Highlights
-
•
A high rate of asymptomatic SARS-CoV-2 carriers was found in healthcare workers.
-
•
Asymptomatic individuals had lower antibody levels in the acute phase.
-
•
Asymptomatic IgG titration reduced during the early convalescent phase.
Keywords: SARS-CoV-2, COVID-19, Epidemiology, Asymptomatic, Pneumonia, Healthcare worker
Abstract
Objectives
To investigate the proportion and characteristics of asymptomatic infection among healthcare workers (HCWs).
Methods
This study retrospectively investigated 1407 HCWs who were screened for COVID-19 by chest computed tomography (CT) scans and nasopharyngeal swabs for SARS-CoV-2 nucleic acid. Demographics, CT features, nasopharyngeal swabs, baseline symptoms, and laboratory data were collected.
Results
Of 1407 HCWs, 235 had symptoms and 1172 were asymptomatic close contacts, of which, 107 were symptomatic cases and 84 were close contacts who had abnormal CT findings. Of 152 symptomatic individuals and 908 close contacts tested for SARS-CoV-2 nucleic acid, 122 symptomatic cases and 38 close contacts had positive reverse-transcriptase real-time polymerase chain (RT-PCR) test results. The rate of confirmed asymptomatic infections was 4.2% (38/908). Both symptomatic and asymptomatic infected cases had high titrations of specific IgG or had ≥four-fold increase in IgG during convalescence compared with the acute phase. Combining the RT-PCR tests and serological findings, the rate of asymptomatic infections was 9.7% (88/908). In terms of the duration of viral shedding, there was no significant difference between symptomatic mild/moderate participants and asymptomatic infections.
Conclusions
The findings demonstrated that a high rate of asymptomatic SARS-CoV-2 carriers existed among healthcare worker close contacts during the outbreak of COVID-19.
Introduction
Coronavirus Disease 2019 (COVID-19) has caused a large number of related deaths and multiple healthcare-associated outbreaks (Zhu et al., 2019, Wang et al., 2020a, Huang et al., 2020, Chen et al., 2020a). Investigators have summarised the clinical characteristics of initial onset in cases (Huang et al., 2020, Chen et al., 2020a): some had atypical clinical manifestations (e.g. severe cases only had moderate or low fever or even no fever), while some mild cases did not have pneumonia and only had low fever or mild fatigue (NHC, 2020, Guan et al., 2020). One report revealed that no radiographic or computed tomography (CT) abnormalities were found in 17.9% of non-severe patients and 2.9% of severe patients (Guan et al., 2020). Radiologic findings may even occur in asymptomatic cases (Shi et al., 2020). However, little is currently known about the prevalence and infectiousness of asymptomatic individuals. People are generally susceptible to the 2019 novel coronavirus (SARS-CoV-2) and asymptomatic infections are becoming a great concern for disease prevention and control (Rothe et al., 2020). Since a clustered outbreak of COVID-19 occurred at the Renmin Hospital of Wuhan University, China, during mid-January 2020, the prevalence rates and clinical features of symptomatic and asymptomatic infections from 1407 healthcare workers (HCWs) at this hospital were investigated.
Methods
Participants
The hospital is a 5000-bed teaching hospital with >12,000 HCWs. During mid-January 2020, a clustered outbreak of HCWs with COVID-19 took place at the hospital. The index patient was a 59-year-old male. On 03 January 2020 he had a fever, headache, chest pain, and myalgias when he visited his wife in a cancer ward, which was shared by four female cancer patients. He initially thought that he had a ‘cold’ and continued to visit his wife's ward until he was admitted to another hospital on 10 January. After his diagnosis was confirmed as COVID-19, he was transferred to a designated hospital and died on 26 January. Being in close contact with the index patient, the four cancer patients subsequently developed fevers and had abnormal CT scans from 09–16 January. All of those patients were quarantined, diagnosed with COVID-19 and treated.
Since 14 January a cluster of HCWs developed fevers, coughs and had abnormal chest CT scans. At that time, HCWs with acute febrile symptoms and their close contacts became fully alerted and were screened for the pandemic disease. From 14 January to 21 February, 1407 HCWs were screened for COVID-19 infections by chest CT scans and reverse-transcriptase real-time polymerase chain reaction (RT-PCR) analysis for SARS-CoV-2 nucleic acid; 235 (16.7%) were symptomatic and the other 1172 (83.3%) were close contacts. Information from all infected employees was collected, including: demography, symptoms, laboratory results, nasopharyngeal swab RT-PCR tests, chest CT scans, and levels of serum antibodies for SARS-CoV-2.
This study received approval from the Ethics Committee of the Renmin Hospital of Wuhan University (No. WDRY2020-K019) and oral consent was obtained from the participants or their family members. Under these exceptional circumstances, a waiver of written informed consent was applied for.
Confirmed criteria for COVID-19 and disease severity
Laboratory-confirmed cases of COVID-19 met the criteria of the Guidelines on the Diagnosis and Treatment of Novel Coronavirus Infected Pneumonia (NHC, 2020). The severity of COVID-19 is classified as mild, moderate, severe, or critical (NHC, 2020).
Definition of asymptomatic infection in the acute phase
Patients who had a mild or worse fever (>37.5 °C), fatigue, and mild or worse dry cough or sore throat were symptomatic (Shaman et al., 2018). Other symptoms were shortness of breath, muscle ache, headache, diarrhoea, and vomiting (NHC, 2020). Therefore, asymptomatic infections refer to those who tested positive for viral nucleic acid but lacked clinical symptoms (NHC, 2020, Guan et al., 2020, Park et al., 2020).
Detection of SARS-CoV-2 nucleic acid
Nasopharyngeal swab specimens were collected, maintained in viral-transport medium and then submitted for inspection by trained technicians. SARS-CoV-2 nucleic acid was detected by RT-PCR, which was performed in the clinical laboratory at the Renmin Hospital of Wuhan University. RT-PCR detection reagents targeting SARS-CoV-2 nucleic acid open reading frame 1ab and nucleocapsid protein were provided by Shanghai GeneoDx Biotech Co., LTD, China.
Serum antibody examinations
Serum specific IgM and IgG antibodies against SARS-CoV-2 were detected by a fully-automatic chemiluminescence immunoassay analyser (UniCel DxI800, Beckman Coulter, Inc. USA). The kits were provided by YHLO Biotech Co., Ltd., Shenzhen, China. A cut-off value of >10.0 AU/mL is considered positive for both antibodies.
Study protocol
Rapid detection for COVID-19 was routinely set up by abnormal CT image-guidance combined with SARS-CoV-2 nucleic acid testing. Figure 1 shows a flowchart of the study design and processing. A total of 235 symptomatic suspected COVID-19 subjects and 1172 close contacts underwent analyses of chest CT radiographs, RT-PCR tests for SARS-CoV-2 and routine blood tests. At the first interview they completed a comprehensive questionnaire, including anthropometric data, epidemiological history, clinical symptoms, and workplace. Regardless of their symptoms, HCWs with positive RT-PCR results and/or CT abnormalities were hospitalised or medically observed, and were tested for viral nucleic acid once every 2 days. Serum antibodies were also examined on Day 2 or Day 3 after admission (the first week after onset) and once every week before discharge. Discharge criteria were in accordance with the requirements (NHC, 2020). After discharge, the infected patients were isolated at a designated place for 14 days, as recommended (NHC, 2020). They were followed-up by primary healthcare facilities and re-tested for viral nucleic acid and serum antibodies on Days 7 and 14. After that, they stayed with their families for the second isolation period of 14 days, and re-visited the hospital for detection of viral nucleic acids and antibodies by the end of the second quarantine period. Each patient's medical information was collected during isolation, which was shared with permission.
Figure 1.
Flowchart of the study design and processing.
Abbreviations: ND, no data; CT, computed tomography; RT-PCR, reverse-transcriptase real-time polymerase chain reaction.
Close contacts with normal CT imaging and negative RT-PCR results were mandated to stay quarantined and followed for 14 days. Nasopharyngeal swabs were collected on Days 7 and 14 to detect viral nucleic acids. If any close contact had a positive test for SARS-CoV-2, they were sent to a designated hospital for isolation and medical observation. Furthermore, all close contacts of SARS-CoV-2 positive cases were traced, including their family members and individuals who had contact with the individuals.
Outcome measures
The end point in this study was to analyse the incidence rate, clinical features and outcomes of asymptomatic COVID-19 infections in HCWs.
Statistical analysis
Continuous variables were presented as medians (IQRs) and categorical variables expressed as absolute numbers and percentages. The normality of the distribution of variables was performed using the Kolmogorov–Smirnov test and statistical comparison was performed using a t-test. The χ 2 test was used to compare dichotomous variables. Categorical variables were expressed as number (n/N%) and compared by the χ 2 test, Fisher's exact test or one-way ANOVA. The time to negative RT-PCR test was developed using the Kaplan–Meier method and compared with a log-rank test. A p ˂ 0.05 was considered statistically significant. All statistical analyses were performed using SAS 9.4 software.
Results
Incidence
A total of 1407 HCWs underwent CT scanning and nucleic acid testing due to symptom(s) (n = 235) or being close contacts (n = 1172). Of those, 13.6% (191/1407) cases had abnormal CT imaging and 15.1% (160/1060) were positive for SARS-CoV-2 nucleic acid. Of individuals tested by RT-PCR, 152 were symptomatic and 908 were close contacts. A total of 347 persons only performed chest CT scanning without nucleic acid testing by RT-PCR.
Figure 2 shows the temporal distribution of 191 HCWs with CT abnormalities, in which daily cases with abnormal CT images are plotted according to the date of CT diagnosis. The epidemic curve of onset of infections peaked from 23 January 23 to 03 February and then gradually declined, which was similar to the reported epidemic trends in China (Epidemiology Working Group, 2020). Geographically, early cases of the clustered distribution were in the Department of Oncology, while the other cases were spread in 38 other branches of the hospital.
Figure 2.
Temporal distribution of healthcare workers (HCWs) with CT abnormality.
A COVID-19 outbreak occurred in a teaching hospital, Wuhan, China, 2020. The epidemic curve of onset of infections peaked from 23 January to 03 February and then gradually declined.
Of suspected HCWs, 210 cases with abnormal CT images and/or positive RT-PCR results were admitted to the hospital for isolation and treatment (Figure 1). According to the presence or absence of symptoms, 122 cases were in the symptomatic group and 88 were in the close contact group. They were also classified into a pneumonia group (n = 191) or a non-pneumonia group (n = 19) according to their CT imaging.
Of the 122 symptomatic patients, 107 had both abnormal CT findings and positive RT-PCR results, while 15 cases had only RT-PCR positive testing. Of 88 close contacts, 34 had both CT abnormalities and positive RT-PCR results, 33 had only CT abnormalities and four had only positive RT-PCR results; 17 cases with CT abnormalities were not tested by RT-PCR. The rate of laboratory-confirmed cases was 80.3% (122/152) in the symptomatic group and 4.2% (38/908) in the close contact group (Figure 1 and Table 1 ).
Table 1.
The results of computed tomography (CT), nucleic acid and antibody tests in 1407 healthcare workers.
| Parameters | Symptomatic individuals n = 235 | Close contacts n = 1172 |
|---|---|---|
| Chest CT, N = 1407 | 235 | 1172 |
| Normal CT, n | 128 | 1088 |
| Abnormal CT, n | 107 | 84 |
| SARS-CoV-2 nucleic acid, N = 1060 | 152 | 908 |
| Positive, n (%) | 122 (80.3%) | 38 (4.2%) |
| Symptomatic cases, N | 122 | 0 |
| Mild/moderate, n | 109 | 0 |
| Severe, n | 10 | 0 |
| Critical, n | 3 | 0 |
| Death, n | 2a | 0 |
| Antibody examinations, N = 1060 | 152 | 908 |
| Positive, n (%) | 127 (83.6%) | 88 (9.7%) |
| Asymptomatic infections, N (%) | 0 | 88 (9.7%) |
| Hospitalisation or medical observation, N | 127 | 88 |
The two deaths are classified as critical cases.
Comparison of clinical characteristics
Table 2 summarises the data of the two groups regarding their demographics, laboratory results, clinical features, and outcomes. There was no significant difference between the two groups in terms of gender; however, the median age of symptomatic severe and critical cases was higher than the mild/moderate cases or the close contacts. The severe/critical patients on admission in the symptomatic group had a progressive decrease in counts of peripheral leukocytes and lymphocytes, and a significant increase in CRP and D-dimer. Those counts in the close contact group were in the normal range and were not significantly different compared with the results of the mild cases in the symptomatic group. On admission of the symptomatic patients and the close contacts with pneumonia, the predominant CT imaging patterns were consistent with the results of studies (Guan et al., 2020, Shi et al., 2020).
Table 2.
Demographics, laboratory results and outcomes of infectious cases associated with SARS-CoV-2.
| Parameters | Symptomatic group (n = 122) |
Close contact group (n = 88) |
p value | ||||
|---|---|---|---|---|---|---|---|
| Mild & Moderate |
Severe & Criticala |
N | IQR or n/N | ||||
| N | IQR or n/N | N | IQR or n/N | ||||
| Age (years) | 109 | 31 (29–31) | 13 | 52 (33–56) | 88 | 31 (30–32) | 0.000 |
| Sex, n (M:F) | 109 | 31:78 | 13 | 5:8 | 88 | 21:67 | 0.493 |
| WBC, ×109/L | 108 | 4.72 (3.75–5.98) | 13 | 4.39 (3.34–6.98) | 57 | 5.38 (4.34–6.63) | 0.068 |
| N, ×109/L | 108 | 2.64 (2.06–3.42) | 13 | 3.54 (2.19–5.61) | 57 | 2.99 (2.42–3.93) | 0.002 |
| L, ×109/L | 108 | 1.37 (0.95–1.85) | 13 | 0.51 (0.31–0.91) | 57 | 1.73 (1.29–2.16) | 0.000 |
| Haemoglobin, g/L | 108 | 126 (116–133) | 13 | 113 (104.5–125.5) | 57 | 129 (122–136) | 0.006 |
| Platelet count, × 109/L | 108 | 205 (160.75–248.75) | 13 | 145 (119–205) | 57 | 223 (191–270) | 0.012 |
| CRP, mg/L | 102 | 1.85 (0.5–9.18) | 12 | 58 (20.9–152.3) | 44 | 0.5 (0.5–4.1) | 0.000 |
| Albumin, g/L | 105 | 39 (36–41) | 12 | 30 (27–33) | 37 | 39 (38–43) | 0.000 |
| ALT, U/L | 106 | 20.5 (13.75–44) | 12 | 58 (25.75–136.25) | 39 | 18 (11–28) | 0.003 |
| AST, U/L | 106 | 21 (17–30.25) | 12 | 42.5 (17.5–68.25) | 39 | 19 (14–23) | 0.119 |
| Alkaline phosphatase, U/L | 106 | 57.2 (46.28–70) | 11 | 80.3 (69.9–108) | 38 | 50.5 (43.15–60.33) | 0.000 |
| Bilirubin, mmol/L | 106 | 11.25 (8.23–14.75) | 12 | 21.1 (11.28–29.24) | 38 | 11.2 (9.18–14.1) | 0.001 |
| Potassium, mmol/L | 106 | 4.13 (3.8–3.36) | 11 | 4.06 (3.3–5.02) | 37 | 4.17 (3.81–4.34) | 0.888 |
| Sodium, mmol/L | 106 | 140 (139–141.23) | 11 | 138.1 (133–144) | 37 | 139.7 (139–141.5) | 0.924 |
| Urea, mmol/L | 106 | 4.06 (3.4–5.1) | 12 | 8.08 (5.85–16.15) | 40 | 3.91 (3.24–4.36) | 0.000 |
| Creatinine, umol/L | 106 | 51 (46–59.25) | 12 | 69 (54.75–77.25) | 40 | 53 (46–60.75) | 0.000 |
| Creatine kinase, U/L | 100 | 56 (40.25–79.25) | 8 | 66 (19.75–214.25) | 10 | 56 (43.25–85.25) | 0.068 |
| LDH, U/L | 100 | 179 (161–215.75) | 10 | 324 (216–578.75) | 33 | 166 (153–202) | 0.000 |
| Ultra-TnI, ng/mL | 27 | 0.006 (0.006–0.006) | 10 | 0.012 (0.006–0.268) | 9 | 0.006 (0.006–0.006) | 0.000 |
| D–dimer, mg/L | 37 | 0.24 (0.15–0.6) | 12 | 3.64 (0.81–16.95) | 20 | 0.26 (0.17–0.35) | 0.039 |
| Fibrinogen, g/dL | 37 | 2.89 (2.3–4.2) | 12 | 4.39 (3.70–6.40) | 20 | 2.46 (1.96–3.01) | 0.134 |
| Prothrombin time, s | 37 | 11.5 (10.95–12) | 12 | 11.05 (10.53–12.8) | 20 | 11.35 (10.53–12) | 0.475 |
| APTT, s | 37 | 29 (26.95–31.6) | 12 | 30.4 (29.45–33.85) | 20 | 28.55 (26.87–28.95) | 0.031 |
| Oximetry saturation, % | 31 | 98 (97–99) | 11 | 88 (84–94) | 8 | 99 (98–99.75) | 0.000 |
| Outcomes | |||||||
| Mild/moderate | 109 | 0 | 88 | 0.003 | |||
| Severe | 0 | 10 | 0 | ||||
| Critical | 0 | 3 | 0 | ||||
| Death | 0 | 2 | 0 | ||||
| Length of hospital stay, day | 109 | 17 (10–21) | 13 | 27 (14.5–42) | 38 | 13.5 (6–26.75) | 0.000 |
| Duration of viral shedding (day) | 109 | 16.8 (7–25.2) | 11b | 28 (20.3–35) | 38 | 16.4 (7–28) | 0.013 |
Data are presented as medians and interquartile ranges (IQR) or n/N (%), where N is the total number of patients with available data. p-Values comparing cases with or without symptoms are from χ2, Fisher's exact test or one-way ANOVA. SARS-CoV-2 = 2019 novel coronavirus.
Abbreviations: WBC, white blood cell; N, Neutrophil; L, Lymphocyte; CRP, C-reactive protein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; LDH, lactate dehydrogenase; Ultra-TnI, hypersensitivity cardiac troponin I; APTT, activated partial thromboplastin time.
Three critical cases were incorporated into the severe type for statistics.
Two cases that died had positive RT-PCR testing at the time of death.
Duration of viral shedding and dynamic changes of antibody levels
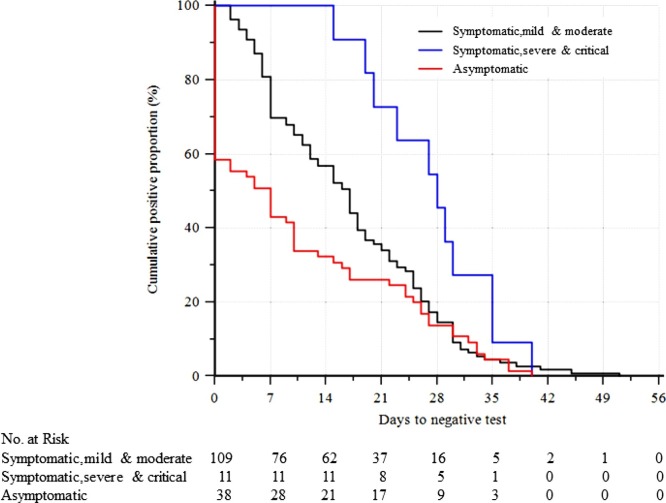
The median (IQR) duration of positive results for RT-PCR analysis after hospital admission was 16.8 (7–25.9) days in 120 cases in the symptomatic group, 16.8 (7–25.2) days in the mild/moderate type (n = 109) and 28 (20.3–35) days in the severe/critical types (n = 11). Two cases that died had positive RT-PCR tests at the time of death. However, 38 asymptomatic individuals had a median (IQR) duration of 16.4 (7–28) days of viral shedding, which was similar to that in the symptomatic mild/moderate group (Table 1). A Kaplan–Meier curve showed that asymptomatic cases cleared the virus at a much earlier time than severe/critical symptomatic patients (Figure 3 ).
Figure 3.
Kaplan–Meier curve showing the time to negative test for symptomatic patients and asymptomatic cases.
The curve shows that asymptomatic cases cleared the virus at a much earlier time than severe/critical symptomatic patients.
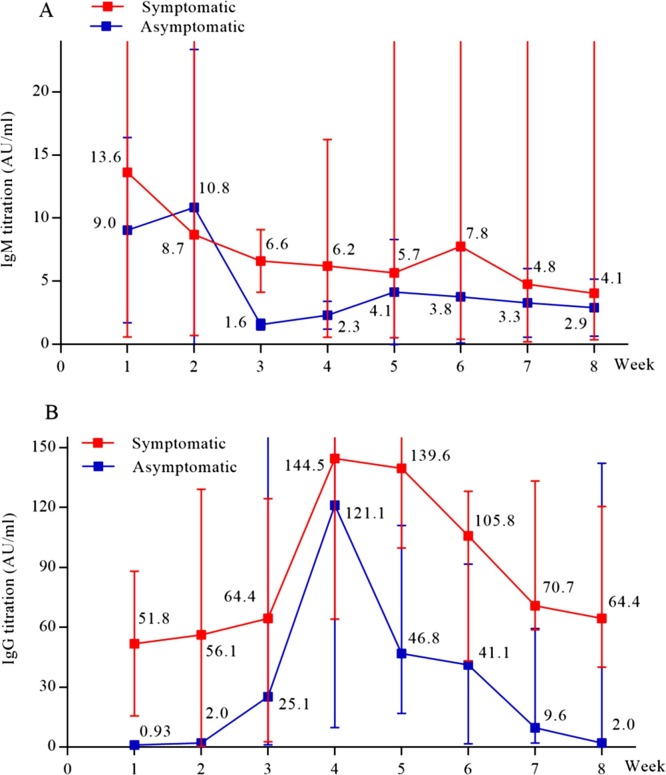
In the course of disease, SARS-CoV-2 specific IgM and IgG in the serum were detectable both in symptomatic and asymptomatic cases, with the titration of specific IgG reaching a ≥four-fold increase during convalescence compared with the acute phase. The positive serological rate in asymptomatic close contacts was 9.7% (88/908). Figure 4 shows temporal changes in the titration of specific antibodies. There was a slight increase in IgM titres at week 1 in the symptomatic group and at week 2 in the asymptomatic group, after which they decreased and remained at a low level. For IgG titres, the symptomatic group significantly increased at week 1 after onset, peaked at week 4, then decreased gradually but remained at a high level at week 8. The asymptomatic group demonstrated a similar seroconversion with more than a four-fold increase in IgG antibody titres at week 4; however, the IgG level decreased below the cut-off value (10 AU/mL) by week 7. Differences in IgG titres between the symptomatic patients and asymptomatic infections were significant for all time points shown (p < 0.05).
Figure 4.
Temporal changes in the titration of specific antibodies against SARS-CoV-2 from illness onset or screening date to convalescence in the symptomatic group and in the asymptomatic group, respectively.
Data show temporal changes of IgM titres (A) and IgG titres (B).
(A) A slight increase of IgM titres occurred at week 1 in the symptomatic group and week 2 in the asymptomatic group, after which the titres decreased and remained at a low level.
(B) Differences in IgG titres between symptomatic patients and asymptomatic infections were significant for all time-points shown (p < 0.05). The symptomatic group significantly increased at week 1, peaked at week 4, then gradually decreased and remained at a high level at week 8. The asymptomatic group demonstrated similar seroconversion with more than a four-fold increase in IgG antibody titres; however, the level decreased below the cut-off value (10 AU/mL) since week 7.
Outcome
Of the 122 symptomatic patients, the percentage of mild/moderate, severe and critical illness was 89.3%, 8.2% and 2.5%, respectively. Two critical cases died: both were re-employed staff members after retirement, one of which was a 61-year-old female and the other was a 67-year-old male with a very severe airflow limitation. As of 29 March 2020, all 120 symptomatic patients were discharged after meeting the discharge criteria (NHC, 2020). The length of hospital stays ranged from 2–51 days, with a median (IQR) of 17 (10–23) days.
Of the 38 confirmed asymptomatic cases, none developed into severe or critical cases during their courses. Eleven cases were pre-symptomatic at the time of screening but later developed symptoms during hospitalisation; four cases remained asymptomatic with normal CT images during hospitalisation and the two periods of 14 days of quarantine. The median (IQR) length of stay in the hospital was 13.5 (6–26.75) days.
Up to 26 April, all symptomatic and asymptomatic cases ended their two periods of 14 days of isolation. None of their close contacts acquired secondary or tertiary infections.
Discussion
In this cohort of HCWs, 9.7% were identified as asymptomatic infections by positive RT-PCR testing and serological results; however, the positive rate of detecting nucleic acid was 4.2%. This finding indicates that a significant level of asymptomatic SARS-CoV-2 shedding may have existed during the epidemic among the hospital population.
Consistent with a report that 41% of the 138 hospitalised patients at a tertiary hospital in Wuhan were hospital-related transmissions (Wang et al., 2020b), a large number of HCWs were infected with COVID-19 in the current cohort. One potential contribution to the disease transmission was close contact with the index patient and the secondary infections early in the outbreak. Cancer patients were allowed to walk around without restrictions and were in close contact with HCWs for examinations and treatments. These findings suggest that serious nosocomial transmission occurred in the early stage of this outbreak and significantly contributed to the widespread nature of the disease (Gandhi et al., 2020).
Many viral infections are associated with asymptomatic, subclinical or very mild symptoms (Al-Tawfiq and Gautret, 2019). Of the 2228 confirmed cases with MERS-CoV 21% were reported to have no or mild symptoms (World Health Organization, 2018). The occurrence of asymptomatic infections was acquired via healthcare-associated transmission (Song et al., 2018) and the possibility of transmission to other individuals was of particular importance in healthcare settings (Al-Tawfiq and Gautret, 2019, Al-Tawfiq and Memish, 2016). Based on serology, 7.5% of exposed HCWs were asymptomatic SARS-CoV-positive cases (Wilder-Smith et al., 2005). MERS-CoV infections among asymptomatic contacts were reported as 1–3.9% (Al-Tawfiq and Gautret, 2019). Based on positive RT-PCR testing and serological results, it was found that asymptomatic infections of SARS-CoV-2 accounted for 9.7% of cases in this study, which was higher than reported results (1.2%) (Epidemiology Working Group, 2020, Wu and McGoogan, 2020). However, the 1.2% was not real-world data, but was extracted from China's Infectious Disease Information System (Wu and McGoogan, 2020). Most observations coming from medical surveillance represent only the symptomatic patients of the total infected population (Birger et al., 2018). The difference in infections between community-acquired and hospital-acquired may be another explanation for the current findings.
This study found that some asymptomatic infections and symptomatic people had pneumonia-like infiltrates on CT imaging, with negative RT-PCR results. However, they produced high levels of specific IgG during convalescence and met the laboratory-confirmed criteria, which suggested that it was not enough for asymptomatic pneumonia by current nucleic acid testing of respiratory specimens. However, CT scans can serve as a rapid assessment method in the COVID-19 pandemic with typical findings (Shi et al., 2020, Ai et al., 2020), permitting an early quarantine and providing valuable clinical clues towards diagnosis. In some cases, chest CT scans have been shown to be more sensitive than RT-PCR analysis, particularly in the earliest stage of infection (likely asymptomatic) (Ai et al., 2020). Certainly, the detection of sample on bronchoalveolar lavage fluid can increase the positive rate of nucleic acid testing. Furthermore, with a titration of a ≥four-fold increase in specific IgG during convalescence than in the acute phase, serologic diagnosis remains an indispensable means for confirming viral infections (Jääskeläinen et al., 2019). The seroprevalence of 9.7% in this cohort of HCWs was much higher than the 0.23% reported in HCWs and close contacts during or after the SARS epidemic (Leung et al., 2006). Due to strict isolation measures of patients, dynamic changes in the titration of specific antibodies can be serially detected (Figure 4). The IgG titres of symptomatic patients increased significantly at week 1 after onset, and peaked at week 4 with more than a four-fold increase. Asymptomatic infections demonstrated a similar seroconversion, but that serological response appeared late and faded fast. These findings are in agreement with the results published by Long et al. (2020). Both showed that the asymptomatic individuals had lower antibody levels relative to the symptomatic cases in the acute phase and reduction in IgG titration during the early convalescent phase. These data might have implications for an immunity strategy for asymptomatic cases who had a weaker immune response to SARS-CoV-2 infection (Long et al., 2020).
From the current limited data, no severe cases of asymptomatic infections were found during the course of the disease. Late symptoms occurred in some cases and the CT images could have changed. However, the results suggest that attention should be paid to the diagnosis of asymptomatic infections, due to their similar duration of viral shedding as mild symptomatic people, lack of clinical symptoms and normal routine laboratory results.
The transmissibility of asymptomatic COVID-19 cases has been studied but without consistent conclusions. Chen et al. (2020b) showed that the transmissibility of asymptomatic cases may be comparable to that of symptomatic patients. However, that contrasts with a study by He et al., which concluded that it could be significantly smaller than that of the symptomatic cases (He et al., 2020). Tertiary infections might not have been found in this cohort, and it is therefore difficult to determine the transmissibility of asymptomatic infections. Since the duration of exposure was likely the main facilitator for spreading SARS-CoV-2 (Park et al., 2020), secondary exposures could be significantly reduced by being fully alert to avoid contact, shorten interactions and comply with self-protection by HCWs. Furthermore, screening all potentially exposed persons was strictly enforced in Wuhan, and any exposed staff were rapidly notified, tested in a timely manner, and strictly isolated and quarantined. The current results confirmed that such control measures for COVID-19 were extremely effective.
The transmission of SARS-CoV-2 from asymptomatic carriers with abnormalities on CT scans (Chan et al., 2020) or normal CT findings (Bai et al., 2020) was previously reported, and it was recently found that asymptomatic cases contributed to SARS-CoV-2 transmission with faster outbreaks in different countries (Park et al., 2020, Arons et al., 2020, Jiang et al., 2020, Pan et al., 2020). Asymptomatic transmission may be the Achilles heel of COVID-19 pandemic control through public health strategies (Wang et al., 2020b). Infection-control strategies that focused only on symptomatic patients were insufficient to prevent transmission. For asymptomatic close contacts, it is critically important to implement strict interventions.
Study limitations
First, this was a single-centre research study. Second, the enrolled participants were from a hospital rather than a community population and, therefore, there were no data about the incidence of asymptomatic infections in the community population. Third, only a crude duration of viral shedding was calculated because nasopharyngeal swab specimens were not analysed on a daily basis.
Conclusions
The findings from this large series of participants in a hospital demonstrated that a high rate of asymptomatic SARS-CoV-2 shedding existed during the outbreak of COVID-19. Managing transmission by asymptomatic carriers is becoming a serious challenge for control of the disease pandemic.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding authors had full access to all data in the study and had final responsibility for the decision to submit for publication.
Contributors
DZ, MMW, YZ, ZSZ, XCL, YTZ, TW, SLZ and WZY collected the epidemiological and clinical data. MW was responsible for data related to the virus. DZ and WHH were responsible for statistical data. DZ and KH drafted the manuscript. KH was responsible for funding, study conception and design, and revising the final manuscript.
Declaration of interests
Dong Zhao contributed equally with Mengmei Wang and Ming Wang. The authors have no competing interest to declare for this study.
Acknowledgments
This study was supported by the Key Technology Project on Novel Coronavirus Pneumonia, Wuhan, China (No. 2020020101010005) and the Science and Technology Key Project on Novel Coronavirus Pneumonia, Hubei province, China (Number 2020FCA002). We thank all the healthcare workers involved in the study.
References
- Ai T., Yang Z., Hou H., Zhan C., Chen C., Lv W. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020;296(2):E32–E40. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Tawfiq J.A., Gautret P. Asymptomatic Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infection: extent and implications for infection control: a systematic review. Travel Med Infect Dis. 2019;27:27–32. doi: 10.1016/j.tmaid.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Tawfiq J.A., Memish Z.A. Drivers of MERS-CoV transmission: what do we know? Expert Rev Respir Med. 2016;10(3):331–338. doi: 10.1586/17476348.2016.1150784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arons M.M., Hatfield K.M., Reddy S.C., Kimball A., James A., Jacobs J.R. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382(22):2081–2090. doi: 10.1056/NEJMoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y., Yao L., Wei T., Tian F., Jin D.Y., Chen L. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323(14):1406–1407. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birger R., Morita H., Comito D., Filip I., Galanti M., Lane B. Asymptomatic shedding of respiratory virus among an ambulatory population across seasons. mSphere. 2018;3(4) doi: 10.1128/mSphere.00249-18. pii: e00249-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.F., Yuan S., Kok K.H., To K.K., Chu H., Yang J. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Wang A., Yi B., Ding K., Wang H., Wang J. The epidemiological characteristics of infection in close contacts of COVID-19 in Ningbo city. Chin J Epidemiol. 2020:41. doi: 10.3760/cma.j.cn112338-20200304-00251. [in Chinese] [DOI] [PubMed] [Google Scholar]
- Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Chin J Epidemiol. 2020;41(2):145–151. [in Chinese] [Google Scholar]
- Gandhi M., Yokoe D.S., Havlir D.V. Asymptomatic transmission, the Achilles’ heel of current strategies to control Covid-19. N Engl J Med. 2020;382(22):2158–2160. doi: 10.1056/NEJMe2009758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- China Medical Treatment Expert Group for Covid-19. Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He D., Zhao S., Lin Q., Zhuang Z., Cao P., Wang M.H. The relative transmissibility of asymptomatic COVID-19 infections among close contacts. Int J Infect Dis. 2020;94:145–147. doi: 10.1016/j.ijid.2020.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jääskeläinen A.J., Korhonen E.M., Huhtamo E., Lappalainen M., Vapalahti O., Kallio-Kokko H. Validation of serological and molecular methods for diagnosis of zika virus infections. J Virol Methods. 2019;263:68–74. doi: 10.1016/j.jviromet.2018.10.011. [DOI] [PubMed] [Google Scholar]
- Jiang X.L., Zhang X.L., Zhao X.N., Li C.B., Lei J., Kou Z.Q. Transmission potential of asymptomatic and paucisymptomatic SARS-CoV-2 infections: a three-family cluster study in China. J Infect Dis. 2020;221(12):1948–1952. doi: 10.1093/infdis/jiaa206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung G.M., Lim W.W., Ho L.M., Lam T.H., Ghani A.C., Donnelly C.A. Seroprevalence of IgG antibodies to SARS-coronavirus in asymptomatic or subclinical population groups. Epidemiol Infect. 2006;134(2):211–221. doi: 10.1017/S0950268805004826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Q.X., Tang X.J., Shi Q.L., Li Q., Deng H.J., Yuan J. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020 doi: 10.1038/s41591-020-0965-6. [Online Ahead of Print] [DOI] [PubMed] [Google Scholar]
- National Health Commission of the People’s Republic of China . 2020. Diagnosis and treatment of novel coronavirus infected pneumonia (trial 7th edition) [EB/OL] http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989/files/ce3e6945832a438eaae415350a8ce964.pdf. [Google Scholar]
- Pan X., Chen D., Xia Y., Wu X., Li T., Ou X. Asymptomatic cases in a family cluster with SARS-CoV-2 infection. Lancet Infect Dis. 2020;20(4):410–411. doi: 10.1016/S1473-3099(20)30114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.Y., Kim Y.M., Yi S., Lee S., Na B.J., Kim C.B. Coronavirus disease outbreak in call center, South Korea. Emerg Infect Dis. 2020;26(8):1666–1670. doi: 10.3201/eid2608.201274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothe C., Schunk M., Sothmann P., Bretzel G., Froeschl G., Wallrauch C. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382:970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaman J., Morita H., Birger R., Boyle M., Comito D., Lane B. Asymptomatic summertime shedding of respiratory viruses. J Infect Dis. 2018;217(7):1074–1077. doi: 10.1093/infdis/jix685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Han X., Jiang N., Cao Y., Alwalid O., Gu J. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20(4):425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y.J., Yang J.S., Yoon H.J., Nam H.S., Lee S.Y., Cheong H.K. Asymptomatic Middle East Respiratory Syndrome coronavirus infection using a serologic survey in Korea. Epidemiol Health. 2018;40:e2018014. doi: 10.4178/epih.e2018014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder-Smith A., Teleman M.D., Heng B.H., Earnest A., Ling A.E., Leo Y.S. Asymptomatic SARS coronavirus infection among healthcare workers, Singapore. Emerg Infect Dis. 2005;11(7):1142–1145. doi: 10.3201/eid1107.041165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . 2018. Middle East respiratory syndrome coronavirus (MERS-CoV). WHO MERS global summary and assessment of risk. August 2018. http://www.who.int/csr/disease/coronavirus_infections/risk-assessment-august-2018.pdf?ua=1. [Google Scholar]
- Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China. N Engl J Med. 2019;382(8):727–733. doi: 10.1056/NEJMoa2001017. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]