxoSmithKline, Gilead, InnoLife, Luitpold/American Reagent, Novartis, Otsuka, ResMed, and the U.S. National Institutes of Health grants U01HL125511-01A1 and R01AG045551-01A1. Dr. Solomon has received research grants from Alnylam, Amgen, AstraZeneca, Bellerophon, Bristol Myers Squibb, Celladon, Cytokinetics, Eidos, Gilead, GlaxoSmithKline, Ionis, Lone Star Heart, Mesoblast, MyoKardia, U.S. National Institutes of Health/National Heart, Lung, and Blood Institute, Novartis, Sanofi Pasteur, and Theracos; and has received consulting fees from Akros, Alnylam, Amgen, AstraZeneca, Bayer, Bristol Myers Squibb, Cardior, Corvia, Cytokinetics, Gilead, GlaxoSmithKline, Ironwood, Merck Sharp & Dohme, Novartis, Roche, Takeda, Theracos, Quantum Genetics, Cardurion, AoBiome, Janssen, and Cardiac Dimensions. Dr. Teerlink has received consulting fees from Amgen, Bayer, Cytokinetics, Novartis, and Stealth Health; and has received research funding from Abbott, Amgen, Bayer, Bristol Myers Squibb, Novartis, and scPharmaceuticals. Dr. Vaduganathan is supported by the KL2/Catalyst Medical Research Investigator Training award from Harvard Catalyst (NIH/NCATS Award UL 1TR002541); and has served on Advisory Boards for Amgen, AstraZeneca, Baxter Healthcare, Bayer AG, Boehringer Ingelheim, Cytokinetics, and Relypsa. Dr. Vardeny has received consulting fees from Novartis; and has received research grants from AstraZeneca and Sanofi Pasteur. Dr. Whellan has received grant support from the U.S. National Institutes of Health (R01AG045551). Dr. Wittes reports that the company for which she works, Statistics Collaborative, has contracts with many companies, some of which are developing treatments for heart failure. Dr. Anker has received clinical trial–related fees from Abbott Vascular, Servier, Vifor, Bayer, Boehringer Ingelheim, Thermo Fisher, Respicardia, and Novartis. Dr. O’Connor has received research support from Roche Diagnostics and Merck; has been a consultant for Bristol Myers Squibb, Merck, Windtree, and Neurotronik; and is a co-owner of Biscardia. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The views expressed in this paper are those of the authors and do not necessarily represent the views of the U.S. Food and Drug Administration or the U.S. Department of Health and Human Services. P.K. Shah, MD served as Guest Editor-in-Chief for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACCauthor instructions page.
Appendix
For an expanded Methods and Reference section as well as a supplemental figure and table, please see the online version of this paper.
Appendix
References
- 1.WCG Resource Center: COVID-19 and Clinical Trial Operations. https://www.wcgclinical.com/covid-19/ Available at:
- 2.Medidata . Release 4.0. 2020. COVID 19 and Clinical Trials: The Medidata Perspective.https://www.medidata.com/en/insight/covid-19-and-clinical-trials-the-medidata-perspective/ Available at: [Google Scholar]
- 3.U.S. Food and Drug Administration FDA Guidance on Conduct of Clinical Trials of Medical Products During COVID-19 Public Health Emergency. 2020. https://www.fda.gov/media/136238/download Available at:
- 4.National Institutes of Health Guidance for NIH-funded Clinical Trials and Human Subjects Studies Affected by COVID-19. 2020. https://grants.nih.gov/grants/guide/notice-files/NOT-OD-20-087.html Available at:
- 5.European Medicines Agency Guidance on the Management of Clinical Trials during the COVID-19 (Coronavirus) Pandemic. 2020. https://ec.europa.eu/health/sites/health/files/files/eudralex/vol-10/guidanceclinicaltrials_covid19_en.pdf Available at:
- 6.Anker S.D., Butler J., Khan M.S. Conducting clinical trials in heart failure during (and after) the COVID-19 pandemic: an Expert Consensus Position Paper from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC) Eur Heart J. 2020;41:2109–2117. doi: 10.1093/eurheartj/ehaa461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abraham W.T., Fiuzat M., Psotka M.A., O'Connor C.M. Heart Failure Collaboratory statement on clinical trials in the landscape of COVID-19. J Am Coll Cardiol HF. 2020;8:423–425. doi: 10.1016/j.jchf.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WCG Centerwatch Remote Monitoring, Protocol Amendments Spike With COVID-19 Spread. 2020. https://www.centerwatch.com/articles/24590-remote-monitoring-protocol-amendments-spike-with-covid-19-spread Available at:
- 9.O'Connor C.M., Psotka M.A., Fiuzat M. Improving heart failure therapeutics development in the United States: the Heart Failure Collaboratory. J Am Coll Cardiol. 2018;71:443–453. doi: 10.1016/j.jacc.2017.11.048. [DOI] [PubMed] [Google Scholar]
- 10.U.S. Department of Health and Human Services, Food and Drug Administration E6(R2) Good Clinical Practice: Integrated Addendum to ICH E6(R1): Guidance for Industry. 2018. https://www.fda.gov/media/93884/download Available at:
- 11.Thompson N., Nazir N., Cox L.S. Unannounced telephone pill counts for assessing varenicline adherence in a pilot clinical trial. Patient Prefer Adherence. 2011;5:475–482. doi: 10.2147/PPA.S24023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Venet D., Doffagne E., Burzykowski T. A statistical approach to central monitoring of data quality in clinical trials. Clin Trials. 2012;9:705–713. doi: 10.1177/1740774512447898. [DOI] [PubMed] [Google Scholar]
- 13.Psotka M.A., von Maltzahn R., Anatchkova M. Patient-reported outcomes in chronic heart failure: applicability for regulatory approval. J Am Coll Cardiol HF. 2016;4:791–804. doi: 10.1016/j.jchf.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 14.U.S. Food and Drug Administration Medical Device Development Tools (MDDT). 2018. https://www.fda.gov/medicaldevices/scienceandresearch/medicaldevicedevelopmenttoolsmddt/ Available at:
- 15.Jayaram N.M., Khariton Y., Krumholz H.M. Impact of telemonitoring on health status. Circ Cardiovasc Qual Outcomes. 2017;10 doi: 10.1161/CIRCOUTCOMES.117.004148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butler J., Khan M.S., Mori C. Minimal clinically important difference in quality of life scores for patients with heart failure and reduced ejection fraction. Eur J Heart Fail. 2020;22:999–1005. doi: 10.1002/ejhf.1810. [DOI] [PubMed] [Google Scholar]
- 17.Smithline H.A., Caglar S., Blank F.S. Physician vs patient assessment of dyspnea during acute decompensated heart failure. Congest Heart Fail. 2010;16:60–64. doi: 10.1111/j.1751-7133.2009.00127.x. [DOI] [PubMed] [Google Scholar]
- 18.Anker S.D., Comin Colet J., Filippatos G. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009;361:2436–2448. doi: 10.1056/NEJMoa0908355. [DOI] [PubMed] [Google Scholar]
- 19.Epis O.M., Casu C., Belloli L. Pixel or paper? Validation of a mobile technology for collecting patient-reported outcomes in rheumatoid arthritis. JMIR Res Protoc. 2016;5:e219. doi: 10.2196/resprot.5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ekman I., Kjork E., Andersson B. Self-assessed symptoms in chronic heart failure--important information for clinical management. Eur J Heart Fail. 2007;9:424–428. doi: 10.1016/j.ejheart.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 21.Redfield M.M., Anstrom K.J., Levine J.A. Isosorbide mononitrate in heart failure with preserved ejection fraction. N Engl J Med. 2015;373:2314–2324. doi: 10.1056/NEJMoa1510774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brooks G.C., Vittinghoff E., Iyer S. Accuracy and usability of a self-administered 6-minute walk test smartphone application. Circ Heart Fail. 2015;8:905–913. doi: 10.1161/CIRCHEARTFAILURE.115.002062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salvi D., Poffley E., Orchard E., Tarassenko L. The mobile-based 6-minute walk test: usability study and algorithm development and validation. JMIR Mhealth Uhealth. 2020;8 doi: 10.2196/13756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.John D., Morton A., Arguello D., Lyden K., Bassett D. “What is a step?” Differences in how a step is detected among three popular activity monitors that have impacted physical activity research. Sensors (Basel) 2018;18:1206. doi: 10.3390/s18041206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly J.P., Ballew N.G., Lin L. Association of implantable device measured physical activity with hospitalization for heart failure. J Am Coll Cardiol HF. 2020;8:280–288. doi: 10.1016/j.jchf.2019.10.009. [DOI] [PubMed] [Google Scholar]
- 26.Kirkpatrick J.N., Grimm R., Johri A.M. Recommendations for echocardiography laboratories participating in cardiac point of care cardiac ultrasound (pocus) and critical care echocardiography training: report from the American Society of Echocardiography. J Am Soc Echocardiogr. 2020;33:40922.e4. doi: 10.1016/j.echo.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Ibrahim N.E., Burnett J.C., Jr., Butler J. Natriuretic peptides as inclusion criteria in clinical trials: a JACC: heart failure position paper. J Am Coll Cardiol HF. 2020;8:347–358. doi: 10.1016/j.jchf.2019.12.010. [DOI] [PubMed] [Google Scholar]
- 28.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo T., Fan Y., Chen M. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tavazzi G., Pellegrini C., Maurelli M. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020;22:911–915. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thygesen K., Alpert J.S., Jaffe A.S. Fourth Universal Definition of Myocardial Infarction (2018) J Am Coll Cardiol. 2018;72:2231–2264. doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- 32.Garg S., Kim L., Whitaker M. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 - COVID-NET, 14 states, March 1-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piccolo R., Bruzzese D., Mauro C. Population trends in rates of percutaneous coronary revascularization for acute coronary syndromes associated with the COVID-19 outbreak. Circulation. 2020;141:2035–2037. doi: 10.1161/CIRCULATIONAHA.120.047457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garcia S., Albaghdadi M.S., Meraj P.M. Reduction in ST-segment elevation cardiac catheterization laboratory activations in the United States during COVID-19 pandemic. J Am Coll Cardiol. 2020;75:2871–2872. doi: 10.1016/j.jacc.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hall M.E., Vaduganathan M., Khan M.S. Reductions in heart failure hospitalizations during the COVID-19 pandemic. J Card Fail. 2020;26:462–463. doi: 10.1016/j.cardfail.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baldi E., Sechi G.M., Mare C. Out-of-hospital cardiac arrest during the Covid-19 outbreak in Italy. N Engl J Med. 2020;383:496–498. doi: 10.1056/NEJMc2010418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pew Research Center Internet/Broadband Fact Sheet. 2019. https://www.pewresearch.org/internet/fact-sheet/internet-broadband/ Available at:
- 38.U.S. Food and Drug Administration Determining the Extent of Safety Data Collection Needed in Late-Stage Premarket and Postapproval Clinical Investigations - Guidance for Industry. 2016. https://www.fda.gov/media/82664/download Available at:
- 39.Wadhera R.K., Wadhera P., Gaba P. Variation in COVID-19 Hospitalizations and Deaths Across New York City Boroughs. JAMA. 2020;323:2192–2195. doi: 10.1001/jama.2020.7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.NYC Health . 2020. Age Adjusted Rate of Fatal Lab Confirmed COVID-19 Cases per 100,000 by Race/Ethnicity Group.https://www1.nyc.gov/assets/doh/downloads/pdf/imm/covid-19-deaths-race-ethnicity-04082020-1.pdf Available at: [Google Scholar]
- 41.Verity R., Okell L.C., Dorigatti I. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. 2020;323:2192–2195. doi: 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.