Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in Wuhan, China in December 2019. On February 11, the World Health Organization (WHO) announced the name for the new illness caused by SARS-CoV-2: COVID-19. By March 11, the outbreak of COVID-19 was declared a pandemic by the WHO. This virus has extensively altered daily life for many across the globe, while claiming hundreds of thousands of lives. While fundamentally a respiratory illness, many infected individuals experience symptoms that involve the central nervous system (CNS). It is likely that many of these symptoms are the result of the virus residing outside of the CNS. However, the current evidence does indicate that the SARS-CoV-2 virus can use olfactory neurons (or other nerve tracts) to travel from the periphery into the CNS, and that the virus may also enter the brain through the blood–brain barrier (BBB). We discuss how the virus may use established infection mechanisms (ACE2, NRP1, TMPRSS2, furin and Cathepsin L), as well mechanisms still under consideration (BASIGIN) to infect and spread throughout the CNS. Confirming the impact of the virus on the CNS will be crucial in dealing with the long-term consequences of the epidemic.
1. Introduction
The symptoms initially attributed to COVID-19 included fever, cough, abnormal chest x-ray and shortness of breath, with some patients developing rales and requiring supplemental oxygen or mechanical ventilation. In the most severe cases, the estimated duration from onset of symptoms to death was ∼17.8 days (Verity et al., 2020). A surprisingly high proportion of COVID-19 patients also reported CNS symptoms during infection (Mao et al., 2020). Initial CNS manifestations include dizziness, headache, impaired consciousness, ataxia, and seizures. Interestingly, in a small subset of COVID-19 positive patients from an early study in Wuhan, 8.9% displayed peripheral nervous system manifestations and the most common of those were anosmia, or lack of smell, at 5.1% (Mao et al., 2020). Neurological manifestations were increasingly reported in confirmed positive patients (36.4% of 214 patients) and were most pronounced in the early stages for patients with severe infection (Mao et al., 2020). However, other studies have reported neurological manifestations occurring late in COVID-19 infection (De Felice et al., 2020, Wu et al., 2020). Of those experiencing anosmia, most reported symptom resolution with clinical resolution of the illness; a small subset, however, reported no return of olfaction (Yan, 2020 April 14). Because COVID-19 is such an aggressive disease, often resulting in hypoxia, severe inflammation, and abnormal clotting, it has been thought that most of the CNS symptoms are manifestations of peripheral pathologies. This view has been reinforced by the fact that angiotensin converting enzyme 2 (ACE2) and transmembrane serine protease 2 (TMPRSS2), proteins thought to be crucial for the infection and spread of SARS-CoV-2 throughout the body, have low levels of expression in the human brain (Hamming et al., 2004). However new evidence indicates that the virus can infect cells via Neuropilin 1 (NRP1) (Cantuti-Castelvetri et al., 2020, Daly et al., 2020) or (preprint) BASIGIN (BSG) (Wang, Chen et al. 2020) and that Cathepsin L (CTSL) and furin (Coutard et al., 2020) can also modify SARS-CoV-1 (Simmons et al., 2005, Huang et al., 2006) and SARS-CoV-2 (Ou et al., 2020) to facilitate infection. All these proteins have higher and broader patterns of expression (Atlas, 2020) in the human brain than ACE2 or TMPRSS2. This would allow the virus to propagate in the brain, once reaching it2. Such an infection could occur in patients with mild or severe peripheral COVID-19 symptoms. Understanding the nature and extent of CNS infection in COVID-19 is crucial for managing acute (medulla oblongata associated respiratory dysfunction) and long-term (cognitive dysfunction/impairment) consequences of COVID-19. It is clear that the CNS is being impacted by the pathological inflammation and the cytokine storm that is triggered outside of the brain by COVID-19 (Barnes et al., 2020, Chen et al., 2020, Lagunas-Rangel and Chavez-Valencia, 2020, Mehta et al., 2020, Zhao et al., 2020). Here, we describe the evidence for a direct infection of the brain, hypothesis how the virus may enter the brain, and how the virus may spread throughout the brain.
2. CNS expression of key viral infection factors
COVID-19 is primarily a pulmonary disease, and this has driven the consensus that the virus infects cells via ACE2 (Hoffmann et al., 2020, Rothan and Byrareddy, 2020) after being modified by TMPRSS2 (Bilinska et al., 2020, Butowt and Bilinska, 2020, Hoffmann et al., 2020). This focus certainly makes sense as both proteins are expressed at very high levels in the lung making them crucial to the pulmonary pathology of COVID-19 (Atlas, 2020). ACE2 and TMPRSS2 are likely expressed in the brain at low levels (Harmer et al., 2002, Doobay et al., 2007, Xia and Lazartigues, 2008, Baig et al., 2020, Bilinska et al., 2020), meaning the virus should in theory be able infect brain cells, and potentially spread throughout the brain. This is in line with preliminary (preprint) (Song et al., 2020) and published data showing that SARS-CoV-2 can infect organoid models of the human brain (Ramani et al., 2020). Based off work done in organoid models, the virus should be capable of infecting and spreading in the brain if it can gain access to it. However, the total number of cells affected would be limited by the low expression of ACE2, and proximity to TMPRSS2 expressing cells. Unfortunately, evidence indicates that the virus can be modified by multiple proteins such as CTSL (Ou et al., 2020, Shang et al., 2020) and furin (Coutard et al., 2020). While CTSL and furin may be of minimal importance for pulmonary pathology, they are expressed at much higher levels in the brain than ACE2 or TMPRSS2 (Atlas, 2020). This allows for greater viral modification in the brain than would be predicted by the pattern of ACE2 or TMPRSS2 expression alone. Additionally, it is possible that viral entry mechanisms other than ACE2 exist. For example, it has been shown that SARS-CoV-1 could enter cells via BSG (Chen et al., 2005), and preliminary evidence (preprint) suggests that the same may be true for SARS-CoV-2 (Wang et al., 2020) 1. Furthermore, NRP1 has recently been described as a novel entry mechanism for SARS-CoV-2 (Cantuti-Castelvetri et al., 2020, Daly et al., 2020). Both proteins are expressed in the brain, including the olfactory bulb (OB), at higher levels than either ACE2 or TMPRSS2 (Atlas, 2020, Iadecola et al., 2020). Additionally, NRP1 is expressed in the olfactory epithelium (OE; humans). Accordingly, SARS-CoV-2 could infect the brain if the virus reaches the brain, potentially via the OE, vagus nerve, blood brain barrier (BBB), or cerebral spinal fluid (CSF).
3. The olfactory nerve and bulb as a conduit for CNS infection
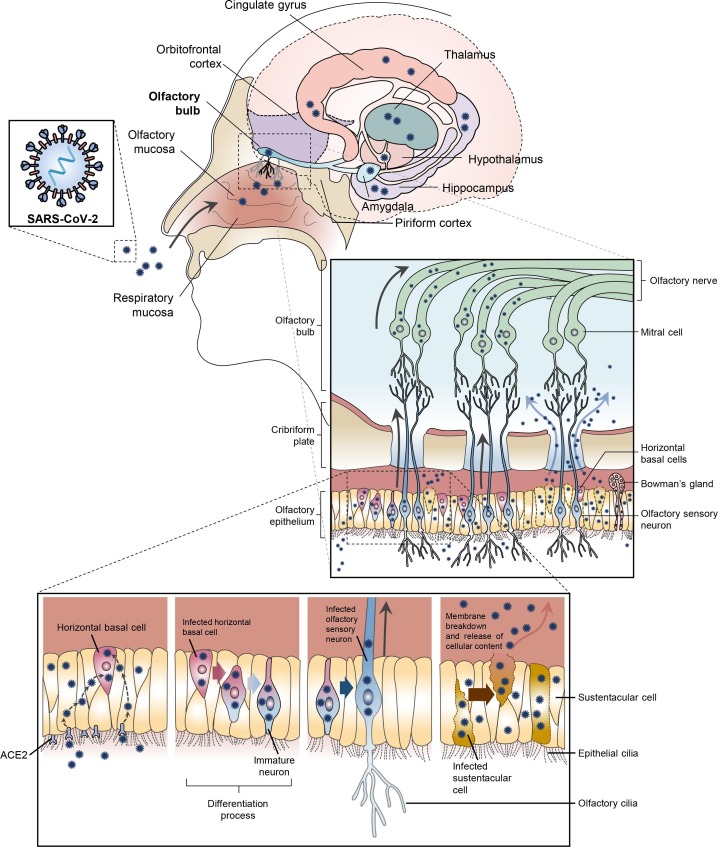
The olfactory mucosa of the nasal cavity is made up of OE containing neurons, two types of basal cells, Bowman’s glands, and epithelial cilia (Moran et al., 1982, Iwai et al., 2008, Harkema et al., 2018, Liang, 2020). Olfactory sensory neurons (OSN), also known as olfactory receptor neurons, extend through the cribriform plate and target the mitral cells of the OB. Mitral cells have connections to the largest part of the olfactory cortex, the piriform cortex, subsequently projecting to the hippocampus, hypothalamus, and orbital frontal cortex (Fig. 1 ) (Mori et al., 1999, Haberly, 2001, Shepherd, 2004, Shepherd, 2007, Buck, 2005, Heydel et al., 2013). It is thought that “new” OSNs arise from a population of immature neurons, horizontal basal cells (HBCs) (Brann and Firestein, 2014). OSNs are thought to reach maturity within 30 days (Brann and Firestein, 2010), have an average life span of 40 days, and a turnover time of 6 days (Liang, 2020). These cells are frequently maturing, dying, and being reborn. HBCs are attached directly to the basal lamina and are the progenitors of OSNs and globus basal cells, which are located above the HBC (Iwai et al., 2008, Harkema et al., 2018). Basal cells also give rise to the sustentacular cells that span the entire thickness of the OE (Harkema et al., 2018). Sustentacular cells are thought to be a mix of glial and epithelial cells. These cells support the OE and OSN metabolism, making them crucial in olfaction (Liang, 2020). When compared to HBCs, sustentacular cells are thought to have a longer life span and a slower turnover time (Arslan, 2014). It has been shown that sustentacular cells (human) express ACE2 and TMPRSS2 at high levels (Bilinska et al., 2020). These cells are vulnerable to SARS-CoV-2 infection, (Bryche et al., 2020), which may explain the high incidence of anosmia in COVID-19 (Bilinska and Butowt, 2020, Gupta et al., 2020). Once infected, damage to sustentacular cells is likely to give rise to anosmia (Brann et al., 2020, Moein et al., 2020). These infected sustentacular cells would also bath HBCs in virus, providing a second, and potentially more worrisome route to anosmia arising from infection of the OB.
Fig. 1.
Proposed mechanisms for an olfactory nerve mediated infection of the CNS. SARS-CoV-2 infects the olfactory epithelium via the ACE2 receptor. The olfactory epithelium surrounds ACE2 receptor containing horizontal basal cells. Human horizontal basal cells express ACE2, while in mice they have been shown to express NRP1 (human data is unavailable), suggesting they can be infected by SARS-CoV-2. Horizontal basal cells can also mature into olfactory neurons. We propose that infected horizontal basal cells mature into SARS-CoV-2 infected olfactory neurons. These infected olfactory neurons share a synaptic connection with neurons in the olfactory bulb (OB). This allows for viral spread from the periphery into the CNS. The OB has many connections throughout the brain. This allows for rapid viral transit to many areas in the brain. Alternatively, the infected olfactory epithelial cells release SARS-CoV-2 at the cribriform plate. The high concentration of cells combined with localized trauma (cells damaged by infection and frequent sneezing) results in viral particles being pushed through the cribriform plate. The virus can then infect local cells (mitral cells / olfactory bulb) or migrate and cause infection elsewhere.
Although ACE2 has not been reported in mature olfactory nerves (human) and is likely present at low levels in neurons (Harmer et al., 2002) and is believed to be expressed in HBCs which mature into OSNs (Hamming et al., 2004, Hamming et al., 2007, Fletcher et al., 2017, Bilinska et al., 2020, Butowt and Bilinska, 2020) 2. HBCs are surrounded by OE. An infection of the OE would likely spread to HBCs and subsequently immature and mature olfactory neurons as observed in the hamster (Zhang et al., 2020). As infected HBCs mature into OSNs, they have a synaptic path to the OB, thereby a potential route of CNS infection (Barnett et al., 1993). It would take relatively few infected OSNs to spread the infection to the OB. Through this route, a peripheral infection could reach the OB and from there, move throughout the brain. Once in the brain, a cycle of viral budding and neuronal damage is possible. Additionally, damage to the BBB, neuronal inflammation triggered by systemic infection, and stroke/hemorrhage may contribute to the outcomes of COVID-19 patients presenting with CNS symptoms.
The critical role of the olfactory nerve and OB as a transient in CNS viral transmission has been demonstrated for multiple viruses (Monath et al., 1983, Lafay et al., 1991, Mori et al., 1995, Yamada et al., 2009), including the mouse hepatitis virus (MHV), which is also a coronavirus (Perlman et al., 1990). Ablation of the OB blocked the development of CNS infection in mice that received an intranasal inoculation with MHV (Perlman et al., 1990). MHV, however, is thought to bind with carcinoembryonic antigen-related cell adhesion molecule 1 rather than ACE2. Regardless, those data show the spread of infection throughout the CNS is, in some instances, predicated on an infection of the OB. The OB also plays a key role in the spread of ACE2-dependent coronavirus infections (SARS-CoV-1). Mice expressing the human ACE2 receptor were intra-nasally treated with SARS-CoV-1, and soon after (60–66 h) antibodies were detected in the OB, some cortical areas, and parts of the basal ganglia (Netland et al., 2008). Four days after the initial exposure, the infection had spread to the medulla, pons, midbrain, thalamus, hypothalamus, amygdala, hippocampus, basal ganglia, cortex, and through most of the OB. These data demonstrate that a coronavirus will “pass through” the OB in the process of infecting more distal regions of the brain (Netland et al., 2008). Moreover, in the hamster, SARS-CoV-2 has been shown to infect olfactory nerves (Sia et al., 2020, Zhang et al., 2020) and the brain (Bryche et al., 2020, Chan et al., 2020, Zhang et al., 2020). Taken together, these data show that an infection which reaches the OB can quickly spread throughout the brain. Low levels of ACE2 in the human brain would limit the impact on the CNS. However, NRP1 is expressed at higher levels throughout the brain than ACE2, particularly in the hippocampus formation (Atlas, 2020), increasing the potential for viral mediated CNS damage.
Several other potential routes for nerve driven infection of the brain exist, such as infection via the vagus, trigeminal, and nasopharyngeal nerves. The vagus nerve, for example, as part of the enteric nervous system is connected to the gastrointestinal tract, which is high in ACE2 and NRP1 (Atlas, 2020). It has been reported that intestinal enteric neurons and glia express ACE2 and TMPRRSS2 (Deffner et al., 2020) providing a mechanism for these cells to become infected with the virus. While data in humans is lacking, the vagal complex is known to express ACE2 in rodents (Ruchaya et al., 2016). If the human vagal complex expresses an appropriate combination of SARS-COV-2 related proteins, then it is probably that an infection from the intestine could reach the brain. Similarly, the trigeminal and nasopharyngeal nerves, are both positioned (gums, tongue, pharynx) such that they would be exposed to the virus. The possibility of an infection of the trigeminal or nasopharyngeal nerve is supported by a recent case report of a COVID-19 patient developing dysphagia (Aoyagi et al., 2020). Additional information about the relative distribution of ACE2, NRP1, and other proteins is necessary to determine if these mechanisms are likely to produce CNS infections in humans.
Human data on SARS-CoV-1 or 2 brain infections are less extensive than that in the rodent literature. The first reported case of a CNS SARS-CoV-1 infection is from 2003 (Hung et al., 2003) when the SARS-CoV-1 virus was detected in the CSF of a patient. Several other case reports of CNS infection in SARS-CoV-1 infections soon followed (Lau et al., 2004, Yeh et al., 2004). A post-mortem study of individuals who died from SARS-CoV-1 found evidence of the virus in the brains of all four individuals investigated (Ding et al., 2004). Data are very limited on SARS-CoV-2 infections in the human CNS. The first description of the virus in the human CNS was in an individual who tested negative for the virus via nasal swab (Moriguchi et al., 2020), highlighting the potential limitations of this diagnostic approach. A larger post-mortem study investigating the multiorgan tropism of SARS-CoV-2 reported that 8 of 27 patients had signs of CNS infection, but in only two of those instances was the virus also detected in the blood (Puelles et al., 2020). While the sample was small and comprised of individuals who died from the disease, these data suggest that instances of CNS infection can occur via the hematogenous route in a limited number of cases (via the BBB). It is also likely that patients who died from the disorder may not be representative of the general population as the overall fatality rate, which varies with age (Wiersinga et al., 2020), is 1.4% (Salzberger et al., 2020) and the number of patients included in COVID-19 post-mortem studies up to this point is relatively small (Chen et al., 2020, Xu et al., 2020).
4. SARS-CoV-2 and the blood brain barrier
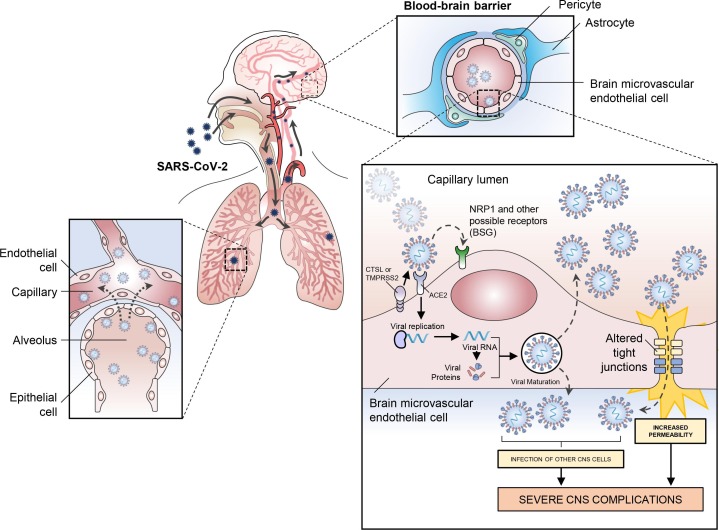
In addition to potential brain infection via the olfactory route, it is likely that SARS-CoV-2 can access the brain via the hematogenous route (Desforges et al., 2019). Reports indicating damage to lung blood vessels caused by SARS-CoV-2 have emerged (Chen et al., 2020, Lang et al., 2020, Magro et al., 2020). Post-mortem analysis of the lungs of COVID-19 patients revealed that endothelial necrosis and capillary injury can occur after SARS-CoV-2 infection with viral proteins detected in the lung capillaries (Chen et al., 2020, Magro et al., 2020). This suggests that SARS-CoV-2 can be translocated from the lungs to the pulmonary microcirculation, and then spread throughout the body and potentially infect other organs, including the brain (Fig. 2 ). Although reported in only a small number of patients, SARS-CoV-2 has been detected in the blood of COVID-19 patients indicating that it can reach the bloodstream and potentially infect other organs (Puelles et al., 2020).
Fig. 2.
Proposed mechanism of SARS-CoV-2 brain infection via the hematogenous route. Viral particles of SARS-CoV-2 present in the lungs can infect the lung capillaries and be translocated to the pulmonary microcirculation. Viral particles in the bloodstream can then reach the brain through the blood–brain barrier (BBB) by infecting and replicating inside brain microvascular endothelial cells. Infection of neurons by SARS-CoV-2 as well as an increase in BBB permeability could be responsible for severe neurological symptoms in COVID 19.
Once in the bloodstream, the virus can quickly infect endothelial cells throughout the vasculature as they express ACE2 and NRP1 (Hamming et al., 2004, Hamming et al., 2007, Wang et al., 2016). Post-mortem analyses of patients who died from COVID-19 have shown that SARS-CoV-2 can infect endothelial cells and produce endotheliitis in the lungs, heart, kidney, liver and small intestine (Varga et al., 2020). Moreover, experimental studies have also shown that SARS-CoV-2 can infect and replicate in human blood vessel organoids (Monteil et al., 2020), strengthening the hypothesis that blood-borne viruses can reach the brain through the endothelial cells of the brain. Even if bloodborne virus and bacteria are present in the circulatory system, infections of the brain are not common occurrences due to the presence of the BBB. The function of the BBB, with brain microvascular endothelial cells (BMVECs) being one of its principal components, is to protect the brain and maintain homeostasis by regulating the traffic of substances into and out of the brain and preventing the entrance of pathogens present in the bloodstream (Hawkins and Davis, 2005). To infect the brain via the hematogenous route, the virus must first bypass the BBB. Expression of the ACE2 receptor (Hamming et al., 2004), and NRP1 (Wang et al., 2016) has also been confirmed in human BMVECs, making them potential targets for SARS-CoV-2 infection (although TMPRSS2 has not yet been identified in these tissues). To date, at least one study has described via post-mortem analysis of the brain by transmission electron microscopy, the presence of viral-like proteins inside BMVECs in the frontal lobe of a patient who died from COVID-19 (Paniz‐Mondolfi et al., 2020). This provides the first direct evidence that SARS-CoV-2 can infect BMVECs of the BBB, although the origin of the infection (brain or blood) is unclear. ACE2 and TMPRSS2 have also been identified in the human choroid plexus (Deffner et al., 2020). Because the choroid plexus has a more permeable blood-CSF barrier, instead of a tightly regulated BBB, this may be a potential site of viral invasion to the CNS. A recent study in an organoid system that modeled the human choroid plexus showed that SARS-CoV-2 can infect cells in the choroid plexus and induce disruption of the blood-CSF barrier, opening another path for viral invasion to the brain (Pellegrini et al., 2020). While it is assumed that the viral particles found in the CSF are evidence of an infection of the CNS, it is also possible that viral particles in the CSF could be entering the brain via the choroid plexus or by penetrating the cribriform plate.
Because SARS-CoV-2 is generally present at only low levels in the blood, it is unlikely that in most patients a choroid plexus mediated infection would occur. In contrast, the immediate proximity of the cribriform plate to a tissue particularly susceptible to SARS-CoV-2 (nasal epithelium), and the trauma likely to occur to the region because of continual sneezing, would offer ample opportunity for CSF infiltration to occur. Infiltration of the brain via the cribriform plate is well established for amebic infection, most notably Naegleria fowleri (Jarolim et al., 2000), and more recently for Rift valley fever encephalitis (Boyles et al., 2020). Ultimately, any virus that entered the CSF through the cribriform plate would still need to penetrate or infect the BBB. However, an infection arising from infiltration at the cribriform plate could occur in any COVID-19 patient suffering from an infection of the nasal cavity. Infiltration of the CSF offers another route to CNS infection with patients who have comparatively minor symptoms. The ability of SARS-CoV-2 to infect immune cells in the periphery that subsequently invade the CNS also warrants consideration. Infected macrophages have been reported (Yao et al., 2020), strengthening the concern that immune cells may act as reservoirs of latent SARS-CoV-2 infection (Beach et al., 2020, Troyer et al., 2020).
Limited evidence exists about the effects of other types of coronaviruses on the BBB. Laboratory animal studies have shown that MHV induces BBB breakdown regulated by a decrease in the tight junction proteins occludin and ZO-1, resulting in a CNS infection (Bleau et al., 2015). In the owl monkey (Aotus trivirgatus), infection with the coronavirus JHM OMP1 via intracerebral, intranasal or intravenous inoculation resulted in CNS infection, as evidenced by the detection of viral products in the brains of all animals, predominantly in the blood vessels and perivascular regions; this suggests that a coronavirus can infect and replicate in endothelial cells to bypass the BBB (Cabirac et al., 1993, Cabirac et al., 1994). Moreover, in vitro studies later demonstrated that coronavirus JHM OMP1 can infect cultured BMVECs isolated from humans and rhesus macaques (Cabirac et al., 1995), providing further evidence that certain coronaviruses can infect BMVECs of the BBB.
Preliminary evidence suggests SARS-CoV-2 can invade BMVECs in humans (Paniz‐Mondolfi et al., 2020). In addition, a recent study, indicates that the spike protein of SARS-CoV-2 can induce an increase in BBB permeability in a BBB-on-a-chip in vitro system (Buzhdygan et al., 2020). Most importantly, emerging evidence from human studies indicates that SARS-CoV-2 induces BBB dysfunction in humans. Bellon and colleagues recently reported that, from 31 COVID-19 patients with neurological manifestations, 58% presented an increase in BBB permeability (Bellon et al., 2020), providing the first-in-human evidence that SARS-CoV-2 induces BBB dysfunction. In this study, it was unclear if the disruption of the BBB was a direct result of SARS-CoV-2 infection, or if this damage was a secondary response to neuroinflammation. More research is needed to elucidate the mechanisms by which this virus bypasses the BBB and enters the brain and whether patients with underlying conditions that affect the BBB are at an increased risk of brain infections. For example, brain expression of NRP1 increases after ischemic (Zhang et al., 2001), vascular (Klagsbrun et al., 2002) and mechanical injuries (Sköld et al., 2000), which would make these patients potentially more susceptible to brain infection. Once inside the brain, SARS-CoV-2 may be able to cause CNS infection, as receptors for virus entry (NRP1, ACE2 and BSG) and viral modification (CTSL, TMPRSS2 and others) are present in the human brain (Atlas, 2020) and viral-like particles have also been detected in neurons of postmortem COVID-19 brains (Paniz‐Mondolfi et al., 2020).
5. Conclusions
Multiple lines of evidence indicate that infection with SARS-CoV-2 can have a direct CNS-invasive component. It is unclear if this is part of the “core pathology” or if it is restricted to a small number of patients with the most advanced symptoms. CNS invasion via the OSNs or cribriform plate could both occur in patients with relatively mild symptoms. Unfortunately, the common occurrence of hypoxia in advanced cases, as well as blood clots, and cytokine “storms” makes it difficult to determine what CNS complications are the result of a CNS infection, as opposed to having a peripheral origin. Furthermore, BMVECs express the ACE2 and NRP1 receptors, implying that viruses which reach the bloodstream have the potential to infect and damage the BBB. This can lead to an infection of the brain, “leakage” in the BBB, and could disrupt vital nutrient exchange between the brain and bloodstream. Unfortunately, animal models may be of little value in evaluating the contribution of CNS infection to CNS pathology in cases of COVID-19. Protein data suggests that humans express little ACE2 and TMPRSS2 in the CNS, whereas rodents (Atlas, 2020) and humanized mice are known to display higher levels than humans (Tseng et al., 2007, Netland et al., 2008). Modeling a non-ACE2/TMPRSS2 CNS infection would require the manipulation of multiple genes in a spatially restricted manner. Differences in the density and distribution of ACE2, TMPRSS2, CTSL, NRP1, and BSG in the brain and periphery mean that humanized rodent models cannot be used to accurately predict the spread of SARS-CoV-2 in the CNS. Moreover, traditional post-mortem analysis (immunohistochemistry) of brains from individuals who died of COVID-19 may also be of limited value. It may prove more effective to quantify the number of cells expressing entry proteins and correlate with the number of dead or dying neurons.
The high incidence of hypoxia induced brain damage and other peripheral events which can damage the brain, makes it very difficult to detected evidence of SARS-CoV-2 related CNS infection. Also, in post-mortem studies the interval from infection to tissue collection is not consistent. Further, an absence of the virus in the brain does not necessary rule out a CNS infection; it rules out a CNS infection as the time of death. The distribution of entry proteins will ultimately determine the extent to which the virus can impact cells within the CNS. For instance, if only 1 in 1000 cells expresses an entry protein, and these cells are scattered throughout the brain in multiple cell types, then the virus may not have a major lasting impact on CNS function in a healthy adult. However, if its presence is restricted to a cellular subtype or region, the impact of infection could be much greater even if relatively few cells actually die from infection. Owing to the pattern of expression of NRP1, the hippocampal formation would be an area of particular concern (Atlas, 2020). The most effective approach to determining the potential damage caused by a CNS infection of SARS-CoV-2 may be to determine the relative distribution of ACE2 and NRP1, as well as other potential entry candidates (BSG) in the human brain, in addition to what type(s) of brain cells express these proteins. It may be difficult to detect viral mediated cell death against a background of neuronal insult, especially in brain regions where the expression level of ACE2 and NRP1 is low. The absence of these cells types in post-mortem tissue may represent the most definitive data on the impact of viral mediated cell death in COVID-19. Similarly, it will be necessary to determine the location of modifying proteins (TMPRSS2, CTSL) to determine if the conditions for post-shedding viral modification exist. This could be done with selective immunohistopathology on post-mortem tissue. Better understanding the pattern of viral mediated cell damage would also help determine how virus is getting into the brain, as each entry mechanism is likely to result in a different pattern of damage. For example, an OSN or cribriform plate mediated infection should preferentially impact cortical regions, while infections originating in the vagus, trigeminal, and nasopharyngeal nerves would be expected to initially impact the hindbrain and an infection due to a compromised BBB would result in wide-spread brain damage. Understanding this is crucial, as a forebrain-based infection would be expected to initially impact cognition, whereas a hindbrain infection would be expected to initially impact autonomic function, including respiration.
Currently, fighting the spread and respiratory complications of infection are the greatest priority in the battle against COVID-19. However, as the pandemic shifts and survivors of the infection increasingly outnumber those with active infections, it will become increasingly important to understand the other ways COVID-19 may have resulted in lasting effects on the human body, including the brain.
6. Disclaimer
The authors declare no competing interests, are employed solely by the U.S. government, and are not affiliated or employed with any company having any financial interests relating to the research presented or its implications.
This study did not generate/analyze datasets/code This manuscript has been processed for clearing/consent for publication via the FDA document tracking system. However, the contents of this manuscript do not necessarily reflect the views and policies or position of the U.S. Food and Drug Administration, nor does the mention of trade names or commercial products constitute the endorsement or any recommendation for use. The information in these materials is not a formal dissemination of information by FDA.
Acknowledgments
Acknowledgements
We dedicate this manuscript to all the medical professionals who put themselves in harm’s way during the SARS and COVID-19 outbreaks. Thank you.
This work was funded by the National Center for Toxicological Research.
We would like to thank Sarah Sabatelli for her timely assistance in document translation. We would like to thank Drs. Sherry Ferguson, Tim Flanigan, and Amy Inselman for their comments on this manuscript.
Figures were prepared with the Motifolio PPT-Toolkits-Biology-Bundle (Motifolio Inc., Ellicott City, USA).
Footnotes
Pre-print data should be interpreted with caution. However, given the rapidly evolving amount of information on SARS-CoV-2, completely abstaining from referring to preprints in this field runs the risk of delaying important and potentially impactful cellular mechanisms. They are included where they impact the potential for SARS-CoV-2 infection within the CNS.
Expression of NRP1 has been reported in OSNs in the mouse olfactory system (Imai, Suzuki et al. 2006, Imai, Yamazaki et al. 2009). ; we are aware of no data on its presence in the human OSN at this time
References
- Aoyagi Y., Ohashi M., Funahashi R., Otaka Y., Saitoh E. Oropharyngeal dysphagia and aspiration pneumonia following coronavirus disease 2019: a case report. Dysphagia. 2020;35(4):545–548. doi: 10.1007/s00455-020-10140-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arslan O. second ed. CRC Press; 2014. Olfactory System. Neuroanatomical Basis of Clinical Neurology; pp. 377–386. [Google Scholar]
- Atlas, H. P., 2020. “The Human Protein Atlas.” Retrieved Sept 3, 2020, from https://www.proteinatlas.org/.
- Baig A.M., Khaleeq A., Ali U., Syeda H. Evidence of the COVID-19 Virus Targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem. Neurosci. 2020;11(7):995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- Barnes B.J., Adrover J.M., Baxter-Stoltzfus A., Borczuk A., Cools-Lartigue J., Crawford J.M., Dassler-Plenker J., Guerci P., Huynh C., Knight J.S., Loda M., Looney M.R., McAllister F., Rayes R., Renaud S., Rousseau S., Salvatore S., Schwartz R.E., Spicer J.D., Yost C.C., Weber A., Zuo Y., Egeblad M. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J. Exp. Med. 2020;217(6) doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett E.M., Cassell M.D., Perlman S. Two neurotropic viruses, herpes simplex virus type 1 and mouse hepatitis virus, spread along different neural pathways from the main olfactory bulb. Neuroscience. 1993;57(4):1007–1025. doi: 10.1016/0306-4522(93)90045-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach S.R., Praschan N.C., Hogan C., Dotson S., Merideth F., Kontos N., Fricchione G.L., Smith F.A. Delirium in COVID-19: a case series and exploration of potential mechanisms for central nervous system involvement. Gen. Hosp. Psychiatry. 2020;65:47–53. doi: 10.1016/j.genhosppsych.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellon M., Schweblin C., Lambeng N., Cherpillod P., Vazquez J., Lalive P.H., Schibler M., Deffert C. Cerebrospinal fluid features in SARS-CoV-2 RT-PCR positive patients. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilinska K., Butowt R. Anosmia in COVID-19: a bumpy road to establishing a cellular mechanism. ACS Chem. Neurosci. 2020;11(15):2152–2155. doi: 10.1021/acschemneuro.0c00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilinska K., Jakubowska P., Von Bartheld C.S., Butowt R. Expression of the SARS-CoV-2 entry proteins, ACE2 and TMPRSS2, in cells of the olfactory epithelium: identification of cell types and trends with age. ACS Chem. Neurosci. 2020;11(11):1555–1562. doi: 10.1021/acschemneuro.0c00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleau C., Filliol A., Samson M., Lamontagne L., Perlman S. Brain invasion by mouse hepatitis virus depends on impairment of tight junctions and beta interferon production in brain microvascular endothelial cells. J. Virol. 2015;89(19):9896–9908. doi: 10.1128/JVI.01501-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyles D.A., Schwarz M.M., Albe J.R., McMillen C.M., O'Malley K.J., Reed D.S., Hartman A.L. Development of Rift valley fever encephalitis in rats is mediated by early infection of olfactory epithelium and neuroinvasion across the cribriform plate. J. Gen. Virol. 2020 doi: 10.1099/jgv.0.001522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brann J.H., Firestein S. Regeneration of new neurons is preserved in aged vomeronasal epithelia. J. Neurosci. 2010;30(46):15686–15694. doi: 10.1523/JNEUROSCI.4316-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brann J.H., Firestein S.J. A lifetime of neurogenesis in the olfactory system. Front. Neurosci. 2014;8:182. doi: 10.3389/fnins.2014.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brann D.H., Tsukahara T., Weinreb C., Lipovsek M., Van den Berge K., Gong B., Chance R., Macaulay I.C., Chou H.-J., Fletcher R.B., Das D., Street K., de Bezieux H.R., Choi Y.-G., Risso D., Dudoit S., Purdom E., Mill J., Hachem R.A., Matsunami H., Logan D.W., Goldstein B.J., Grubb M.S., Ngai J., Datta S.R. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci. Adv. 2020;6(31) doi: 10.1126/sciadv.abc5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryche B., St Albin A., Murri S., Lacote S., Pulido C., Ar Gouilh M., Lesellier S., Servat A., Wasniewski M., Picard-Meyer E., Monchatre-Leroy E., Volmer R., Rampin O., Le Goffic R., Marianneau P., Meunier N. Massive transient damage of the olfactory epithelium associated with infection of sustentacular cells by SARS-CoV-2 in golden Syrian hamsters. Brain Behav. Immun. 2020 doi: 10.1016/j.bbi.2020.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck L.B. Unraveling the sense of smell (Nobel lecture) Angew. Chem. Int. Ed. Engl. 2005;44(38):6128–6140. doi: 10.1002/anie.200501120. [DOI] [PubMed] [Google Scholar]
- Butowt R., Bilinska K. SARS-CoV-2: olfaction, brain infection, and the urgent need for clinical samples allowing earlier virus detection. ACS Chem. Neurosci. 2020;11(9):1200–1203. doi: 10.1021/acschemneuro.0c00172. [DOI] [PubMed] [Google Scholar]
- Buzhdygan T.P., DeOre B.J., Baldwin-Leclair A., Bullock T.A., McGary H.M., Khan J.A., Razmpour R., Hale J.F., Galie P.A., Potula R., Andrews A.M., Ramirez S.H. The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in-vitro models of the human blood-brain barrier. Neurobiol. Dis. 2020;146 doi: 10.1016/j.nbd.2020.105131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabirac G.F., Soike K.F., Butunoi C., Hoel K., Johnson S., Cai G.Y., Murray R.S. Coronavirus JHM OMP1 pathogenesis in owl monkey CNS and coronavirus infection of owl monkey CNS via peripheral routes. Adv. Exp. Med. Biol. 1993;342:347–352. doi: 10.1007/978-1-4615-2996-5_53. [DOI] [PubMed] [Google Scholar]
- Cabirac G.F., Soike K.F., Zhang J.-Y., Hoel K., Butunoi C., Cai G.-Y., Johnson S., Murray R.S. Entry of coronavirus into primate CNS following peripheral infection. Microb. Pathog. 1994;16(5):349–357. doi: 10.1006/mpat.1994.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabirac G.F., Murray R.S., McLaughlin L.B., Skolnick D.M., Hogue B., Dorovini-Zis K., Didier P.J. In vitro interaction of coronaviruses with primate and human brain microvascular endothelial cells. Adv. Exp. Med. Biol. 1995;380:79–88. doi: 10.1007/978-1-4615-1899-0_11. [DOI] [PubMed] [Google Scholar]
- Cantuti-Castelvetri L., Ojha R., Pedro L.D., Djannatian M., Franz J., Kuivanen S., van der Meer F., Kallio K., Kaya T., Anastasina M., Smura T., Levanov L., Szirovicza L., Tobi A., Kallio-Kokko H., Osterlund P., Joensuu M., Meunier F.A., Butcher S.J., Winkler M.S., Mollenhauer B., Helenius A., Gokce O., Teesalu T., Hepojoki J., Vapalahti O., Stadelmann C., Balistreri G., Simons M. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science. 2020 doi: 10.1126/science.abd2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.F., Zhang A.J., Yuan S., Poon V.K., Chan C.C., Lee A.C., Chan W.M., Fan Z., Tsoi H.W., Wen L., Liang R., Cao J., Chen Y., Tang K., Luo C., Cai J.P., Kok K.H., Chu H., Chan K.H., Sridhar S., Chen Z., Chen H., To K.K., Yuen K.Y. Simulation of the clinical and pathological manifestations of Coronavirus Disease 2019 (COVID-19) in golden Syrian hamster model: implications for disease pathogenesis and transmissibility. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Mi L.i., Xu J., Yu J., Wang X., Jiang J., Xing J., Shang P., Qian A., Li Y.u., Shaw P., Wang J., Duan S., Ding J., Fan C., Zhang Y., Yang Y., Yu X., Feng Q., Li B., Yao X., Zhang Z., Li L., Xue X., Zhu P. Function of HAb18G/CD147 in invasion of host cells by severe acute respiratory syndrome coronavirus. J. Infect. Dis. 2005;191(5):755–760. doi: 10.1086/427811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T., Wu D., Chen H., Yan W., Yang D., Chen G., Ma K., Xu D., Yu H., Wang H., Wang T., Guo W., Chen J., Ding C., Zhang X., Huang J., Han M., Li S., Luo X., Zhao J., Ning Q. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368 doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutard B., Valle C., de Lamballerie X., Canard B., Seidah N.G., Decroly E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antiviral Res. 2020;176 doi: 10.1016/j.antiviral.2020.104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly J.L., Simonetti B., Klein K., Chen K.E., Williamson M.K., Anton-Plagaro C., Shoemark D.K., Simon-Gracia L., Bauer M., Hollandi R., Greber U.F., Horvath P., Sessions R.B., Helenius A., Hiscox J.A., Teesalu T., Matthews D.A., Davidson A.D., Collins B.M., Cullen P.J., Yamauchi Y. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science. 2020 doi: 10.1126/science.abd3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice F.G., Tovar-Moll F., Moll J., Munoz D.P., Ferreira S.T. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the central nervous system. Cell Press Rev. (Trends Neurosci.) 2020;3 doi: 10.1016/j.tins.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deffner F., Scharr M., Klingenstein S., Klingenstein M., Milazzo A., Scherer S., Wagner A., Hirt B., Mack A.F., Neckel P.H. Histological evidence for the enteric nervous system and the choroid plexus as alternative routes of neuroinvasion by SARS-CoV2. Front. Neuroanat. 2020;14 doi: 10.3389/fnana.2020.596439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desforges M., Le Coupanec A., Dubeau P., Bourgouin A., Lajoie L., Dubé M., Talbot P.J. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2019;12(1) doi: 10.3390/v12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y., He L.i., Zhang Q., Huang Z., Che X., Hou J., Wang H., Shen H., Qiu L., Li Z., Geng J., Cai J., Han H., Li X., Kang W., Weng D., Liang P., Jiang S. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J. Pathol. 2004;203(2):622–630. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doobay M.F., Talman L.S., Obr T.D., Tian X., Davisson R.L., Lazartigues E. Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin-angiotensin system. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292(1):R373–381. doi: 10.1152/ajpregu.00292.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher R.B., Das D., Gadye L., Street K.N., Baudhuin A., Wagner A., Cole M.B., Flores Q., Choi Y.G., Yosef N., Purdom E., Dudoit S., Risso D., Ngai J. Deconstructing olfactory stem cell trajectories at single-cell resolution. Cell Stem Cell. 2017;20(6):817–830 e818. doi: 10.1016/j.stem.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta K., Mohanty S.K., Mittal A., Kalra S., Kumar S., Mishra T., Ahuja J., Sengupta D., Ahuja G. The cellular basis of the loss of smell in 2019-nCoV-infected individuals. Brief Bioinform. 2020 doi: 10.1093/bib/bbaa168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberly L.B. Parallel-distributed processing in olfactory cortex: new insights from morphological and physiological analysis of neuronal circuitry. Chem. Senses. 2001;26(5):551–576. doi: 10.1093/chemse/26.5.551. [DOI] [PubMed] [Google Scholar]
- Hamming I., Timens W., Bulthuis M.L.C., Lely A.T., Navis G.J., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming I., Cooper M.E., Haagmans B.L., Hooper N.M., Korstanje R., Osterhaus A.D.M.E., Timens W., Turner A.J., Navis G., van Goor H. The emerging role of ACE2 in physiology and disease. J. Pathol. 2007;212(1):1–11. doi: 10.1002/path.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkema, J., Carey, S., and Wagner, J., 2018. Nose, Sinus, Pharynx, and Larynx. in: Treutine, P., D. S. and Montine, K. (eds.) Comparative Anatomy and Histology, 2nd Edition. , Academic Press: 89-114.
- Harmer D., Gilbert M., Borman R., Clark K.L. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett. 2002;532(1–2):107–110. doi: 10.1016/s0014-5793(02)03640-2. [DOI] [PubMed] [Google Scholar]
- Hawkins B.T., Davis T.P. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol. Rev. 2005;57(2):173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- Heydel J.M., Coelho A., Thiebaud N., Legendre A., Le Bon A.M., Faure P., Neiers F., Artur Y., Golebiowski J., Briand L. Odorant-binding proteins and xenobiotic metabolizing enzymes: implications in olfactory perireceptor events. Anat. Rec. (Hoboken) 2013;296(9):1333–1345. doi: 10.1002/ar.22735. [DOI] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Muller M.A., Drosten C., Pohlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280 e278. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang I.-C., Bosch B.J., Li F., Li W., Lee K.H., Ghiran S., Vasilieva N., Dermody T.S., Harrison S.C., Dormitzer P.R., Farzan M., Rottier P.J.M., Choe H. SARS coronavirus, but not human coronavirus NL63, utilizes cathepsin L to infect ACE2-expressing cells. J. Biol. Chem. 2006;281(6):3198–3203. doi: 10.1074/jbc.M508381200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung E.C., Chim S.S., Chan P.K., Tong Y.K., Ng E.K., Chiu R.W., Leung C.B., Sung J.J., Tam J.S., Lo Y.M. Detection of SARS coronavirus RNA in the cerebrospinal fluid of a patient with severe acute respiratory syndrome. Clin. Chem. 2003;49(12):2108–2109. doi: 10.1373/clinchem.2003.025437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C., Anrather J., Kamel H. Effects of COVID-19 on the nervous system. Cell. 2020;183(1):16–27.e1. doi: 10.1016/j.cell.2020.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai N., Zhou Z., Roop D.R., Behringer R.R. Horizontal basal cells are multipotent progenitors in normal and injured adult olfactory epithelium. Stem Cells. 2008;26(5):1298–1306. doi: 10.1634/stemcells.2007-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarolim K.L., McCosh J.K., Howard M.J., John D.T. A light microscopy study of the migration of Naegleria fowleri from the nasal submucosa to the central nervous system during the early stage of primary amebic meningoencephalitis in mice. J. Parasitol. 2000;86(1):50–55. doi: 10.1645/0022-3395(2000)086[0050:ALMSOT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Klagsbrun M., Takashima S., Mamluk R. The role of neuropilin in vascular and tumor biology. Adv. Exp. Med. Biol. 2002;515:33–48. doi: 10.1007/978-1-4615-0119-0_3. [DOI] [PubMed] [Google Scholar]
- Lafay F., Coulon P., Astic L., Saucier D., Riche D., Holley A., Flamand A. Spread of the CVS strain of rabies virus and of the avirulent mutant AvO1 along the olfactory pathways of the mouse after intranasal inoculation. Virology. 1991;183(1):320–330. doi: 10.1016/0042-6822(91)90145-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagunas-Rangel F.A., Chavez-Valencia V. High IL-6/IFN-gamma ratio could be associated with severe disease in COVID-19 patients. J. Med. Virol. 2020 doi: 10.1002/jmv.25900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang M., Som A., Mendoza D.P., Flores E.J., Reid N., Carey D., Li M.D., Witkin A., Rodriguez-Lopez J.M., Shepard J.-A., Little B.P. Hypoxaemia related to COVID-19: vascular and perfusion abnormalities on dual-energy CT. Lancet Infect. Dis. 2020;20(12):1365–1366. doi: 10.1016/S1473-3099(20)30367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau K.K., Yu W.C., Chu C.M., Lau S.T., Sheng B., Yuen K.Y. Possible central nervous system infection by SARS coronavirus. Emerg. Infect. Dis. 2004;10(2):342–344. doi: 10.3201/eid1002.030638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang F. Sustentacular cell enwrapment of olfactory receptor neuronal dendrites: an update. Genes (Basel) 2020;11(5) doi: 10.3390/genes11050493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magro C., Mulvey J.J., Berlin D., Nuovo G., Salvatore S., Harp J., Baxter-Stoltzfus A., Laurence J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl. Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., Chang J., Hong C., Zhou Y., Wang D., Miao X., Li Y., Hu B. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J., U. K. Hlh Across Speciality Collaboration COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moein, S. T., Hashemian, S. M. R., Mansourafshar, B., Khorram-Tousi, A., Tabarsi, P. and Doty, R. L., 2020. “Smell dysfunction: a biomarker for COVID-19.” Int Forum Allergy Rhinol. [DOI] [PMC free article] [PubMed]
- Monath T.P., Cropp C.B., Harrison A.K. Mode of entry of a neurotropic arbovirus into the central nervous system. Reinvestigation of an old controversy. Lab. Invest. 1983;48(4):399–410. [PubMed] [Google Scholar]
- Monteil V., Kwon H., Prado P., Hagelkruys A., Wimmer R.A., Stahl M., Leopoldi A., Garreta E., Hurtado Del Pozo C., Prosper F., Romero J.P., Wirnsberger G., Zhang H., Slutsky A.S., Conder R., Montserrat N., Mirazimi A., Penninger J.M. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181(4):905–913 e907. doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran D.T., Rowley J.C., Jafek B.W., Lovell M.A. The fine structure of the olfactory mucosa in man. J. Neurocytol. 1982;11(5):721–746. doi: 10.1007/BF01153516. [DOI] [PubMed] [Google Scholar]
- Mori I., Komatsu T., Takeuchi K., Nakakuki K., Sudo M., Kimura Y. Parainfluenza virus type 1 infects olfactory neurons and establishes long-term persistence in the nerve tissue. J. Gen. Virol. 1995;76(Pt 5):1251–1254. doi: 10.1099/0022-1317-76-5-1251. [DOI] [PubMed] [Google Scholar]
- Mori K., Nagao H., Yoshihara Y. The olfactory bulb: coding and processing of odor molecule information. Science. 1999;286(5440):711–715. doi: 10.1126/science.286.5440.711. [DOI] [PubMed] [Google Scholar]
- Moriguchi T., Harii N., Goto J., Harada D., Sugawara H., Takamino J., Ueno M., Sakata H., Kondo K., Myose N., Nakao A., Takeda M., Haro H., Inoue O., Suzuki-Inoue K., Kubokawa K., Ogihara S., Sasaki T., Kinouchi H., Kojin H., Ito M., Onishi H., Shimizu T., Sasaki Y., Enomoto N., Ishihara H., Furuya S., Yamamoto T., Shimada S. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int. J. Infect. Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netland J., Meyerholz D.K., Moore S., Cassell M., Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J. Virol. 2008;82(15):7264–7275. doi: 10.1128/JVI.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., Guo L., Guo R., Chen T., Hu J., Xiang Z., Mu Z., Chen X., Chen J., Hu K., Jin Q., Wang J., Qian Z. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020;11(1):1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paniz‐Mondolfi A., Bryce C., Grimes Z., Gordon R.E., Reidy J., Lednicky J., Sordillo E.M., Fowkes M. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) J. Med. Virol. 2020;92(7):699–702. doi: 10.1002/jmv.25915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini L., Albecka A., Mallery D.L., Kellner M.J., Paul D., Carter A.P., James L.C., Lancaster M.A. SARS-CoV-2 infects the brain choroid plexus and disrupts the blood-CSF barrier in human brain organoids. Cell Stem Cell. 2020;27(6):951–961.e5. doi: 10.1016/j.stem.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman S., Evans G., Afifi A. Effect of olfactory bulb ablation on spread of a neurotropic coronavirus into the mouse brain. J. Exp. Med. 1990;172(4):1127–1132. doi: 10.1084/jem.172.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puelles V.G., Lütgehetmann M., Lindenmeyer M.T., Sperhake J.P., Wong M.N., Allweiss L., Chilla S., Heinemann A., Wanner N., Liu S., Braun F., Lu S., Pfefferle S., Schröder A.S., Edler C., Gross O., Glatzel M., Wichmann D., Wiech T., Kluge S., Pueschel K., Aepfelbacher M., Huber T.B. Multiorgan and renal tropism of SARS-CoV-2. N. Engl. J. Med. 2020;383(6):590–592. doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramani A., Muller L., Niklas Ostermann P., Gabriel E., Abida-Islam P., Muller-Schiffmann A., Mariappan A., Goureau O., Gruell H., Walker A., Andree M., Hauka S., Houwaart T., Dilthey A., Wohlgemuth K., Omran H., Klein F., Wieczorek D., Adams O., Timm J., Korth C., Schaal H., Gopalakrishnan J. SARS-CoV-2 targets neurons of 3D human brain organoids. EMBO J. 2020;e2020106230 doi: 10.15252/embj.2020106230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruchaya P.J., Speretta G.F., Blanch G.T., Li H., Sumners C., Menani J.V., Colombari E., Colombari D.S. Overexpression of AT2R in the solitary-vagal complex improves baroreflex in the spontaneously hypertensive rat. Neuropeptides. 2016;60:29–36. doi: 10.1016/j.npep.2016.06.006. [DOI] [PubMed] [Google Scholar]
- Salzberger B., Buder F., Lampl B., Ehrenstein B., Hitzenbichler F., Hanses F. Epidemiology of SARS-CoV-2 infection and COVID-19. Internist (Berl) 2020;61(8):782–788. doi: 10.1007/s00108-020-00834-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang J., Ye G., Shi K., Wan Y., Luo C., Aihara H., Geng Q., Auerbach A., Li F. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581(7807):221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd G.M. The human sense of smell: are we better than we think? PLoS Biol. 2004;2(5):e146. doi: 10.1371/journal.pbio.0020146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd G.M. Perspectives on olfactory processing, conscious perception, and orbitofrontal cortex. Ann. N. Y. Acad. Sci. 2007;1121:87–101. doi: 10.1196/annals.1401.032. [DOI] [PubMed] [Google Scholar]
- Sia S.F., Yan L.M., Chin A.W.H., Fung K., Choy K.T., Wong A.Y.L., Kaewpreedee P., Perera R., Poon L.L.M., Nicholls J.M., Peiris M., Yen H.L. Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature. 2020 doi: 10.1038/s41586-020-2342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons G., Gosalia D.N., Rennekamp A.J., Reeves J.D., Diamond S.L., Bates P. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc. Natl. Acad. Sci. U.S.A. 2005;102(33):11876–11881. doi: 10.1073/pnas.0505577102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sköld M., Cullheim S., Hammarberg H., Piehl F., Suneson A., Lake S., Sjögren A., Walum E., Risling M. Induction of VEGF and VEGF receptors in the spinal cord after mechanical spinal injury and prostaglandin administration. Eur. J. Neurosci. 2000;12(10):3675–3686. doi: 10.1046/j.1460-9568.2000.00263.x. [DOI] [PubMed] [Google Scholar]
- Song E., Zhang C., Israelow B., Lu P., Weizman O.-E., Liu F., Dai Y., Szigeti-Buck K., Yasumoto Y., Wang G., Castaldi C., Heltke J., Ng E., Wheeler J., Alfajaro M.M., Fontes B., Ravindra N.G., Van Dijk D., Mane S., Gunel M., Ring A., Wilen C.B., Horvath T.L., Louvi A., Farhadian S.F., Bilguvar K., Iwasaki A. Neuroinvasive potential of SARS-CoV-2 revealed in a human brain organoid model. bioRxiv. 2020 2020.2006.2025.169946. [Google Scholar]
- Troyer E.A., Kohn J.N., Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav. Immun. 2020;87:34–39. doi: 10.1016/j.bbi.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng C.-T., Huang C., Newman P., Wang N., Narayanan K., Watts D.M., Makino S., Packard M.M., Zaki S.R., Chan T.-S., Peters C.J. Severe acute respiratory syndrome coronavirus infection of mice transgenic for the human Angiotensin-converting enzyme 2 virus receptor. J. Virol. 2007;81(3):1162–1173. doi: 10.1128/JVI.01702-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., Mehra M.R., Schuepbach R.A., Ruschitzka F., Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verity R., Okell L.C., Dorigatti I., Winskill P., Whittaker C., Imai N., Cuomo-Dannenburg G., Thompson H., Walker P.G.T., Fu H., Dighe A., Griffin J.T., Baguelin M., Bhatia S., Boonyasiri A., Cori A., Cucunubá Z., FitzJohn R., Gaythorpe K., Green W., Hamlet A., Hinsley W., Laydon D., Nedjati-Gilani G., Riley S., van Elsland S., Volz E., Wang H., Wang Y., Xi X., Donnelly C.A., Ghani A.C., Ferguson N.M. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect. Dis. 2020;20(6):669–677. doi: 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Cao Y., Mangalam A.K., Guo Y., LaFrance-Corey R.G., Gamez J.D., Atanga P.A., Clarkson B.D., Zhang Y., Wang E., Angom R.S., Dutta K., Ji B., Pirko I., Lucchinetti C.F., Howe C.L., Mukhopadhyay D. Neuropilin-1 modulates interferon-gamma-stimulated signaling in brain microvascular endothelial cells. J. Cell Sci. 2016;129(20):3911–3921. doi: 10.1242/jcs.190702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Chen W., Zhou Y.-S., Lian J.-Q., Zhang Z., Du P., Gong L., Zhang Y., Cui H.-Y., Geng J.-J., Wang B., Sun X.-X., Wang C.-F., Yang X., Lin P., Deng Y.-Q., Wei D., Yang X.-M., Zhu Y.-M., Zhang K., Zheng Z.-H., Miao J.-L., Guo T., Shi Y., Zhang J., Fu L., Wang Q.-Y., Bian H., Zhu P., Chen Z.-N. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cellls. Signal Transduct Target Ther. 2020;5(1):283. doi: 10.1038/s41392-020-00426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- Wu Y., Xu X., Chen Z., Duan J., Hashimoto K., Yang L., Liu C., Yang C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav. Immun. 2020;87:18–22. doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia H., Lazartigues E. Angiotensin-converting enzyme 2 in the brain: properties and future directions. J. Neurochem. 2008;107(6):1482–1494. doi: 10.1111/j.1471-4159.2008.05723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., Tai Y., Bai C., Gao T., Song J., Xia P., Dong J., Zhao J., Wang F.-S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M., Nakamura K., Yoshii M., Kaku Y., Narita M. Brain lesions induced by experimental intranasal infection of Japanese encephalitis virus in piglets. J. Comp. Pathol. 2009;141(2–3):156–162. doi: 10.1016/j.jcpa.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Yan, W., 2020. “Coronavirus Tests Science’s Need for Speed Limits.” The New York Times.
- Yao X.H., Li T.Y., He Z.C., Ping Y.F., Liu H.W., Yu S.C., Mou H.M., Wang L.H., Zhang H.R., Fu W.J., Luo T., Liu F., Guo Q.N., Chen C., Xiao H.L., Guo H.T., Lin S., Xiang D.F., Shi Y., Pan G.Q., Li Q.R., Huang X., Cui Y., Liu X.Z., Tang W., Pan P.F., Huang X.Q., Ding Y.Q., Bian X.W. A pathological report of three COVID-19 cases by minimal invasive autopsies. Zhonghua Bing Li Xue Za Zhi. 2020;49(5):411–417. doi: 10.3760/cma.j.cn112151-20200312-00193. [DOI] [PubMed] [Google Scholar]
- Yeh E.A., Collins A., Cohen M.E., Duffner P.K., Faden H. Detection of coronavirus in the central nervous system of a child with acute disseminated encephalomyelitis. Pediatrics. 2004;113(1 Pt 1):e73–76. doi: 10.1542/peds.113.1.e73. [DOI] [PubMed] [Google Scholar]
- Zhang A.J., Lee A.C., Chu H., Chan J.F., Fan Z., Li C., Liu F., Chen Y., Yuan S., Poon V.K., Chan C.C., Cai J.P., Wu K.L., Sridhar S., Chan Y.S., Yuen K.Y. SARS-CoV-2 infects and damages the mature and immature olfactory sensory neurons of hamsters. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z.G., Tsang W., Zhang L., Powers C., Chopp M. Up-regulation of neuropilin-1 in neovasculature after focal cerebral ischemia in the adult rat. J. Cereb. Blood Flow Metab. 2001;21(5):541–549. doi: 10.1097/00004647-200105000-00008. [DOI] [PubMed] [Google Scholar]
- Zhao J.Y., Yan J.Y., Qu J.M. Interpretations of “Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7)”. Chin. Med. J. (Engl.) 2020 doi: 10.1097/CM9.0000000000000866. [DOI] [PMC free article] [PubMed] [Google Scholar]