Abstract
Objective
Previous studies mainly reported the clinical characteristics of novel coronavirus 2019 (COVID-19) infections, but the research on clinical characteristics and treatment outcomes of COVID-19 patients with stroke is still rare.
Methods
A multi-center retrospective study was conducted at 11 hospitals in 4 provinces of China, and COVID-19 patients with stroke were enrolled from February 24 to May 4, 2020. We analyzed epidemiological, demographic, and clinical characteristics of cases as well as the laboratory test results, treatment regimens and outcomes, and the clinical characteristics and therapeutic outcomes were compared between severe and nonsevere patients, and by age group, respectively.
Results
A total of 27 patients [mean age: 66.41 (SD 12.1) years] were enrolled. Among them, 9 (33.3%) were severe patients and 18 (66.7%) were nonsevere patients; 17 (63.0%) were female; 19 (70.4%) were aged 60 years and above. The most common symptoms were fever [19 (70.4%)], fatigue [12 (44.4%)] and cough [11 (40.7%)], respectively. Abnormal laboratory findings of COVID-19 patients with stroke included high levels of C-reactive protein [19 (73.1%)], D-dimer [14 (58.3%)], blood glucose [14 (53.8%)], fibrinogen [13 (50.0%)], and decreased lymphocytes [12 (44.4%)]. Comparing to nonsevere cases with stroke, severe patients with stroke were likely to be older, susceptible to receiving oxygen inhalation, and had more complications (p < 0.05). In addition, there were significant differences in lymphocytes, neutrophils, lactate dehydrogenase, C-reactive protein, creatine kinase between the severe cases and nonsevere cases (p < 0.05). The older patients had a decreased platelet count and elevated fibrinogen, compared with the younger (p < 0.05). All patients (100%) received antiviral treatment, 12 (44.4%) received antibiotics treatment, 26 (96.3%) received Traditional Chinese Medicine (Lung cleansing & detoxifying decoction), and oxygen inhalation was in 18 (66.7%). The median duration of hospitalization was 16 days. By May 4, 2020, a total of 26 (96.3%) patients were cured and discharged, and 1 (3.7%) patients died.
Conclusion
COVID-19 patients with stroke had poor indicators of coagulation system, and severe and older patients might have a higher risk of complications and unfavorable coagulation system. However, the overall treatment outcome is favorable.
Keywords: COVID-19, Clinical characteristics, Stroke, Treatment outcome
Abbreviations: COVID-19, novel coronavirus disease 2019; CT, computed tomography; ICU, intensive care unit; LDH, lactate dehydrogenase; PaO2, arterial partial pressure of oxygen; SARS-CoV, severe acute respiratory syndrome coronavirus; TIA, transient ischemic attack
Graphical abstract
Introduction
The novel coronavirus disease 2019 (COVID-19) has posed a significant global health threat (Huang et al., 2020, Phelan et al., 2020, Zhu et al., 2020). As of 22 October 2020, there have been over 40 million confirmed COVID-19 cases and more than 1 million deaths reported across the world (World Health Organization WHO, 2020). The most prominent symptoms of COVID-19 are fever, cough, and fatigue (Chen et al., 2020; Huang et al., 2020). The neurologic manifestations of COVID-19 infections have also received increasing attention, including acute cerebrovascular diseases, and impaired consciousness (Mao et al., 2020a).
A previous retrospective study demonstrated that there were 30 (1.9%) patients suffering cerebrovascular diseases among all hospitalized COVID-19 patients (Guan et al., 2020a), and another study from China also found that 5.7% severe infections among 214 hospitalized COVID-19 patients had suffered stroke (Mao et al., 2020b). Furthermore, a higher incidence of thromboembolic complications including stroke were also found in the severe acute respiratory syndrome (SARS) infections in 2003 (Umapathi et al., 2004). One of the emerging clinical characteristics of severe SARS-CoV-2 infections was coagulopathy with high levels of D-dimer and fibrinogen (Tang et al., 2020a). Thrombosis is a key mechanism for many acute ischemic strokes, and a higher basal plasma D-dimer concentration is a risk marker for ischemic stroke (Folsom et al., 2016) in the general population. Studies revealed that hypercoagulability associated with COVID-19 were more likely to have stroke (Hess et al., 2020, Connors and Levy, 2020). According to autopsy findings of COVID-19 patients, the high incidence of thromboembolic events suggests an important role of COVID-19-induced coagulopathy (Wichmann et al., 2020). In addition, previous literature revealed that COVID-19 patients with aged 50 years presented with symptoms of large-vessel ischemic stroke within 2 weeks after illness onset, with a risk much higher than other times (Oxley et al., 2020). Some experts also explored the potential influence of racial background in stroke outcomes in this pandemic (Dmytriw et al., 2020). Therefore, the stroke with COVID-19 or potentially caused by SARS-CoV-2 infections has attracted more and more attention (Avula et al., 2020; Beyrouti et al., 2020; Markus and Brainin, 2020).
However, our current understanding of COVID-19 patients with stroke history or acute stroke remains limited. Especially, so far, there are few studies that have systematically compared clinical characteristics, laboratory and radiologic findings, and treatment outcome of COVID-19 patients with stroke by severity and age group. Therefore, we performed a retrospective study to investigate the clinical characteristics and therapeutic outcomes of COVID-19 patients with stroke, with the comparison between severe and nonsevere infections, and patients aged < 60 years and aged ≥ 60 years. The findings of our study can provide useful information for designing novel strategy for stroke patient treatment under the ongoing and future waves of COVID-19 pandemic.
METHODS
Study population
The multicenter retrospective study was conducted at 11 hospitals in 4 provinces of China from February 24 to May 4, 2020. All stroke patients with laboratory confirmed COVID-19 were enrolled. The diagnosis of COVID-19 was based on guidelines issued by the National Health Commission of the People's Republic of China (China., 2020.). This study was approved by the National Administration of Traditional Chinese Medicine, the Administration of Traditional Chinese Medicine in 4 provinces and the institutional board of 11 participating hospitals. Based on the urgent need to collect data and treat COVID-19 patients, the written informed consent was replaced by verbal consent.
Definition
Fever was defined as axillary temperature of at least 37.3 °C. The diagnosis of stroke was made by clinicians according to the health history of patients and images of brain chest computed tomography (CT) on admission. The severity of the disease was categorized by using the guidelines for diagnosis and treatment of COVID-19 (seventh edition) issued by the National Health Commission of China (China., 2020.). Definitions of the severity for SARS-CoV-2 infections are described as follows. 1) Mild cases: The clinical symptoms were mild, and there was no sign of pneumonia on imaging; 2) Moderate cases: Showing fever and respiratory symptoms with radiological findings of pneumonia; 3) Severe cases: (i) Respiratory distress (≥ 30 breaths/min); (ii) Oxygen saturation ≤ 93% at rest; (iii) Arterial partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2) ≤ 300mmHg (l mmHg = 0.133kPa). In high-altitude areas (at an altitude of over 1,000 meters above the sea level), PaO2/FiO2 should be corrected by the following formula: PaO2/FiO2 [Atmospheric pressure (mmHg)/760]. Cases with the chest imaging that shows obvious lesion progression within 24 - 48 hours >50% should be managed as severe cases. Nonsevere patients included mild and moderate cases.
Data collection
Epidemiological, demographic, clinical, laboratorial, radiological, and treatment data were collected and obtained from patients’ electronic medical records provided by each hospital. Two researchers independently checked the eligibility of patients for this study and extracted data. Data were entered into a database and cross-checked. If the core data such as clinical characteristics, laboratory and radiologic findings, treatment outcome were unclear or missing, we would send requests for clarification to the coordinator for this project in each hospital, who subsequently contacted the cliniciansinvolved in this study.
Statistical Analysis
Continuous variables were described using mean with standard deviation (SD) and median with interquartile range (IQR), as appropriate. Categorical data were presented as counts and percentages. Continuous variables were compared by t tests if the data were assumed with a normal distribution; otherwise, by using the Mann-Whitney test. Proportions for categorical variables were compared using the χ2 test or Fisher exact test between severe and non-severe groups, patients aged < 60 years and aged ≥ 60 years. Statistical analysis was performed with R software version 3.5.3 (R Foundation for Statistical Computing, Vienna, Austria). A two-sided α of less than 0.05 was considered to be statistically significant.
RESULTS
Demographic and clinical characteristics of COVID-19 patients
A total of 27 patients with stroke and confirmed SARS-CoV-2 infection were identified at 11 hospitals, including 3 (11.1%) mild cases, 15 (55.6%) moderate, and 9 (33.3%) severe. Among them, 5 cases were acute stroke patients within 30 days of illness onset of SARS-CoV-2 infection, and 22 patients had a history of stroke. The demographic and clinical characteristics of patients are shown in Table 1 . The mean age of the patients was 66.4 years (SD: 12.1; range: 43 – 86 years), and 17 (63.0%) patients were female. Eight patients (29.6%) were current or former smokers, and nine patients (33.3%) were drinkers. The most patients had either a history of exposure to epidemic areas [7 (25.9%) cases] or close contact with a person known to have been ill [15 (55.6%)].
Table 1.
Characteristics of patients by clinical severity and age group.
| Characteristic | Total (N = 27) | Clinical severity | Age group | ||||
|---|---|---|---|---|---|---|---|
| Nonsevere | Severe | p | ≥ 60 years | < 60 years | p | ||
| (N = 18) | (N = 9) | (N = 19) | (N = 8) | ||||
| Age, mean (SD), yr | 66.4 ± 12.1 | 62.4 ± 12.0 | 74.3 ± 8.0 | 0.006 | 72.5 ± 8.3 | 51.9 ± 5.4 | <0.001 |
| Age (categorized) | |||||||
| ≥ 60 yr - no./total no. (%) | 19 (70.4) | 10 (55.6) | 9 (100) | 0.026 | - | - | - |
| < 60 yr - no./total no. (%) | 8 (29.6) | 8 (44.4) | 0 (0) | - | - | - | |
| Sex - no. (%) | |||||||
| Female | 17 (63.0) | 11 (61.1) | 6 (66.7) | 1.000 | 12 (63.2) | 5 (62.5) | 1.000 |
| Male | 10 (37.0) | 7 (38.9) | 3 (33.3) | 7 (36.8) | 3 (37.5) | ||
| Smoking history - no./total no. (%) | 8 (29.6) | 6 (33.3) | 2 (22.2) | 0.676 | 7 (36.8) | 1 (12.5) | 0.364 |
| Drinking history - no./total no. (%) | 9 (33.3) | 7 (38.9) | 2 (22.2) | 0.667 | 6 (31.6) | 3 (37.5) | 1.000 |
| Profession - no./total no. (%) | |||||||
| Farmers | 8 (29.6) | 8 (44.4) | 0 (0) | 0.034 | 5 (26.3) | 3 (37.5) | 0.304 |
| Non-salary employee | 13 (48.2) | 6 (33.3) | 7 (77.8) | 11 (57.9) | 2 (25) | ||
| Salary employee | 6 (22.2) | 4 (22.2) | 2 (22.2) | 3 (15.8) | 3 (37.5) | ||
| History of travel and contact - no. (%) | |||||||
| Exposure to source of transmission within past 14 days | 7 (25.9) | 5 (27.8) | 2 (22.2) | 1.000 | 5 (26.3) | 2 (25) | 1.000 |
| Contact with COVID-19 patients | 15 (55.6) | 10 (55.6) | 5 (55.6) | 1.000 | 11 (57.9) | 4 (50) | 1.000 |
| Coexisting disorder -no./total no. (%) | |||||||
| Any | 17 (63.0) | 8 (44.4) | 9 (100) | 0.009 | 13 (68.4) | 4 (50) | 0.415 |
| Hypertension | 13 (48.2) | 5 (27.8) | 8 (88.9) | 0.004 | 10 (52.6) | 3 (37.5) | 0.678 |
| Diabetes | 7 (25.9) | 5 (27.8) | 2 (22.2) | 1.000 | 5 (26.3) | 2 (25) | 1.000 |
| Cardiovascular disease | 2 (7.4) | 0 (0) | 2 (22.2) | 0.103 | 2 (10.5) | 0 (0) | 1.000 |
| Symptoms - no. (%) | |||||||
| Body temperature, mean (SD), °C | 37.5±0.7 | 37.6±0.8 | 37.4±0.4 | 0.655 | 37.4±0.6 | 37.7±0.8 | 0.356 |
| Fever | 19 (70.4) | 13 (72.2) | 6 (66.7) | 1.000 | 13 (68.4) | 6 (75) | 1.000 |
| Fatigue | 12 (44.4) | 9 (50) | 3 (33.3) | 0.683 | 9 (47.4) | 3 (37.5) | 0.696 |
| Cough | 11 (40.7) | 7 (38.9) | 4 (44.4) | 1.000 | 9 (47.4) | 2 (25) | 0.405 |
| Dyspnoea or tachypnoea | 5 (18.5) | 4 (22.2) | 1 (11.1) | 0.636 | 4 (21.1) | 1 (12.5) | 1.000 |
| Sore throat | 2 (7.4) | 2 (11.1) | 0 (0) | 0.538 | 0 (0) | 2 (25) | 0.080 |
| Diarrhoea | 2 (7.4) | 0 (0) | 2 (22.2) | 0.103 | 2 (10.5) | 0 (0) | 1.000 |
| Complications - no. (%) | |||||||
| Any | 8 (29.6) | 2 (11.1) | 6 (66.7) | 0.006 | 8 (42.1) | 0 (0) | 0.040 |
| Onset of symptom to hospital admission, median (IQR), d | 3 (1, 7.5) | 3 (2, 6.5) | 5 (0, 8) | 0.897 | 3 (0.5, 7) | 4.5 (2, 10.3) | 0.251 |
| Hospital admission, median (IQR), d | 16 (14, 22.50) | 16 (13.3, 20.8) | 19.5 (14.8, 26.8) | 0.387 | 20 (15, 25.5) | 13.5 (11, 15.5) | 0.015 |
| Days of first viral shedding, mean (SD), d | 13.9±6.4 | 12.2±5 | 17.9±7.8 | 0.087 | 15.1±7 | 11.4±4.4 | 0.120 |
| Treatment - no. (%) | |||||||
| Oxygen inhalation | 18 (66.7) | 9 (50) | 9 (100) | 0.012 | 14 (73.7) | 4 (50) | 0.375 |
| Antiviral drugs | 27 (100) | 19 (100) | 8 (100) | - | 19 (100) | 8 (100) | - |
| Antibiotics | 12 (44.4) | 8 (44.4) | 4 (44.4) | 1.000 | 1 (5.3) | 2 (25) | 0.201 |
| Traditional Chinese medicine | 26 (96.3) | 18 (100) | 8 (88.9) | 0.333 | 18 (94.7) | 8 (100) | 1.000 |
Data are median (IQR), mean (SD), n (%), or n/N (%), where N is the total number of patients with available data.
P values indicate differences between clinical severity and age group. p < 0.05 was considered statistically significant.
COVID-19 = coronavirus disease 2019.
Chronic medical conditions were common among patients in this study. Apart from the coexisting disease of stroke, 17 (63.0%) patients had other chronic diseases including hypertension [13 (48.2%) cases], diabetes [7 (25.9%)], and cardiovascular disease [2 (7.4%)]. On admission, the most common symptoms reported were fever [19 (70.4%)], fatigue [12 (44.4%)], and cough [11 (40.7%)], respectively. Other symptoms were recorded infrequently, including dyspnoea or tachypnoea [5 (18.5%)], sore throat [2 (7.4%)], and diarrhoea [2 (7.4%)]. In addition, compared with nonsevere cases, severe cases were likely to be older (p = 0.006), and most patients were older than 60 years (p = 0.026) and famers were less (p = 0.034). Severe cases were also more likely to have complications (p = 0.006) and hypertension (p = 0.004).
Laboratory and radiologic findings
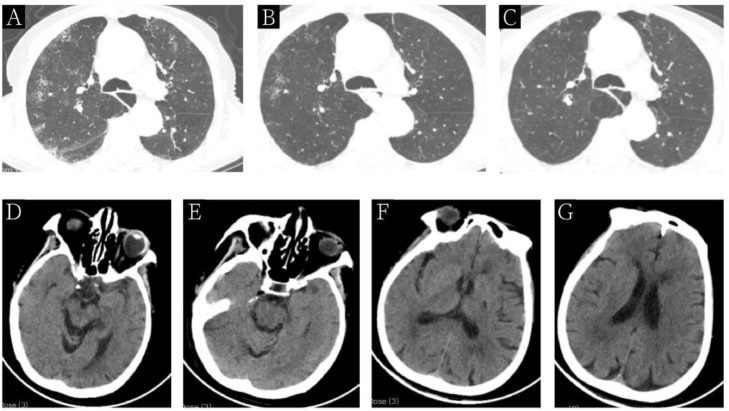
Table 2 shows the main laboratory and radiologic results of COVID-19 patients. Abnormal findings in patients with stroke included increases in C-reactive protein [19 (73.1%)], D-dimer [14 (58.3%)], blood sugar [14 (53.8%)], fibrinogen [13 (50%)], and lactate dehydrogenase [10 (38.5%)], along with the decrease in lymphocytes [12 (44.4%)]. The severe cases and non-severe cases had significantly differences in neutrophils (p = 0.012), lymphocytes (p = 0.027), lactate dehydrogenase (p = 0.011), C-reactive protein (p = 0.029), and Creatine kinase (p = 0.029). There were 23 (85.2%) patients with bilateral pulmonary opacities. Fig 1 . presents typical chest and brain CT images of patients.
Table 2.
Laboratory and radiologic findings between severe and nonsevere COVID-19 patients with stroke.
| Laboratory and radiologic findings | Total (N=27) | Non-severe (N = 18) | Severe (N = 9) | p |
|---|---|---|---|---|
| Laboratory tests (reference values) | ||||
| White blood cells (4-10 × 10⁹cells per l) | 6.2±1.9 | 5.6±1.6 | 7.3±2.2 | 0.055 |
| Increased (n/N; %) a | 3/27 (11.1) | 1/18 (5.6) | 2/9 (22.2) | |
| Lymphocytes (× 10⁹ per l; normal range 1.1-3.2) | 1.1±0.6 | 1.2±0.7 | 0.7±0.4 | 0.027 |
| Decreased (n/N; %) b | 12/27 (44.4) | 7/18 (38.9) | 5/9 (55.6) | |
| Neutrophils count (× 10⁹cells per l; normal range 1.8-6.3) | 4.6±1.9 | 3.9±1.3 | 6.1±2 | 0.012 |
| Increased (n/N; %) | 5/27 (18.5) | 1/18 (5.6) | 4/9 (44.4) | |
| Haemoglobin (g/l; normal range 130.0-175.0) | 130.2±17 | 132.9±17.9 | 124.7±14.3 | 0.211 |
| Decreased (n/N; %) | 3/27 (11.1) | 1/18 (5.6) | 2/9 (22.2) | |
| Platelet count (× 10⁹ per l; normal range 125.0–350.0) | 194.8±60.5 | 198.4±59 | 187.6±66.5 | 0.685 |
| Increased (n/N; %) | 2/27 (7.4) | 1/18 (5.6) | 1/9 (11.1) | |
| Alanine aminotransferase (U/l; normal range 9.0-50.0) | 23 (17.5,30.8) | 25 (21,31) | 21 (16,25) | 0.402 |
| Increased (n/N; %) | 5/26 (19.2) | 3/17 (17.6) | 2/9 (22.2) | |
| Aspartate aminotrandferase (U/l; normal range 15.0-40.0) | 25.5 (18.5, 37.8) | 24 (17, 37) | 27 (25, 38) | 0.403 |
| Increased (n/N; %) | 6/26 (23.1) | 3/17 (17.6) | 3/9 (33.3) | |
| Lactate dehydrogenase (U/l; normal range 120.0-250.0) | 241.4±66.1 | 214.6±47.1 | 292.1±69.2 | 0.011 |
| Increased (n/N; %) | 10/26 (38.5) | 4/17 (23.5) | 6/9 (66.7) | |
| Creatine kinase (U/l; normal range 50.0-170.0) | 65 (47, 119.8) | 56 (42.9, 68) | 122 (66.9, 222) | 0.029 |
| Increased (n/N; %) | 4/26 (15.4) | 1/17 (5.9) | 3/9 (33.3) | |
| Creatine kinase MB (U/l; normal range<18) | 15.7±6.9 | 14.8±6.9 | 17.4±6.8 | 0.380 |
| Increased (n/N; %) | 1/25 (4) | 1/16 (6.3) | 0/9 (0) | |
| C-reactive protein (mg/l; normal range 0.0–5.0) | 14.5 (5.2, 46.8) | 11.9 (4.9, 16.2) | 47.7 (23.5, 50.5) | 0.029 |
| Increased (n/N; %) | 19/26 (73.1) | 10/17 (58.8) | 9/9 (100) | |
| Prothrombin time (s; normal range 10.5-13.5) | 12.6 (11.7, 13.2) | 12.4 (11.5, 13) | 12.7 (12.1, 13.9) | 0.161 |
| Increased (n/N; %) | 4/26 (15.4) | 3/17 (17.6) | 1/9 (11.1) | |
| Fibrinogen (g/l; normal range 2-4) | 3.6±1.2 | 3.4±1.2 | 4.1±0.9 | 0.128 |
| Increased (n/N; %) | 13/26 (50) | 9/17 (52.9) | 4/9 (44.4) | |
| D-dimer (µg/l; normal range 0.0-0.5) | 0.7 (0.3, 1.4) | 0.4 (0.2, 1) | 1.2 (0.7, 5.5) | 0.095 |
| Increased (n/N; %) | 14/24 (58.3) | 8/15 (53.3) | 6/9 (66.7) | |
| blood glucose (mmol/l; normal range 3.9-6.1) | 6.5 (5.2, 7.3) | 6.2 (5, 7) | 7.2 (5.3, 9.6) | 0.336 |
| Increased (n/N; %) | 14/26 (53.8) | 8/17 (47.1) | 6/9 (33.3) | |
| CT findings —no./total no. (%) | ||||
| bilateral pulmonary opacities | 23/27 (85.2) | 15/18 (83.3) | 8/9 (88.9) | 1.000 |
| Unilateral pulmonary opacities | 3/27 (11.1) | 2/18 (11.1) | 1/9 (11.1) |
Note: Data are presented with median (IQR), mean (SD), n (%), or n/N (%), where N is the total number of patients with available data. P values indicate differences between nonsevere patients and severe patients, and p < 0.05 was considered as statistically significance.
Increased means over the upper limit of the normal range.
Decreased means below the lower limit of the normal range.
Figure 1.
Chest and brain CT images of a 79-Year-Old COVID-19 patient with stroke. Figures A, B, and C show chest CT images before treatment (day 0), after 10 days of treatment (day 10), and before discharge, respectively. Figures D, E, F, and G show the brain CT images on admission to demonstrate the infarction of basal ganglia in the left frontal lobe and bilateral corona radiata.
Additionally, Table 3 demonstrates the laboratory and radiologic findings by age group. Of 27 patients, 8 (29.6%) were aged < 60 years and 19 (70.4%) were aged ≥ 60 years. The older patients had a decrease of platelet counts (p = 0.025) and an increase of fibrinogen (p = 0.013), compared to the younger patients.
Table 3.
Laboratory and radiologic findings between aged ≥60 years and aged <60 years among COVID-19 patients with stroke.
| Laboratory and radiologic findings | Aged ≥ 60 years (N = 19) | Aged < 60 years (N = 8) | p |
|---|---|---|---|
| Laboratory tests (reference values) | |||
| White blood cells (4–10 × 10⁹ cells per l) | 6.2±2.0 | 6.1±2.0 | 0.904 |
| Increased (n/N; %) a | 2/19 (10.5) | 1/8 (12.5) | |
| Lymphocytes (1.1–3.2 × 10⁹ cells per l) | 1±0.5 | 1.3±0.8 | 0.312 |
| Decreased (n/N; %) b | 8/19 (42.1) | 4/8 (50) | |
| Neutrophils count (1.8-6.3 × 10⁹ cells per l) | 4.8±2 | 4.3±1.6 | 0.542 |
| Increased (n/N; %) | 4/19 (21.1) | 1/8 (12.5) | |
| Platelet count (× 10⁹ per l; normal range 125.0–350.0) | 179.4±60.2 | 231.4±45.7 | 0.025 |
| Decreased (n/N; %) | 2/19 (10.5) | 0/8 (0) | |
| Lactate dehydrogenase (U/l; normal range 120.0-250.0) | 256.9±66 | 206.6±54.8 | 0.060 |
| Increased (n/N; %) | 8/18 (44.4) | 2/8 (25) | |
| C-reactive protein (mg/l; normal range 0.0-5.0) | 24.1 (12, 49) | 5.4 (4.9, 12.6) | 0.085 |
| Increased (n/N; %) | 15/18 (83.3) | 4/8 (50) | |
| Prothrombin time (s; normal range 10.5-13.5) | 12.6 (12, 13.5) | 11.5 (10.5, 13.1) | 0.140 |
| Increased (n/N; %) | 4/19 (21.1) | 0/7 (0) | |
| Fibrinogen (g/l; normal range 2-4) | 4±1.1 | 2.8±0.8 | 0.013 |
| Increased (n/N; %) | 11/19 (57.9) | 1/7 (14.3) | |
| D-dimer (µg/l; normal range 0.0-0.5) | 0.7 (0.4, 1.2) | 0.7 (0.2, 1.6) | 0.689 |
| Increased (n/N; %) | 10/18 (55.6) | 3/6 (50) | |
| CT findings - no./total no. (%) | |||
| bilateral pulmonary opacities | 17/19 (89.5) | 6/8 (75) | 0.448 |
| Unilateral pulmonary opacities | 1/19 (5.3) | 2/8 (25) |
Note: Data are presented with median (IQR), mean (SD), n (%), or n/N (%), where N is the total number of patients with available data. P values indicate differences between different age groups (<60 years vs. older than 60 years), and p < 0.05 was considered as statistically significance.
Increased means over the upper limit of the normal range.
Decreased means below the lower limit of the normal range.
Treatment regimens and outcomes
In our study, all patients received antiviral treatment, 12 (44.4%) received antibiotics treatment, 26 (96.3%) received Traditional Chinese Medicine (Lung cleansing & detoxifying decoction), and 18 (66.7%) received oxygen inhalation. The median duration of hospitalization was 16 days. Compared with nonsevere patients, severe patients had more complications such as atherosclerotic plaque initiation [4 (44.4%)], respiratory failure [3 (33.3%)], shock [1 (11.1%)], myocardial injury [1 (11.1%)], and ARDS [1 (11.1%)]. As of May 4, 2020, a total of 26 patients were cured and discharged, and 1 case died.
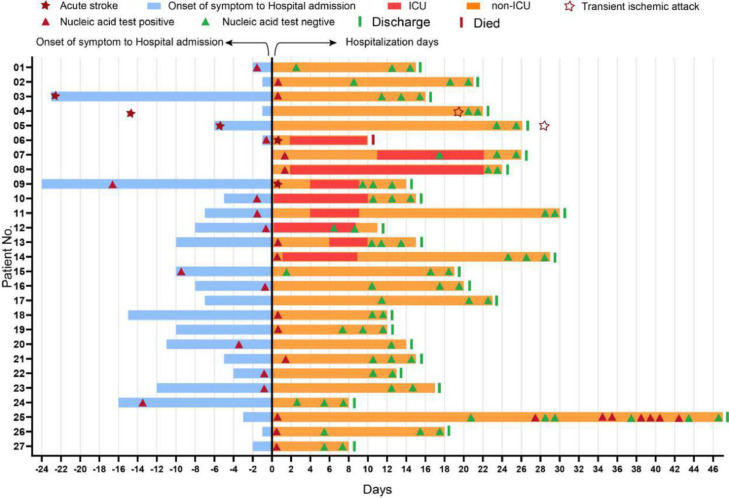
The Fig 2 . has detailed the epidemiological history and treatment outcomes of these 27 patients. Among all patients, 4 cases (14.8%) were referred from the sites for quarantine and medical observation or other hospitals to the hospitals participated in this study. In addition, patients (no. 6 and 9) had developed acute stroke after admission to hospital; and the acute stroke of patients (no. 3, 4 and 5) occurred within 1 month before COVID-19 onset, while two of them (no. 4 and 5) developed a transient ischemic attack (TIA) during hospitalization after illness onset.
Figure 2.
Epidemiological feature and treatment outcomes of 27 COVID-19 infections with stroke. The cases are successively listed according to the date of diagnosis. Date of diagnosis was defined as origin point, and the history of acute stroke and contact was reviewed. Nucleic acid tests were recorded during hospitalization. As of May 4, one (3.7%) patient died and 26 patients had been cured and discharged from hospitals. All patients were followed up for at least 28 days.
DISCUSSION
The COVID-19 has posed major public health threats affecting billions of people worldwide with considerable impacts on stroke (Markus and Brainin, 2020). According to a report in 2014, China reported the largest number of cases of stroke in the world (Feigin et al., 2014). COVID-19 patients with stroke had more severe clinical symptoms and poorer outcomes compared to patients without stroke(Qin et al., 2020). Thus, it is of importance to understand clinical feature, laboratory findings, and treatment outcome of COVID-19 patients with stroke by clinical severity and age group.
In our study, we found that severe cases had high levels of neutrophils count and C-reactive protein. Additionally, the increasing levels of lactate dehydrogenase (LDH) and creatine kinase were also observed in severe patients. The LDH was an inflammatory predictor in many pulmonary diseases (Inamura et al., 2014) and significantly higher in refractory COVID-19 pneumonia (Mo et al., 2020). These findings suggest that a cytokine storm and infections might be associated with the severity of COVID-19.
In laboratory tests, C-reactive protein was elevated in two-thirds of patients, with 38.5% for increasing lactate dehydrogenase, reflecting a progress of inflammatory and infection. More than half patients had increased D-dimer, which is consistent with previous studies (Avula et al., 2020; Breakey and Escher, 2020, Qin et al., 2020). On admission, COVID-19 patients with low activated partial thromboplastin time and elevated fibrinogen in therapy demonstrated that patients with stroke should pay attention to pre-clotting state. A recent study showed that the deceased cases had significantly higher D-dimer and fibrin degradation product (FDP) levels, which might indicate the presence of an abnormal clot (Tang et al., 2020b). These include hypercoagulability as evidenced by raised D-dimer levels (Tang et al., 2020a). The indicator of D-dimer higher than 1 μg/ml might help physicians to identify patients with poor prognosis at the early stage of infections (Zhou et al., 2020a).
In addition, the elderly were more susceptible to suffer severe illness and be admitted to the intensive care unit (ICU), and the mortality of patients aged ≥ 60 years were higher (Liu et al., 2020, Guan et al., 2020b, Pan et al., 2020). Our data demonstrated that lower platelet counts and higher levels of fibrinogen were observed in old persons of over 60 years. According to a primary prevention trial, among Chinese hypertensive adults, low platelet counts had a highest risk of first stroke (Kong et al., 2018). Severe thrombocytopenia likely contributed to the cerebral hemorrhage (Dixon et al., 2020). Moreover, fibrinogen is an essential hemostatic factor and primary phase inflammation marker, which is primarily involved in platelet–platelet interactions in thrombus formation (Davalos and Akassoglou, 2012, Petersen et al., 2018). These results suggest that the antithrombotic medication might be adjusted for COVID-19 patients with stroke according to the platelet counts or coagulation function.
Among 27 patients, severe cases have more complications, two patients had developed acute stroke, and three patients with acute stroke had received systemic treatment within one month before COVID-19 admission. Subsequently, the three patients then presented to the hospital again while they had a close contact with COVID-19 patient, and two patients developed TIA on admission. The early initiation of existing treatments after TIA or minor stroke was associated with an 80% reduction of the risk of early recurrent stroke (Rothwell et al., 2007). However, the underlying mechanisms remain unknown. Studies demonstrated that most coronaviruses are neurotropic, and others speculated that SARS-CoV-2 has same features (Steardo et al., 2020). The SARS-CoV-2 uses the same cell entry receptor-angiotensin converting enzyme II (ACE2) as SARS-CoV (Zhou et al., 2020b). The ACE2 is present in the brain stem (Steardo et al., 2020), potentially allowing SARS-CoV-2 to cross the blood-brain barrier and affect the the central nervous system (Chen et al., 2014). The experimental animal studies for SARS-CoV have shown that virus particles could be detected in specific brain areas (Hu et al., 2018; Li et al., 2016). In this case, an underlying inflammatory and hypercoagulable state might incite cerebrovascular disease without the disruption of blood–brain barrier (Al Saiegh et al., 2020).
In the present study we also found that, similar to patients with aged ≥ 60 years, severe COVID-19 patients had more comorbidities, poorer laboratory and radiologic findings, longer days of first viral shedding and hospital admission, compared to the non-severe cases. However, the overall treatment outcome was favorable with a cure rate of 96.3% (26/27).
There were several limitations in our study. First, although our study were conducted at 11 hospitals in 4 provinces, the small sample size of 27 patients might cause a high biases or uncertainty on the conclusions regarding factors associated with clinical outcome. Thus, further multi-center and long-term studies with a bigger sample size might be needed to draw a clear and robust conclusion. Second, given the need to treat patients at urgent timeline and outbreak response, some of clinical laboratory tests and data were not available for each patient. Despite these limitations, there are some notable strengths. We found that COVID-19 patients with stroke had both elevated C-reactive protein and D-dimer. Severe patients had increased inflammatory state, and older patients had an upregulation of fibrinogen and D-dimer. We also revealed that complications such as vascular embolism occurred more frequently in severe patients, and the coagulation system of elderly stroke patients was worse, which might lead to poor prognosis. This study improves our understanding on the clinical feature, laboratory and radiologic findings, and treatment outcome of COVID-19 patients with stroke, which can provide important evidence for clinicians to optimize treatment regimens and improve favorable outcome during the ongoing and future waves of COVID-19 pandemic.
Author contributions
Y.Y.W., Y.P.W., H.M.Z., Y.M., and X.Y.J. designed the study. X.Y.J. and Y.M. carried out the statistical analysis, drew the tables and pictures. X.Y.J., Y.M., and N.N.S. wrote the manuscript. N.L., R.B.C., S.H.L., and S.S. conducted data extraction and helped to draft the manuscript. G.H.W., H.C., J.W.W., H.N., Y.C. Z., M.Q.L., Y.D.W., X.M.H., Y.H.H., Z.L., H.J.X., and L.S.Z. recruited patients. Y.Y.W., Y.P.W., H.M.Z., Y.M., N.N.S., and X.Y.J. revised the final paper. All data were generated in-house, and no paper mill was used. All authors agree to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of Competing Interest
The authors declare that they have no competing interests.
Acknowledgments
We would like to thank all participants in the study, and we thank Prof Shenjie Lai for language editing of the manuscript. This study was supported by the National Science and Technology Major Project of China (2018ZX10101001-005-003, 2018ZX10101001-005-004). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Reference
- Al Saiegh F., Ghosh R., Leibold A., Avery M.B., Schmidt R.F., Theofanis T., Mouchtouris N., Philipp L., Peiper S.C., Wang Z.X., Rincon F., Tjoumakaris S.I., Jabbour P., Rosenwasser R.H., Gooch M.R. Status of SARS-CoV-2 in cerebrospinal fluid of patients with COVID-19 and stroke. Journal of neurology, neurosurgery, and psychiatry. 2020 doi: 10.1136/jnnp-2020-323522. [DOI] [PubMed] [Google Scholar]
- Avula A., Nalleballe K., Narula N., Sapozhnikov S., Dandu V., Toom S., Glaser A., Elsayegh D. COVID-19 presenting as stroke. Brain, behavior, and immunity. 2020 doi: 10.1016/j.bbi.2020.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyrouti R., Adams M.E., Benjamin L., Cohen H., Farmer S.F., Goh Y.Y., Humphries F., Jäger H.R., Losseff N.A., Perry R.J., Shah S., Simister R.J., Turner D., Chandratheva A., Werring D.J. Characteristics of ischaemic stroke associated with COVID-19. Journal of neurology, neurosurgery, and psychiatry. 2020 doi: 10.1136/jnnp-2020-323586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breakey N., Escher R. D-dimer and mortality in COVID-19: a self-fulfilling prophecy or a pathophysiological clue? Swiss medical weekly. 2020;150:w20293. doi: 10.4414/smw.2020.20293. [DOI] [PubMed] [Google Scholar]
- Chen J., Zhao Y., Chen S., Wang J., Xiao X., Ma X., Penchikala M., Xia H., Lazartigues E., Zhao B., Chen Y. Neuronal over-expression of ACE2 protects brain from ischemia-induced damage. Neuropharmacology. 2014;79:550–558. doi: 10.1016/j.neuropharm.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet (London, England) 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- China., N.H.a.H.C.o.t.P.s.R.o., 2020. Diagnosis and treatment guidelines for 2019 novel coronavirus pneumonia (draft version 5). China, p. http://www.nhc.gov.cn/yzygj/s7653p/202003/202046c209294a202007dfe202004cef202080dc202007f205912eb201989.shtml.
- Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos D., Akassoglou K. Fibrinogen as a key regulator of inflammation in disease. Seminars in immunopathology. 2012;34:43–62. doi: 10.1007/s00281-011-0290-8. [DOI] [PubMed] [Google Scholar]
- Dixon L., Varley J., Gontsarova A., Mallon D., Tona F., Muir D., Luqmani A., Jenkins I.H., Nicholas R., Jones B., Everitt A. COVID-19-related acute necrotizing encephalopathy with brain stem involvement in a patient with aplastic anemia. Neurology(R) neuroimmunology & neuroinflammation. 2020;7 doi: 10.1212/NXI.0000000000000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmytriw A.A., Phan K., Schirmer C., Settecase F., Heran M.K.S., Efendizade A., Kühn A.L., Puri A.S., Menon B.K., Dibas M., Sivakumar S., Mowla A., Leung L.Y., Malek A.M., Voetsch B., Sehgal S., Wakhloo A.K., Wu H., Xavier A., Tiwari A. Ischaemic stroke associated with COVID-19 and racial outcome disparity in North America. Journal of neurology, neurosurgery, and psychiatry. 2020 doi: 10.1136/jnnp-2020-324653. [DOI] [PubMed] [Google Scholar]
- Feigin V.L., Forouzanfar M.H., Krishnamurthi R., Mensah G.A., Connor M., Bennett D.A., Moran A.E., Sacco R.L., Anderson L., Truelsen T., O'Donnell M., Venketasubramanian N., Barker-Collo S., Lawes C.M., Wang W., Shinohara Y., Witt E., Ezzati M., Naghavi M., Murray C. Global and regional burden of stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet (London, England) 2014;383:245–254. doi: 10.1016/s0140-6736(13)61953-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folsom A.R., Gottesman R.F., Appiah D., Shahar E., Mosley T.H. Plasma d-Dimer and Incident Ischemic Stroke and Coronary Heart Disease: The Atherosclerosis Risk in Communities Study. Stroke. 2016;47:18–23. doi: 10.1161/STROKEAHA.115.011035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W.J., Liang W.H., Zhao Y., Liang H.R., Chen Z.S., Li Y.M., Liu X.Q., Chen R.C., Tang C.L., Wang T., Ou C.Q., Li L., Chen P.Y., Sang L., Wang W., Li J.F., Li C.C., Ou L.M., Cheng B., Xiong S., Ni Z.Y., Xiang J., Hu Y., Liu L., Shan H., Lei C.L., Peng Y.X., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.M., Liu J.Y., Chen Z., Li G., Zheng Z.J., Qiu S.Q., Luo J., Ye C.J., Zhu S.Y., Cheng L.L., Ye F., Li S.Y., Zheng J.P., Zhang N.F., Zhong N.S., He J.X. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. The European respiratory journal. 2020;55 doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., Du B., Li L.J., Zeng G., Yuen K.Y., Chen R.C., Tang C.L., Wang T., Chen P.Y., Xiang J., Li S.Y., Wang J.L., Liang Z.J., Peng Y.X., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.M., Liu J.Y., Chen Z., Li G., Zheng Z.J., Qiu S.Q., Luo J., Ye C.J., Zhu S.Y., Zhong N.S. Clinical Characteristics of Coronavirus Disease 2019 in China. The New England journal of medicine. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess D.C., Eldahshan W., Rutkowski E. COVID-19-Related Stroke. Translational stroke research. 2020;11:322–325. doi: 10.1007/s12975-020-00818-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D., Zhu C., Ai L., He T., Wang Y., Ye F., Yang L., Ding C., Zhu X., Lv R., Zhu J., Hassan B., Feng Y., Tan W., Wang C. Genomic characterization and infectivity of a novel SARS-like coronavirus in Chinese bats. Emerging microbes & infections. 2018;7:154. doi: 10.1038/s41426-018-0155-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England) 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamura N., Miyashita N., Hasegawa S., Kato A., Fukuda Y., Saitoh A., Kondo E., Teranishi H., Wakabayashi T., Akaike H., Tanaka T., Ogita S., Nakano T., Terada K., Ouchi K. Management of refractory Mycoplasma pneumoniae pneumonia: utility of measuring serum lactate dehydrogenase level. Journal of infection and chemotherapy: official journal of the Japan Society of Chemotherapy. 2014;20:270–273. doi: 10.1016/j.jiac.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Kong X., Huang X., Zhao M., Xu B., Xu R., Song Y., Yu Y., Yang W., Zhang J., Liu L., Zhang Y., Tang G., Wang B., Hou F.F., Li P., Cheng X., Zhao S., Wang X., Qin X., Li J., Huo Y. Platelet Count Affects Efficacy of Folic Acid in Preventing First Stroke. Journal of the American College of Cardiology. 2018;71:2136–2146. doi: 10.1016/j.jacc.2018.02.072. [DOI] [PubMed] [Google Scholar]
- Li K., Wohlford-Lenane C., Perlman S., Zhao J., Jewell A.K., Reznikov L.R., Gibson-Corley K.N., Meyerholz D.K., McCray P.B., Jr. Middle East Respiratory Syndrome Coronavirus Causes Multiple Organ Damage and Lethal Disease in Mice Transgenic for Human Dipeptidyl Peptidase 4. The Journal of infectious diseases. 2016;213:712–722. doi: 10.1093/infdis/jiv499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K., Chen Y., Lin R., Han K. Clinical features of COVID-19 in elderly patients: A comparison with young and middle-aged patients. The Journal of infection. 2020;80:e14–e18. doi: 10.1016/j.jinf.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., Chang J., Hong C., Zhou Y., Wang D., Miao X., Li Y., Hu B. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA neurology. 2020 doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., Chang J., Hong C., Zhou Y., Wang D., Miao X., Li Y., Hu B. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA neurology. 2020;77:1–9. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus H.S., Brainin M. COVID-19 and stroke-A global World Stroke Organization perspective. International journal of stroke: official journal of the International Stroke Society. 2020;15:361–364. doi: 10.1177/1747493020923472. [DOI] [PubMed] [Google Scholar]
- Mo P., Xing Y., Xiao Y., Deng L., Zhao Q., Wang H., Xiong Y., Cheng Z., Gao S., Liang K., Luo M., Chen T., Song S., Ma Z., Chen X., Zheng R., Cao Q., Wang F., Zhang Y. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2020 doi: 10.1093/cid/ciaa270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxley T.J., Mocco J., Majidi S., Kellner C.P., Shoirah H., Singh I.P., De Leacy R.A., Shigematsu T., Ladner T.R., Yaeger K.A., Skliut M., Weinberger J., Dangayach N.S., Bederson J.B., Tuhrim S., Fifi J.T. Large-Vessel Stroke as a Presenting Feature of Covid-19 in the Young. The New England journal of medicine. 2020;382:e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan A., Liu L., Wang C., Guo H., Hao X., Wang Q., Huang J., He N., Yu H., Lin X., Wei S., Wu T. Association of Public Health Interventions With the Epidemiology of the COVID-19 Outbreak in Wuhan, China. Jama. 2020;323:1–9. doi: 10.1001/jama.2020.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen M.A., Ryu J.K., Akassoglou K. Fibrinogen in neurological diseases: mechanisms, imaging and therapeutics. Nature reviews. Neuroscience. 2018;19:283–301. doi: 10.1038/nrn.2018.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan A.L., Katz R., Gostin L.O. The Novel Coronavirus Originating in Wuhan, China: Challenges for Global Health Governance. Jama. 2020 doi: 10.1001/jama.2020.1097. [DOI] [PubMed] [Google Scholar]
- Qin C., Zhou L., Hu Z., Yang S., Zhang S., Chen M., Yu H., Tian D.S., Wang W. Clinical Characteristics and Outcomes of COVID-19 Patients With a History of Stroke in Wuhan, China. Stroke. 2020;51:2219–2223. doi: 10.1161/STROKEAHA.120.030365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell P.M., Giles M.F., Chandratheva A., Marquardt L., Geraghty O., Redgrave J.N., Lovelock C.E., Binney L.E., Bull L.M., Cuthbertson F.C., Welch S.J., Bosch S., Alexander F.C., Silver L.E., Gutnikov S.A., Mehta Z. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet (London, England) 2007;370:1432–1442. doi: 10.1016/S0140-6736(07)61448-2. [DOI] [PubMed] [Google Scholar]
- Steardo L., Steardo L., Jr., Zorec R., Verkhratsky A. Neuroinfection may contribute to pathophysiology and clinical manifestations of COVID-19. Acta physiologica (Oxford, England) 2020:e13473. doi: 10.1111/apha.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. Journal of thrombosis and haemostasis: JTH. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. Journal of thrombosis and haemostasis: JTH. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umapathi T., Kor A.C., Venketasubramanian N., Lim C.C., Pang B.C., Yeo T.T., Lee C.C., Lim P.L., Ponnudurai K., Chuah K.L., Tan P.H., Tai D.Y., Ang S.P. Large artery ischaemic stroke in severe acute respiratory syndrome (SARS) Journal of neurology. 2004;251:1227–1231. doi: 10.1007/s00415-004-0519-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann D., Sperhake J.P., Lütgehetmann M., Steurer S., Edler C., Heinemann A., Heinrich F., Mushumba H., Kniep I., Schröder A.S., Burdelski C., de Heer G., Nierhaus A., Frings D., Pfefferle S., Becker H., Bredereke-Wiedling H., de Weerth A., Paschen H.R., Sheikhzadeh-Eggers S., Stang A., Schmiedel S., Bokemeyer C., Addo M.M., Aepfelbacher M., Püschel K., Kluge S. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19. Annals of internal medicine. 2020 doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet (London, England) 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. A Novel Coronavirus from Patients with Pneumonia in China, 2019. The New England journal of medicine. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO). https://covid19.who.int/. (Accessed 4 November 2020).