Abstract
Background
Coronavirus disease 2019 (COVID-19) infection can cause alterations in the coagulation mechanism conditioning thrombotic phenomena such as acute limb ischemia (ALI) as the only manifestation of the infection. The aim of the study was to describe clinical and surgical characteristics of a group of patients infected with severe acute respiratory syndrome coronavirus 2 who presented ALI in the context of the COVID-19 pandemic at Lima, Peru.
Methods
A multicenter, observational, and retrospective study was performed in six general hospitals, from March to July 2020. The variables considered were the pathological history and associated habits, laboratory tests, the severity of COVID-19 infection and ALI, the anatomic location of the lesion, treatment, evolution, and discharge conditions.
Results
Thirty patients with ALI infected with COVID-19 were evaluated. Their mean age was 60 ± 15 years, the condition being more frequent in men (76.6%). The main comorbidities were arterial hypertension (33.3%), obesity (33.3%), and diabetes mellitus 2 (26.6%). There were 23.3% asymptomatic patients, and their only manifestation was ALI. Rutherford IIA and IIB stage included 93.2% of patients. The most frequent location of the thrombosis was the lower limbs (73.3% vs. 26.6%). Thrombectomy was performed in 76.6% of the patients, and amputation (primary and secondary) was performed in 30% of the patients. The mortality rate was 23.3%, all of it because of acute respiratory distress syndrome.
Conclusions
ALI is a vascular pathology associated with embolic and thrombotic processes. COVID-19 infection can cause severe alterations in coagulation mechanisms, leading some patients to present severe acute arterial complications such as thrombosis, as the only associated manifestation. We report a younger cohort than those described in other studies and with a high frequency of amputations despite adequate surgical treatment.
Introduction
COVID-19 is a pathological condition caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.1 Since the first cases reported in Wuhan (China), the symptoms of the infection have not been specific and are frequently associated with pulmonary complications.1 , 2
Different extrapulmonary manifestations have been described related to acute thromboembolic phenomena in the context of a systemic inflammatory state, endothelial injury, and platelet dysfunction at cardiopulmonary, cerebrovascular, and peripheral venous and arterial level, affecting as high as 49% of patients and overshadowing the prognosis of the disease.3, 4, 5 Recent publications have described a sudden increase in cases of arterial thrombosis in patients with COVID-19, conditioning acute limb ischemia (ALI) and constituting a surgical emergency.6 , 7
Peru is one of the most affected countries in the region by the pandemic, with a cumulative incidence of 23.57 per 1,000 habitants.8 These arterial complications, which have not been reported so far in Latin America, have been observed in our country, identifying an increase in cases of ALI in patients with COVID-19.
The objective of this study was to describe the clinical and surgical characteristics in a cohort of patients with ALI infected with COVID-19 in the main Centers of Vascular and Endovascular Surgery in Peru.
Methods
Design, Population, and Sample Size
This was a multicentered, descriptive, observational, and retrospective study. We analyzed 30 patients infected with COVID-19, diagnosed by a molecular test of nasopharyngeal smear or qualitative serological test, associated with ALI. These patients were admitted and evaluated by the vascular and endovascular surgery areas in 6 general hospitals in Lima, Peru: Guillermo Almenara Irigoyen National Hospital, Edgardo Rebagliati Martins National Hospital, Cayetano Heredia National Hospital, Hipolito Unanue National Hospital, Dos de Mayo National Hospital, and Luis N. Saenz National Police Hospital, from March to July 2020. These hospitals had a Vascular Activity Condition (VASCCON) of 2, and the surgeries that treated life-threatening emergencies and limbs of patients were prioritized.
Data Collection and Study Variables
The primary source of information were medical records and operative reports of patients treated for ALI and infected with COVID-19 during the period established by the study. The information collected was selected and arranged retrospectively according to the chronological order of their clinical and surgical care. The categorical variables considered were sex, age, body mass index (BMI), pathological history, risk factors, the severity of COVID-19 infection, anatomic location, stage of ischemia, surgery performed, and postoperative events, and the continuous variables considered were the laboratory tests obtained of the patients during hospital admission. The ALI assessment was based on the Rutherford classification system,9 and the severity of COVID-19 by the proposal of the World Health Organization.10 Primary amputation was defined as that performed without previous revascularization and secondary amputation as that performed after revascularization within the first 30 days after ALI diagnosis.
Surgical Technique
Surgeries were performed under general or regional anesthesia as required by the patient, using intravenous unfractionated heparin (UFH) before the arterial clamping. In patients with aortoiliac or femoropopliteal occlusion, an inguinal incision was used to expose the femoral bifurcation, and a brachial exposure was performed on the upper limb. When the tibial arteries were compromised, an infrageniculate approach was performed. Thromboembolectomy was executed using an embolectomy catheter, sized according to the site of occlusion. Patients were anticoagulated during hospitalization with low-molecular-weight heparin (LMWH) and UFH and at discharge with LMWH as bridging therapy to warfarin; the latter was left prescribed according to its clinical evolution and control by outpatient clinic. All embolectomy surgeries performed on our patients were technically successful, and their definition was based on the complete removal of the intraluminal clot from the affected vessel with the subsequent presence of adequate runoff and backflow.
Statistical Analysis
All statistical calculations were performed as descriptive analysis. Continuous variables were expressed as medians and interquartile ranges. The categorical variables were summarized as counts and percentages; likewise, the distribution of the frequencies in absolute values and relative values was made. In all cases, the data analysis was performed using the statistical programs Microsoft Excel 2016 and STATA MP v16 for the Windows 10 version.
Ethical Aspects
The study protocol followed standard norms of the Declaration of Helsinki and was approved by the Guillermo Almenara Irigoyen National Hospital Specialized Surgery Department (NIT 1142-2020-0237). The names and addresses of the people included in the study were not used. Because of the retrospective nature of the work, informed consent was not required, and the authors signed a letter of commitment to the confidentiality of the data.
Results
Data from 30 patients diagnosed with ALI and SARS-CoV-2 infection were evaluated. The mean age was 60 ± 15 years (32–99 years), being more frequent in males (76.6%). An average BMI of 28.6 ± 5.9 kg/m2 was found. The main antecedents were arterial hypertension (33.3%), obesity (33.3%), and diabetes mellitus 2 (26.6%). No patients with myxoma, heart failure, a history of neoplasia, cerebrovascular disease, or vascular surgical history were found. The diagnosis of COVID-19 infection was made through molecular (26.6%) and serological (73.3%) tests. There were 7 patients (23.3%) who came to the hospital only because of ALI. Anticoagulation therapy (LMWH) previous to the ALI was received in 23.3% of the patients. The laboratory tests requested are shown in Table I .
Table I.
Presurgical characteristics of patients infected by COVID-19 and ALI
| Variables | Value (n = 30) | Porcentage (%) |
|---|---|---|
| Sex | ||
| Female | 7 | 23.3 |
| Male | 23 | 76.6 |
| Age (years) | ||
| 31–45 | 5 | 16.6 |
| 46–60 | 7 | 23.3 |
| 61–75 | 23 | 76.6 |
| 76–90 | 4 | 13.3 |
| 91–105 | 1 | 3.3 |
| BMI (kg/m2) | ||
| Normal | 8 | 26.6 |
| Overweight | 12 | 40 |
| Obesity I | 4 | 13.3 |
| Obesity II | 4 | 13.3 |
| Obesity III | 2 | 6.6 |
| Background | ||
| Arterial hypertension | 10 | 33.3 |
| Obesity | 10 | 33.3 |
| Diabetes mellitus 2 | 8 | 26.6 |
| Peripheral arterial disease | 4 | 13.3 |
| Chronic coronary disease | 4 | 13.3 |
| Sedentary lifestyle | 4 | 13.3 |
| Atrial fibrillation | 3 | 10 |
| Smoking | 3 | 10 |
| Dyslipidemia | 1 | 3.3 |
| Chronic kidney disease | 1 | 3.3 |
| COVID-19 diagnosis | ||
| Molecular test | 8 | 26.6 |
| Serological test | 22 | 73.3 |
| IgM | 8 | 26.6 |
| IgG | 1 | 3.3 |
| IgM + IgG | 13 | 43.3 |
| Severity of COVID-19 | ||
| Asymptomatic | 7 | 23.3 |
| Mild | 3 | 10 |
| Moderate | 4 | 13.3 |
| Severe | 16 | 53.3 |
| Anticoagulation before to ALI | 7 | 23.3 |
| ICU stay before to ALI (days) | 5 | 16.6 |
| Laboratory exams | ||
| Hemoglobin (12–14 g/dL) | 12.9 (11.6–14.5) | |
| Leukocytes (×1,000 cells/mm3) | 11.6 (9.7–16.1) | |
| Lymphocytes (×1,000 cells/mm3) | 1.76 (1.1–3.4) | |
| Platelets (×1,000 cells/mm3) | 284 (220–371) | |
| Glucose (mg/dL) | 110 (82.5–124.5) | |
| Creatinine (mg/dL) | 0.8 (0.6–1.2) | |
| C-reactive protein (mg/dL) | 35.5 (24–61) | |
| INR (0.8–1.2) | 1.06 (0.92–1.26) | |
| aPTT (27–41) | 32.5 (28.3–37.8) | |
| Fibrinogen (2–4.2 mg/dL) | 4.8 (4.7–6.3) | |
| AST (15–46 U/L) | 56 (38–76) | |
| ALT (<50 U/L) | 55 (40–81) | |
| Albumin (3.5–5 g/dL) | 3.2 (2.9–3.8) | |
| D-dimer (up to 0.25 mg/L) | 3.2 (1.6–4.3) | |
| Ferritin (5–148 ng/mL) | 520.7 (396–862) |
ALI, acute limb ischemia; ALT, alanine transaminase; aPTT, activated partial thromboplastin time; AST, aspartate transaminase; ICU, intensive care unit; INR, international normalized ratio.
The mean ischemic time was 74.7 hr; the leading symptoms were absence of pulse (100%), pain (93.3%), and cyanosis (89.2%) (Fig. 1 ). The majority of patients (93.2%) were classified on Rutherford Stage IIA and IIB (Table II ). The anatomic location of thrombosis was 73.3% on lower limbs and 26.6% on the upper ones, with popliteal (10.7%) and brachial (8.7%) arteries being the most affected (Fig. 2 ).
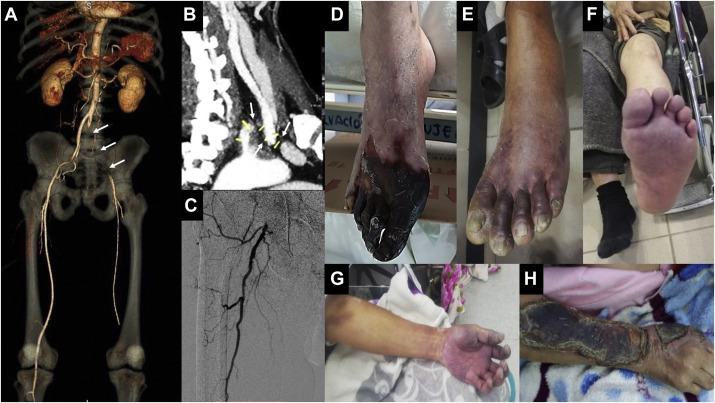
Fig. 1.
(A) CT angiography with three-dimensional reconstruction showing an intraluminal thrombus affecting the common, external, and left internal iliac arteries with distal recanalization (Arrows). (B) CT angiography showing a thrombosis at the level of the origin of the left subclavian artery (Arrow). (C) Arteriography with occlusion of the common and superficial right femoral artery. (D–H) Various clinical manifestations of ALI in patients infected by COVID-19.
Table II.
Clinical-surgical characteristics of patients infected by COVID-19 and ALI
| Variables | Value (n = 30) | Percentage (%) |
|---|---|---|
| ALI time (hr) | 39 (12–72) | |
| Clinical manifestations of the ALI | ||
| No pulse | 30 | 100 |
| Pain | 28 | 93.3 |
| Cyanosis | 25 | 89.2 |
| Paresthesia | 25 | 89.2 |
| Pallor | 23 | 76.6 |
| Motor deficit | 7 | 23.3 |
| Rutherford staging | ||
| I | 0 | 0 |
| IIA | 23 | 76.6 |
| IIB | 5 | 16.6 |
| III | 2 | 6.6 |
| Surgical treatment of the ALI | ||
| Thrombectomy | 23 | 76.6 |
| Superior limb | 6 | 20 |
| Lower limb | 17 | 46.6 |
| Amputation | 9 | 30 |
| Primary | 5 | 16.6 |
| Superior limb | 1 | 3.3 |
| Lower limb | 4 | 13.3 |
| Secondary | 4 | 13.3 |
| Superior limb | 1 | 3.3 |
| Lower limb | 3 | 10 |
| No surgical treatment | 2 | 6.6 |
ALI, acute limb ischemia.
Fig. 2.
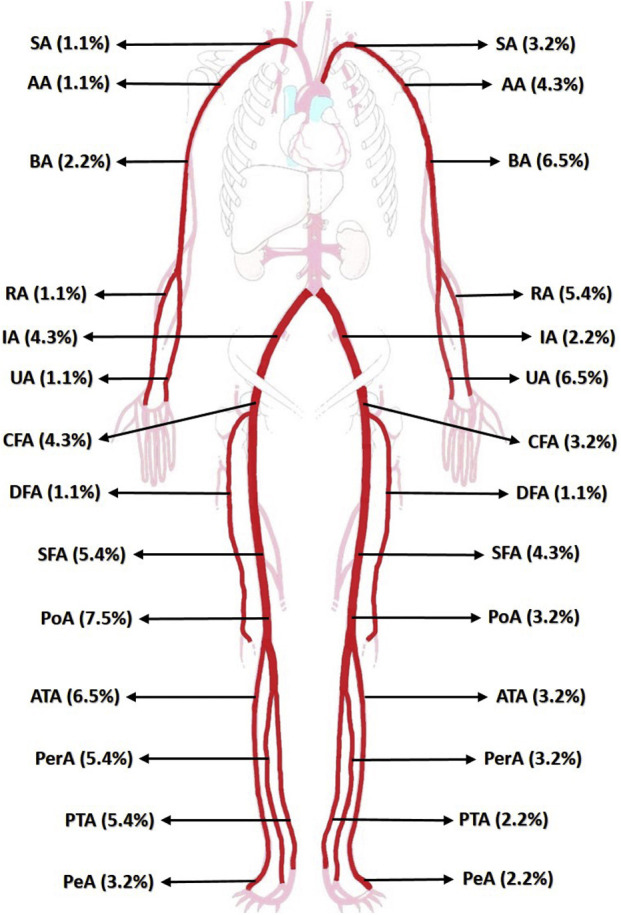
Location of the affected arterial vessel in patients infected by COVID-19 and ALI. SA, subclavian artery; AA, axillary artery; BA, braquial artery; RA, radial artery; UA, ulnar artery; IA, iliac artery; CFA, common femoral artery; DFA, deep femoral artery; SFA, superficial femoral artery; PoA, popliteal artery; ATA, anterior tibial artery; PerA, peroneal artery; PTA, posterior popliteal artery; PeA, pedia artery.
Thrombectomy and fasciotomy were performed in 23 (76.6%) and 6 (20%) patients, respectively, two of them required reoperation 120 hr average after the initial surgical treatment. Postoperative anticoagulation started at 5.7 ± 1.3 hr, and there were no postoperative complications such as bleeding, pseudoaneurysm, or infection of the operative site. Two patients (6.6%) were not operated on because of unfavorable evolution of COVID-19 (Table II). There were nine amputations (30%), five of them were primary and four were secondary. Rutherford classification patients presented mainly Stage IIA and IIB, 44.4% and 33.3%, respectively. On primary amputations, the mean ischemia time was 206.4 hr, and the surgical indication was the prolonged ischemia time. On secondary amputations, the mean ischemia time was 23.5 hr, and the reason for the surgical indication was poor clinical evolution after initial thrombectomy (Table III ).
Table III.
Amputation in patients infected by COVID-19 and ALI
| Variables | Amputation |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Primary |
Secondary |
||||||||
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 9 | |
| Sex | Female | Male | Male | Male | Female | Male | Female | Male | Male |
| Age (years) | 99 | 51 | 69 | 62 | 65 | 61 | 32 | 68 | 81 |
| Severity of COVID-19 infection | Asymptomatic | Moderate | Moderate | Severe | Moderate | Mild | Severe | Severe | Severe |
| ICU requirement | Not | Not | Not | Yes | Not | Not | Yes | Yes | Yes |
| Location of thrombosis | Infrainguinal | Iliac and infrainguinal | Infrainguinal | Infrainguinal | Upper limb | Infrainguinal | Upper limb | Infrainguinal | Infrainguinal |
| Rutherford staging | IIA | IIA | III | IIA | III | IIA | IIB | IIB | IIB |
| ALI time (hr) | 144 | 288 | 312 | 48 | 240 | 72 | 6 | 8 | 6 |
| Hospital stay (days) | 32 | 35 | 32 | 46 | 10 | 32 | 28 | 11 | 23 |
| Reason for amputation | Chronic prostration | Prolonged ALI | Prolonged ALI | ARDS | Infected limb | Unfavorable clinical course | Unfavorable clinical course | Unfavorable clinical course | Unfavorable clinical course |
ALI, acute limb ischemia; ARDS, acute respiratory distress syndrome; COVID-19, coronavirus disease 2019; ICU, intensive care unit.
The hospital stay was 16.6 ± 11.5 days, and 76.6% of the patients were discharged with ambulatory anticoagulant therapy. We reported a mortality of 23.3%, and the main cause was severe COVID-19 infection (85.7%), and a Rutherford IIB staging (71.4%) was associated (Table IV ).
Table IV.
Mortality in patients infected by COVID-19 and ALI
| Variables | Mortality |
||||||
|---|---|---|---|---|---|---|---|
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | |
| Age (years) | 65 | 63 | 32 | 68 | 81 | 58 | 52 |
| Sex | Male | Male | Female | Male | Male | Male | Male |
| Severity of COVID-19 infection | Severe | Severe | Severe | Severe | Severe | Moderate | Severe |
| ICU requirement | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Location of the ALI | Infrainguinal | Upper limb | Upper limb | Infrainguinal | Infrainguinal | Iliac and infrainguinal | Infrainguinal |
| Rutherford staging | IIA | IIA | IIB | IIB | IIB | IIB | IIB |
| Surgical treatment of ALI | Not | Not | Yes | Yes | Yes | Yes | Yes |
| Amputation | Not | Not | Yes | Yes | Yes | Yes | Not |
| Hospital stay (days) | 7 | 8 | 28 | 11 | 23 | 10 | 8 |
| Death cause | ARDS | ARDS | ARDS | ARDS | ARDS | ARDS | ARDS |
ALI, acute limb ischemia; ARDS, acute respiratory distress syndrome; COVID-19, coronavirus disease 2019; ICU, intensive care unit.
Discussion
The relationship between COVID-19 and arterial thrombosis of limbs is widely described, and it has been frequently reported on male patients especially on older patients,10 although it has also been described on younger patients without comorbidities.11
Several studies have sought the risk factors associated with the development of arterial thrombosis related to COVID-19, mainly hypertension, diabetes, dyslipidemia, heart failure, history of heart attack, and smoking,6 , 12 , 13 finding a significant association between older age and coronary disease with ALI (p < 0.001).14 In our findings, 18 patients (60%) presented only one or no risk factors, presuming that the mechanism of action of arterial thrombosis would not be directly associated with the aforementioned risk factors.15
Many of the seriously ill patients infected with COVID-19 and admitted to intensive care unit (ICU) have increased risk of presenting arterial and venous thromboembolic complications. Klok et al. described that in 184 patients with severe COVID-19, the prevalence of arterial thrombosis was 3.7%, and venous thrombosis was 28%16; however, Nicole et al. reported that the primary presentation was an acute thrombotic event, without respiratory symptoms, or mild respiratory discomfort.15 We have evidenced 14 cases between asymptomatic (23.3%), mild symptoms (10%), and moderate symptoms (13.3%), reinforcing the possibility that other pathophysiological mechanisms explain the prothrombotic state associated with COVID-19, excluding the severe condition of patients on ICU.
There is increasing evidence that associates the relationship between a prothrombotic state and COVID-19, as described in different studies that report an increase in D-dimer and fibrinogen values. Likewise, a series of 1,099 patients in China showed that 46% of the patients presented an elevated D-dimer17 , 18 and were associated with poor prognosis and higher mortality.19 The mean fibrinogen concentrations in COVID-19 patients have been reported to be usually elevated and associated with an acute phase response,20 as described in our work. The prolongation of the prothrombin time and the decrease in platelets in severe cases show that the coagulation mechanisms are significantly altered compared with uninfected people, allowing these laboratory evaluations to be classified as early predictors of severe forms of the disease.18 , 21 , 22
ALI in COVID-19 can occur in two situations. During the in-hospital evolution of severe COVID-19 infection,6 , 23, 24, 25, 26 Zhan et al. has reported a median of 19 (11–23 days) from the appearance of symptoms of the infection to the installation of ischemia27 or they can be admitted to the emergency room for this vascular condition with mild or no respiratory symptoms.28, 29, 30
We report an ischemic time of 39 hr, defined from the apparent onset of symptoms to surgical intervention. The factors that determined this time were related to the absence of an early and timely diagnosis, fear of patients going to the hospital, care focused on other more urgent symptoms of COVID-19 infection, availability of beds for management in critical areas, emergency operating rooms, and transfer of the patient to hospital emergency rooms with adequate clinical suspicion. In contrast, Bellosta et al. reported a delay in the transfer of patients with suspected ischemic symptoms of 120 min.6
The Rutherford staging found in our series was predominantly IIA (75.9%) and IIB (17.2%), a result that differs from that reported by Bellosta et al. (IIB 75% and IIA 10%).6 This could be related to the magnitude of the inflammatory response and hypercoagulability that patients develop.31 Likewise, that most of the reported patients were hospitalized (76.6%), where they were clinically evaluated and detected as having ALI by the health team. It should be noted that the diagnosis of ALI in this type of patient was not based on any proposed protocol but mainly on the comprehensive clinical evaluation of the critical patient. Bellosta series found a mortality rate of 40% compared with 24% in our series.6
Conventional catheter embolectomy was the only revascularization treatment in our series (75.9%), as most reports in both upper and lower limbs.6 , 23 , 24 , 28 Pharmacological thrombolysis was applied occasionally in some reports, associated with thrombectomy6 , 17 or as the only procedure.28 Other treatments described were angioplasties with or without a stent or bypass.6 , 26
Recently published studies, mostly from Asia, Europe, and the United States, have usually described venous involvement above the arterial since the beginning of the pandemic.7 , 31 Between short and isolated reports, more frequent arterial involvement was shown in the lower limbs, predominantly the popliteal, anterior, and posterior tibial and superficial femoral, iliac, and distal aorta.7 , 12 , 23 , 24 , 26 , 28 , 32, 33, 34 This distribution coincides with our findings where these arteries were mainly counting about 50% of the total of vessels involved.
Concerning the upper limbs, frequent involvement of the subclavian, brachial, radial, and ulnar arteries and an isolated case of the brachiocephalic trunk have been reported.24 , 29 , 32 , 34 , 35 In our series, the subclavian artery and the brachial and ulnar arteries constituted more than 25% of the total of vessels affected. On the other hand, infrequent cases of thrombosis affecting aortic arch, descending thoracic aorta, aortobifemoral bypass, femoropopliteal bypass and stent, renal artery, mesenteric, and splenic arteries associated with a dark clinical course of the disease have been reported.6 , 7 , 12
Our series reported a 30% amputation rate between primary and secondary. These results are higher than those described by Bellosta and Goldman, who reported rates of 6.3% and 25%, respectively.6 , 36 This amputation rate is higher than in patients with acute arterial thrombosis without COVID-19 infection reported in the United States and England.37 , 38 Our high amputation rate could be explained because of elevated number of patients with severe COVID-19 infection (53.3%) and a high number of moderate and severe infected patients among the amputated ones (77.7%).
In our series, 60% of patients who developed acute arterial thrombosis during their stay in the ICU required amputation because of poor clinical evolution after thrombectomy; this fact was described by Klok et al., showing a high incidence of arterial thromboembolic complications and amputations in patients who required intensive medicine and mechanical ventilatory support.16
The decision of primary amputation in our series was based on different factors such as general condition, time of ischemia, or infection of the affected limb, and not on Rutherford's staging, contrary to what was proposed by Hardman et al.39 Goldman et al. described that coagulation disorders observed in COVID-19 are usually more extensive and severe, finding worse evolution if they are associated with respiratory symptoms and leading to a high frequency of amputations.36 These findings share a relationship with ours because we found that 78% of the amputations performed in our series were on patients with moderate to severe respiratory compromise. Secondary amputation was performed on 17.4% of patients who underwent thrombectomies, a finding much higher than reported (3–5%)39 and one which could be related to the hypercoagulability state in patients infected with COVID-19,40 thus conditioning adverse results after initial revascularization and higher amputation rates in young patients with acute arterial thrombosis.41
The mortality rate in our series was 23.3%, similar to other studies with mortality rates between 23 and 40%.6 , 14 , 36 , 42 , 43 The average age of the deceased was 59 years, corresponding to the age group most affected in our population.44 It is expected that adults aged >80 years are the main affected group, with a higher rate in men in whom hypercoagulable states predominate.6 , 42 , 43 , 45 Thromboembolic phenomena and mortality correlate with the severity of the infection.6 , 14 , 35 In all the patients who died, they had greater clinical severity of COVID-19 infection, and mortality was attributed to acute respiratory distress syndrome. In deceased patients, the anatomic location of thrombosis was mainly infrainguinal (71.4%), similar to what was observed in several case reports.6 , 43
The main limitations of the present work were related to the availability of auxiliary diagnostic studies in the context of the health emergency, such as transthoracic or transesophageal echocardiography, angiographic studies and among others, which could help us to identify the etiology of ALI (embolic vs. thrombotic primary) in the context of COVID-19 infection. On the other hand, we believe that the lack of a clear and standardized care protocol for the management of these cases was one of the main limitations that weighed on the prognosis and morbidity and mortality of the disease. Finally, the descriptive nature of our study has not allowed us to conclusively conclude or affirm statistically significant premises, which is why there is an urgent need for further analytical studies to help us understand the behavior of this pathology and its respective predictive factors of poor evolution.
Conclusions
ALI is a vascular pathology associated with embolic and thrombotic processes. COVID-19 infection can cause severe alterations in coagulation mechanisms, leading some patients to present severe acute arterial complications such as thrombosis, as the only associated manifestation. We report a cohort of 30 patients younger than those described in other studies and with a high frequency of amputations despite adequate surgical treatment. Our series is one of the first reported in Latin America, with a considerable number of patients that allowed us to describe relevant characteristics of arterial thrombosis in patients infected with COVID-19. At the close of this series, we are still admitting a significant number of patients with this condition, who could be added for future research.
Footnotes
Authors’ contributions: All authors contributed equally in the design, collection, analysis, writing, and final approval of the manuscript.
Conflicts of interest: None.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Zhu N., Zhang D., Wang W., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu R., Zhao X., Li J., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta A., Madhavan M.V., Sehgal K., et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26:1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PubMed] [Google Scholar]
- 4.The Lancet H. COVID-19 coagulopathy: an evolving story. Lancet Haematol. 2020;7:e425. doi: 10.1016/S2352-3026(20)30151-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ackermann M., Verleden S.E., Kuehnel M., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellosta R., Luzzani L., Natalini G., et al. Acute limb ischemia in patients with COVID-19 pneumonia. J Vasc Surg. 2020;6:1864–1872. doi: 10.1016/j.jvs.2020.04.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Etkin Y., Conway A.M., Silpe J., et al. Acute arterial thromboembolism in patients with COVID-19 in the New York City area. Ann Vasc Surg. 2020;70:290–294. doi: 10.1016/j.avsg.2020.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Center for Epidemiology, Prevention and Control of Diseases-Ministry of Health of Peru (MHP) 2020. Daily Report "COVID-19". Lima Peru.https://www.dge.gob.pe/portalnuevo/categoria/covid-19/ [cited September 27, 2020] [Google Scholar]
- 9.Björck M., Earnshaw J.J., Acosta S., et al. Editor's choice - European Society for Vascular Surgery (ESVS) 2020 Clinical Practice Guidelines on the management of acute limb ischaemia. Eur J Vasc Endovasc Surg. 2020;59:173–218. doi: 10.1016/j.ejvs.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization (WHO) 2020. Coronavirus disease 2019 (COVID-19): situation report, 82.https://www.paho.org/es/tag/enfermedad-por-coronavirus-covid-19/ [cited September 15, 2020] [Google Scholar]
- 11.National Institute of Statistics and Informatics (NISI) 2020. XII National Census of Population and Housing 2017. Demographic Bulletin Nº39, Lima, Peru.https://www.paho.org/es/tag/enfe [cited September 20, 2020] [Google Scholar]
- 12.Kashi M., Jacquin A., Dakhil B., et al. Severe arterial thrombosis associated with Covid-19 infection. Thromb Res. 2020;192:75–77. doi: 10.1016/j.thromres.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carranza M., Salazar D.-E., Troya J., et al. Aortic thrombus in patients with severe COVID-19: review of three cases. J Thromb Thrombolysis. 2020;9:1–6. doi: 10.1007/s11239-020-02219-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bilaloglu S., Aphinyanaphongs Y., Jones S., et al. Thrombosis in hospitalized patients with COVID-19 in a New York City health system. JAMA. 2020;324:799–801. doi: 10.1001/jama.2020.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ilonzo N., Rao A., Berger K., et al. Acute thrombotic events as initial presentation of patients with COVID-19 infection. J Vasc Surg Cases Innov Tech. 2020;6:381–383. doi: 10.1016/j.jvscit.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guan W-j, Ni Z-y, Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lippi G., Favaloro E. D-dimer is Associated with Severity of Coronavirus Disease 2019: A Pooled Analysis. Thromb Haemost. 2020;120:876–878. doi: 10.1055/s-0040-1709650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levi M., Thachil J., Iba T., et al. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7:e438–e440. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baccellieri D., Bilman V., Apruzzi L., et al. A case of Covid-19 patient with acute limb ischemia and heparin resistance. Ann Vasc Surg. 2020;68:88–92. doi: 10.1016/j.avsg.2020.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perini P., Nabulsi B., Massoni C.B., et al. Acute limb ischaemia in two young, non-atherosclerotic patients with COVID-19. The Lancet. 2020;395:1546. doi: 10.1016/S0140-6736(20)31051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giacomelli E., Dorigo W., Fargion A., et al. Acute thrombosis of an aortic prosthetic graft in a patient with severe COVID-19-related pneumonia. Ann Vasc Surg. 2020;66:8–10. doi: 10.1016/j.avsg.2020.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anwar S., Acharya S., Shabih S., et al. Acute limb ischemia in COVID-19 disease: a mysterious coagulopathy. Cureus. 2020;12:e9167–e. doi: 10.7759/cureus.9167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schultz K., Wolf J.M. Digital ischemia in COVID-19 patients: case report. J Hand Surg Am. 2020;45:518–522. doi: 10.1016/j.jhsa.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mietto C., Salice V., Ferraris M., et al. Acute lower limb ischemia as clinical presentation of COVID-19 infection. Ann Vasc Surg. 2020;69:80–84. doi: 10.1016/j.avsg.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shao T., In-Bok Lee C., Jabori S., et al. Acute upper limb ischemia as the first manifestation in a patient with COVID-19. J Vasc Surg Cases Innov Tech. 2020;6:674–677. doi: 10.1016/j.jvscit.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaur P., Posimreddy S., Singh B., et al. COVID-19 presenting as acute limb ischaemia. Eur J Case Rep Intern Med. 2020;7:001724. doi: 10.12890/2020_001724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mestres G., Puigmacià R., Blanco C., et al. Risk of peripheral arterial thrombosis in COVID-19. J Vasc Surg. 2020;72:756–757. doi: 10.1016/j.jvs.2020.04.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vacirca A., Faggioli G., Pini R., et al. Unheralded lower limb threatening ischemia in a COVID-19 patient. Int J Infect Dis. 2020;96:590–592. doi: 10.1016/j.ijid.2020.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaur P., Qaqa F., Ramahi A., et al. Acute upper limb ischemia in a patient with COVID-19. Hematol Oncol Stem Cell Ther. 2020 doi: 10.1016/j.hemonc.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galanis N., Stavraka C., Agathangelidis F., et al. Coagulopathy in COVID-19 infection: a case of acute upper limb ischemia. Int J Surg Case Rep. 2020;2020:204. doi: 10.1093/jscr/rjaa204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldman I., Ye K., Scheinfeld M. Lower extremity arterial thrombosis associated with COVID-19 is characterized by greater thrombus burden and increased rate of amputation and death. Radiology. 2020;297:E263–E269. doi: 10.1148/radiol.2020202348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Genovese E.A., Chaer R.A., Taha A.G., et al. Risk factors for long-term mortality and amputation after open and endovascular treatment of acute limb ischemia. Ann Vasc Surg. 2016;30:82–92. doi: 10.1016/j.avsg.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Earnshaw J.J., Whitman B., Foy C. National audit of Thrombolysis for acute leg ischemia (NATALI): clinical factors associated with early outcome. J Vasc Surg. 2004;39:1018–1025. doi: 10.1016/j.jvs.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 39.Hardman R.L., Jazaeri O., Yi J., et al. Overview of classification systems in peripheral artery disease. Semin Interv Radiol. 2014;31:378–388. doi: 10.1055/s-0034-1393976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han H., Yang L., Liu R., et al. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020;58:1116–1120. doi: 10.1515/cclm-2020-0188. [DOI] [PubMed] [Google Scholar]
- 41.Torrealba J.I., Osman M., Kelso R. Hypercoagulability predicts worse outcomes in young patients undergoing lower extremity revascularization. J Vasc Surg. 2019;70:175–180. doi: 10.1016/j.jvs.2018.09.062. [DOI] [PubMed] [Google Scholar]
- 42.Cantador E., Núñez A., Sobrino P., et al. Incidence and consequences of systemic arterial thrombotic events in COVID-19 patients. J Thromb Thrombolysis. 2020;50:543–547. doi: 10.1007/s11239-020-02176-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheruiyot I., Kipkorir V., Kariuki B., et al. Arterial thrombosis in coronavirus disease 2019 (COVID-19) patients: a rapid systematic review. Ann Vasc Surg. 2020;70:273–281. doi: 10.1016/j.avsg.2020.08.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mejía F., Medina C., Cornejo E., et al. 2020. Clinical features and prognostic factors related to mortality in hospitalized adult patients with COVID-19 in a public hospital in Lima, Peru. [cited 27/09/2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Capuano A., Rossi F., Paolisso G. Covid-19 kills more men than women: an overview of possible reasons. Front Cardiovasc Med. 2020;7:131. doi: 10.3389/fcvm.2020.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]