Abstract
Objective
Obese patients with rheumatoid arthritis (RA) may be more likely to discontinue therapy than non-obese patients, possibly signifying a more refractory phenotype. The purpose of this study was to examine the association between body mass index (BMI) and discontinuation rates for different RA treatments accounting for confounding factors.
Methods
Veterans Affairs administrative databases were used to define initial courses of methotrexate (MTX), hydroxychloroquine, sulfasalazine, prednisone, and self-injectable tumour necrosis factor inhibitors (TNFi). Discontinuation was defined as a lapse in drug refill >90 days. Using overweight BMI (25–30 kg/m2) as the referent group, multivariable Cox proportional hazards models were used to evaluate associations between BMI category and time to treatment discontinuation.
Results
There were 46,970 initial RA treatment courses identified from 2005–2014 among 23,669 Veterans with RA. In multivariable models, severe obesity (BMI >35 kg/m2), compared to overweight BMI, was not associated with treatment discontinuation with the exception of prednisone [HR 1.10 (1.04, 1.17) p<0.001]. Patients with low (<20 kg/m2) and normal BMI (20–25 kg/m2) were more likely to discontinue MTX, TNFi, and HCQ compared to overweight patients. Other factors associated with earlier MTX and/or TNFi discontinuation included female sex, black race, greater comorbidity, depression, malignancy, congestive heart failure, current smoking, and more recent calendar year.
Conclusions
Obesity was not associated with therapy discontinuation among veterans with RA after accounting for confounding factors, suggesting that obesity is not a biological mediator of more refractory disease. Conversely, low BMI, comorbidity, and depression were identified as important predictors of drug discontinuation.
Keywords: rheumatoid arthritis, obesity, anti-TNF drugs, DMARDs, drug persistence
Introduction
A growing literature reports associations between body mass index (BMI) and rheumatoid arthritis (RA) disease phenotype, including disease activity, progressive joint damage, disability, and treatment response. For example, several studies found that higher BMI was associated with a reduced risk of radiographic joint damage and extraarticular disease manifestations (1–3). Other studies, however, have demonstrated that patients with overweight or obese BMI tend to have higher levels of clinical disease activity and lower likelihood of achieving low disease activity compared to patients with normal or low BMI (4–7).
In addition to demonstrating lower likelihood of achieving remission among obese RA patients (4–8), a number of studies have also noted reduced clinical responses to tumour necrosis factor inhibitors (TNFi) in RA patients with high BMI (9–11), raising concern for a refractory RA treatment phenotype associated with obesity. Accordingly, greater therapy discontinuation might be expected among overweight and/or obese patients. To our knowledge, only one study has specifically evaluated the association between BMI and therapy discontinuation in RA (2). In this study, obese RA patients were >60% more likely to discontinue their initial TNFi [HR 1.64 (95% CI 1.02, 2.62)] and demonstrated the lowest rates of disease remission. With second TNFi exposures, the risk of discontinuation was even greater among obese patients, nearly three times greater than those with normal weight [HR 2.90 (95% CI 1.08, 8.45)].
It has been suggested that the chronic inflammation produced directly by excess adipose tissue could mitigate TNFi effectiveness and lead to more refractory disease in obese patients (9, 10, 12). However, given the lower rate of progressive radiographic damage reported in this group, alternative hypotheses also warrant consideration. Osteoarthritis, fibromyalgia, depression, and additional comorbid conditions and other factors that are more common in obesity may affect perceived RA disease activity and affect drug discontinuation.
In other words, it is possible that suboptimal therapeutic responses and earlier treatment discontinuations might be related to comorbid conditions that reduce perceived benefit and increase the risk of adverse events. This hypothesis is in contrast to that of a proposed direct biologic effect from adipose tissue. Previous studies that suggest a refractory disease phenotype have not adequately accounted for these potential confounders. The purpose of this study was to examine the association between BMI and disease-modifying anti-rheumatic drug (DMARD)/TNFi persistence, accounting for factors that may confound the relationship, including comorbidity.
Patients and methods
Study setting
The study design is a retrospective cohort study using real-world clinical data from 2005 to 2014 derived from Veterans Affairs (VA) administrative databases of US Veterans with RA. Analyses were performed among patients with at least one diagnosis code for RA in the 12 months prior to initiation of the course of RA therapy (International Classification of Diseases, 9th edition, 714.xx). Similar definitions of RA have demonstrated an 81–97% positive predictive value (13). Methods relevant to the identification of pharmacy drug courses and cohort derivation have been previously described (14). Briefly, pharmacy dispensing records were used to define the duration of unique initial drug courses of methotrexate (MTX), hydroxychloroquine (HCQ), sulfasalazine, prednisone, and self-injectable TNFi (adalimumab, etanercept, golimumab, certolizumab). This analysis focused on self-injectable rather than intravenous TNFi preparations given greater difficulty in accurately characterising length of drug courses for intravenous formulations. We focused on TNFi rather than non-TNFi biologic agents as these are routinely considered first-line among currently available biologics and have the most frequent clinical use in RA. Likewise, the above listed DMARDs were analysed as these represent the majority of conventional DMARDs used to treat RA. Combination therapy was assessed by evaluating overlapping courses, and concomitant DMARDs were included as covariables. Only the initial course of each drug for each patient was defined as a drug course and a unique observation (15). Thus, a patient could contribute initial VA-based courses for multiple drugs but each patient would only contribute one course for each of the DMARDs under study.
Definition of treatment course length
For each dispensing episode, the amount of drug dispensed and the expected duration of the treatment episode were determined. The expected days of supply were determined based on dosing instructions and the number pills/units dispensed. A drug course was defined as the duration of time in which dispensing episodes did not have a 90-day gap from the end of the days’ supply of the last dispensing episode to the start of the next dispensing episode (14). Duration of treatment was calculated as the time from the date of first treatment until the date of the expected end of the last dispensing episode for the course. The primary outcome of interest was the course length (or persistence) of the therapy, censoring for death or end of follow-up. If patients had multiple treatment courses of the same medication, only the first treatment course was included. Patients could contribute multiple distinct treatment courses for different medications.
Body mass index
Weight and height were extracted from vital sign packages in the VA electronic medical record. The closest value for weight within 30 days of the start date was used. When multiple different values for height were used, the modal value was imputed. BMI [weight (kg)/ height(m)2] was categorised per the World Health Organization (WHO) as underweight (<20 kg/m2), normal weight (≥20–25 kg/m2), over-weight (≥25–30 kg/m2), obese (≥30–35 kg/m2), and severely obese (≥35 kg/m2) groups (16). The overweight group was found to demonstrate the lowest likelihood of discontinuation across agents examined. This group was chosen as the reference category to enable comparisons of other BMI categories to the most extreme category and therefore minimise the likelihood of missing important associations. Courses missing BMI values within 30 days were excluded.
Potential confounders
Covariates of interest were derived from administrative and laboratory databases from the medical record. These included potential confounders hypothesised to influence RA disease activity or treatment tolerability, including RA disease duration, current smoking, calendar year (2005–2009 vs. 2010–2014), age, sex, race (Black vs. White/Other), anti-cyclic citrullinated peptide antibody (ACPA) status, and C-reactive protein (CRP) concentration. The calendar years 2005–2009 and 2010–2014 were divided as such and evaluated separately due to the increased number of available biologics for RA treatment in later years, which was thought to potentially impact medication persistence. Comorbidities were defined by previously validated and published algorithms using diagnosis codes for diabetes, hypertension, congestive heart failure (CHF), history of malignancy, anxiety, and depression (17, 18). The Rheumatic Disease Comorbidity Index (RDCI) was also calculated for each observation. The RDCI is validated and was designed specifically to predict outcomes, including death, physical functioning and disability, medical costs, social security disability, and hospitalisations among patients with rheumatic diseases (19).
Statistical analysis
Differences in patient characteristics across BMI categories were tested using analysis of variance (ANOVA) or Kruskal-Wallis tests for non-parametric data. To avoid dropping observations, multiple imputation (5 imputations) was performed to account for missing values for CRP (77%), ACPA status (27%), and disease duration (> or ≤ five years) (27%). Multivariable Cox proportional hazards models were used to evaluate associations between BMI category and time to treatment discontinuation before and after considering potential confounding factors. Follow-up time for each course was censored at the first of 1) the end of the period of observation, 2) the last vital signs data recorded in the VA, or 3) death. We performed sensitivity analyses after clustering by patient and found no impact on the results (not shown). The proportional hazards assumption was tested by visualising log-log plots and Kaplan-Meier curves. We also performed sensitivity analyses looking at drug discontinuation and limiting the follow-up period to 2 years. All analyses were performed using Stata 14.0 software (StataCorp, LP, College Station, TX) within the VA Informatics and Computing Infrastructure (VINCI).
Results
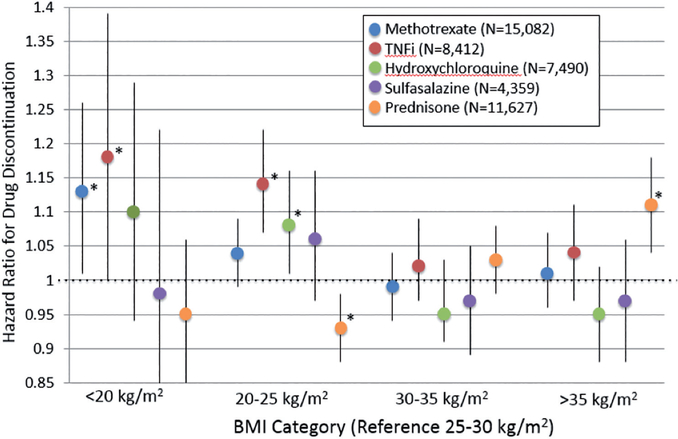
There were 46,970 total unique initial DMARD or TNFi courses among 23,669 unique patients with RA between 2005–2014. Demographics of the study population across BMI categories are presented in Table I. There were a number of significant differences in covariates across BMI categories. For example, patients with normal BMI were more likely to be ACPA-positive (70%) compared to the severely obese group (57%). There were also a number of differences in comorbid conditions across BMI categories. Obese patients had higher comorbidity scores and a greater likelihood of having CHF compared to normal weight (12% vs. 7%, respectively). Patients with low BMI had the highest frequency of a history of malignancy (17%), while severely obese patients had the highest frequency of anxiety (22%) and depression (44%). In models adjusted only for age, sex, race, and calendar year, patients with severe obesity were more likely to discontinue MTX, TNFi, and prednisone versus overweight patients (Table II). However, the associations between severe obesity and drug discontinuation for MTX and TNFi in fully-adjusted multivariable models were completely attenuated (Table II, Fig. 1), Among those receiving TNFi, there was a numerical trend towards a greater risk of drug discontinuation among those with a disease duration >5 years [HR 1.14 (1.00, 1.29) p=0.056; n=4320]. This trend was not present in those with early disease [HR 0.95 (0.87, 1.04) p=0.29; n=2747]. In sensitivity analyses limiting the analysis to 2 years of follow-up, severe obesity was not associated with drug discontinuation in adjusted models except for among those receiving prednisone [HR 1.23 (1.10, 1.37) p<0.001]. In contrast to MTX and TNFi, the association of severe obesity with discontinuation of prednisone remained significant [HR 1.11 (1.04, 1.18) p<0.001] in fully adjusted models. Obesity was not associated with the discontinuation of HCQ or sulfasalazine in either univariate or multivariate analyses.
Table I.
Patient characteristics according to BMI category.
| Patient characteristic | Underweight | Normal | Overweight | Obese | Severely Obese |
|---|---|---|---|---|---|
| <20 kg/m2 | 20–25 kg/m2 | 25–30 kg/m2 | 30–35 kg/m2 | ≥35 kg/m2 | |
| n. | 1,242 | 9,329 | 17,183 | 11,818 | 7,398 |
| Age, yrs | 64.2 (12.6) | 64.1 (12.5) | 63.2 (11.2) | 60.9 (10.4) | 59.0 (9.5) |
| Male, n. (%) | 1,064 (86%) | 8,238 (88%) | 15,448 (90%) | 10,348 (88%) | 6,118 (83%) |
| Black, n. (%) | 196 (16%) | 1,257 (13%) | 2,235 (13%) | 1,681 (14%) | 1,113 (15%) |
| ACPA-positive (%)** | 72% | 70% | 65% | 60% | 57% |
| Calendar year 2005–2009* | 797 (64%) | 5,886 (63%) | 10,525 (61%) | 6,857 (58%) | 3,967 (54%) |
| (vs. 2010–2014), n. (%) | |||||
| Current smoking | 242 (19%) | 1,669 (18%) | 2,791 (16%) | 1,920 (16%) | 1,256 (17%) |
| CHF | 93 (7%) | 683 (7%) | 1,244 (7%) | 1,000 (8%) | 887 (12%) |
| Diabetes | 187 (15%) | 1,638 (18%) | 4,194 (24%) | 3,850 (33%) | 3,240 (44%) |
| HTN | 665 (54%) | 5,462 (59%) | 11,488 (67%) | 8,760 (74%) | 5,899 (80%) |
| History of malignancy | 209 (17%) | 1,492 (16%) | 2,511 (15%) | 1,456 (12%) | 774 (10%) |
| Anxiety | 238 (19%) | 1,442 (15%) | 3,012 (18%) | 2,394 (20%) | 1,642 (22%) |
| Depression | 439 (35%) | 2,891 (31%) | 5,795 (34%) | 4,453 (38%) | 3,248 (44%) |
| RDCI | 1 (0,2) | 1 (1,2) | 1 (1,3) | 2 (1,3) | 2 (1,3) |
| CRP** | 3.1 (3.9) | 2.5 (3.5) | 2.2 (3.4) | 2.0 (3.4) | 1.7 (3.2) |
| Disease >5 yrs** | 40% | 37% | 35% | 31% | 29% |
Values are represented as mean (SD) or median (IQR) for skewed data unless otherwise noted. All p-values based on ANOVA and Kruskal-Wallis tests of significance at <0.001.
Calendar date of the start of the course observation
Includes imputed values.
SD: standard deviation; RDCI: Rheumatic Disease Comorbidity Index; ACPA: anti-citrullinated peptide antibodies; CHF: congestive heart failure; HTN: hypertension.
Table II.
Associations between BMI category and earlier drug discontinuation.
| Methotrexate | TNFi | Prednisone | HCQ | SSZ | |
|---|---|---|---|---|---|
| n=15,082 | n=8,412 | n=11,627 | n=7,490 | n=4,359 | |
| Discontinued: 14,770 | Discontinued: 8,313 | Discontinued: 11,349 | Discontinue: 7,287 | Discontinued: 4,299 | |
| Partially-adjusted† | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) |
| BMI Category <20 kg/m2 | 1.14 (1.02, 1.27)** | 1.13 (0.96, 1.33) | 0.96 (0.85, 1.07) | 1.12 (0.96, 1.30) | 1.00 (0.81, 1.24) |
| 20–25 kg/m2 | 1.02 (0.97, 1.07) | 1.13 (1.06, 1.21)*** | 0.92 (0.87, 0.97)** | 1.09 (1.02, 1.16)** | 1.06 (0.97, 1.16) |
| 25–30 kg/m2 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| 30–35 kg/m2 | 1.00 (0.96, 1.05) | 1.03 (0.98, 1.10) | 1.03 (0.98, 1.08) | 0.95 (0.89, 1.02) | 0.96 (0.89, 1.04) |
| ≥35 kg/m2 | 1.06 (1.00, 1.11)* | 1.07 (1.00, 1.14)* | 1.10 (1.04, 1.17)*** | 0.92 (0.85, 0.99)* | 0.96 (0.88, 1.06) |
| Fully-adjusted‡ | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) |
| BMI Category <20 kg/m2 | 1.13 (1.01, 1.26)*** | 1.18 (1.00, 1.39) | 0.95 (0.84, 1.06) | 1.10 (0.94, 1.29) | 0.98 (0.79, 1.22) |
| 20–25 kg/m2 | 1.04 (0.99, 1.09)* | 1.14 (1.07, 1.22)*** | 0.93 (0.88, 0.98)* | 1.08 (1.01, 1.16)** | 1.06 (0.97, 1.16) |
| 25–30 kg/m2 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| 30–35 kg/m2 | 0.99 (0.94, 1.03) | 1.02 (0.97, 1.09) | 1.03 (0.98, 1.08) | 0.97 (0.91, 1.03) | 0.97 (0.89, 1.05) |
| ≥35 kg/m2 | 1.01 (0.96, 1.07) | 1.04 (0.97, 1.11) | 1.11 (1.04, 1.18)*** | 0.95 (0.88, 1.02) | 0.97 (0.88, 1.06) |
p<0.05
p<0.01
p<0.001
Adjusted for calendar date, age, sex, black race. For TNFi: also adjusted for drug name and initial vs. subsequent biologic course.
Also adjusted for: Concurrent medication use (HCQ, SSZ, MTX, prednisone, TNFi), RDCI, CRP, ever ACPA-positive, disease duration >5 years, diabetes, HTN, CHF, cancer, anxiety, depression, current smoking. For TNFi: also adjusted for first biologic use and therapy.
BMI: body mass index; HR: hazard ratio; TNFi: tumour necrosis factor inhibitor; RDCI: Rheumatic Disease Comorbidity Index; CRP: C-reactive protein; ACPA: anti-citrullinated peptide antibody; HTN: hypertension; CHF: congestive heart failure.
Fig. 1.
All models adjusted for calendar date, age, sex, black race, concurrent medication use, RDCI, CRP, ever CCP positive, disease duration >5 years, diabetes, HTN, CHF, cancer, anxiety, depression, current smoking. For TNFi: also adjusted for first biologic use and therapy. *p<0.05.
Patients in low and normal BMI categories generally were more likely to discontinue MTX, HCQ, and TNFi, compared to overweight patients, and these associations persisted in fully-adjusted multivariable models (Table II). In contrast, patients with normal BMI were less likely than overweight patients to discontinue prednisone, even in fully-adjusted models.
Table III shows other factors associated with DMARD discontinuation for MTX, TNFi, prednisone, HCQ, and sulfasalazine. Factors associated with earlier MTX discontinuation included female sex, black race, greater comorbidity, depression, anxiety, CHF, active smoking, ACPA-negativity, and more recent calendar year (Table III). Among TNFi users, female sex, older age, greater comorbidity, depression, malignancy, smoking, concurrent prednisone use, and recent calendar year were independently associated with a greater likelihood of discontinuation. Concurrent MTX use was associated with a lower likelihood of TNFi discontinuation [HR 0.89 (0.85, 0.93) p<0.001]. Initial TNFi users were less likely to discontinue therapy than those starting a subsequent TNFi.
Table III.
Association between covariates of interest and earlier drug discontinuation.
| Methotrexate | TNFi | Prednisone | HCQ | SSZ | |
|---|---|---|---|---|---|
| n=15,082 | n=8,412 | n=11,627 | n=7,490 | n=4,359 | |
| Discontinued: 14,770 | Discontinued: 8,313 | Discontinued: 11,349 | Discontinued: 7,287 | Discontinued: 4,299 | |
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Age | |||||
| <60 yrs | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| 60–70 yrs | 0.82 (0.79, 0.86)*** | 0.89 (0.84, 0.94)** | 0.98 (0.93, 1.03) | 0.92 (0.86, 0.97)* | 0.90 (0.83, 0.97)** |
| 70–80 yrs | 0.88 (0.83, 0.93)* | 0.92 (0.85, 0.99) | 0.98 (0.93, 1.04) | 0.94 (0.87, 1.02) | 0.96 (0.86, 1.06) |
| >80 yrs | 0.94 (0.87, 1.02) | 1.14 (0.99, 1.32)** | 0.88 (0.81, 0.96)* | 1.12 (1.05, 1.34)*** | 0.97 (0.82, 1.14) |
| Male | 0.90 (0.85, 0.95)*** | 0.80 (0.75, 0.86)*** | 0.86 (0.81, 0.91)*** | 0.93 (0.86, 1.00) | 0.86 (0.78, 0.96)** |
| Black | 1.13 (1.07, 1.19)*** | 1.08 (1.00, 1.16) | 1.05 (0.99, 1.11) | 1.31 (1.22, 1.41)*** | 1.17 (1.07, 1.28)* |
| Depression | 1.09 (1.04, 1.14)*** | 1.14 (1.09, 1.21)*** | 1.08 (1.03, 1.13)** | 1.14 (1.08, 1.21)*** | 1.13 (1.05, 1.22)** |
| Anxiety | 1.11 (1.06, 1.17)*** | 1.06 (1.00, 1.13) | 1.16 (1.09, 1.22)*** | 1.01 (0.95, 1.09) | 0.98 (0.90, 1.07) |
| CHF | 1.09 (1.02, 1.17)* | 1.06 (0.96, 1.17) | 1.06 (0.99, 1.14) | 0.96 (0.87, 1.06) | 1.01 (0.90, 1.13) |
| Malignancy | 1.05 (1.00, 1.11) | 1.11 (1.03, 1.20)* | 1.00 (0.94, 1.06) | 1.03 (0.95, 1.10) | 1.01 (0.92, 1.11) |
| Comorbidity Score (RDCI) | 1.03 (1.01, 1.04)** | 1.03 (1.01, 1.06)** | 0.99 (0.97, 1.01) | 0.97 (0.95, 0.99)* | 0.98 (0.95, 1.01) |
| 2010–2014 vs. 2005–2009 | 1.10 (1.05, 1.15)*** | 1.05 (0.99, 1.12) | 1.29 (1.23, 1.36)*** | 1.09 (1.03, 1.16)*** | 0.98 (0.90, 1.06) |
| Current smoker | 1.07 (1.00, 1.13)* | 1.11 (1.04, 1.20)*** | 1.14 (1.07, 1.22)*** | 1.07 (0.99, 1.15) | 1.02 (0.92, 1.12) |
| ACPA-positive | 0.87 (0.83, 0.91)*** | 0.94 (0.87, 1.01) | 0.90 (0.86, 0.95)** | 0.95 (0.89, 1.02) | 0.97 (0.88, 1.06) |
| Concurrent Prednisone | 0.97 (0.93, 1.01) | 1.13 (1.07, 1.18)*** | N/A | 1.00 (0.95, 1.05) | 1.07 (1.00, 1.15)* |
| Concurrent MTX use | N/A | 0.89 (0.85, 0.93)*** | 0.97 (0.93, 1.00) | 0.98 (0.93, 1.03) | 0.94 (0.88, 1.01) |
| Concurrent TNFi use | 1.07 (1.00, 1.13)* | N/A | 1.14 (1.07, 1.21)*** | 1.09 (0.99, 1.20) | 1.07 (0.95, 1.20) |
| Concurrent SSZ use | 1.05 (0.96, 1.14) | 0.96 (0.88, 1.05) | 1.13 (1.03, 1.25)* | 1.11 (1.02, 1.22)* | N/A |
| Initial Biologic | N/A | 0.87 (0.82, 0.91)*** | N/A | N/A | N/A |
p<0.05
p<0.01
p<0.001.
Additional variables were included in the models and are not shown (BMI category) or non-significant (CRP, disease duration >5 years, concurrent HCQ, diabetes, hypertension).
CHF: congestive heart failure; RDCI: Rheumatic Disease Comorobidity Index; ACPA: anti-cyclic citrullinated peptide antibody; MTX: methotrexate; TNFi: tumour necrosis factor inhibitor; SSZ: sulfasalazine; CRP: C-reactive protein; HCQ: hydroxychloroquine.
Among prednisone users, greater use of other therapies, female sex, more recent calendar year, depression, anxiety, and smoking were each associated with a greater likelihood of discontinuation. Patients who were ACPA-positive were less likely to discontinue prednisone.
Discussion
This large, real-world, national study demonstrated that obesity was not associated with discontinuation of conventional DMARDs and/or TNFi’s when considering differences in other factors including comorbid conditions. While there was modestly greater discontinuation among the severely obese in crude analyses, there was no association between obesity and DMARD/ TNFi discontinuation in adjusted models. These data refute the hypothesis that obesity and excess adiposity directly interfere with the biological effect of commonly prescribed RA therapies. Given the growing number of treatments available for rheumatoid arthritis (20), consideration of comorbidities is increasingly important to guide clinical decision making and treatment choice.
A decreased response rate and increased discontinuation rates of DMARD/TNFi among obese RA patients have been demonstrated in several smaller studies (2, 9, 10, 21) and among patients with other types of inflammatory arthritis (22). The current study confirms greater drug discontinuation among the severely obese, but the lack of association after multivariable adjustment suggests that this phenomenon is not likely to be a causal effect of excess weight. Rather, the slightly higher discontinuation rates among severely obese patients in this study were not independent of confounding factors.
We hypothesise that comorbid conditions and other factors among obese individuals, and not refractory RA disease activity, drive more frequent DMARD/TNFi changes. More frequent medication changes in obese individuals may be appropriate in certain contexts (i.e. when a co-morbidity is a contraindication for a specific therapy), but might also occur for other reasons (i.e. false elevation in estimation of disease activity which may reflect other disease processes such as fibromyalgia, depression or osteoarthritis). Heimans and colleagues demonstrated this trend in the BeSt (Treatment Strategies for Rheumatoid Arthritis) trial, in which they found that patients with high BMI had higher DAS scores that were driven primarily by higher pain and joint tenderness scores, resulting in significantly more treatment steps over 8 years compared to patients with low/normal BMI (12). Swollen joint counts, however, are not associated with BMI and do not apparently perform worse in RA patients with greater BMI (23).
In contrast to the lack of association between obesity and drug discontinuation observed in this study, low BMI was consistently associated with greater DMARD and TNFi therapy discontinuation after adjustment for confounders. This finding may align with evidence that RA patients with low BMI tend to have more erosive and severe disease as demonstrated on MRI and radiographic imaging (24, 25), and supports the hypothesis that patients with a low BMI have a refractory phenotype of disease. In support of this hypothesis, prednisone is less likely to be discontinued among patients with a normal BMI compared to those that were overweight or obese. However, it is possible that unmeasured confounding, discontinuation of therapy due to worsening health status, and more frequent side-effects in this group may play a role in explaining these associations.
Prednisone was generally discontinued earlier among severely obese patients, in contrast to other DMARDs. Complications of prednisone use may be more frequent in the severely obese (or physicians and patients may be more fearful or have a lower tolerance of such complications). The authors are not aware of studies that examine adverse glucocorticoid effects in obese patients compared to normal or underweight patients. The adverse effects of long-term glucocorticoid use, however, including morphologic changes in adipose distribution, hyperglycemia, infection risk, and cardiovascular risk, among others, might be hypothesised to be of greatest relevance in this group (26).
A limitation of this retrospective analysis of health records data is that it does not provide the opportunity to evaluate individual patients on a more granular level. This national VA database does not include disease activity scores, so this potentially important clinical information was unavailable in these analyses. While a strength is the presence of laboratory data in this administrative database, ACPA status was missing for many patients and thus necessitated imputing values to allow incorporation of ACPA into multivariable models. Drug discontinuation was determined using pharmacy data, which may not accurately reflect actual use of therapies in every case. For example, prednisone use is often not utilised exactly as directed. Additionally, it is not possible to determine whether individual discontinuations occurred because of lack of efficacy, adverse reactions or intolerance, patient preference, poor adherence, socioeconomic factors, or other reasons. However, medication persistence is a well-established proxy for efficacy. The methodology does not capture temporary medication discontinuations either.
Additionally, data are derived from the US Veteran population, with over-representation of Caucasian and male patients. However, no interactions between sex or racial groups were identified, suggesting that these findings may be generalisable beyond the VA health system. Additionally, it can be challenging to identify true synovitis in obese patients, and it is possible that an equivocal physical exam may overestimate disease activity and subsequently affect medication persistence. The reasons for discontinuation in the different BMI categories cannot be assessed here, and this is an important limitation. Furthermore, our analysis only looks at BMI at a single point in time, and it is known that weight changes over the lifespan and that these changes may be informative. Finally, dual care from the VA and the public sector could result in prescriptions that are not captured in VA databases, although recent work suggests that this is uncommon in part related to generally better health benefits through the VA compared to civilian benefits (27).
The study has several notable strengths. First, the cohort was large, including over 45,000 unique initial DMARD or TNFi courses in the analysis. The study is an advancement over prior studies in its use of robust clinical data from the electronic medical record as well as well-defined drug courses using pharmacy records, allowing for a comprehensive and well-powered analysis. Our findings also capture national data as opposed to one region of the country. This study is among the first to comprehensively analyse the association between BMI and drug discontinuation while accounting for potentially significant confounding factors in a nationwide sample.
In conclusion, this study did not demonstrate an association between BMI and DMARD/TNFi discontinuation rates after adjusting for important covariates. While these data do not support the hypothesis that obesity contributes to poor treatment efficacy or tolerability, we cannot rule out biologic differences in response to therapy. However, other factors including co-morbidities were identified as important predictors of drug discontinuation.
Acknowledgements
Dr Baker would like to acknowledge funding through a Veterans Affairs Clinical Science Research & Development Career Development Award (IK2 CX000955). Dr Caplan is supported, in part, by VA HSR&D IIR 14-048-3. Dr Mikuls is supported by a VA CSR&D Merit Award (CX000896) and grants from the NIH/NIAAA (R25AA020818) and NIGMS (U54GM115458). Dr Barton is supported by the NIH/NIAMS (K23-AR-064372). This material is also based on work supported by Specialty Care Center of Innovation, Veterans Health Administration and Department of Veterans Affairs, Health Services Research and Development. The contents of this work do not represent the views of the Department of the Veterans Affairs or the United States Government.
Competing interests: T.R. Mikuls is supported by a VA CSR&D Merit Award (CX000896) and grants from the NIH/NAAA (R25AA020818) and NIGMS (U54GM115458).
G.W. Cannon is partially supported by Specialty Care Center of Innovation, Veterans Health Administration and Department of Veterans Affairs, Health Services Research and Development.
J.F. Baker is supported by a Clinical Science Research & Development Career Development Award (IK2 CX000955). J.L. Barton is supported by the NIH/ NIAMS (K23-AR-064372).
L. Caplan is supported by the Department of Veterans Affairs HSR&D Merit IIR 14-048-3.
Footnotes
The other co-authors have declared no competing interests.
References
- 1.BAKER JF, BILLIG E, MICHAUD K et al. : Weight loss, the obesity paradox, and the risk of death in rheumatoid arthritis. Arthritis Rheumatol 2015; 67: 1711–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.IANNONE F, FANIZZI R, NOTARNICOLA A, SCIOSCIA C, ANELLI MG, LAPADULA G: Obesity reduces the drug survival of second line biological drugs following a first TNF-alpha inhibitor in rheumatoid arthritis patients. Joint Bone Spine 2015; 82: 187–91. [DOI] [PubMed] [Google Scholar]
- 3.VELPULA UD, AGRAWAL S, THOMAS J, PRABU VN, RAJASEKHAR L, NARSIMULU G: Low body mass index is adversely associated with radiographic joint damage in Indian patients with early rheumatoid arthritis. J Rheumatol 2011; 38: 434–8. [DOI] [PubMed] [Google Scholar]
- 4.LUPOLI R, PIZZICATO P, SCALERA A et al. : Impact of body weight on the achievement of minimal disease activity in patients with rheumatic diseases: a systematic review and meta-analysis. Arthritis Res Ther 2016; 18: 297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.VIDAL C, BARNETCHE T, MOREL J, COMBE B, DAIEN C: Association of body mass index categories with disease activity and radiographic joint damage in rheumatoid arthritis: a systematic review and metaanalysis. J Rheumatol 2015; 42: 2261–9. [DOI] [PubMed] [Google Scholar]
- 6.LIU Y, HAZLEWOOD GS, KAPLAN GG, EKSTEEN B, BARNABE C: Impact of obesity on remission and disease activity in rheumatoid arthritis: a systematic review and meta-analysis. Arthritis Care Res (Hoboken) 2017; 69: 157–65. [DOI] [PubMed] [Google Scholar]
- 7.JAWAHEER D, OLSEN J, LAHIFF M et al. : Gender, body mass index and rheumatoid arthritis disease activity: results from the QUEST-RA Study. Clin Exp Rheumatol 2010; 28: 454–61. [PMC free article] [PubMed] [Google Scholar]
- 8.GEORGE MD, OSTERGAARD M, CONAGHAN PG, EMERY P, BAKER DG, BAKER JF: Obesity and rates of clinical remission and low MRI inflammation in rheumatoid arthritis. Ann Rheum Dis 2017; 76: 1743–6. [DOI] [PubMed] [Google Scholar]
- 9.GREMESE E, CARLETTO A, PADOVAN M et al. : Obesity and reduction of the response rate to anti-tumor necrosis factor alpha in rheumatoid arthritis: an approach to a personalized medicine. Arthritis Care Res (Hoboken) 2013; 65: 94–100. [DOI] [PubMed] [Google Scholar]
- 10.KLAASEN R, WIJBRANDTS CA, GERLAG DM, TAK PP: Body mass index and clinical response to infliximab in rheumatoid arthritis. Arthritis Rheum 2011; 63: 359–64. [DOI] [PubMed] [Google Scholar]
- 11.OTTAVIANI S, GARDETTE A, TUBACH F et al. : Body mass index and response to infliximab in rheumatoid arthritis. Clin Exp Rheumatol 2015; 33: 478–83. [PubMed] [Google Scholar]
- 12.HEIMANS L, VAN DEN BROEK M, LE CESSIE S et al. : Association of high body mass index with decreased treatment response to combination therapy in recent-onset rheumatoid arthritis patients. Arthritis Care Res (Hoboken) 2013; 65: 1235–42. [DOI] [PubMed] [Google Scholar]
- 13.SINGH JA, HOLMGREN AR, NOORBALOOCHI S: Accuracy of Veterans Administration databases for a diagnosis of rheumatoid arthritis. Arthritis Rheum 2004; 51: 952–7. [DOI] [PubMed] [Google Scholar]
- 14.BAKER JF, SAUER BC, CANNON GW et al. : Changes in body mass related to the initiation of disease-modifying therapies in rheumatoid arthritis. Arthritis Rheumatol 2016; 68: 1818–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.CANNON GW, MIKULS TR, HAYDEN CL et al. : Merging Veterans Affairs rheumatoid arthritis registry and pharmacy data to assess methotrexate adherence and disease activity in clinical practice. Arthritis Care Res 2011; 63: 1680–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.COMMITTEE WE: Physical status: the use and interpretation of anthropometry Report of a WHO Expert Committee. World Health Organization; 1995. [PubMed] [Google Scholar]
- 17.BIRMAN-DEYCH E, WATERMAN AD, YAN Y, NILASENA DS, RADFORD MJ, GAGE BF: Accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Med Care 2005; 43: 480–5. [DOI] [PubMed] [Google Scholar]
- 18.QUAN H, SUNDARARAJAN V, HALFON P et al. : Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005; 43: 1130–9. [DOI] [PubMed] [Google Scholar]
- 19.ENGLAND BR, SAYLES H, MIKULS TR, JOHNSON DS, MICHAUD K: Validation of the rheumatic disease comorbidity index. Arthritis Care Res (Hoboken) 2015; 67: 865–72. [DOI] [PubMed] [Google Scholar]
- 20.BORTOLUZZI A, FURINI F, GENERALI E, SILVAGNI E, LUCIANO N, SCIRE CA: One year in review 2018: novelties in the treatment of rheumatoid arthritis. Clin Exp Rheumatol 2018; 36: 347–61. [PubMed] [Google Scholar]
- 21.IANNONE F, LOPALCO G, RIGANTE D, ORLANDO I, CANTARINI L, LAPADULA G: Impact of obesity on the clinical outcome of rheumatologic patients in biotherapy. Auto-immun Rev 2016; 15: 447–50. [DOI] [PubMed] [Google Scholar]
- 22.ROSAS J, LLINARES-TELLO F, SENABREGALLEGO JM et al. : Obesity decreases clinical efficacy and levels of adalimumab in patients with ankylosing spondylitis. Clin Exp Rheumatol 2017; 35: 145–8. [PubMed] [Google Scholar]
- 23.CAPLAN L, DAVIS LA, BRIGHT CM et al. : Body mass index and the rheumatoid arthritis swollen joint count: an observational study. Arthritis Care Res (Hoboken) 2013; 65: 101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.BAKER JF, GEORGE M, BAKER DG, TOEDTER G, VON FELDT JM, LEONARD MB: Associations between body mass, radiographic joint damage, adipokines and risk factors for bone loss in rheumatoid arthritis. Rheumatology (Oxford) 2011; 50: 2100–7. [DOI] [PubMed] [Google Scholar]
- 25.KAUFMANN J, KIELSTEIN V, KILIAN S, STEIN G, HEIN G: Relation between body mass index and radiological progression in patients with rheumatoid arthritis. J Rheumatol 2003; 30: 2350–5. [PubMed] [Google Scholar]
- 26.POETKER DM, REH DD: A comprehensive review of the adverse effects of systemic corticosteroids. Otolaryngol Clin North Am 2010; 43: 753–68. [DOI] [PubMed] [Google Scholar]
- 27.SCHWAB P, SAYLES H, BERGMAN D et al. : Utilization of care outside the Veterans Affairs health care system by US Veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2017; 69: 776–82. [DOI] [PMC free article] [PubMed] [Google Scholar]