Abstract
Objective
To analyze findings and trends on serial electrocardiograms (ECGs) in multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease taken during the course of illness and at follow-up.
Study design
We included all children presenting with MIS-C at a single center with 3 or more ECGs taken during the course of their illness. We measured ECG intervals (PR, QRSd, and QTc) and amplitudes (R-, S-, and T-waves) on each ECG and documented any arrhythmias and ST-segment changes.
Results
A majority of children (n = 42, 67%) showed ECG changes. The most common findings were low QRS amplitudes and transient T-wave inversion. ST changes were uncommon and included ST-segment elevation consistent with pericarditis in 1 child and acute coronary ischemia in 1 child. Arrhythmias were seen in 13 children (21%) but were benign with the exception of 1 child who was compromised by an atrial tachycardia requiring support with extracorporeal membrane oxygenation. No children were found to have high-grade atrioventricular block.
Conclusions
MIS-C is associated with electrocardiographic changes over the course of the illness, with low amplitude ECGs on presentation, followed by transient T-wave inversion, particularly in the precordial leads. There was a low prevalence of ST-segment changes and tachyarrhythmias.
Keywords: coronavirus, SARS-CoV-2, arrhythmias, MIS-C, PIMS, pediatric
Abbreviations: COVID-19, Coronavirus disease 2019; ECG, Electrocardiogram; ECMO, Extracorporeal membrane oxygenation; ICU, Intensive care unit; MIS-C, Multisystem inflammatory syndrome in children; PICU, Pediatric intensive care unit; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; TWI, T-wave inversion
See editorial, p 10
Initial reports of the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) suggested that children were largely spared of the worst effects of the disease termed coronavirus disease 2019 (COVID-19). However, since April 2020 the emergence of cohorts of children and young adults presenting with a hyperinflammatory syndrome with multiorgan involvement and features similar to Kawasaki disease shock syndrome, toxic shock syndrome, hemophagocytic lymphohistiocytosis, and macrophage activation syndrome induced by a cytokine storm has been reported.1, 2, 3, 4, 5, 6, 7, 8, 9, 10 This new condition appeared after the peak of the SARS-CoV-2 pandemic in the respective countries. The novel syndrome has since been defined in the United Kingdom, the US, and the World Health Organization11, 12, 13 using several terminologies, including multisystem inflammatory syndrome in children (MIS-C) and pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 infection.
Data have now been published from around the world detailing outcomes of children with this condition with emphasis on cardiac imaging to assess cardiac dysfunction and demonstrating the presence of widespread myocardial edema and pancarditis.2, 3, 4, 5, 6, 7 , 14 One small cohort study noted 9 of 15 children (60%) with abnormal electrocardiogram (ECG) findings.5 A large US cohort reported a 12% rate of arrhythmia, but with no further details of type or outcome,9 and 1 case report described a transient, high-grade AV block requiring pacing in a child with MIS-C.15
The aim of our study was to describe findings and review trends on serial ECGs in a single-center cohort of children presenting with MIS-C.
Methods
We retrospectively collected 12-lead ECGs from children presenting with MIS-C to Evelina London Children's Hospital between April and June 2020. ECGs were recorded at admission, discharge, and on follow-up, with additional ECGs recorded at serial intervals of around 48 hours during their inpatient stay, or if clinically indicated. We included those patients with 3 or more ECGs covering the inpatient stay and follow-up. All ECGs were recorded at standard 25 mm/second paper speed and gain setting of 10 mm/mV. The ECGs were scanned in high definition (400 dpi) and Adobe Photoshop (Adobe Systems) was used with a calibrated digital calliper tool, to measure ECG intervals (PR, QRSd, RR, QTc [3 consecutive QT intervals in leads II and V5, with preceding RR interval, corrected using the Bazett formula]) and amplitudes (R- and S-wave amplitudes in V1, V4, and V6 and T-wave amplitudes in leads I and V6). Computer-generated measurements for heart rate, QRS duration, and QRS axis were used and visually checked for accuracy.
Definitions
First degree AV block is the PR interval over 98th percentile for age and sex.16 In patients with a ≥5 bpm difference in heart rate in ≥2 ECGs, a positive slope, with paradoxical lengthening of PR interval at increasing heart rates, was defined as abnormal PR:HR slope.17 QRS prolongation is the QRS duration over 98th percentile for age and sex.16 Prolonged corrected QT interval is the QTc over 98th percentile for age and sex.16 ST-segment depression was defined as ≥0.05 mV in ≥2 contiguous leads. ST-elevation was defined as ≥0.2mV in leads V2/3, or ≥0.1mV in other leads, when present in ≥2 contiguous leads. T-wave inversion (TWI) is defined on any single ECG if negative T-waves of ≥0.1 mV amplitude are seen in ≥2 contiguous leads (excluding leads aVR, III, and V1-3). Transient TWI is the transient development of negative T-waves in ≥2 contiguous precordial leads, which later resolved on serial ECGs. Negative T-waves in the anterior leads (V1-3) were initially considered a normal juvenile pattern and excluded; however, if subsequent ECGs showed upright T-waves that persisted into follow-up, they were then included. We chose ≥2 contiguous leads to help mitigate against differences in positioning of individual electrodes. Biphasic T-waves were not included in the definition of transient TWI.
Demographic details (including the UK government ethnicity groupings), imaging data, biochemistry, length of hospital stay, need for and length of pediatric intensive care unit (PICU) admission, and need for inotropic or extracorporeal membrane oxygenation (ECMO) support were collected from the hospital records. Peak levels of inflammatory markers (C-reactive protein and ferritin) and cardiac biomarkers (N-terminal pro-brain-natriuretic peptide and troponin-I) were included. Echocardiographic data used were worst fractional shortening percentage and worst global longitudinal strain measurements from admission through to follow-up after discharge, as described previously.14
Statistical analyses were performed using SPSS v 26.0 (SPSS, Inc). Variables were tested for normality using the Kolmogorov-Smirnov test. Values are expressed as either mean ± SD or percentages. Differences in group means were compared using independent t tests or Mann-Whitney U tests (for normally and non-normally distributed variables, respectively). A χ2 test or Fisher exact test was used to test group differences of proportions. Repeated measures ANOVA was performed to evaluate changes of electrocardiographic measures from admission, during hospitalization, at discharge, and on follow-up. Statistical significance was defined as a P value of <.05.
Local ethics approval was received for retrospective analysis of patient data for this cohort (08/H0810/058).
Results
We included all 63 children (Black 23 [37%], White 13 [21%], Asian 5 [8%], other 1 [2%], and unknown 21 [33%]) with suitable serial ECGs for analysis. Six children were excluded due to lack of serial ECGs during their inpatient stay. Those excluded did not have any notable difference in their presenting ECG, clinical course, or outcomes. In total, 312 ECGs were included in the analysis with a median of 5 ECGs per patient.
The demographic data, level of intensive care unit (ICU) support, and hospital length of stay are summarized in Table I . None of the children was on QT prolonging medications such as hydroxychloroquine or erythromycin. Table II (available at www.jpeds.com) summarizes the peak levels of biochemical markers and worst echocardiographic measurements of heart function and outlines the timeline of these changes in relation to date of symptom onset.
Table I.
Summary of patient characteristics
| Patient characteristic | No. (%) |
|---|---|
| Age in y – median (range) | 10 (0.3,16) |
| Male | 43 (68) |
| ICU admission | 43 (68) |
| Inotropic support | 32 (51) |
| ECMO support | 2 (3) |
| Length of stay hospital in d – median (range) | 7 (2,22) |
| Length of stay ICU in d – median (range) | 3 (0,11) |
Although there were statistically significant changes in the PR interval, the QRS duration and axis, and the corrected QT intervals (Table III ), the mean values of ECG variables still largely fell within normal limits. Only small changes in cardiac conduction and repolarization were observed during the course of the illness (Table IV ). First-degree AV block was seen in 16 (25%) children, peaking at the midpoint of admission with 9 (17%) children affected, and persisting in only 2 (3%) by follow-up. There was an abnormal (positive) PR:HR slope response with paradoxical prolongation of the PR interval at increasing heart rates in 22 (37%). In 6 children (9.5%), there was a prolongation of the QRS duration, with normalization prior to discharge in 5 of 6. In the child discussed later with an atrial tachycardia requiring ECMO, this did not recover. The corrected QT interval peaked at a mean of 412 msec at the midpoint of inpatient stay, reducing to 401 msec by discharge (P = .019). There were 14 (22%) children demonstrating transient prolongation of the QTc above 98th percentile for age (maximum of 490msec), with normalization by discharge in a majority.
Table III.
Summary of serial measurements on ECGs
| Measurement | Admission | P value | Midpoint | P value | Discharge | P value | Follow-up |
|---|---|---|---|---|---|---|---|
| Number of patients | 63 | 55 | 60 | 60 | |||
| ECG parameters: | |||||||
| Heart rate (bpm) | 108 ± 22 | <.001 | 80 ± 22 | .171 | 77 ± 20 | <.001 | 88 ± 15 |
| PR interval (msec) | 136 ± 24 | .023 | 146 ± 28 | .295 | 142 ± 26 | .013 | 133 ± 18 |
| QRS duration (msec) | 84 ± 12 | .374 | 88 ± 13 | .048 | 84 ± 10 | .376 | 84 ± 11 |
| QRS axis (degrees) | 71 ± 36 | .014 | 63 ± 35 | .596 | 64 ± 32 | .004 | 57 ± 28 |
| QTc (msec) | 410 ± 30 | .909 | 412 ± 29 | .019 | 401 ± 25 | .720 | 403 ± 22 |
| Conduction/repolarization delay: | |||||||
| First degree AV block, number (% of children) | 5 (8%) | n/a | 9 (17%) | n/a | 7 (12%) | n/a | 2 (3%) |
| QRS prolongation, number (% of children) | 4 (6%) | n/a | 4 (7%) | n/a | 1 (2%) | n/a | 0 |
| QTc prolongation, number (% of children) | 7 (11%) | n/a | 8 (15%) | n/a | 2 (3%) | n/a | 1 (2%) |
| QRS amplitude (mV): | |||||||
| R wave V1 | 0.473 ± 0.44 | .789 | 0.469 ± 0.39 | .104 | 0.555 ± 0.45 | .086 | 0.686 ± 0.44 |
| S wave V1 | 0.761 ± 0.59 | 0.390 | 0.789 ± 0.53 | .169 | 0.917 ± 0.62 | <.001 | 1.231 ± 0.64 |
| R wave V4 | 1.663 ± 0.84 | 0.841 | 1.603 ± 0.81 | .381 | 1.728 ± 0.74 | .022 | 2.080 ± 0.78 |
| R wave V6 | 1.296 ± 0.50 | 0.166 | 1.410 ± 0.55 | .222 | 1.523 ± 0.60 | .564 | 1.629 ± 0.46 |
| T-wave amplitude (mV): | |||||||
| T wave amplitude (mV) Lead I | 0.197 ± 0.14 | 0.153 | 0.233 ± 0.16 | .909 | 0.240 ± 0.16 | <.001 | 0.347 ± 0.12 |
| T wave amplitude (mV) V6 | 0.214 ± 0.13 | 0.214 | 0.243 ± 0.20 | .071 | 0.297 ± 0.17 | <.001 | 0.436 ± 0.12 |
| T/R ratio V6 | 0.175 ± 0.10 | 0.502 | 0.188 ± 0.14 | .116 | 0.212 ± 0.13 | <.001 | 0.280 ± 0.08 |
| T-wave changes: | |||||||
| Number negative T-waves in chest leads | 1.52 ± 1.36 | 0.181 | 1.75 ± 1.61 | .010 | 1.23 ± 1.20 | .054 | 0.95 ± 0.86 |
| TWI present in V4-6, number (% of children) | 7 (11%) | n/a | 9 (16%) | n/a | 3 (5%) | n/a | 1 (2%) |
n/a, not applicable.
Values are given as mean ± SD, or number (percentage). P values are comparisons between time points represented in adjacent columns.
P values are represented in bold if statistically significant (P < .05).
Table IV.
Summary of ECG findings and heart rhythm abnormalities
| Electrocardiographic findings | Number | % |
|---|---|---|
| No significant ECG changes throughout illness | 21 | 33.3 |
| Transient first-degree AV block | 16 | 25.4 |
| Positive (abnormal) PR:HR slope | 22 | 37 |
| Transient QRS prolongation | 6 | 9.5 |
| RBBB pattern | 3 | 4.8 |
| LBBB pattern | 1 | 1.6 |
| Non-specific intraventricular delay | 2 | 3.2 |
| Transient QTc prolongation | 14 | 22.2 |
| ST segment changes | 5 | 7.9 |
| Pericarditis type ST elevation | 1 | 1.6 |
| Ischaemic (regional) ST elevation | 1 | 1.6 |
| ST depression | 3 | 4.8 |
| Transient TWI | ||
| All precordial leads (V1-6) | 32 | 51.0 |
| Lateral precordial leads (V4-6) | 15 | 23.8 |
| Inferior leads (II, aVF) | 13 | 20.6 |
| Tachyarrhythmias | 2 | 3.2 |
| Non-sustained atrial ectopic tachycardia | 1 | 1.6 |
| Broad complex tachycardia (atrial tachycardia) | 1 | 1.6 |
| Bradyarrhythmias | 11 | 17.5 |
| First degree AV block +/- AV Wenckebach | 4 | 6.3 |
| Junctional rhythm | 4 | 6.3 |
| Low atrial rhythm | 3 | 4.8 |
LBBB, left bundle branch block; RBBB, right bundle branch block.
ST-segment changes were uncommon (n = 5, 7.9%), with 3 children having transient ST depression and 1 child showed a typical pericarditis pattern of ST elevation (this child required ECMO support because of refractory hypotension during a long PICU admission). Importantly, 1 child showed ST elevation in the lateral chest leads during an episode of chest pain, consistent with acute coronary ischemia. Acute CT- and later cardiac magnetic resonance imaging (MRI) confirmed dilated left anterior descending and circumflex arteries, and a subendocardial myocardial infarction in the territory supplied by the dilated left anterior descending and left circumflex coronary arteries.14
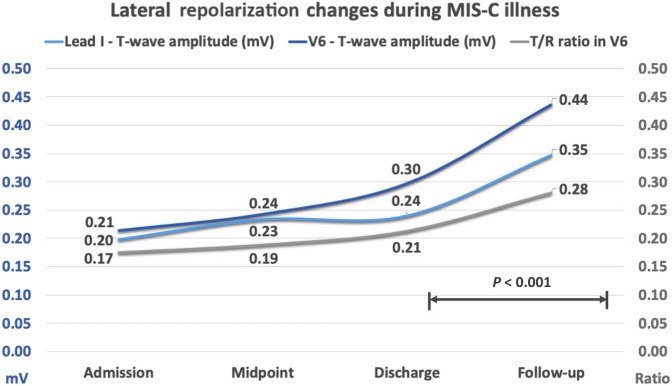
Changes in ECG amplitudes were common, with children presenting with lower amplitudes in precordial QRS complexes (S-wave V1 and R-wave in V4) and flat lateral T-waves (lead I and V6) persisting during their admission, and that recovered significantly between discharge and follow-up ECGs (P < .001) (Table III and Figure 1 [available at www.jpeds.com]). T-wave flattening was more striking than the reduction of the QRS amplitude in the lateral leads as demonstrated by the change in T/R ratio in V6. The majority of children (n = 40, 63%) showed abnormal findings at some point during their illness and these are summarized in Table IV. Transient TWI was the most common finding and was particularly prevalent in the anterior precordial leads, less commonly extending to inferolateral leads. In children where transient TWI was observed during their illness, the peak number of negative T-waves in the precordial leads occurred a median of 6 days following symptom onset and typically around 24 hours after their admission to hospital. Resolution of TWI typically occurred between the midpoint of stay and discharge (P = .01), equivalent to median days 8 and 11 following symptom onset, respectively.
Figure 1.
Lateral repolarization changes during MIS-C illness. T-wave amplitudes over course of illness in V6 and Lead I (mv), with T/R ratio in V6 demonstrating the predominant amplitude changes are seen in T-waves in this lead.
In total, 13 children (21%) were noted to have arrhythmias. The majority (n = 11) were transient bradyarrhythmias with no hemodynamic consequence. One 6-year-old child receiving inotropic support on PICU had several episodes of nonsustained atrial ectopic tachycardia. He remained asymptomatic and required no treatment. One child deteriorated with a broad complex tachycardia. This 14-year-old presented in sinus rhythm with a narrow QRS complex, however became increasingly unstable with refractory hypotension supported with multiple inotropic agents and mechanical ventilation. He then decompensated further with a broad complex tachycardia at 140-160 bpm with a right bundle branch block morphology. After a failed direct current-cardioversion, he was immediately placed on ECMO support and rate control was achieved with an amiodarone infusion. Analysis of serial ECGs showed the mechanism to be an atrial ectopic tachycardia conducted with aberration. Unfortunately, he died following complications from the ECMO support.
None of the ECG findings including transient TWI, TWI in V4-6, or abnormal PR:HR slope showed statistical correlation with markers of disease severity (inotropic use or ICU admission), or echocardiographic markers of left ventricular function (P values of >.05). The most common findings were of transient TWI that largely resolved prior to discharge, and QRS and T-wave amplitude changes, which improved during inpatient stay, then significantly prior to follow-up.
Discussion
MIS-C is associated with significant cardiac dysfunction. We describe changes in serial ECGs in a single-center cohort, specifically transient changes in ECG voltages and T-wave polarity, as well as a low incidence of ST-segment changes and arrhythmias.
Lower QRS amplitudes in precordial leads (V1 and V4) and T-wave flattening in lateral leads (lead I and V6) on presentation improved over time. As the lateral T-waves were rarely affected by transient TWI and were comparatively more affected than R-wave amplitudes, we found the trend in amplitude in these leads was a helpful objective measure to quantify recovery of ‘flattened’ T-waves over the course of the illness. Lower T-wave amplitudes in lead I and V6 have previously been described in acute pericarditis,18 and the changes we have seen in our cohort may be reflective of myopericarditis seen in patients with MIS-C.
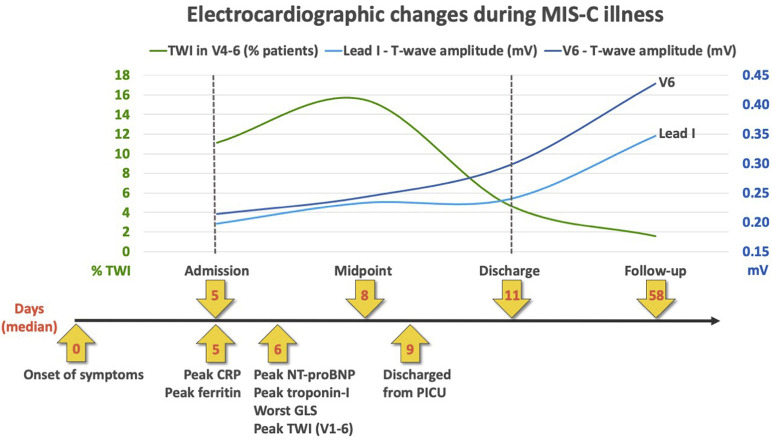
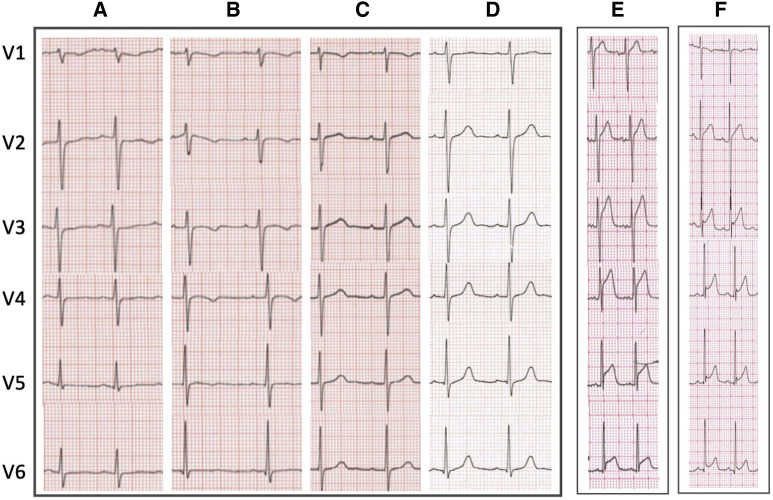
Transient TWI in the precordial leads was seen in one-half of patients, with the peak number of negative T-waves seen at a median of day 6 following onset of symptoms, the same timing as peak markers of cardiac dysfunction (raised cardiac enzymes and lowered global longitudinal strain on echocardiogram) (Figure 2; available at www.jpeds.com). A typical sequence seen in this cohort (Figure 3 ) was of a low QRS amplitude, but otherwise unremarkable ECG on admission, followed by precordial T-wave flattening and inversion in one-half of the patients, which then normalized before discharge.
Figure 2.
Summary of electrocardiographic changes during MIS-C illness. T-wave changes seen during illness, represented across timeline of clinical events and investigations (x-axis showing median days since symptom onset). GLS, global longitudinal strain.
Figure 3.
Representative ECG changes. Typical pattern of transient T-wave flattening and inversion with QRS amplitude changes A, on admission, B, midpoint, C, discharge, and D, follow-up in same patient. ST-segment elevation in 2 children E, lateral ST-segment elevation with chest pain because of subendocardial myocardial infarction in the territory supplied by left anterior descending and circumflex arteries. F, Pericarditis pattern of ST-segment elevation with concave ST-segment elevation.
The mean QTc during the course of the illness was still well within normal limits, with only 14 children showing a transient prolongation above 98th percentile for age, and only 5 with QTc recorded over 470 msec. We did observe, however, a longer corrected QT interval on ECGs recorded on admission and at the midpoint of stay, to those recorded prior to discharge. This delay in ventricular repolarization during the initial phases of the illness, correlated in time with the observed transient TWI in precordial leads.
Transient TWI in the anterior precordial leads and prolongation of the QTc, in adults with reversible left ventricular dysfunction, has been linked to myocardial edema on cardiac MRI in a variety of clinical conditions.19 In a smaller cohort of children presenting with MIS-C our group has shown 50% to have evidence of myocardial edema on cardiac MRI.14 The findings of transient TWI and an increase in the QTc may also reflect a delay, or dispersion, of ventricular repolarization in the context of myocardial inflammation and edema. These observations may provide electrocardiographic markers of cardiac involvement and recovery in MIS-C.
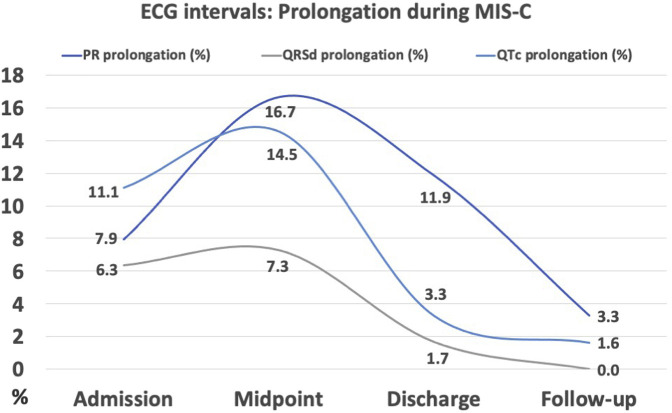
Finally, there was evidence of effects on the cardiac conduction system during the MIS-C illness with transient first degree AV block, prolongation of the QRS duration, and prolongation of QTc seen in this cohort, illustrated in Figure 4 (available at www.jpeds.com). Pavri et al have recently shown around 50% of adult patients with COVID-19 had an abnormal PR:HR slope, which correlated with the worst clinical outcomes.17 This finding occurred in 25% of our cohort, however, our data showed no correlation with either ICU admission or inotropic support.
Figure 4.
ECG intervals: Prolongation during MIS-C. ECG intervals (PR, QRS duration [QRSd], and corrected QT [QTc]) showing prolongation (>98th percentile for age), expressed as percentage of cohort affected at each time point.
An inflammatory response within the myocardium and cardiac conduction system would be expected to be reflected in electrocardiographic changes. We had anticipated a limited overlap with findings in children and adult patients presenting with polymerase chain reaction-positive COVID-19 where arrhythmias had been seen in up to 16.7% of patients, but of particular concern in those with pre-existing cardiovascular morbidity.20, 21, 22, 23 We therefore undertook a systematic analysis of ECGs in this novel disease to focus on findings previously described in children affected by myopericarditis and Kawasaki disease. Indeed, with the exception of high-grade AV block, we showed significant overlap with these diseases. Myocarditis in children having previously been associated with TWI, ST-segment changes, low voltages on the ECG, arrhythmias, varying between 11% and 45%, and less commonly atrioventricular block.24, 25, 26, 27 In Kawasaki disease, changes in ventricular repolarization, flattened T-waves, arrhythmias, and AV node dysfunction have been described.28, 29, 30, 31
The study is limited by retrospective analysis in a single center. We used the follow-up ECG as a baseline for each child and compared paired results for each individual to establish change over the course of the illness, but could not compare with the true pre-illness ECG. Thus, some changes persisting after the illness may not have been recognized. The small sample size consisting of a range of ethnic backgrounds makes comparison with “normal population” values difficult in our cohort. The number of patients included means caution should be taken in drawing conclusions on the apparently low risk of malignant arrhythmias or high-grade AV block. A multicenter study with prospective data collection is needed to confirm the cardiovascular impact of MIS-C and possible predictive or diagnostic ECG changes and arrhythmia risk in this group.
Acknowledgments
We are grateful to the multidisciplinary team who contributed their expertise to the management and care of these children, at Evelina London Children's Hospital, including pediatric intensive care, infectious diseases, rheumatology, nursing, and cardiac physiology staff.
Footnotes
The authors declare no conflicts of interest.
Supplementary Data
Appendix
Table II.
Summary and timings of biochemical and echocardiographic markers in relation to events during MIS-C illness
| Biochemical markers (peak value) | Median (IQR) | Ref. Range |
|---|---|---|
| CRP (mg/L) | 187 (118, 287.5) | 0-4 |
| Ferritin (μg/L) | 631 (354, 1120) | 22-275 |
| Troponin (ng/L) | 38 (8, 118.5) | 0-13 |
| NT-pro BNP (ng/L) | 2613 (1100, 6485) | <100 |
| Echocardiographic measurements (worst value) | ||
| Global longitudinal strain (-%) | 15 (18.35, 12) | >18% |
| Fractional shortening (%) | 29.6 (24.95, 33.7) | 28%-45% |
| Timeline of illness - Events timed from symptom onset: | Days (median, IQR): | |
| Admission to hospital | 5 (3, 5) | |
| Peak CRP | 5 (4, 7) | |
| Peak ferritin | 5 (4, 7) | |
| Peak troponin | 6 (4, 7) | |
| Peak NT-proBNP | 6 (5, 7) | |
| Peak number of negative T-waves in chest leads (ECG) | 6 (5, 7) | |
| Worst global longitudinal strain (echocardiogram) | 6 (5, 8) | |
| Discharge from PICU (those admitted) | 9 (6.5, 10.5) | |
| Discharge from hospital | 11 (10, 14) | |
| Follow-up length | 58 (44, 67) | |
CRP, C-reactive protein; Nt-pro BNP, N-terminal pro-brain-natriuretic peptide.
References
- 1.Riphagen S., Gomez X., Gonzalez-martinez C., Wilkinson N., Theocharis P. Correspondence Hyperinflammatory shock in children during COVID-19 : PCR screening of asymptomatic health- hospital. Lancet. 2020;395:1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Licciardi F., Pruccoli G., Denina M., Parodi E. SARS-CoV-2–induced Kawasaki-like hyperinflammatory syndrome: a novel COVID phenotype in children. Pediatrics. 2020;146:e20201711. doi: 10.1542/peds.2020-1711. [DOI] [PubMed] [Google Scholar]
- 3.Toubiana J., Poirault C., Corsia A., Bajolle F., Fourgeaud J., Angoulvant F., et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ. 2020;369:m2094. doi: 10.1136/bmj.m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaushik S., Aydin S.I., Derespina K.R., Bansal P.B., Kowalsky S., Trachtman R., et al. Multisystem Inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2 infection (MIS-C): a multi-institutional study from New York City. J Pediatr. 2020;224:24–29. doi: 10.1016/j.jpeds.2020.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramcharan T., Nolan O., Lai C.Y., Prabhu N., Krishnamurthy R., Richter A.G., et al. Paediatric inflammatory multisystem syndrome: temporally associated with SARS-CoV-2 (PIMS-TS): cardiac features, management and short-term outcomes at a UK tertiary paediatric hospital. Pediatr Cardiol. 2020;41:1391–1401. doi: 10.1007/s00246-020-02391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belhadjer Z., Bonnet D. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142:429–436. doi: 10.1161/CIRCULATIONAHA.120.048360. [DOI] [PubMed] [Google Scholar]
- 7.Whittaker E., Bamford A., Kenny J., Kaforou M., Jones C.E., Shah P., et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259–269. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies P., Evans C., Kanthimathinathan H.K., Lillie J., Brierley J., Waters G., et al. Intensive care admissions of children with paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) in the UK: a multicentre observational study. Lancet Child Adolesc Heal. 2020;2:1–9. doi: 10.1016/S2352-4642(20)30215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feldstein L.R., Rose E.B., Horwitz S.M., Collins J.P., Newhams M.M., Son M.B.F., et al. Multisystem inflammatory syndrome in U.S. Children and adolescents. N Engl J Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simpson J.M., Newburger J.W. Multisystem inflammatory syndrome in children in association with COVID-19. Circulation. 2020;142:437–440. doi: 10.1161/CIRCULATIONAHA.120.048726. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention Multisystem inflammatory syndrome in children (MIS-C) asociated with coronavirus disease 2019 (COVID-19) 2020. https://emergency.cdc.gov/han/2020/han00432.asp
- 12.Royal College of Paediatrics and Child Health Guidance: Paediatric multisystem inflammatory syndrome temporally associated with COVID-19. 2020. https://www.rcpch.ac.uk/sites/default/files/2020-05/COVID-19-Paediatric-multisystem-%20inflammatory%20syndrome-20200501.pdf
- 13.WHO . 2020. Multisystem inflammatory syndrome in children and adolescents with COVID-19; pp. 1–3. [Google Scholar]
- 14.Theocharis P., Wong J., Pushparajah K., Mathur S.K., Simpson J.M., Pascall E., et al. Multimodality cardiac evaluation in children and young adults with multisystem inflammation associated with COVID-19. Eur Hear J Cardiovasc Imaging. 2020;0:1–8. doi: 10.1093/ehjci/jeaa212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Domico M., McCanta A.C., Hunt J.L., Ashouri N., Nugent D., Kelly R.B. High-grade heart block requiring transvenous pacing associated with multisystem inflammatory syndrome in children during the COVID-19 pandemic. Heart Rhythm Case Rep. 2020;6:811–814. doi: 10.1016/j.hrcr.2020.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rijnbeek P.R., Witsenburg M., Schrama E., Hess J., Kors J.A. New normal limits for the paediatric electrocardiogram. Eur Heart J. 2001;22:702–711. doi: 10.1053/euhj.2000.2399. [DOI] [PubMed] [Google Scholar]
- 17.Pavri B.B., Kloo J., Farzad D., Riley J.M. Behavior of the PR interval with increasing heart rate in patients with COVID-19. Hear Rhythm. 2020;17:1–25. doi: 10.1016/j.hrthm.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ginzton L.E., Laks M.M. The differential diagnosis of acute pericarditis from the normal variant: new electrocardiographic criteria. Circulation. 1982;65:1004–1009. doi: 10.1161/01.cir.65.5.1004. [DOI] [PubMed] [Google Scholar]
- 19.Migliore F., Zorzi A., Marra M.P., Basso C., Corbetti F., De Lazzari M., et al. Myocardial edema underlies dynamic T-wave inversion (Wellens’ ECG pattern) in patients with reversible left ventricular dysfunction. Hear Rhythm. 2011;8:1629–1634. doi: 10.1016/j.hrthm.2011.04.035. [DOI] [PubMed] [Google Scholar]
- 20.Kochi A.N., Tagliari A.P., Forleo G.B., Fassini G.M., Tondo C. Cardiac and arrhythmic complications in patients with COVID-19. J Cardiovasc Electrophysiol. 2020;31:1003–1008. doi: 10.1111/jce.14479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T., et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samuel S., Friedman R.A., Sharma C., Ganigara M., Mitchell E., Schleien C., et al. Incidence of arrhythmias and electrocardiographic abnormalities in symptomatic pediatric patients with PCR-positive SARS-CoV-2 infection, including drug-induced changes in the corrected QT interval. Heart Rhythm. 2020;17:1960–1966. doi: 10.1016/j.hrthm.2020.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson B.R., Silver E.S., Richmond M.E., Liberman L. Usefulness of arrhythmias as predictors of death and resource utilization in children with myocarditis. Am J Cardiol. 2014;114:1400–1405. doi: 10.1016/j.amjcard.2014.07.074. [DOI] [PubMed] [Google Scholar]
- 25.Wang J.N., Tsai Y.C., Lee W.L., Lin C.S., Wu J.M. Complete atrioventricular block following myocarditis in children. Pediatr Cardiol. 2002;23:518–521. doi: 10.1007/s00246-002-0129-0. [DOI] [PubMed] [Google Scholar]
- 26.Miyake C.Y., Teele S.A., Chen L., Motonaga K.S., Dubin A.M., Balasubramanian S., et al. In-hospital arrhythmia development and outcomes in pediatric patients with acute myocarditis. Am J Cardiol. 2014;113:535–540. doi: 10.1016/j.amjcard.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 27.Dasgupta S., Iannucci G., Mao C., Clabby M., Oster M.E. Myocarditis in the pediatric population: A review. Congenit Heart Dis. 2019;14:868–877. doi: 10.1111/chd.12835. [DOI] [PubMed] [Google Scholar]
- 28.Sumitomo N., Karasawa K., Taniguchi K., Ichikawa R., Fukuhara J., Abe O., et al. Association of sinus node dysfunction, atrioventricular node conduction abnormality and ventricular arrhythmia in patients with Kawasaki disease and coronary involvement. Circ J. 2008;72:274–280. doi: 10.1253/circj.72.274. [DOI] [PubMed] [Google Scholar]
- 29.Reddy S., Rai M., Singh Chouhan R.R., Rao S., Kamath N. Electrocardiographic analysis of repolarization changes in South Indian Children with Kawasaki disease after the acute phase of illness. Int J Pediatr. 2018;2018:1–5. doi: 10.1155/2018/1062154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujino M., Hata T., Kuriki M., Horio K., Uchida H., Eryu Y., et al. Inflammation aggravates heterogeneity of ventricular repolarization in children with kawasaki disease. Pediatr Cardiol. 2014;35:1268–1272. doi: 10.1007/s00246-014-0926-2. [DOI] [PubMed] [Google Scholar]
- 31.Ichida F., Fatica N.S., O’Loughlin J.E., Snyder M.S., Ehlers K.H., Engle M.A. Correlation of electrocardiographic and echocardiographic changes in Kawasaki syndrome. Am Heart J. 1988;116:812–819. doi: 10.1016/0002-8703(88)90342-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.