Graphical abstract
Abbreviations: IPF, Idiopathic pulmonary fibrosis; COVID-19, Coronavirus Disease 2019; TCM, Traditional Chinese Medicine; PDDS, Pulmonary drug delivery system; TGF, Transforming growth factor; α-SMA, alpha smooth muscle actin; HYP, Hydroxyproline; TNF-α, Tumor necrosis factor-α; INF-γ, Increasing interferon-γ; Smad3, Sekelsky mothers against dpp3; TIMP-1, Tissue inhibitor of metalloproteinases-1; ECM, Extracellular matrix; Nrf2, Nuclear factor erythroid 2-related factor 2; NOX4, Nicotinamide adenine dinucleotide phosphate oxidase 4; MAPK, Mitogen-activated protein kinase; PI3K, Phosphatidylinositol 3 kinase; AKt, Protein kinase B; EMT, Epithelial-mesenchymal transition; LPO, Lipid peroxidation; HSP90, Heat shock protein 90; VASH, Vasohibin; HLF, Human lung fibroblasts; CUR, Curcumin; MMP, Matrix metalloproteinase; QE, Quercetin; HELF, Human embryonic lung fibroblast; SphK1, Sphingosine kinase 1; S1P, sphinogosine-1-phosphate; GA, Gambogic acid; PDGF, Platelet-derived growth factor; FGF, fibroblast growth factor; ASV, Astragaloside IV; Col III, Collagen III; LN, Laminin; HA, Hyaluronic acid; FOXO3a, Forkhead box O3a; SAB, Salvianolic acid B; JNK, c-Jun amino-terminal kinase; ACE-2, Angiotensin-converting enzyme-2; ANG-(1-7), Angiotensin-(1-7); IL-6, Interleukin-6; HSP-47, Heat shock protein-47; MDA, Malondialdehyde; GSH, Glutathione; ERK, Extracellular signal-regulated kinases; MDI, Metered dose inhalers; PMDI, Pressurized metered-dose inhalers; NEB, Nebulizer; DPI, Dry powder inhalers
Keywords: Traditional Chinese medicine, Pulmonary drug delivery system, COVID-19, Idiopathic pulmonary fibrosis
Highlights
-
•
Pathogenesis and characteristics of idiopathic pulmonary fibrosis (IPF) are presented.
-
•
The history and current situation of traditional Chinese medicine (TCM) in treating lung diseases are introduced.
-
•
Therapeutic mechanisms of different TCM to treat IPF are summarized.
-
•
Advantages and types of pulmonary drug delivery systems (PDDS) are emphasized.
-
•
Combining TCM with PDDS is a potential strategy to treat IPF.
Abstract
Idiopathic pulmonary fibrosis (IPF) is a progressive pulmonary interstitial inflammatory disease of unknown etiology, and is also a sequela in severe patients with the Coronavirus Disease 2019 (COVID-19). Nintedanib and pirfenidone are the only two known drugs which are conditionally recommended for the treatment of IPF by the FDA. However, these drugs pose some adverse side effects such as nausea and diarrhoea during clinical applications. Therefore, it is of great value and significance to identify effective and safe therapeutic drugs to solve the clinical problems associated with intake of western medicine. As a unique medical treatment, Traditional Chinese Medicine (TCM) has gradually exerted its advantages in the treatment of IPF worldwide through a multi-level and multi-target approach. Further, to overcome the current clinical problems of oral and injectable intakes of TCM, pulmonary drug delivery system (PDDS) could be designed to reduce the systemic metabolism and adverse reactions of the drug and to improve the bioavailability of drugs. Through PubMed, Google Scholar, Web of Science, and CNKI, we retrieved articles published in related fields in recent years, and this paper has summarized twenty-seven Chinese compound prescriptions, ten single TCM, and ten active ingredients for effective prevention and treatment of IPF. We also introduce three kinds of inhaling PDDS, which supports further research of TCM combined with PDDS to treat IPF.
1. Introduction
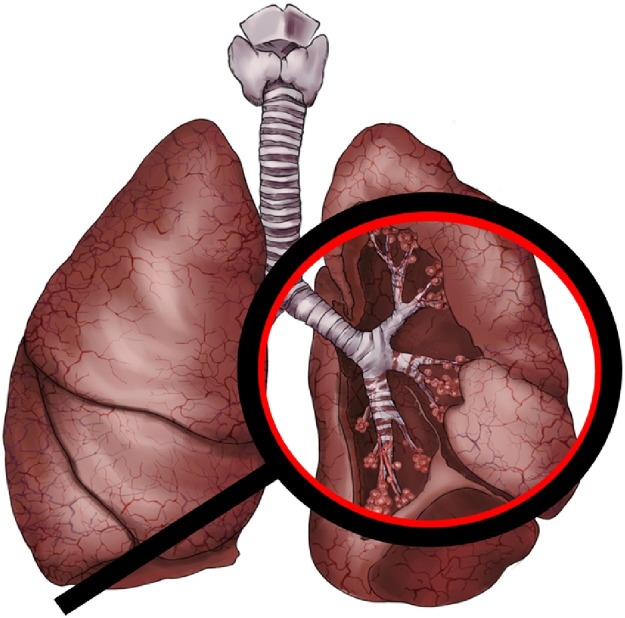
IPF is a chronic and irreversible lung disease with diffuse alveolitis leading to structural damage to the alveoli, as shown as Fig. 1 . The etiology of IPF is unclear, with high incidence among the elderly and the condition progressively worsens with old age. [1]. The median survival is only 2–5 years after diagnosis. The symptoms in the early stage are not visible, which mainly manifests as cough and sputum, and the dyspnea will gradually accentuate in the later stages. Further, the pulmonary function deteriorates and fibrosis leads to acute respiratory failure and death [2]. The mortality rate of IPF is higher than that of most tumors, so it sometimes identified as tumor-like disease [3].
Fig. 1.
Diffuse alveolitis and damage to alveolar structure in IPF.
As of July 2020, more than ten million people have been diagnosed with COVID-19 [4]. The Guideline on Diagnosis and Treatment of Coronavirus Disease 2019 (Trial Version 7th) officially issued by National Health Commission of the People’s Republic of China indicated that pulmonary interstitial fibrosis might occur among patients in the severe stages of COVID-19. The first anatomy report of the patient with COVID-19 indicated that IPF and its complications were not as severe as those observed in patients SARS (the genetic sequence homology with 2019-nCoV is over 85 %), but the severe pulmonary fibrosis still remained [5].
Glucocorticoids [6], immunosuppressive agents [7] and antioxidants [8] are used in combination with western medicine, which reduce the early lung injury and fibrosis by targeting growth factors and cytokines. But they can also only slow down decline lung function rather than reverse the process of fibrosis, and there are many toxic and side effects associated with this treatment strategy [9,10]. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: Treatment of Idiopathic Pulmonary Fibrosis recommended conditional administration of only two drugs, Nintedanib and Pirfenidone [11]. However, their effects are not ideal and may produce many complications such as nausea, diarrhea, dyspepsia and rash. [12]. Currently, lung transplantations are the only effective western medicine treatment strategy, however, it is difficult to achieve effective treatment for IPF due to its complexity, management and limited supply of donor organs [13].
Fortunately, TCM has a long history in treating epidemics and accumulated rich experience, which plays a specific advantage in the current process of treating IPF. TCM considers that IPF is the obstruction of qi and the stagnation of blood circulation, so IPF needs to be managed from the aspects of qi and blood circulation [14]. Clinically, many TCMs have been identified to be efficient as anti-fibrosis compounds and also seems to improve lung function along with reduction of dyspnea. Routine clinical treatment of IPF using TCM mainly include decoctions [15], pills, granules [16] and injections [17]. But all of these have limitations, such as poor drug tolerance, inefficient targeted therapy effect, low bioavailability in lungs and hepatoenteric first-pass effect [18].
PDDS can accurately deliver drugs to the lungs and produce a local or systemic therapeutic effect in a non-injectable route. Additionally, this route can avoid the absorption and metabolism of drugs by liver and intestine [19]. Besides, the lungs have a large surface area (about 100−140 m2), rich capillary network and thin alveolar epidermal cell layer, which act as unique advantages for systemic delivery.
PDDS has been widely used in the treatment of lung diseases as a unique way of administration [20], and have received much attention in the recent decade. On the one hand, this paper summarizes, some useful TCM compounds, single TCM, and active ingredients by consulting a large number of documents, which provides ideas and directions for the clinical therapy of IPF. On the other hand, the application of PDDS as a targeted treatment of lung diseases such as IPF can improve the bioavailability of drugs, reduce the toxic and side effects, which have potential application value.
2. The long history of TCM in the treatment of IPF
In the ancient kinds of literatures of TCM, there was no disease name corresponding to IPF. However, due to its clinical manifestations of cough, expectoration, chronic, progressive aggravation of dyspnea and recurrent symptoms, it was classified as "feiwei"(means pulmonary fistula) and "feibi" (means pulmonary arthralgia) by TCM.
"Feiwei" was first appeared in Zhang Zhongjing's (CE 150-CE 215) Jinkui Yaolue (the earliest extant book on diagnosis and treatment of miscellaneous diseases in China), which put forward that "Maimendong Decoction" was the primary treatment for deficiency heat of "feiwei", and "Gancao Ganjiang Decoction" was used to treat deficiency cold of "feiwei". In the Tang Dynasty (CE 618-CE 907), Sun Simiao (CE 541-CE 682) used "Shengjiang Gancao Decoction" to treat asthenia cold in Qianjin Yaofang • Feiwei. Zhu Danxi of the Yuan Dynasty (CE 1271-CE 1368) believed that "feiwei" were mainly concerned with nourishing blood, nourishing lungs and nourishing qi. In the Ming Dynasty (CE 1368-CE 1644), the Mingyi Zhizhang (a comprehensive medical book) put forward the principle of treatment of "feiwei". Li Yongcui's syndrome differentiation of "feiwei" in Qing Dynasty (A.D.1636-A.D.1912) concluded that they used "Erdi Erdong Decoction" to nourish yin, and add "Mendong Qingfei Drink" to put it over.
"Feibi" was first appeared in Suwen·Yuji Zhenzang Lun of Huangdi 's Internal Classic, but there was no prescription record of treating "feibi" in it. It was not until the Song Dynasty (A.D.960-A.D.1279) that there was the first prescription for "feibi" in Songji Zonglun, such as "Danggui Decoction", orange peel pill, almond pill, etc. In the Ming Dynasty, "Shengmaisan Jiawei", "Xie Bai San" and "Renshen Pingfei San" were created in Zheng Yin Mai Zhi. The "Shigao Decoction" mentioned in Pujifang. "Wubi Decoction" was mentioned in Zhengzhi Zhunsheng and "Feibi Decoction" recorded in Bianzheng Lun of the Qing Dynasty supplemented the treatment of "feibi".
3. Compound prescriptions of TCM and single TCM in the IPF treatment
TCM treatment is based on syndrome differentiation. It is continuous and slow which has advantages like low toxicity, multi-level and multi-target. Different from western medicine, TCM has more comprehensive ideas and methods in the treatment of IPF, such as staged treatment, prescription treatment, collateral treatment and acupuncture combined with internal and external treatment.
At present, most of the TCM clinical researches take invigorating qi and promoting blood circulation as the principles of formulating prescription. They use decoction, Chinese patent medicine and TCM injections to perform treatment in patients at different stages of IPF by adjusting the match of the monarch, minister, assistant and guide in TCM. Through the collection and research of a large number of literatures, twenty-seven compound prescriptions of TCM with good curative effect for the treatment of IPF are shown in Table 1 [[21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47]].
Table 1.
Prescription drugs for idiopathic pulmonary fibrosis treatment.
| Prescription | Dosage form | References | Prescription | Dosage form | References |
|---|---|---|---|---|---|
| Fufang Biejia Ruangan Pills | Pills | [21] | Ciwujia Injection | Injection | [35] |
| Jinkui Shenqi Wan | Pills | [22] | Danhong Injection | Injection | [36] |
| Dahuang Zhechong Wan | Pills | [23] | Danshen Injection | Injection | [37] |
| Qishen Yiqi Dripping Pills | Pills | [24] | Chuan xiong qin Injection | Injection | [38] |
| Yangyin Yifei Tongluo Wan | Pills | [25] | Shenmai Injection | Injection | [39] |
| Biejia Jianwan | Pills | [26] | Baihe Gujin Decoction | Decoction | [40] |
| Baihe Gujin Wan | Pills | [27] | Qingzao Jiufei Decoction | Decoction | [41] |
| Yangyin Qingfei Wan | Pills | [28] | Maimendong Decoction | Decoction | [42] |
| Danlou Prescription | Pills | [29] | Kangfuxin Oral liquid | Oral liquid | [43] |
| Kangxian Yifei capsules | Capsules | [30] | Jinbei Oral liquid | Oral liquid | [44] |
| Ke Fei Ning capsules | Capsules | [31] | Shengmai San | Granules | [45] |
| Ke Chuan Kang | Capsules | [32] | Yiqi Huoxue Granules | Granules | [46] |
| Gancao Suangan Injection | Injection | [33] | Qingxuan Granules | Granules | [47] |
| Re du ning Injection | Injection | [34] |
3.1. Danlou prescription
Danlou Prescription (DLP) is developed from the TCM formula Gualou Xiebai Decoction [48]. It is composed of ten herbs: Trichosanthes kirilowii Maxim (Gualoupi), Allium macrostemon Bunge, Puerariae Lobatae Radix, Salvia miltiorrhiza Bunge, Astragalus mongholicus Bunge, Davallia trichomanoides Blume, Paeonia lactiflora Pall, Alisma plantago-aquatica L., Ligusticum chuanxiong Hort and Curcuma aromatica Salisb. Danlou tablet is a national-level new drug. Modern research shows that it has a wide range of pharmacological effects, such as anti-myocardial ischemia, anti-inflammatory, antioxidant and improvement of blood lipid metabolism [49].
Shao et al. [50] found that DLP could alleviate bleomycin (BLM)-induced IPF by inhibiting the TGF signalling activated myofibroblast diff ;erentiation and α-SAM expression. Besides, DLP could not only regulate endocytosis-related genes and alveolar macrophage activation, but also regulate myofibroblast differentiation and collagen secretion-related genes. Therefore, DLP can simultaneously inhibit the pro-inflammatory and pro-fibrotic pathways. It has a good development prospect in the treatment of IPF.
3.2. Yangqing kangxian formula
The main compositions of Yangqing Kangxian Formula (YKF) include Ophiopogon japonicas, Adenophorae Ae Radix, Panax quinquefolius Radix, Trichosanthes kirilowii Maxim, Fritillariae thunbergii Bulbus and Radix Paeoniae Rubra.
Li et al. [51] summarized that BLM induced rats’ lungs might occur inflammatory cells infiltration, collagen deposition and HYP level elevation, and the YKF can regulate the release of certain inflammatory factors, such as decreasing TNF-α and IFN-γ in the lungs of BLM-induced rats.
3.3. Shenmai kaifei san
Shenmai Kaifei San (Shenks) is a TCM preparation with the functions of "tonifying qi and yin". The ingredients of this recipe include Panax quinquefolius L., Ophiopogon japonicus (L. f.) Ker-Gawl., Salvia miltiorrhiza Bunge, Gynostemma pentaphyllum (Thumb.) Makino, Perillafrutescens, Amygdalus Communis Vas, Scutellaria baicalensis Georgi, Desmodium styracifolium (Osbeck.) Merr. and Perilla frutescens (L.) Britt..
The prescription conforms to the standard of "monarch, minister, assistant and guide" in TCM. Panax quinquefolius L. is the "monarch" medicine, which has the main therapeutic effect. Ophiopogon japonicus (L. f.) Ker-Gawl., Salvia miltiorrhizae Bunge and Gynostemma pentaphyllum (Thumb.) Makino are "minister" medicines, and they are the secondary ingredients that used to enhance or supplement the main ingredients. The other ingredients are "assistant" and "guide" components of the formula, which mainly treat the accompanying symptoms [52]. Chu et al. [53] found that Shenks could reduce the phosphorylation level of Smad3 and Smad-binding element activity, thereby inhibiting the activity of TGF-α signalling.
Single herbs used in IPF treatment are shown in Table 2 [[54], [55], [56], [57], [58], [59], [60], [61], [62], [63]]. Under the guidance of the theory of TCM, compound preparations and single TCM act on all aspects of lung disease treatment through multiple channels and multiple targets, especially show the unique advantages in the treatment of IPF.
Table 2.
Single TCM used in IPF treatment.
| Single TCM | Efficacy in TCM | References |
|---|---|---|
| Rheum palmatum L. | It can inhibit TIMP-1 expression, reduce collagen production and ECM deposition. |
[54] |
| Curcuma longa L. | It can inhibit type I collagen synthesis and deposition, and TGF-β1 expression. | [55] |
| Salvia miltiorrhiza Bunge | It can inhibit Smad-dependent signalling and Smad-dependent MAPK pathway, and restore the balance of Nrf2-NOX4. | [56] |
| Angelica sinensis (Oliv.) Diels | It can down-regulate the expression of proinflammatory cytokines TNF-α and TGF-β1 to inhibit the process of radiation-induced IPF. | [57] |
| Astragalus mongholicus Bunge | It can inhibit TGF-β1/PI3K/Akt-induced FOXO3a process of hyperphosphorylation and down-regulate EMT reversal in the fibrosis. |
[58] |
| Rhodomyrtus tomentosa (Ait.) Hassk. | It can inhibit inflammation, reduce pulmonary fibrosis and HP, and affect LPO and catalase activity. | [59] |
| Tripterygium wilfordii Hook. f. | It can regulate the transition of epithelial cells to mesenchymal and fibroblasts cells through the inhibition of HSP90. | [60] |
| Garcinia hanburyi Hook. f. | It can regulate VASH-2/VASH-1 and inhibit the TGF-β1/Smad3 | [61] |
| pathway reversed the proliferation of EMT and EndoMT and HLF-1 in vitro. |
||
| Rhodiola rosea L. | It can inhibit phosphorylation of Smad3 caused by reduced TGF-β1. | [62] |
| Glycyrrhiza uralensis Fisch. | It can adjust the oxidation/antioxidation balance when the level of lipid peroxidase decreases and the level of catalase increases in lung tissue. | [63] |
4. Active components of TCM in the IPF treatment
The active components of TCM refer to a certain chemical component extracted, separated, and purified from a single medicine or a compound prescription of TCM. It can be expressed by molecular formula and structural formula, and has certain pharmacological activity and therapeutic effect. This paper summarized mainly ten active ingredients of TCM from a large number of studies, which have significant therapeutic effects on IPF.
4.1. Curcumin
Curcumin (CUR), an active ingredient isolated from turmeric, is a diketone compound which is a rare pigment in the plant kingdom. The main chains of it are unsaturated esters and aromatic groups, so it has low solubility in the water. Studies have found that CUR has many pharmacological activities such as anti-inflammatory [64], antibacterial [65], anti-oxidation [66], hypolipidemic [67], anticancer [68] and anti-fibrosis properties [69], etc. It has low toxicity and small adverse reactions.
CUR can inhibit the differentiation of lung fibroblasts driven by TGF-β2 into myofibroblasts, and it is effective for treating IPF [70,71]. On the other hand, CUR can also inhibit the development of mouse IPF model by reducing the activity of MMP-9. It is a potentially effective active ingredient for the treatment of IPF [72].
4.2. Quercetin
Quercetin (QE) is the most common flavonoid in plants, it can be extracted and separated from vegetables, fruits and Chinese herbs. QE has the advantages of wide sources, low price, high safety and few adverse reactions. It has a variety of pharmacological effects, such as antitumor [73], antiviral [74], anti-inflammatory [75], anti-oxidant [76], anti-thrombotic [77], etc.
Zhang [78] found that QE could ameliorate BLM-induced pulmonary fibrosis and TGF-β-induced fibrosis in HELF cells through inhibiting SphK1/S1P signalling. And Veith’s [79] study indicated that the characteristic of IPF is a disorder of pulmonary redox balance associated with inflammation. And QE, as an exogenous antioxidant, can effectively restore redox disorders by increasing the expression of Nrf2 and Nrf2-regulated genes.
4.3. Gambogic acid
Gamboge is a dry resin secreted by Garcinia hanburyi Hook.f. in Southeast Asia, and Gambogic acid (GA) is the main active ingredient of it. Studies have found that GA has anti-tumor cell proliferation [80], anti-inflammatory [81], anti-bacterial [82] and neuroprotective effects [83]. As a pure natural Chinese herbal medicine, GA has the advantages of low toxicity and low residue, and it is not easy to develop drug resistance.
Qu et al. [84] found that GA could adjust the rate of VASH-/VASH-2, inhibit TGF-β1 and CoCl2 stimulated HLF-1 proliferation with reduction of PDGF and FGF-2 in vitro. Therefore, GA can be used as a new multi-target drug for the early and fibrotic treatment of IPF.
4.4. Astragaloside IV
Astragaloside IV (ASV) is the main active ingredient of the dried roots of the legume perennial herb Mongolian Astragalus or Astragalus membranaceus. It has the functions of immunoregulation [85], anti-inflammatory [86], anti-fibrosis [87] and regulating apoptosis [88]. Meanwhile, it can improve lipid metabolism, reduce blood viscosity and improve renal function.
Li et al. [89] found that ASV could reduce the level of Col III, LN, HA and HYP in lung tissue homogenate. Qian et al. [90] concluded that ASV could inhibit TGF-β1/PI3K/Akt-induced FOXO3a hyperphosphorylation and downregulate epithelial-mesenchymal transition’s role of the process in fibrosis.
4.5. Salvianolic acid B
Salvianolic acid B (SAB) is obtained from the roots and rhizomes of Salvia miltiorrhiza Bunge. SAB has anti-oxidation [91] and protection functions of brain damage [92], especially plays an essential role in protecting cardiovascular.
Liu et al. [93] confirmed in the experiment that SAB exerted a therapeutic effect by inhibiting cell infiltration, destruction of alveolar structure and collagen deposition of IPF. He also pointed out that SAB through inhibiting Smad-dependent signalling and Smad-independent MAPK pathway to inhibit TGF-induced myofibroblast differentiation of MRC-5 cells and TGF-mediated epithelial-mesenchymal transition of A549 cells. Another study confirmed the anti-pulmonary fibrosis effect of SAB through the following aspects. First, the protective effect of it on oxidative stress in vitro fibrosis was verified. Then Westblot and PCR results showed that SAB induced Nrf2 nuclear translocation in vitro. Finally, immunohistochemical results showed that SAB treatment could increase Nrf2 expression in lung tissue [94].
4.6. Gallic acid
Gallic acid is considered to be a polyphenol compound extracted from plants such as Rheum palmatum L. and Eucalyptus robusta Smith, and it is the purest natural polyphenol compound. Gallic acid has a variety of biological activities such as anti-inflammatory [95], antibacterial [96], etc.
Gallic acid has preventive and therapeutic effects on cardiovascular diseases, neurological diseases, diabetes, liver fibrosis, and tumors, which are strictly related to its anti-inflammatory and antioxidant activities. It provides a broad prospect for the treatment of diseases [97].
Chen et al. [98] verified that Gallic acid mediated hydrogen peroxide formation was a promoter of the JNK signal pathway, which led to the activation of human tumor suppressor gene p53 and the apoptosis of mouse pulmonary fibroblasts to treat IPF. Rong [99] concluded that Gallic acid derivative could reduce the activation of embryonic development to some extent and exerted its effect through TGF-1/Smad2 signalling pathway and balancing NOX4/Nrf2, which has an excellent therapeutic effect on IPF.
4.7. Tanshinone IIA
Tanshinone IIA (TSIIA) is a representative type of fat-soluble diterpenoid active ingredient extracted from Salvia miltiorrhiza Bunge. It is fuchsia needle-like crystal, and is insoluble or slightly soluble in water. By reviewing the literature, it was concluded that TSIIA has the following pharmacological activities, such as anti-inflammatory, anti-oxidant, etc. It is a potentially effective drug to improve blood microcirculation and prevent cerebrovascular diseases [100,101].
He et al. [102] found that administration of TSIIA could reduce BLM-induced rat embryonic cell infiltration, the release of embryonic cytokines and excessive collagen deposition. In addition, TSIIA could also inhibit BLM-induced abnormal oxidation and NO production in rats. Another study of Wu [103] found that TSIIA could reduce TGF-β overexpression, and reverse the reduction of ACE-2 and ANG-(1−7) in lung tissue. That is, TSIIA has protective effects on IPF.
4.8. Emodin
Emodin is mainly derived from Polygonaceae and is the main active ingredient of Reynoutria japonica Houtt. and Rheum palmatum L.. Pharmacological studies showed that emodin has antibacterial, antiviral, antitumor and liver protection effects [104,105].
The antiviral mechanism of Emodin is inhibiting the virus's absorption and penetration process, thereby preventing the virus from replicating. At present, it has become a broad-spectrum antiviral drug component. Studies have shown that Emodin has an inhibitory effect on SARS virus by blocking the interaction between S-protein and ACE-2 protein, and it could inhibit S-protein retrovirus infection. So it was considered as a potential principal therapeutic agent for the treatment of the SARS [106].
In the treatment of IPF, Emodin can reduce pulmonary oedema and fibrosis, decrease the collagen deposition, and inhibit the infiltration of myofibroblasts and inflammatory cells. At the same time, Emodin can also reduce TNF-α, IL-6, TGF-β1 and HSP-47 levels in lung tissue after BLM treatment [107].
4.9. Andrographolide
Andrographis paniculata (Burm. F.) Nees is an Acanthaceae Andrographis plant, and the main active ingredient is Andrographolide (AND). It has many pharmacological effects such as anti-inflammatory, anti-viral, antibacterial, anti-cancer. Since the last century, Andrographis paniculata has been widely used to treat sore throats, flu, and upper respiratory infections [[108], [109], [110]].
AND can inhibit oxidative stress, reduce MDA content and increase the GSH ratio. It also can improve the BLM-mediated changes in the ratio of MMP-1/TIMP-1 [111]. Therefore, AND has a potential therapeutic effect on preventing IPF.
4.10. Resveratrol
Resveratrol (RES) is a non-flavonoid polyphenol compound which is an antitoxin produced by many plants when they are stimulated. It is mainly found in the peels of grapes, peanuts and mulberries. RES has a wide range of pharmacological effects. In addition to anti-cardiovascular diseases [112], it has important effects on anti-cancer [113], antibacterial [114] and anti-inflammatory [115].
Studies have shown that RES played a protective role in BLM-induced IPF by reducing oxidative damage and fibrosis, and it could inhibit the remodelling of vascular smooth muscle cells and the growth, and proliferation of cardiac fibroblasts. Fagone [116] concluded that RES could inhibit the expression of α-SMA at both the mRNA and protein levels, and it could attenuate the deposition of collagen, as well as show the effect of inhibiting TGF-β-induced phosphorylation of ERK1/2 and Akt.
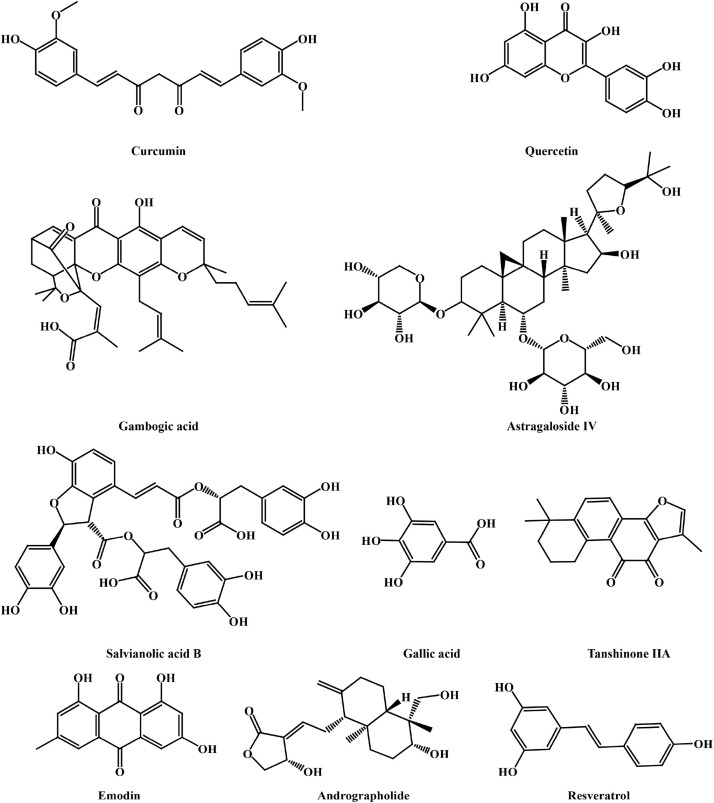
The structures of the above active ingredients of TCM are shown in Fig. 1. And the mechanisms of them in treating IPF are summarized in Table 3 .
Table 3.
Active components of TCM for IPF and machanisms.
| Active components | Source | Mechanisms | References |
|---|---|---|---|
| CUR | Curcuma longa L. | It can reduce the activity of MMP-9 and inhibit the differentiation of lung fibroblasts driven by TGF-β2 into myofibroblasts. |
[70,71,72] |
| QUE | Begonia dryadis Irmsch. | It can reduce the level of S1P in lung tissue and HELF cells, as well as the SphK1 and degradation enzyme S1PL, and restore redox disorders. | [78,79] |
| GA | Garcinia hanburyi Hook. f. | It can attenuate epithelial-mesenchymal transition, inhibit the proliferation of HLF-1 stimulated and reduce PDGF and FGF-2 expression. | [84] |
| ASV | Astragalus mongholicus Bunge | It can reduce the level of Col III, LN, HA and HYP. And inhibit TGFβ1/PI3K/Akt-induced FOXO3a hyperphosphorylation and downregulate epithelial-mesenchymal transition. | [89] |
| SAB | Salvia miltiorrhiza Bunge | It can inhibit Smad-dependent signalling and Smad-independent MAPK pathway, increase Nrf2 expression in lung tissue. | [93,94] |
| Gallic acid | Rhus chinensis Mill. | It can activate p53 and induce apoptosis of fibroblasts and transform growth factor1/Smad2 signalling pathway and balance NOX4 / Nrf2. | [97,98,99] |
| TSIIA | Salvia miltiorrhiza Bunge | It can inhibit abnormal oxidation and NO production and reduce TGF-β | [102,103] |
| overexpression, reverse the reduction of | |||
| ACE-2 and ANG- (1−7). | |||
| Emodin | Rheum palmatum L. | It can decrease the collagen deposition and inhibit the TNF-α, infiltration of myofibroblasts and inflammatory cells, reduce IL-6, TGF-β1 and HSP -47 levels. |
[106,107] |
| AND | Andrographis paniculata (Burm.Nees F.) | It can inhibit oxidative stress and increases the GSH/GSSG ratio, and improve the change of MMP-1/TIMP-1 ratio. | [111] |
| RES | Reynoutria japonica Houtt. | It can inhibit the expression of α-SMA, and attenuate the deposition of collagen and inhibit TGF-β-induced phosphorylation of ERK1/2 and Akt. | [116] |
In recent years, with the continuous in-depth study of the pathological mechanism of pulmonary fibrosis and the exploration of treatment methods, it has been concluded that the pathogenesis of IPF mainly involves inflammation, immunity, oxidative stress and other pathways, and it closely related to inflammatory factors, cytokines, chemokines and growth factors. The treatment of IPF is a slow and gradual process of multi-factor, multi-link and multi-path interaction. TCM active components have multiple targets and multiple mechanisms of pharmacological activity. Except for the above active ingredients, such as flavonoids (Baicalin [117], Hydroxysaffl [118]), terpenes (Triptolide [119], Paclitaxel [120]), glycosides (Paeoniflorin [121], Ginsenoside [122]) and alkaloids (Matrine [123], Isoliensinine [124]) also have shown different degrees of preventive or therapeutic effects on IPF, and involved multiple protective mechanisms.
Although many TCM active ingredients play a functional role in the treatment of IPF, they also have limitations. For example, CUR has many functions such as anti-inflammatory, scavenging free radicals and anti-pulmonary fibrosis in the treatment of lung diseases. Still, when it is treated directly, it has low solubility, poor stability and rapid metabolism in the body which leads to low blood concentration. They all make the lesions difficult to maintain adequate drug concentration [125]. Another example is the poor absorption of SAB, the drug prototype and metabolites are mainly excreted through the bile, where has apparent hepato-enteric circulation, and the protein binding rate is high, all of which lead to SAB low oral bioavailability [126]. These shortcomings make the TCM still have many clinical problems in the treatment of IPF.
Fortunately, PDDS provides an effective way to make the drug concentrate in the lesion and enhance drug bioavailability, increase drug stability and reduce the first-pass effect, and it has a potentially essential meaning in the clinical researches for the treatment of IPF.
5. Pulmonary drug delivery system for TCM to treat IPF
The PDDS refers to a drug delivery method which allows direct entry of the drugs by breathing in with the aid of a unique delivery device and exert local or systemic treatment. Its unique physiological structure can reduce lung metabolism after the drug is absorbed, and directly pass to the blood and thus avoid the first-pass effect of the liver [127,128]. Although, till now, there have been no definitive or effective drugs that have been developed for the treament of COVID-19, many scholars have explored some feasible solutions for the treatments through PDDS [129]. For example, Sai [130] conceived a salinomycin nanostructured lipid carrier, which exerts its antiviral effect through inhalation. And Isabella [131] designed delivery of fenretinide into the lungs at a high concentration using PDDS, to efficiently utilize the anti-inflammatory effect of fenretinide (Fig. 2 ).
Fig. 2.
Chemical structures of the active components in TCM.
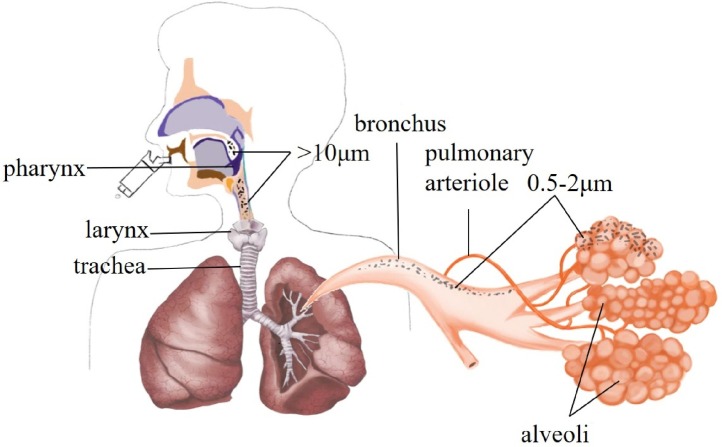
The particle size of the lung inhaled particles will affect the deposition form and location of the drug in the lungs. Large particles (>10 μm) are deposited in the throat and upper respiratory tract. Small particles (0.5–2.0 μm) are deposited in the respiratory bronchioles and alveoli wall. 80 % particles (<0.5 μm) are exhaled from the body due to Brownian motion. Particle with a size of 1.0–3.0 μm have the highest sedimentation rates in bronchioles and alveoli, and are generally selected as the main component of pulmonary inhalation preparations [132,133]. The position of particles of varied size inhaled through PDDS in lungs are shown in Fig. 3 .
Fig. 3.
The picture of the particle size and position of inhaled particles in the PDDS.
There have been some clinical applications to treat IPF by PDDS in western medicine. For example, Rasool [134] prepared an ultrasonic nebulizer of pirfenidone, and Vartiainena with l-leucine coated tilorone dry particles [135], which all provide the basis for the treatment of IPF.
The inhalation of TCM has a long history in China. As early as in the Huangdi's Internal Classic, there has been a record of treatment through nasal administration. A large number of records in the Compendium of Materia Medica of the Ming Dynasty used inhalation of TCM to treat cough, headache, malaria, etc. Wu Shangxian's Theoretical Essays indicated that inhalation of hot tea through the respiratory tract could treat sore throat. All of these studies have administered treatment strategies directly into the bloodstream through the respiratory tract to exert a therapeutic effect. And, modern TCM includes those also used for local treatment of the lungs and the respiratory tract by pulmonary inhalational ways, such as Shuanghuanglian aerosol, aerosolized Houttuynia cordata preparation, and they all show the advantages of PDDS in TCM [136].
The WHO and other health organizations in thedeveloped countries have recommended lung drug therapy as the first choice for respiratory diseases such as asthma and chronic obstructive pulmonary disease (COPD). They are now gradually being used for systemic drugs such as insulin. There are three main types of pulmonary inhalation: metered dose inhalers (MDI), nebulizer (NEB) and dry powder inhalers (DPI) [137]. The strengths, weaknesses and methods of the three pulmonary inhalation techniques are summarized in Table 4 .
Table 4.
Advandages, disadvantages and instructions of MDI, NEB and DPI.
| Species | Advantages | Disadvantages | Instructions | References |
|---|---|---|---|---|
| MDI | Multiple dose | Requires patient synergy | mouth type / mask type | [138,139,140] |
| Easy to use | Requires propellant | |||
| cheap price | Easy to cough | |||
| Easy to carry | Big side effects | |||
| NEB | Simple operation | Needs atomizing pump and power | mouth type /mask type | [143,144,145] |
| High inhalation | Not easy to carry | |||
| High drug deposition concentration | High cost | |||
| No irritant | Low efficiency | |||
| DPI | Low synergy required | Flow-dependent | mouth type | [148,149,150,151,152,153] |
| No propellant, no irritation | more expensive than MDI | |||
| Suitable for macromolecular drugs | Susceptible to moisture | |||
| Large delivery dose, few side effects | Different devices |
5.1. MDI
MDI refers to mixing a drug solution or suspension with a suitable propellant to form a mixed liquid, which is packaged together in a pressure tank with a specific valve device. The propellant pressure is used to spray the liquid in the container into an aerosol. Quantitative pMDI has become a widely used form of non-injected pulmonary administration method in recent years due to its effectiveness, low cost, and relatively simple process of use [138].
But the drug crystals suspended in the propellant may form larger floc, which will affect the stability and uniformity of the drug. Therefore, surfactants (such as oleic acid or lecithin) are usually added to the suspension to reduce the agglomeration of particles [139]. The triggering of pMDI needs to be matched with the patient's breathing. TTherefore, the elderly and little children who cannot provide sufficient inspiratory flow rates have poor coordination issues associated with the use of the device [140]. To overcome these difficulties, it is usually used in conjunction with a spacer. A mini mist reservoir designed by Anderson [141] increased the transmission time and distance between the pMDI actuator and the patient's mouth, and it allowed the aerosol particle size to be reduced. This not only can slow down the spray speed and facilitate inhalation, but can also guarantee the complete evaporation of the propellant, and excipients can minimize the particle size of the mist and improve the efficiency of lung delivery.
Liu et al. [142] prepared ligustrazine combined with nebulized inhaled budesonide to treat IPF by inhibiting the migration and activation of inflammatory cells, division and proliferation of human fibroblasts, and promoting apoptosis and inhibiting collagen production. Compared with drug administration by injection, the drug is deposited in the alveoli in the form of aerosol after inhalation by atomization, forming a higher drug concentration in the lung tissue which exerts a strong anti-inflammatory effect with a strong local influence, and the advantages of administration are low dosage and few adverse reactions.
5.2. NEB
NEB refers to dissolution of the drug substance in water or dispersing it in the corresponding medium to prepare an aqueous solution or suspension, and then using a nebulizer to convert the liquid into an aerosol of the required size. These aerosols can be successfully inhaled into the respiratory tract to reach the lungs for a local or systemic effect [143].
NEB has the advantage over MDI that it does not require the use of propellant, which avoids the aggregation of drug particles in the propellant and improves the stability of the drug. It can deliver high drug doses, and the operation is simple, and it is suitable for patients of any age or emergency. But it also has drawbacks, as it allows only single dosage and requires prolonged treatment time, complicated and bulky device, a nebulizer pump and power supply, and is very expensive [144]. The therapeutic effect of atomized inhalation is related to many factors, such as the size of inhaled particles, the performance of the atomizer and patient compliance [145]. The current atomizers are divided into three categories: jet atomizers, ultrasonic atomizers and vibrating screen atomizers [146].
Su et al. [147] prepared an NEB of TET-HP-β-CD, which used tetrandrine to prevent the fibroblasts proliferation, collagen synthesis and reverse fibrosis to treat IPF. Compared to the injection, it can reduce systemic exposure of the drug and toxic and side effects, reduce the number of administrations and increase patient compliance.
5.3. DPI
DPI refers to the preparation and utilization of solid micronized drug substance alone or mixed with a suitable carrier, using a special DPI device to actively inhale the atomized drug into the lungs without synergy to produce a local or systemic therapeutic effect. This method allows administration of high dosage of the inhaled product with dosages ranging from less than 10 μg to more than 20 mg [148].
One of the biggest advantages of DPI is that it does not require the use of propellants compared with MDI, and thus, reducing the ecological damage. It also has superior advantages such as high administration dose, multiple drugs deliveries at the same time, high inhalation efficiency, less auxiliary materials, excellent stability, no need for hand lung inhalation coordination, noninvasive and easy to use [149].
It is necessary for DPI drug delivery devices to be protected against moisture absorption. Inhalation devices have a significant impact on the inhaled dose, efficacy and clinical effectiveness of DPI. With the improvement of micronization technology and the development of DPI devices, the inhalation device has developed from the original capsule type, vesicle type, to a reservoir type drug delivery device [150,151].
There are two potential problems in DPI. One is the limitation of carrier types, which only lactose having been approved to be used for inhalation administration as excipient by FDA at present. So the problems associated patients’ lactose intolerance and lactose allergy need to be solved. Second, because the size of micronized particles are too small, the cohesion between the powder particles is too strong (such as van der Waals force, electrostatic adsorption, etc.), the dispersion efficiency and fluidity of particles are low [152,153].
To solve the above problems, it is possible to adjust the types of excipients in the prescription or use new carriers and modify them to improve the performance of the formulation and the physical and chemical properties of the drugs, increase the solubility of the drugs in the cells, and increase the stability of the medicines, prolong the residence time of the drugs in the lung and improve the bioavailability of the drug. For example, Elisa et al. [154] prepared a paclitaxel-loaded dry powder nanocomposite microparticle which improved the dispersion of the drug after it reached the lungs and increased the active uptake by the cells. V. Levet [155] used a high-pressure homogenization method to compress cisplatin to micron size and mix it with lipids to prepare a DPI preparation, which increased the exposure of the drug in the lungs and reduces systemic toxicity.
However, there have been some studies and reports on the treatment of IPF. Hu et al. [156] prepared curcumin macroporous micro powder DPI with dependence on curcumin’s mechanism of action such as inhibiting the activation of the upstream pathway of TGF-β1 to inhibit the release of type Ⅰcollagen, reducing the level of TNF-α and thereby reducing the release of proinflammatory cytokines to achieve local diminishment of IPF. Another example is the use of salvianolic acid and tanshinone powder mixture as a DPI [157], which mainly reduced BLM-induced IPF by inhibiting the TGF-β1 signaling pathway. Further, it was found that compared with oral and intravenous injection, DPI can avoid the first-pass effect of the liver and enterohepatic circulation, improve bioavailability and patient compliance, and ensure the efficient release of drugs in the lungs.
DPI has many obvious advantages in exerting drug effects and reducing side effects, especially in improving the bioavailability of peptide and protein macromolecular drugs [158,159]. A survey found that 40 % of patients with asthma and chronic obstructive pulmonary disease in Europe use DPI [160,161]. The development and progress of DPI provides a more effective clinical treatment approach for PDDS, and also provides ideas for the utilization of TCM in the treatment of IPF.
5.4. Limitations of PDDS
Although the PDDS shows increased targeting, stability and high bioavailability in the treatment of local or systemic diseases, there are many issues that needs to be addressed: (1) There are few kinds of excipients approved by FDA for the preparation of pulmonary drug inhalation devices. The safety research process of new excipients is long and complicated, so the excipients have certain limitations in the PDDS. (2) The complexity and diversity of PDDS also causes inconvenience to patients. Matching the ideal inhalation device for different categories of patients is also a problem yet to be solved [162]. (3) For drugs such as proteins and polypeptides, long-term administration may lead to safety problems such as lung injury and toxicity. Therefore, using the PDDS to treat IPF and other lung diseases, it is necessary to conduct in-depth studies from inside and outside the body to ensure a safer and more effective use of PDDS to treat IPF and other lung diseases [163].
6. Conclusions and future perspectives
IPF is a progressive pulmonary interstitial inflammatory disease of unknown cause, which mostly is prevalent among the elderly. Presently, the treatment strategies using western medicine can only alleviate the disease process but cannot reverse it. Additionally, they also cause many other adverse reactions. As an only effective way, lung transplantation also has certain limitations. With the outbreak of the COVID-19, the emergence of complications like IPF among severely sick patients made it one of the urgent problems that needed to be prevented and treated effectively. TCM is an ideal drug development strategy through multi-level and multi-target differentiation and treatment. Through the screening of some effective compound prescriptions, herbs and active ingredients and even combined drug therapy, which in combination with lung inhalation targeted drug delivery system improves drug targeting, safety, effectiveness and have become an effective way to treat IPF.
The unique physiological structure of the lungs provides convenience for PDDS, and the targeted therapy by inhaling effective drugs into the lungs in the form of aerosols is the main treatment for lung diseases currently. This article aims at assessing the use of TCM with PDDS, so that high concentration of drugs can be concentrated on the lesion which would allow achievement of an effective treatment for IPF. As the most widely used dosage form in PDDS, DPI also has its limitations. Therefore, there is also need for development of potential treatment strategies which are much safer and more effective inhalation preparations by either changing the prescription or modifying the drugs for the treatment of IPF.
Author contributions
Yukun Zhang conceived the manuscript and figures.
Yukun Zhang and Peng Lu wrote the manuscript.
Yueling Zhang made the figures.
Huan Qin and Xunan Song reviewed and edited the manuscript.
Zhidong Liu supervised and edited the manuscript.
Declaration of Competing Interest
The authors declare no conflict of interest for publishing this manuscript.
Acknowledgments
This work was supported by Scientific Research Project of Tianjin Municipal Education Commission (number: 2019KJ083).
References
- 1.Selman M., Pardo A. The leading role of epithelial cells in the pathogenesis of idiopathic pulmonary fibrosis. Cell. Signal. 2020;66:109482. doi: 10.1016/j.cellsig.2019.109482. [DOI] [PubMed] [Google Scholar]
- 2.Aryal S., Nathan S.D. An update on emerging drugs for the treatment of idiopathic pulmonary fibrosis. J. Expert Opin. Emerg. Drugs. 2018;23(2):159–172. doi: 10.1080/14728214.2018.1471465. [DOI] [PubMed] [Google Scholar]
- 3.Zhao C.P., Li H., Liu X.H. Dissecting the underlying pharmaceutical mechanism of Danggui Buxue decoction acting on idiopathic pulmonary fibrosis with network pharmacology. Tradit. Med. Res. 2020;5(4):238–251. [Google Scholar]
- 4.Guo Y.R., Gao Q.D., Hong Z.S. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak-an update on the status. Mil. Med. Res. 2020;7(1):11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang J., Wang B.J., Yang J.C., Wang M.Y. Advances in the research of mechanism of pulmonary fibrosis induced by Corona Virus Disease 2019 and the corresponding therapeutic measures. Chin. J.Burns. 2020;36(0):E006. doi: 10.3760/cma.j.cn501120-20200307-00132. [DOI] [PubMed] [Google Scholar]
- 6.Chennakesavulu S., Mishra A., Sudheer A. Pulmonary delivery of liposomal dry powder inhaler formulation for effective treatment of idiopathic pulmonary fibrosis. J. Asian J. Pharm. Sci. 2018;13(1):91–100. doi: 10.1016/j.ajps.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naccache J.M., Montil M., Cadranel J. Study protocol: exploring the efficacy of cyclophosphamide added to corticosteroids for treating acute exacerbation of idiopathic pulmonary fibrosis; a randomized double-blind, placebo-controlled, multi-center phase III trial (EXAFIP) J. BMC Pulm. Med. 2019;19(1):75. doi: 10.1186/s12890-019-0830-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fusco R., Cordaro M., Genovese T. Adelmidrol: a new promising antioxidant and anti-inflammatory therapeutic tool in pulmonary fibrosis. J. Antioxid. (Basel) 2020;9(7):601. doi: 10.3390/antiox9070601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Q., Zhou Y., Feng F.C., Zhou X.M. Effectiveness and safety of chinese medicine for idiopathic pulmonary fibrosis: a systematic review and meta-analysis. J. Chin. J. Integr. Med. 2019;25(10):778–784. doi: 10.1007/s11655-017-2429-5. [DOI] [PubMed] [Google Scholar]
- 10.Bahri S., Ben Alic R., Abidia A., Jameleddine S. The efficacy of plant extract and bioactive compounds approaches in the treatment of pulmonary fibrosis: a systematic review. J. Biomed. Pharmacother. 2017;93:666–673. doi: 10.1016/j.biopha.2017.06.052. [DOI] [PubMed] [Google Scholar]
- 11.Pang L.J., Liu J.P., Lv X.D. Comparative effectiveness of 3 Traditional Chinese Medicine treatment methods for idiopathic pulmonary fibrosis: a systematic review and network meta-analysis protocol. J. Med. (Baltim.) 2019;98(30):e16325. doi: 10.1097/MD.0000000000016325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo J., Li B., Wu W.B. Chinese herbal medicines compared with N-Acetylcysteine for the treatment of idiopathic pulmonary fibrosis: a systematic review of randomized controlled trials. J. Evid Based Complement Alternat Med. 2019;2019:5170638. doi: 10.1155/2019/5170638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schäfer S.C., Funke-Chambour M., Berezowska S. Idiopathic pulmonary fibrosis-epidemiology, causes, and clinical course. J. Pathologe. 2020;41(1):46–51. doi: 10.1007/s00292-019-00747-x. [DOI] [PubMed] [Google Scholar]
- 14.Zhang S.X., Wu H., Liu J. Medication regularity of pulmonary fibrosis treatment by contemporary traditional Chinese medicine experts based on data mining. J. Thorac. Dis. 2018;10(3):1775–1787. doi: 10.21037/jtd.2018.03.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu X.L., Zhang Y.X., Yang X.H., Zhang X.M. The influence of BuqiHuoxueTongluo formula on histopathology and pulmonary function test in bleomycin-induced idiopathic pulmonary fibrosis in rats. J. Evid Based Complement Alternat Med. 2018;2018:8903021. doi: 10.1155/2018/8903021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qing Y.X., Guang Y.S., Yang X. Traditional Chinese medicine in the treatment of idiopathic pulmonary fibrosis based on syndrome differentiation: study protocol of an exploratory trial. J. Integr. Med. 2020;18(2):163–168. doi: 10.1016/j.joim.2019.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Xin L.L., Jiang M., Zhang G., Gong J.N. Efficacy and safety of Danhong injection for idiopathic pulmonary fibrosis: Meta-analysis. J. Chin. Materia Medica. 2016;1(20):3859–3865. doi: 10.4268/cjcmm20162024. [DOI] [PubMed] [Google Scholar]
- 18.Lu P., Xing Y., Xue Z.F. Pharmacokinetics of salvianolic acid B, rosmarinic acid and Danshensu in rat after pulmonary administration of Salvia miltiorrhiza polyphenolic acid solution. Biomed. Chromatogr. 2019;33(8):e4561. doi: 10.1002/bmc.4561. [DOI] [PubMed] [Google Scholar]
- 19.Zhang G.L., Mo S.Y., Fang B.R., Zeng R., Wang J. Pulmonary delivery of therapeutic proteins based on zwitterionic chitosan-based nanocarriers for treatment on bleomycin-induced pulmonary fibrosis. J. Int. J. Biol. Macromol. 2019;133:58–66. doi: 10.1016/j.ijbiomac.2019.04.066. [DOI] [PubMed] [Google Scholar]
- 20.Gandhimathi Chinnasamy, Venugopal Jayarama Reddy, Sundarrajan Subramanian, Sridhar Radhakrishnan, Tay Samuel Sam Wah, Ramakrishna Seeram, Kumar Srinivasan Dinesh. Breathable medicine: pulmonary mode of drug delivery. J. Nanosci. Nanotechnol. 2015;15:2591–2604. doi: 10.1166/jnn.2015.10341. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y.Q., Mao X., Chen W.J. A discovery of clinically approved formula FBRP for repositioning to treat HCC by inhibiting PI3K/AKT/NF-kappa B activation. Mol. Ther-Nucl. Acids. 2019;19:890–904. doi: 10.1016/j.omtn.2019.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song J.P., Li W., Li R.Q. Effects of different TCM prescriptions of JinKuiYaoLve on the levels of Noradrenaline(NE), Dopamine(DA) and 5-hydroxytryptamine(5-HT) in lung and brain tissues of pulmonary fibrosis in the early stages. Chin. J. Tradit. Chin. Med. Pharm. 2009;24(5):568–571. [Google Scholar]
- 23.Song J.P., Xie Z.L., Li W. Influence of Dahuang Zhechong Pills on neurotransmitter in the lung and brain tissues in the formation stage of pulmonary fibrosis of rats. J. Tradit. Chin. Med. 2011;52(19):1676–1678. [Google Scholar]
- 24.Geng S.H., Shan Q.Y., Xu M.X. Effect of Qishen Yiqi droplet on adenylate contents in myocardium of cor pulmonale rats. Chin. J. Chin. Mater. Med. 2017;42(1):170–174. doi: 10.19540/j.cnki.cjcmm.20161222.022. [DOI] [PubMed] [Google Scholar]
- 25.Li Z.H., Dong R., Xin F.R. Observation of curative effect of Yangyin Yifei Tongluo Wan on patients with idiopathic pulmonary fibrosis. J. Liaon. Univ. Tradit. Chin. Med. 2015;17(11):163–165. [Google Scholar]
- 26.Liu X., Chen J., Gu F.H. Study on the improvement effect of Biejiajian pills on paraquat-induced pulmonary fibrosis in rats. World Clin. Drug. 2016;37(3) 160-165+198. [Google Scholar]
- 27.Zhou Z.G., Zhou S., Wang S.Q. Baihe Gujin Pill in the treatment of 20 cases of idiopathic pulmonary fibrosis. Hunan J. Tradit. Chin. Med. 2006;2006(05):15–16. [Google Scholar]
- 28.Zhang Z., Tang Y.Q., Yu B.C. Chemical composition database establishment and metabolite profiling analysis of Yangyin qingfei decoction. Biomed. Chromatogr. 2019;33(9):e4581. doi: 10.1002/bmc.4581. [DOI] [PubMed] [Google Scholar]
- 29.Gao L.N., Zhou X., Lu Y.R. Dan-lou prescription inhibits foam cell formation induced by ox-LDL via the TLR4/NF-κB and PPARγ signaling pathways. J. Front. Physiol. 2018;9:590. doi: 10.3389/fphys.2018.00590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao Q.P., Wang Y., Xue X.M., Guan W. Influences of Kangxian Yifei Capsules on lung histopathological changes and serum TGF-β1 and TNF-αin rats with pulmonary fibrosis. J. Beijing Univ. Tradit. Chin. Med. (Clin. Med.) 2012;19(06):16–19. [Google Scholar]
- 31.Wang Z.X., Yu J.Y., Liu M.Z. Clinical observation on 70 cases of idiopathic pulmonary interstitial fibrosis treated with kefeining capsule. J. Emerg. Tradit. Chin. Med. 2009;18(02) 188-189+216. [Google Scholar]
- 32.Xu Y.L., Qu N.N., Ma L.J., Zhao K.M. Effects of Kechuankang on the expression of TGF-β1 and its receptor mRNA in the lung tissue of rats with pulmonary fibrosis induced by bleomycin A5. Liaon. J. Tradit. Chin. Med. 2009;36(01):145–147. [Google Scholar]
- 33.Dai C.J., Zhang B. The therapeutic effect of compound glycyrrhizin combined with prednisone on idiopathic pulmonary fibrosis. J. Med. Innov. China. 2012;9(29):6–7. [Google Scholar]
- 34.Jiang C.H., Zhong R.L., Zhang J., Wang X.X. Reduning injection ameliorates paraquat-induced acute lung injury by regulating AMPK/MAPK/NF-κB signaling. J. Cell. Biochem. 2019;120(8):12713–12723. doi: 10.1002/jcb.28540. [DOI] [PubMed] [Google Scholar]
- 35.Li R. Acanthopanax senticosus injection for 26 cases of idiopathic pulmonary fibrosis. J. Tradit. Chin. Med. Chin. Materia Medica Jinlin. 2003;23(10):14–15. [Google Scholar]
- 36.Xin L.L., Jiang M., Zhang G., Gong J.N. Efficacy and safety of Danhong injection for idiopathic pulmonary fibrosis: Meta-analysis. J. Tradit. Chin. Med. Chin. Materia Medica. 2016;41(20):3859–3865. doi: 10.4268/cjcmm20162024. [DOI] [PubMed] [Google Scholar]
- 37.Cheng C.H. Efficacy evaluation of compound Danshen injection combined with hormone in the treatment of idiopathic pulmonary fibrosis. J. Asia-Pacific Tradit. Med. 2017;13(08):134–135. [Google Scholar]
- 38.Huang C.L., Wu X., Wang S.P., Wang W.G. Salvia miltiorrhiza combination of and ligustrazine attenuates bleomycin-induced pulmonary fibrosis in rats via modulating TNF-α and TGF-β. J. Chin Med. 2018;13:36. doi: 10.1186/s13020-018-0194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luo X.F., Zhu L.X. Efficacy of Shenmai injection combined with tetrandrine on pneumoconiosis pulmonary fibrosis. J. Shenzhen J. Integ. Tradit. Chin. West. Med. 2018;28(23):46–48. [Google Scholar]
- 40.Zhong W., Wang H. Clinical observation on treatment of idiopathic pulmonary fibrosis with integrated chinese and western medicine. J. North Pharm. 2011;08(10):41. [Google Scholar]
- 41.Wang T., Lin S., Liu R. Acute lung injury therapeutic mechanism exploration for Chinese classic prescription Qingzao Jiufei Decoction by UFLC-MS/MS quantification of bile acids, fatty acids and eicosanoids in rats. J. Pharm. Biomed. Anal. 2020;189:113463. doi: 10.1016/j.jpba.2020.113463. [DOI] [PubMed] [Google Scholar]
- 42.Yang L., Zhu Z.H., Qi Z.H. Comparative analysis of the chemical consistency between the traditional and mixed decoction of Maimendong Decoction by Ultra-Performance Liquid Chromatography coupled to quadrupole with Time-of-Flight Mass Spectrometry (UPLC-QTOF-MS)-based chemical profiling approach. J. Chromatogr. Sci. 2020;58(6):549–561. doi: 10.1093/chromsci/bmz104. [DOI] [PubMed] [Google Scholar]
- 43.Yao H., Wei S.J., Xiang Yo.J. βKangfuxin oral liquid attenuates bleomycin-induced pulmonary fibrosis via the TGF-1/Smad Pathway. J. Evid Based Complement Alternat Med. 2019;2019:5124026. doi: 10.1155/2019/5124026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang C.H., Cui Q.H., Tian J.Z. Effect of Jinbei oral liquid on bleomycin-induced pulmonary fibrosis in rats. J. Pharmacol. Clin. Chin. Materia Medica. 2018;34(06):146–150. [Google Scholar]
- 45.Qi J.H., Li J.P., Ren D.M. The role of Pingfeng Shengmai Powder in improving the cellular immune function in patients with idiopathic pulmonary fibrosis of both qi and yin deficiency. J. World Latest Med. Inform. 2019;19(13):153–154. [Google Scholar]
- 46.Qu N.N., Qin Y.B., Zheng X. Therapeutic effect of Yiqi Yangyin Huoxue Granule on connective tissue disease associated interstitial lung disease, deficiency of both qi and Yin and blood stasis syndrome. Chin. Arch. Tradit. Chin. Med. 2020:1–11. [Google Scholar]
- 47.Liu B., Lü W.H., Ge H.T., Tang H.T., Li R.S., Zhang C.F. Protective effect of the traditional chinese patent medicine qing-xuan granule against bleomycin-induced pulmonary fibrosis in mice. J. Chem. Biodivers. 2019;16(12):e1900467. doi: 10.1002/cbdv.201900467. [DOI] [PubMed] [Google Scholar]
- 48.Yan L.L., Zhang W.Y., Wei X.H. Gualou xiebai decoction, a traditional chinese medicine, prevents cardiac reperfusion injury of hyperlipidemia rat via energy modulation. J. Front Physiol. 2018;9:296. doi: 10.3389/fphys.2018.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang L., Mao S., Qi J.Y. Effect of Danlou Tablet on peri-procedural myocardial injury among patients undergoing percutaneous coronary intervention for non-ST elevation acute coronary syndrome: a study protocol of a multicenter, randomized, controlled trial. Chin. J. Integr. Med. 2015;21(9):662–666. doi: 10.1007/s11655-015-2284-1. [DOI] [PubMed] [Google Scholar]
- 50.Shao R., Wang F.J., Ming Lyu. Ability to suppress TGF-β-Activated myofibroblast differentiation distinguishes the anti-pulmonary fibrosis efficacy of two danshen-containing chinese herbal medicine prescriptions. J. Front Pharmacol. 2019;10:412. doi: 10.3389/fphar.2019.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li M., Li Y., Li J.S. κLong-term effects of TCM yangqing kangxian formula on bleomycin-induced pulmonary fibrosis in rats via regulating nuclear Factor-B signaling. J. Evid. Based Complement Alternat Med. 2017;2017:2089027. doi: 10.1155/2017/2089027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yuan R., Lin Y. Traditional Chinese medicine: an approach to scientific proof and clinical validation. J. Pharmacol. Ther. 2000;86(2):191–198. doi: 10.1016/s0163-7258(00)00039-5. [DOI] [PubMed] [Google Scholar]
- 53.Chu H.Y., Shi Y., Jiang S.A. Treatment effects of the traditional Chinese medicine Shenks in bleomycin-induced lung fibrosis through regulation of TGF-beta/Smad3 signaling and oxidative stress. Sci. Rep. 2017;7:2252. doi: 10.1038/s41598-017-02293-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tian S.L., Yang Y., Liu X.L., Xu Q.B. Emodin attenuates bleomycin-induced pulmonary fibrosis via anti-inflammatory and anti-oxidative activities in rats. Med. Sci. Monit. 2018;24:1–10. doi: 10.12659/MSM.905496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rodriguez L.R., Bui S.N., Beuschel R.T. Curcumin induced oxidative stress attenuation by N-acetylcysteine co-treatment: a fibroblast and epithelial cell in-vitro study in idiopathic pulmonary fibrosis. Mol. Med. 2019;2(1):27. doi: 10.1186/s10020-019-0096-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peng L.Y., An L., Sun N.Y. Salvia miltiorrhiza restrains reactive oxygen species-associated pulmonary fibrosis via targeting Nrf2-Nox4 redox balance. J. Am. J. Chin. Med. 2019;47(5):1113–1131. doi: 10.1142/S0192415X19500575. [DOI] [PubMed] [Google Scholar]
- 57.Qian W., Cai X., Qian Q., Wang D., Zhang L. Angelica sinensis polysaccharide suppresses epithelial-mesenchymal transition and pulmonary fibrosis via a DANCR/AUF-1/FOXO3 regulatory Axis. Aging Dis. 2020;11(1):17–30. doi: 10.14336/AD.2019.0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qian W.B., Cai X.R., Qian Q.H. Astragaloside IV modulates TGF-β1-dependent epithelial-mesenchymal transition in bleomycin-induced pulmonary fibrosis. J. Cell. Mol. Med. 2018;22(9):4354–4365. doi: 10.1111/jcmm.13725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Samareh Fekri M., Mandegary A., Sharififar F. Protective effect of standardized extract of Myrtus communis L. (myrtle) on experimentally bleomycin-induced pulmonary fibrosis: biochemical and histopathological study. Drug Chem. Toxicol. 2018;41(4):408–414. doi: 10.1080/01480545.2018.1459670. [DOI] [PubMed] [Google Scholar]
- 60.Divya T., Velavan B., Sudhandiran G. Regulation of transforming growth Factor-β/Smad-mediated epithelial-mesenchymal transition by celastrol provides protection against bleomycin-induced pulmonary fibrosis. Basic Clin. Pharmacol. Toxicol. 2018;123(2):122–129. doi: 10.1111/bcpt.12975. [DOI] [PubMed] [Google Scholar]
- 61.Qu Y.B., Zhang G.H., Ji Y.X., Zhua H.B. Protective role of gambogic acid in experimental pulmonary fibrosis in vitro and in vivo. Phytomedicine. 2016;23(4):350–358. doi: 10.1016/j.phymed.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 62.Zhang K., Si X.P., Huang J. Preventive effects of Rhodiola rosea L. On bleomycin-induced pulmonary fibrosis in rats. Int. J. Mol. Sci. 2016;17(6):879. doi: 10.3390/ijms17060879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Samareh Fekri M., Poursalehi H.R., Sharififar F. The effects of methanolic extract of Glycyrrhiza glabra on the prevention and treatment of bleomycin-induced pulmonary fibrosis in rat: experimental study. J. Drug Chem. Toxicol. 2019:1–7. doi: 10.1080/01480545.2019.1606232. [DOI] [PubMed] [Google Scholar]
- 64.Chainoglou E., Hadjipavlou-Litina D. Curcumin analogues and derivatives with anti-proliferative and anti-inflammatory activity: structural characteristics and molecular targets. J. Expert Opin. Drug Discov. 2019;14(8):821–842. doi: 10.1080/17460441.2019.1614560. [DOI] [PubMed] [Google Scholar]
- 65.Moghadamtousi S.Z., Kadir H.A., Hassandarvish P. A review on antibacterial, antiviral, and antifungal activity of curcumin. J. Biomed. Res. Int. 2014;2014:186864. doi: 10.1155/2014/186864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kukongviriyapan U., Apaijit K., Kukongviriyapan V. Oxidative stress and cardiovascular dysfunction associated with cadmium exposure: beneficial effects of curcumin and tetrahydrocurcumin. Tohoku J. Exp. Med. 2016;239(1):25–38. doi: 10.1620/tjem.239.25. [DOI] [PubMed] [Google Scholar]
- 67.Panahi Y., Ahmadi Y., Teymouri M. Curcumin as a potential candidate for treating hyperlipidemia: a review of cellular and metabolic mechanisms. J. Cell. Physiol. 2018;233(1):141–152. doi: 10.1002/jcp.25756. [DOI] [PubMed] [Google Scholar]
- 68.Tomeh M.A., Hadianamrei R., Zhao X.B. A review of curcumin and its derivatives as anticancer agents. Int. J. Mol. Sci. 2019;20(5):1033. doi: 10.3390/ijms20051033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hu Y.Z., Li M., Zhang M.G. Inhalation treatment of idiopathic pulmonary fibrosis with curcumin large porous microparticles. Int. J. Pharm. 2018;551:212–222. doi: 10.1016/j.ijpharm.2018.09.031. [DOI] [PubMed] [Google Scholar]
- 70.Liu D.S., Gong L., Zhu H.L. Curcumin inhibits transforming growth factor β induced differentiation of mouse lung fibroblasts to myofibroblasts. J. Front Pharmacol. 2016;7:419. doi: 10.3389/fphar.2016.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smith M.R., Gangireddy S.R., Narala V.R. Curcumin inhibits fibrosis-related effects in IPF fibroblasts and in mice following bleomycin-induced lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2010;298(5):L616–25. doi: 10.1152/ajplung.00002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chauhan P.S., Dash D., Singh R. Intranasal curcumin inhibits pulmonary fibrosis by modulating matrix Metalloproteinase-9 (MMP-9) in ovalbumin-induced chronic asthma. J. Inflam. 2017;40(1):248–258. doi: 10.1007/s10753-016-0475-3. [DOI] [PubMed] [Google Scholar]
- 73.Massi A., Bortolini O., Ragno D. Research progress in the modification of quercetin leading to anticancer agents. J. Mol. 2017;22(8):1270. doi: 10.3390/molecules22081270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ferreira C.G.T., Campos M.G., Felix D.M. Evaluation of the antiviral activities of Bacharis dracunculifolia and quercetin on Equid herpesvirus 1 in a murine model. J. Res. Vet. Sci. 2018;120:70–77. doi: 10.1016/j.rvsc.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 75.Carullo G., Cappello A.R., Frattaruolo L. Quercetin and derivatives: useful tools in inflammation and pain management. J. Future Med Chem. 2017;9(1):79–93. doi: 10.4155/fmc-2016-0186. [DOI] [PubMed] [Google Scholar]
- 76.Pehar M., Beeson G., Beeson C.C. Mitochondria-targeted catalase reverts the neurotoxicity of hSOD1G93A astrocytes without extending the survival of ALS-linked mutant hSOD1 mice. Plos One. 2014;9(7):e103438. doi: 10.1371/journal.pone.0103438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pan W., Chang M.J., Booyse F.M. Quercetin induced tissue-type plasminogen activator expression is mediated through Sp1 and p38 mitogen-activated protein kinase in human endothelial cells. J. Thromb. Haemost. 2008;6(6):976–985. doi: 10.1111/j.1538-7836.2008.02977.x. [DOI] [PubMed] [Google Scholar]
- 78.Zhang X.C., Cai Y.L., Zhang W. Quercetin ameliorates pulmonary fibrosis by inhibiting SphK1/S1P signaling. J. Biochem. Cell Biol. 2018;96(6):742–751. doi: 10.1139/bcb-2017-0302. [DOI] [PubMed] [Google Scholar]
- 79.Veith C., Drent M., Bast A., van Schooten F.J., Boots A.W. The disturbed redox-balance in pulmonary fibrosis is modulated by the plant flavonoid quercetin. J. Toxicol. Appl. Pharmacol. 2017;336:40–48. doi: 10.1016/j.taap.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 80.Zhu M.H., Wang M.J., Jiang Y.F. Gambogic acid induces apoptosis of non-small cell lung Cancer (NSCLC) cells by suppressing notch signaling. J. Med. Sci. Monit. 2018;24:7146–7151. doi: 10.12659/MSM.912563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu X.D., Long L., Liu J. Gambogic acid suppresses inflammation in rheumatoid arthritis rats via PI3K/Akt/mTOR signaling pathway. J. Mol. Med. Rep. 2017;16(5):7112–7118. doi: 10.3892/mmr.2017.7459. [DOI] [PubMed] [Google Scholar]
- 82.Hua X., Jia Y., Yang Q. Staphylococcus aureusTranscriptional analysis of the effects of gambogic acid and neogambogic acid on methicillin-resistant. J. Front Pharmacol. 2019;10:986. doi: 10.3389/fphar.2019.00986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hsieh Y.L., Kan H.W., Chiang H. Distinct TrkA and Ret modulated negative and positive neuropathic behaviors in a mouse model of resiniferatoxin-induced small fiber neuropathy. J. Exp. Neurol. 2018;300:87–99. doi: 10.1016/j.expneurol.2017.10.026. [DOI] [PubMed] [Google Scholar]
- 84.Qu Y.B., Zhang G.H., Ji Y.X. Protective role of gambogic acid in experimental pulmonary fibrosis in vitro and in vivo. J. Phytomedicine. 2016;24(4):350–358. doi: 10.1016/j.phymed.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 85.Qi Y., Gao F., Hou L.F. Anti-inflammatory and immunostimulatory activities of astragalosides. Am. J. Chin. Med. 2017;45(6):1157–1167. doi: 10.1142/S0192415X1750063X. [DOI] [PubMed] [Google Scholar]
- 86.Zhang Z.J., Cheng X.Y., Ge D.J. Protective effects of astragaloside IV combined with budesonide in bronchitis in rats by regulation of Nrf2/Keap1 pathway. J. Med. Sci. Monit. 2018;24:8481–8488. doi: 10.12659/MSM.911150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yuan X.X., Gong Z.Q., Wang B.Y. βAstragaloside inhibits hepatic fibrosis by modulation of TGF-1/Smad signaling pathway. J. Evid Based Complement Alternat Med. 2018;2018:3231647. doi: 10.1155/2018/3231647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shen X.H., Sun H.H., Cui H. Astragaloside attenuates lipopolysaccharide-induced cell apoptosis in human gingiva cells via MAPK signaling pathway. J. Cell. Biochem. 2019;120(8):12273–12279. doi: 10.1002/jcb.28286. [DOI] [PubMed] [Google Scholar]
- 89.Li L.C., Xu L., Hu Y. Astragaloside IV improves bleomycin-induced pulmonary fibrosis in rats by attenuating extracellular matrix deposition. J. Front Pharmacol. 2017;8:513. doi: 10.3389/fphar.2017.00513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Qian W.B., Cai X.R., Qian Q.H. Astragaloside IV modulates TGF-β1-dependent epithelial-mesenchymal transition in bleomycin-induced pulmonary fibrosis. J. Cell. Mol. Med. 2018;22(9):4354–4365. doi: 10.1111/jcmm.13725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang Y.F., Xu L.W., Liang K. Protective effect of salvianolic acid B against oxidative injury associated with cystine stone formation. J. Urolithiasis. 2019;47(6):503–510. doi: 10.1007/s00240-019-01114-4. [DOI] [PubMed] [Google Scholar]
- 92.Fan Y., Luo Q.P., Wei J.J. Mechanism of salvianolic acid B neuroprotection against ischemia/reperfusion induced cerebral injury. J. Brain Res. 2018;1679:125–133. doi: 10.1016/j.brainres.2017.11.027. [DOI] [PubMed] [Google Scholar]
- 93.Liu Q.M., Chu H.Y., Ma Y.Y. Salvianolic acid B attenuates experimental pulmonary fibrosis through inhibition of the TGF-β signaling pathway. J. Sci Rep. 2016;6:27610. doi: 10.1038/srep27610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu M., Xu H.Y., Zhang L. Salvianolic acid B inhibits myofibroblast transdifferentiation in experimental pulmonary fibrosis via the up-regulation of Nrf2. J. Biochem. Biophys. Res. Commun. 2018;495(1):325–331. doi: 10.1016/j.bbrc.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 95.Fan Y.J., Piao C.H., Eunjin Hyeon. Gallic acid alleviates nasal inflammation via activation of Th1 and inhibition of Th2 and Th17 in a mouse model of allergic rhinitis. J. Int. Immunopharmacol. 2019;70:512–519. doi: 10.1016/j.intimp.2019.02.025. [DOI] [PubMed] [Google Scholar]
- 96.Lu J., Wang Z.N., Ren M.R. Antibacterial effect of gallic acid against Aeromonas hydrophila and Aeromonas sobria through damaging membrane integrity. J. Curr. Pharm. Biotechnol. 2016;17(13):1153–1158. doi: 10.2174/1389201017666161022235759. [DOI] [PubMed] [Google Scholar]
- 97.Locatelli C., Filippin-Monteiro F.B., Creczynski-Pasa T.B. Alkyl esters of gallic acid as anticancer agents: a review. Eur. J. Med. Chem. 2013;60:233–239. doi: 10.1016/j.ejmech.2012.10.056. [DOI] [PubMed] [Google Scholar]
- 98.Chen C.Y., Chen K.C., Yang T.Y. Gallic acid induces a reactive oxygen species-provoked c-Jun NH2-Terminal kinase-dependent apoptosis in lung fibroblasts. J. Evid. Based Complement Alternat Med. 2013;2013:613950. doi: 10.1155/2013/613950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rong Y.M., Cao B., Liu B. A novel Gallic acid derivative attenuates BLM-induced pulmonary fibrosis in mice. J. Int. Immunopharmacol. 2018;64:183–191. doi: 10.1016/j.intimp.2018.08.024. [DOI] [PubMed] [Google Scholar]
- 100.Gao H.W., Huang L.T., Ding F. Simultaneous purification of dihydrotanshinone, tanshinone I, cryptotanshinone, and tanshinone IIA from Salvia miltiorrhiza and their anti-inflammatory activities investigation. J. Sci. Rep. 2018;8(1):8460. doi: 10.1038/s41598-018-26828-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhou Z.Y., Zhao W.R., Zhang J. Sodium tanshinone IIA sulfonate: a review of pharmacological activity and pharmacokinetics. Biomed. Pharmacother. 2019;118:109362. doi: 10.1016/j.biopha.2019.109362. [DOI] [PubMed] [Google Scholar]
- 102.He H.Y., Tang H.Y., Gao L.L. Tanshinone IIA attenuates bleomycin-induced pulmonary fibrosis in rats. J. Mol. Med. Rep. 2015;11(6):4190–4196. doi: 10.3892/mmr.2015.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wu H.J., Li Y., Wang Y.X. Tanshinone IIA attenuates bleomycin-induced pulmonary fibrosis via modulating angiotensin-converting enzyme 2/ angiotensin-(1-7) axis in rats. J. Int. J. Med. Sci. 2014;11(6):578–586. doi: 10.7150/ijms.8365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li L., Song X., Yin Z.Q. The antibacterial activity and action mechanism of emodin from Polygonum cuspidatum against Haemophilus parasuis in vitro. J. Microbiol. Res. 2016:139–145. doi: 10.1016/j.micres.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 105.Lin W.F., Wang C., Ling C.Q. Research progress in anti-tumor effect of emodin. J. Chin. Materia Medica. 2015;40(20):3937–3940. [PubMed] [Google Scholar]
- 106.Ho T.Y., Wu S.L., Chen J.C. Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction. J. Antiviral Res. 2007;74(2):92–101. doi: 10.1016/j.antiviral.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Guan R.J., hao Z.X.M., Wang X. Emodin alleviates bleomycin-induced pulmonary fibrosis in rats. J. Toxicol. Lett. 2016;262:161–172. doi: 10.1016/j.toxlet.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 108.Zhu Z.Y., Duan H.B., Jing M. Synthesis and biological evaluation of andrographolide derivatives as anti-inflammatory agent. J. Curr. Pharm. Des. 2018;24(30):3529–3533. doi: 10.2174/1381612824666180724130014. [DOI] [PubMed] [Google Scholar]
- 109.Gupta S., Mishra K.P., Ganju L. Broad-spectrum antiviral properties of andrographolide. J. Arch. Virol. 2017;162(3):611–623. doi: 10.1007/s00705-016-3166-3. [DOI] [PubMed] [Google Scholar]
- 110.Lu J.S., Ma Y.M., Wu J.J. A review for the neuroprotective effects of andrographolide in the central nervous system. J. Biomed. Pharmacother. 2019;117:109078. doi: 10.1016/j.biopha.2019.109078. [DOI] [PubMed] [Google Scholar]
- 111.Yin J.N., Li Y.N., Gao Y. Andrographolide plays an important role in bleomycin-induced pulmonary fibrosis treatment. J. Int. J. Clin. Exp. Med. 2015;8(8):12374–12381. [PMC free article] [PubMed] [Google Scholar]
- 112.Xia N., Daiber A., Förstermann U. Antioxidant effects of resveratrol in the cardiovascular system. Br. J. Pharmacol. 2017;174:1633–1646. doi: 10.1111/bph.13492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang D.L., Gao Z.F., Zhang X. Resveratrol induces apoptosis in murine prostate Cancer cells via Hypoxia-Inducible factor 1-alpha (HIF-1α)/Reactive oxygen species (ROS)/P53 signaling. J. Med. Sci. Monit. 2018;24:8970–8976. doi: 10.12659/MSM.913290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vestergaard M., Ingmer H. Antibacterial and antifungal properties of resveratrol. Int. J. Antimicrob. Agents. 2019;53(6):716–723. doi: 10.1016/j.ijantimicag.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 115.Dull A.M., Moga M.A., Dimienescu O.G. Therapeutic approaches of resveratrol on endometriosis via anti-inflammatory and anti-angiogenic pathways. J. Mol. 2019;24(4):667. doi: 10.3390/molecules24040667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fagone E., Conte E., Gili E. Resveratrol inhibits transforming growth factor-β-induced proliferation and differentiation of ex vivo human lung fibroblasts into myofibroblasts through ERK/Akt inhibition and PTEN restoration. J. Exp. Lung Res. 2011;37(3):162–174. doi: 10.3109/01902148.2010.524722. [DOI] [PubMed] [Google Scholar]
- 117.Huang X.Y., He Y.C., Chen Y.F. Baicalin attenuates bleomycin-induced pulmonary fibrosis via adenosine A2a receptor related TGF-β1-induced ERK1/2 signaling pathway. J. BMC Pulm. Med. 2016;16(1):132. doi: 10.1186/s12890-016-0294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jin M., Wang L., Wu Y. Protective effect of hydroxysafflor yellow A on bleomycin- induced pulmonary inflammation and fibrosis in rats. J. Chin. J. Integr. Med. 2018;24(1):32–39. doi: 10.1007/s11655-017-2094-z. [DOI] [PubMed] [Google Scholar]
- 119.Chen H., Chen Q., Jiang C.M. Triptolide suppresses paraquat induced idiopathic pulmonary fibrosis by inhibiting TGFB1-dependent epithelial mesenchymal transition. J. Toxicol. Lett. 2018;284:1–9. doi: 10.1016/j.toxlet.2017.11.030. [DOI] [PubMed] [Google Scholar]
- 120.Zhou Y., Zhu W.P., Cai X.J. Atomized paclitaxel liposome inhalation treatment of bleomycin-induced pulmonary fibrosis in rats. J. Genet. Mol. Res. 2016;15(2) doi: 10.4238/gmr.15027309. [DOI] [PubMed] [Google Scholar]
- 121.Ji Y., Dou Y.N., Zhao Q.W. Paeoniflorin suppresses TGF-β mediated epithelial-mesenchymal transition in pulmonary fibrosis through a Smad-dependent pathway. J. Acta Pharmacol. Sin. 2016;37(6):794–804. doi: 10.1038/aps.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhan H.Q., Huang F., Ma W.Z. Protective effect of ginsenoside Rg1 on bleomycin-induced pulmonary fibrosis in rats: involvement of Caveolin-1 and TGF-β1 signal pathway. J. Biol. Pharm. Bull. 2016;39(8):1284–1292. doi: 10.1248/bpb.b16-00046. [DOI] [PubMed] [Google Scholar]
- 123.Li L.Y., Ma L.Y., Wang D.C. Design and synthesis of matrine derivatives as novel anti-pulmonary fibrotic agents via repression of the TGFβ/Smad pathway. J. Mol. 2019;24(6):1108. doi: 10.3390/molecules24061108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xiao J.H., Zhang J.H., Chen H.L. Inhibitory effects of isoliensinine on bleomycin-induced pulmonary fibrosis in mice. J. Planta Med. 2005;71(3):225–230. doi: 10.1055/s-2005-837821. [DOI] [PubMed] [Google Scholar]
- 125.Hu Y.Z., Li M., Zhang M.M., Jin Y.G. Inhalation treatment of idiopathic pulmonary fibrosis with curcumin large porous microparticles. J. Int. J. Pharm. 2018;551:212–222. doi: 10.1016/j.ijpharm.2018.09.031. [DOI] [PubMed] [Google Scholar]
- 126.Jin X., Zhang S.B., Li S.M., Liang K., Jia Z.Y. Influence of chitosan nanoparticles as the absorption enhancers on salvianolic acid B in vitro and in vivo evaluation. J. Pharmacogn. Mag. 2016;12(45):57–63. doi: 10.4103/0973-1296.176047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liang Q., Cai W.Y., Zhao Y.X., Xu H.B., Tang H.R., Chen D.J. Lycorine ameliorates bleomycin-induced pulmonary fibrosis via inhibiting NLRP3 inflammasome activation and pyroptosis. J. Pharmacol. Res. 2020;158:104884. doi: 10.1016/j.phrs.2020.104884. [DOI] [PubMed] [Google Scholar]
- 128.Chen L., Yang Y., Peng X.Y. Transcription factor YY1 inhibits the expression of THY1 to promote interstitial pulmonary fibrosis by activating the HSF1/miR-214 axis. J. Aging (Albany NY) 2020;12(9):8339–8351. doi: 10.18632/aging.103142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Weiss C., Carriere M., Fusco L. Toward nanotechnology-enabled approaches against the COVID-19 pandemic. J. ACS Nano. 2020;14(6):6383–6406. doi: 10.1021/acsnano.0c03697. [DOI] [PubMed] [Google Scholar]
- 130.Pindiprolu S.K.S.S., Kumar C.S.P., Kumar Golla V.S. Pulmonary delivery of nanostructured lipid carriers for effective repurposing of salinomycin as an antiviral agent. J. Med. Hypotheses. 2020;143:109858. doi: 10.1016/j.mehy.2020.109858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Orienti I., Gentilomi G.A., Farruggia G. Pulmonary delivery of fenretinide: a possible adjuvant treatment in COVID-19. Int. J. Mol. Sci. 2020;21(11):3812. doi: 10.3390/ijms21113812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Takeuchi I., Koshi Y., Makino K. Drug delivery properties of nanocomposite particles for inhalation: comparison of drug concentrations in lungs and blood. In Vivo. 2020;34(2):543–547. doi: 10.21873/invivo.11806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lu P., Xing Y., Peng H. Physicochemical and pharmacokinetic evaluation of spray-dried coformulation of Salvia miltiorrhiza polyphenolic acid and L-Leucine with improved bioavailability. J. Aerosol. Med. Pulm. D. 2020;33(2):73–82. doi: 10.1089/jamp.2019.1538. [DOI] [PubMed] [Google Scholar]
- 134.Rokhsana R., Hamid R., Abbas P., Ali M. Preference of aerosolized pirfenidone to oral intake: an experimental model of pulmonary fibrosis by paraquat. J. Aerosol Med. Pulm. Drug Deliv. 2018;31(1):25–32. doi: 10.1089/jamp.2016.1342. [DOI] [PubMed] [Google Scholar]
- 135.Vartiainen V., Raulab J., Bimboe L.M. Pulmonary administration of a dry powder formulation of the antifibrotic drug tilorone reduces silica-induced lung fibrosis in mice. J. Int. J. Pharm. 2018;544(1):121–128. doi: 10.1016/j.ijpharm.2018.04.019. [DOI] [PubMed] [Google Scholar]
- 136.Miao X., Zhou J., Li J. Chinese medicine in inhalation therapy: a review of clinical application and formulation development. Curr. Pharm. Des. 2015;21(27):3917–3931. doi: 10.2174/1381612821666150820110550. [DOI] [PubMed] [Google Scholar]
- 137.Chandel A., Goyal A.K., Ghosh G. Recent advances in aerosolised drug delivery. J. Biomed. Pharmacother. 2019;112:108601. doi: 10.1016/j.biopha.2019.108601. [DOI] [PubMed] [Google Scholar]
- 138.Pleasants R.A., Hess D.R. Aerosol delivery devices for obstructive lung diseases. J. Respir Care. 2018;63(6):708–733. doi: 10.4187/respcare.06290. [DOI] [PubMed] [Google Scholar]
- 139.Ferguso G.T., Hickey A.J., Dwived S. Co-suspension delivery technology in pressurized metered-dose inhalers for multi-drug dosing in the treatment of respiratory diseases. J. Respir Med. 2018;134:16–23. doi: 10.1016/j.rmed.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 140.Newman S.P. Drug delivery to the lungs: challenges and opportunities. Ther. Deliv. 2017;8(8):647–661. doi: 10.4155/tde-2017-0037. [DOI] [PubMed] [Google Scholar]
- 141.Anderson G., Johnson N., Mulgirigama A. Use of spacers for patients treated with pressurized metered dose inhalers: focus on the VENTOLINTM Mini Spacer. J. Expert Opin. Drug Deliv. 2018;15(4):419–430. doi: 10.1080/17425247.2018.1437414. [DOI] [PubMed] [Google Scholar]
- 142.Liu Y. Observation of curative effect of ligustrazine combined with inhaled budesonide in the treatment of idiopathic pulmonary fibrosis. Modern J. Integr. Tradit. Chin. West. Med. 2015;24(23) 2536-2538+2557. [Google Scholar]
- 143.Okuda T., Okamoto H. Present situation and future progress of inhaled lung Cancer therapy: necessity of inhaled formulations with drug delivery functions. J. Chem. Pharm. Bull. 2020;68(7):589–602. doi: 10.1248/cpb.c20-00086. [DOI] [PubMed] [Google Scholar]
- 144.Longest W., Spence B., Hindle M. Devices for improved delivery of nebulized pharmaceutical aerosols to the lungs. J. Aerosol Med. Pulm. Drug Deliv. 2019;32(5):317–339. doi: 10.1089/jamp.2018.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xing Y., Lu P., Xue Z.F. Nano-strategies for improving the bioavailability of inhaled pharmaceutical formulations. Mini-Rev. Med. Chem. 2020;20:1258–1271. doi: 10.2174/1389557520666200509235945. [DOI] [PubMed] [Google Scholar]
- 146.Lavorini F., Buttini F., Usmani O.S. 100 years of drug delivery to the lungs. J. Handb. Exp. Pharmacol. 2019;260:143–159. doi: 10.1007/164_2019_335. [DOI] [PubMed] [Google Scholar]
- 147.Su W.Q., Liang Y.M., Meng Z.P., Chen X.Y. Inhalation of Tetrandrine-hydroxypropyl-β-cyclodextrin inclusion complexes for pulmonary fibrosis treatment. J. Mol. Pharm. 2020;17(5):1596–1607. doi: 10.1021/acs.molpharmaceut.0c00026. [DOI] [PubMed] [Google Scholar]
- 148.Li L., Sun S.P., Thaigarajan Parumasivam. L-Leucine as an excipient against moisture on in vitro aerosolization performances of highly hygroscopic spray-dried powders. Eur. J. Pharm. Biopharm. 2016;102:132–141. doi: 10.1016/j.ejpb.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 149.Berkenfeld K., Lamprecht A., McConville J.T. Devices for dry powder drug delivery to the lung. J. AAPS PharmSciTech. 2015;16(3):479–490. doi: 10.1208/s12249-015-0317-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chrystyn H., Lavorini F. The dry powder inhaler features of the Easyhaler that benefit the management of patients. J. Expert Rev. Respir Med. 2020;14(4):345–351. doi: 10.1080/17476348.2020.1721286. [DOI] [PubMed] [Google Scholar]
- 151.Lavorini F. Easyhaler: an overview of an inhaler device for day-to-day use in patients with asthma and chronic obstructive pulmonary disease. J. Drugs Context. 2019;8:212596. doi: 10.7573/dic.212596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Healy A.M., Amaro, Paluch M.I.K.J. Dry powders for oral inhalation free of lactose carrier particles. Adv. Drug Deliv. Rev. 2014;75:32–52. doi: 10.1016/j.addr.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 153.Muralidharan P., Malapi M., Mallory E. Inhalable nanoparticulate powders for respiratory delivery. J. Nanomedicine. 2015;11(5):1189–1199. doi: 10.1016/j.nano.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 154.Guzmán E.A.T., Sun Q.H., Meenach S.A. Development and evaluation of paclitaxel-loaded aerosol nanocomposite microparticles and their efficacy against air-grown lung Cancer tumor spheroids. J. ACS Biomater. Sci. Eng. 2019;5(12):6570–6580. doi: 10.1021/acsbiomaterials.9b00947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Levet V., Rosière R., Merlos R. Development of controlled-release cisplatin dry powders for inhalation against lung cancers. Int. J. Pharm. 2016;515:209–220. doi: 10.1016/j.ijpharm.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 156.Hu Y.Z., Li M., Zhang M.M., Jin Y.G. Inhalation treatment of idiopathic pulmonary fibrosis with curcumin large porous microparticles. Int. J. Pharm. 2018;551:212–222. doi: 10.1016/j.ijpharm.2018.09.031. [DOI] [PubMed] [Google Scholar]
- 157.Wang J.H., Zhai W.W., Yu J.Q., Wang J., Dai J.D. Preparation and quality evaluation of salvianolic acids and tanshinones dry powder inhalation. J. Pharm. Sci. 2018;107(9):2451–2456. doi: 10.1016/j.xphs.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 158.Lechanteur A., Evrard B. Influence of composition and spray-drying process parameters on carrier-free DPI properties and behaviors in the lung: a review. J. Pharm. 2020;12(1):55. doi: 10.3390/pharmaceutics12010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Brunaugh A.D., Smyth H.D.C. Formulation techniques for high dose dry powders. Int. J. Pharm. 2018;547:489–498. doi: 10.1016/j.ijpharm.2018.05.036. [DOI] [PubMed] [Google Scholar]
- 160.Kaur S.S. Pulmonary drug delivery system: newer patents. Pharm. Pat. Anal. 2017;6(5):225–244. doi: 10.4155/ppa-2017-0019. [DOI] [PubMed] [Google Scholar]
- 161.Cazzola M., Cavalli F., Usmani O.S. Advances in pulmonary drug delivery devices for the treatment of chronic obstructive pulmonary disease. J. Expert Opin. Drug Deliv. 2020;17(5):635–646. doi: 10.1080/17425247.2020.1739021. [DOI] [PubMed] [Google Scholar]
- 162.Ari A., Fink J.B. Recent advances in aerosol devices for the delivery of inhaled medications. J. Expert Opin. Drug Deliv. 2020;17(2):133–144. doi: 10.1080/17425247.2020.1712356. [DOI] [PubMed] [Google Scholar]
- 163.Thakur A.K., Chellappan D.K., Dua K. Patented therapeutic drug delivery strategies for targeting pulmonary diseases. J. Expert Opin. Ther Pat. 2020;30(5):375–387. doi: 10.1080/13543776.2020.1741547. [DOI] [PubMed] [Google Scholar]