Abstract
In recent years increased attention is focussed on microorganisms inhabiting the digestive system that provides prophylactic and therapeutic benefits to the host. After Metchnikoff exposed the secret behind Bulgarian peasants' extended longevity, a graze to incorporate the responsible microbes in functional food emerged. Then interest towards microbe-rich food went to the vegetative phase for some time, but now a renaissance to engage these wonder microbes in the healthcare sector is increasing. With a new definition, probiotics, these good microbes have been widely applied in different types of products, either as pharmaceuticals, nutritional supplements, or foods. Probiotics, a significant source in functional dairy products, claims diverse roles such as improving intestinal tract health, enhancing the immune system, synthesizing and enhancing the bioavailability of nutrients, reducing symptoms of lactose intolerance, decreasing the prevalence of allergy in susceptible individuals, and reducing the risk of certain cancers. In the recent COVID-19 issue, searches are going fast to use probiotics as vaccine carriers, dysbiosis balancer, and immunity booster. The high expectation from probiotics expanded the development of bioengineered probiotics as new-generation probiotics. From the animal model and in vitro studies, the probiotic intervention is extrapolated to innate and adaptive immunity inducer against SARS viral infections. The possibility of using it as prophylactic and therapeutic agents in COVID-19 is explored. However, its significant activity against corona virus-induced respiratory syndromes is questioned by a few researchers also. The emerging citations on the research approach and meta-analysis of probiotic intervention against the re-emerging pandemic viral attack on the respiratory and gastrointestinal domains need to be analyzed in this context. As it is essential to understand the reality of recent experimental outcomes in the probiotic approach towards SARS-CoV-2 prevention, management, and control, the recent publications were focused on this review.
Keywords: Lactobacilli, Probiotics, SARS, MERS, SARS-CoV-2
1. Introduction
Respiratory diseases in different forms have become major health challenges in the globalized world. Severe acute respiratory syndrome (SARS), a new generation human illness, appeared in 2002, infected 8098 people in 26 countries, and 774 died (Hon et al., 2003). This viral respiratory illness was recognized as a global threat in March 2003. The causative agent for SARS was sequenced (Marra et al., 2003). Although this pandemic was contained in the initial occurrence; it started resurgence after a few months. By the end of May 2004, more than 2000 SARS related publications were cited (Drosten et al., 2003).
After a long gap, the re-emergence of coronavirus epidemics in the Middle East Saudi Arabian Peninsula [MERS-CoV], subsequently in 27 other countries, including Korea, raised global concern over this health issue. Both SARS and MERS were corona viral (Co - vs) diseases with little difference in epidemiology (Kwok et al., 2019). It is reported that coronaviruses are endemic to humans and infect other mammalian species like bats, masked Palm civet cats, Camels, Pangolins, Raccoon dogs, and these intermediate hosts for COVID-19 (Bell et al., 2004). Coronaviruses have evolved by accumulating point mutations and genome recombination from different strains or species (Chan and Chan, 2013).
The emergence of MERS- – -Co V, SARS-CoV, and the recent SARS-CoV-2 causing COVID-19 within ten years express that they are fast evolving from a zoonotic virus causing COVID-19 infection. SARS-CoV-2 shares nearly 80% of its genome with SARS-CoV. However, WHO announced that the newly discovered seventh member in the coronavirus family SARS-CoV-2, responsible for viral pneumonia, is different from MERS and SARS (WHO, 2020).
As there is no remedy in the form of drugs or vaccines for the recent SARS-CoV-19 pandemic, there is a need to find alternative medicine to take prophylactic and therapeutic measures. Several social and physical precautionary methods are recommended, along with alternative therapy, to derive preventative and supportive roles. As an alternative therapy, probiotics are recommended as they play a regulatory role on the gut-lung axis, innate and adaptive immunity, and help to rebuild the damaged tissues and organs (Sundararaman et al., 2020, Baud et al., 2020, Senapati et al., 2020, Kayode, 2020).
1.1. Corona virus an overview
Coronaviruses (CoV) are the members of enveloped positive-sense RNA viruses that belonged to the order Nidovirales of the family Corona viridae. The CoV was first discovered in 1960 after the advent of electron microscopy. The unique characteristic glycol protein spikes on the virus's surface promote its easy identification and adhesion with host cells. Corona viral RNA is large up to 32 kb (Hulswit et al., 2016). Coronavirus related to human and animal hosts is categorized into four genera. These include α -alpha, β-beta, ϒ-gamma, and δ -delta viruses.
The alpha coronavirus includes the human isolates HCoV-229E and HCoVNL63. The beta corona virus contains HCoV-OC43, HCoV-HKUL, and the recently included SARS-CoV, MERS-CoV, and SARS-CoV-2 (COVID-19). The gamma CoV-includes the avian infectious bronchitis virus – [AIBV]. The delta corona virus is less reported, and it is also discovered as avian origin, particularly in songbirds (Jancovich et al., 2012). The human coronaviruses causing cold, HCoV-229E, and HCoV-OC43 use aminopeptidase receptors for adhesion (Yeager et al., 1992). The beta-human corona viruses, [HCoV-NL63, SARS-CoV and SARS –CoV-2] use angiotensin-converting enzyme – 2 (ACE-2) as their receptor. The other non-human beta corona viruses, HCoV-OC43 and HCoV-HKUL use sialic acid residues as receptors (Vlasak et al., 1988).
2. SARS-CoV-2-COVID-9 infection
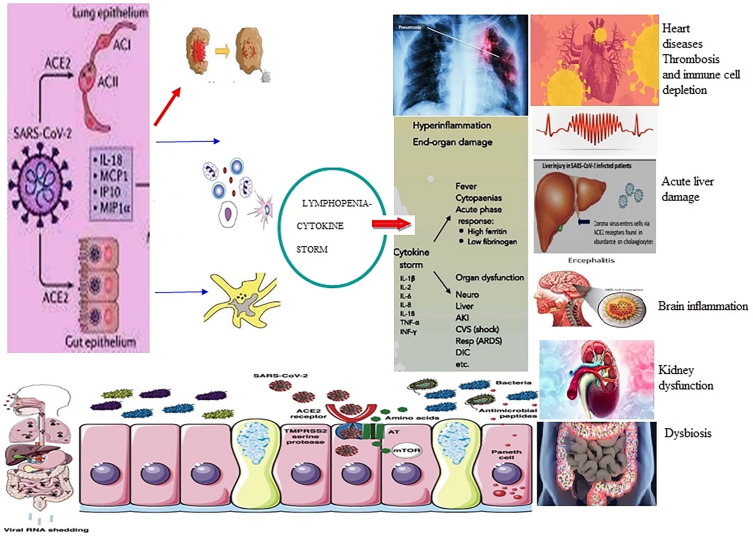
The recent pandemic COVID-19 or SARS-CoV-2 viral emergence from China and its spread to almost all countries infected. Globally, as of 3:59 pm CET, 20 November 2020, there have been 56,623,643 confirmed cases of COVID-19, including 1,355,963 deaths, reported to WHO (WHO-CET, 2020). The global COVID-19 death over 1 million with no sign of slowing, remains a global burden for WHO. The root cause of SARS-CoV-2, whether zoonotic or accidental biotech or bioengineered origin, remains unclear. Researchers worldwide are burning their midnight oil to find effective vaccines, antiviral drugs, and other alternative therapy options like probiotic supplements. On infection, the corona viruses, HCoV-NL63, SARS-CoV, and SARS –CoV-2 adhere to angiotensin-converting enzyme – 2 (ACE-2) receptor on the epithelial cells of the host's respiratory and gastrointestinal tract through their surface glycoprotein spikes, SI and S2. The SI spikes make a strong adhesion; the S2 spikes make changes in epithelial cell surface and transfer viral genome into the host cells (Hoffmann et al., 2020). The viral RNA replicates inside the host cells, and after 2–14 days of incubation, infection symptoms appear, but a few victims are asymptomatic, and scanning can alone confirm the infection. The infected hosts transmit the viral particles to other people through respiratory droplets or other mechanisms that still need research. The SARS-CoV-2 infection leads to the severe acute respiratory syndrome and the alveolar epithelium and associated gas exchange get affected (Fanos et al., 2020). As a result, a battery of immune cells, macrophages, monocytes, dendritic cells, NK cells, granulocytes, B-cells, and T-cells [CD 8] operating innate and adaptive immunity against this viral antigen gets functional impairment. The dysregulation of monocyte and macrophage function promotes a marked reduction in CD16+ monocytes and a little increase in CD14+ monocytes (Wilk et al., 2020). The upregulation of CD14+ monocytes may be due to IL-6 secretion by inflammatory monocytes. In a study with 17 acute and 24 convalescent patients showed an increase in dendritic cells (myeloid mDCs and plasmacytoid [pDCs] in acute infection (Zhou et al., 2020)). The macrophages accumulation at the site of infection stimulates fibroblasts to cause pulmonary fibrosis. The peripheral NK cells also lost their functional roles during severe SARS-CoV-2 infection and got reduced, and Xiong et al. reported an increase in apoptosis and lymphopenia SARS-CoV-2-victims (Xiong et al., 2020). SARS-CoV-2 have induced complications in renal functions, cardiac activities, thrombotic mechanism, hepatocellular control, diabetic management, neural system, vision and skin integrity (Gupta et al., 2020).
3. Dendritic cells [DC] and COVID-19 infection
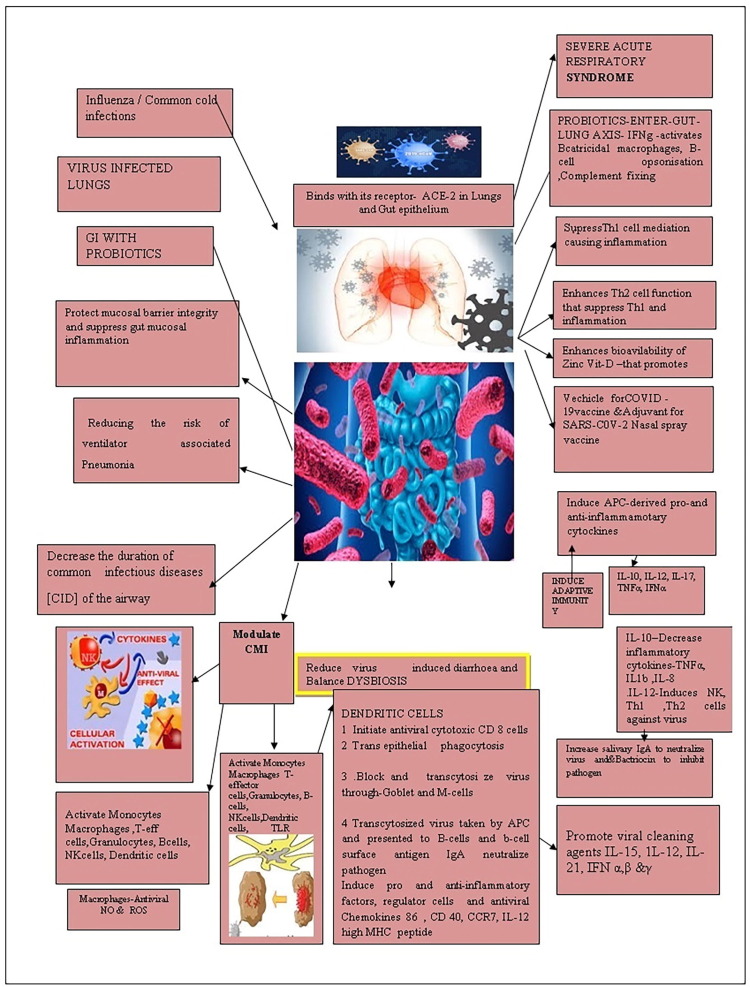
When there is COVID-19 antigenic challenge, the sentinel immature DC cells start to scavenge the viral antigens in Fig. 1 . The dendritic cells then matured and presented viral antigen to T cells present in the mucus epithelium and lymphoid tissues. They also secrete cytokines to regulate immune responses and maintain the homeostatic balance that got disturbed due to virus infection. In addition to phagocytosis, the DC induces adaptive immunity against the viral antigen. Both myeloid [mDC] and plasmacytoid DC[pDC] have triggered antiviral responses by generating a substantial amount of type I interferon. Also, make immune surveillance in the airways and the distal lung through intrinsic, innate receptors, using RIG-1, MDA5, NLRP3 inflammasome, and the RNA sensing TLRs 7 and 8 (Gupta et al., 2020).
Fig. 1.
Illustrating the immune boosting, and antiviral roles played by probiotics.
During the presentation of antigen to T cells, APC is highly specific. When it interacts with CD4+ - T cells, it is differentiated into T-helper cells of different types – Th1, Th 2, Th 17 or other CD4+ T cells. This T-cell process is influenced by its secretions, such as cytokines and chemokine’s. For the differentiation of naive CD4+ T cells, the dendritic cells secrete the cytokine, IL-16. Based on antigen, the dendritic cells activate different cytokine genes. The surface of the dendritic cells has receptors called Toll- like receptors (TLRs). The TLR identify the nature of the pathogen and send signal to turn on specific cytokine genes. The cytokine (interleukin 12-IL-12) induce T cells to differentiate into the subset Th 1 cells. The cytokine (IL-23) helps differentiate T cells into Th 17 cells that deal with extracellular antigens. Also, interleukin 4 (IL-4) converts the T-cells into Th2 Cells that promote antibody production by B cells. The activated dendritic cells also secrete TGF-β and IL-10 that promote T-regulatory cells that induce an immune response. Dendritic cells (DC) initiate innate immune response through Natural killer cells, NKT cells, and Y & T cells. The challenging viral enhances the pDCs population, and it secretes IFN –β in response to viral antigen challenge (Ahmed-Hassan et al., 2020). The pDC cross-prime the naive CD 8 -T cells by transferring antigen to conventional dendritic cells through exosomes. The COVID-19 infection also interferes with the secretory activity of the immune cells in Fig. 2 . Because of acute infection, an upregulation of chemokines leads to a heavy influx of monocytes, macrophages, and neutrophils to the infected sites causing tissue damage and a cytokine storm. In acute cases, the cytokine storm promotes proinflammatory cytokines synthesis [IL-1β, IL-2, and IL-6] that further complicates the disease (Fu et al., 2020). Interferon production also increases in acute cases, and the reason still needs research (McKechnie and Blish, 2020). As the disease progress, changes are reported in the quantum of myeloid cells. The neutrophil and lymphocytes ratio was high. The upregulation of myeloid cells in severe infection increases the neutrophils, granulocytes, and mast cells (Blanco-Melo et al., 2020). Even though complement protein's role in combating viral pathogenesis is unclear, the increase in serum complement level in acute SARS-COv-2 infected (Huang et al., 2020) needs further study. All these biochemical modulations promote inflammation and tissue damage in respiratory alveoli leading to mortality or acute pneumonia. Recently it is observed that the pathogenicity of COVID-19 infection is also high in the gut (Gao et al., 2020). Under such SARS-CoV-2 induced immunity dysregulation, immunomodulators are suggested for the rescue. And several immune modulators are recommended for co-administration with scheduled drugs to block IL-6 or other biochemical factors to get recovery (Meo et al., 2020, Xu et al., 2020). In this situation, research studies with probiotics inform that all these biochemical deterioration leading to tissue architecture and functional mechanism can be remediated through probiotic therapy (Sundararaman et al., 2020, Baud et al., 2020, Senapati et al., 2020).
Fig. 2.
Pathogenesis of COVID-19 in different systems in human body.
4. Probiotic role in SARS-CoV-2 infection
In the wake of COVID-19 pandemic, there has been a sharp increase in inquiries about the immunity boosting probiotic prophylactic agents (Bhimraj et al., 2020, Bahadur Gurung et al., 2020, Tiwari et al., 2020). The global inquisitiveness for a nutritious food reminds Hippocrates, “Let food be thy medicine, and medicine be thy food”. The secret behind the extended longevity among the Bulgarian peasants unearthed by Metchnikoff and Mitchd (1910) paved a way for positive research on probiotics. Probiotics are “Live microorganisms which/that when administered in adequate amounts confer a health benefit on their host. The recent interest in probiotics and COVID-19 enhanced the global market size of probiotics. It is expected to be USD 74.69 billion by the end 2025, and the Saudi Arabia probiotics market is expected to attain a value of USD 199.409 by 2025 (Research and Market, 2020).
4.1. Corona viral infection and probiotics
The entry point of SARS-CoV-2 is mostly the respiratory passage, causing severe acute respiratory illness. However, its impact on the gastrointestinal system is also well pronounced. The affinity of SARS-CoV-2 to the respiratory epithelium and gastrointestinal epithelium is because of the receptor angiotensin-converting enzyme-2[ACE-2] in the brush border of gut enterocytes and lungs alveolar epithelial type II cells (Hoffmann et al., 2020). The presence of viral RNA of SARS-CoV-2 in faecal samples of COVID-19 patients (Ahlawat et al., 2020) confirm their gut inhabitation. The co-infection in the gut impairs the physiology of the gut and eliminates “Good “bacteria making an imbalance between good and bad bacteria (dysbiosis) (Trottein and Sokol, 2020, Lin et al., 2020). Unless the corona virus-induced dysbiosis in the gut microbial biome is set right by probiotic supplementation, further complications may shoot up (Tiwari et al., 2020).
Along with the respiratory tract invasion, the entry of SARS-CoV-2 into the gut infects the gut epithelium and affects it integrity and gut micro biome (Jin et al., 2020). The sentinel cells, macrophages, dendritic cells, and mast cells promote first-line defence on viral entry. Viruses with the specific receptor binding molecules called pathogen-associated molecular pattern [PAMPs] bind with the sentinel cells' receptors. As PAMPs are specific for virus types, the receptor in sentinel cells must be complimentary (Li et al., 2020). In COVID-19 viruses, PAMPs are masked, and the viruses escape sentinel cells' surveillance. Further, in people with a severe form of COVID-19, the lymphocytes [T-cells, B-cells, and natural killer (NK) cells] of the immune system that recognize viruses and secrete antibodies against them are sharply reduced, and immunity is affected (Yang et al., 2020). But probiotics and their secretions stimulate cytokines and other factors that activate the innate and adaptive immunity (Anwar et al., 2020).
Due to SARS-CoV-2 infection, T helper cells and macrophages initiate the uncontrolled release of cytokines. That cytokine storm leads to heavy inflammation-associated acute respiratory distress syndrome, which is often fatal (Taghizadeh-Hesary and Akbari, 2020). Hence, probiotic supplementation may be useful because its secretions induce Th1 and Th2 cells, promoting anti-inflammatory actions to reduce the lungs' viral load (Vlasak et al., 1988). Further, probiotics supplementation with vitamin D and zinc decreases Th1/Th17 T cells and pro-inflammatory cytokines, such as IL-1, IL-6, IL-8, IFNγ, and TNFα, due to Th2 action and the inflammation in COVID-19 infection problem can be avoided in the gut and lungs (Yeager et al., 1992).
Among the older people and immune-compromised patients, the gut probiotics consortium usually is low, and so these people endure the severe impact of COVID-19 disease (Viana et al., 2020). Probiotic anti-inflammatory secretions can regulate this inflammatory reaction by co-supplementing personalized functional food incorporating probiotics (Antunes et al., 2020).
As a therapeutic solution is lingering for COVID-19, attention is focussed on effective nutritional therapy. Several recent reviews highlighted probiotics as an adjunct alternative among the therapies available for the treatment to overcome the new coronavirus (Akour, 2020; Aarti et al., 2020, Liisa et al., 2020). Also, L. gasseri plays a role in purine management as an adjuvant nutritional therapy to help COVID treatment weaken viral replication (Morais et al., 2020). Several experimental studies proved that the probiotic L. rhamnosus GG secrete a protein p40 that reduces TNF, IL-6, IFNγ, and chemoattractant to prevent inflammation in gut epithelium (Yan et al., 2011). A cocktail of L. acidophilus, L. reuteri, L. casei and other probiotics secretions stimulated dendritic cell functions and down-regulated Th1, Th2 and other factors that induced inflammation in the gut and lungs though gut- lung axis link (Yang et al., 2020).
As the development of a vaccine for COVID-19 takes time, alternative measures using immunity-boosting nutritional strategies incorporating probiotics and prebiotics are recommended. As probiotics assert gut homeostasis, regulates the gut–lung axis, and produce several antiviral defensive and offensive mechanisms through innate and adaptive immunity, it stands good (Tiwari et al., 2020). A review of gastrointestinal disorders in patients with COVID-19 emphasizes the need to glorify the gut with probiotics targeting gut dysbiosis, a major impact due to COVID-19 (Mak et al., 2020).
4.2. Probiotic lactobacilli spp. – effective antiviral agent
Lactobacilli are Gram-positive, anaerobic, rod-shaped, non–spore-forming lactic acid-producing bacteria (LAB). Lactobacillus spp (LB) convert hexose sugar into lactic acid and alcohol. The genus LB includes more than 250 species. Among them, few species operating mainly from the gastrointestinal tract and nasal-pharynx are experimentally proved to have a good anti-corona viral activity (Pourhossein and Moravejolahkami, 2020).
5. Probiotics in naso-pharynx and viral Infections
An airway microbiome profiling study revealed Lactobacillus spp. in the naso-pharynx and inhibited several upper respiratory tract pathogens' growth and virulence. The lactobacilli are capable of blocking the attachment of viral particles to human cells. This unique property opened a platform to discover the possibility of using this microbe to develop a local antiviral nasal lactobacilli spray (Hanifi et al., 2020). In the present approach to develop a vaccine against SARA-CoV, several teams use viral spike S protein or its receptor-binding domain as antigen to induce a protective immune response. However, Prof. Sarah Lebeer and his team from University of Antwerp, Belgium, are working on nasal lactobacilli strains with immune-stimulatory effects so as to use it as adjuvants for intranasal SARS-CoV-2 vaccination. Also, attempts to make genetically engineered antigen-producing lactobacilli for vaccine delivery is in progress (Lebeer, 2020). Viruses are responsible for more than 90% of upper RTIs and several reports inform the potential of lactobacilli probiotics on the prevention of upper RTIs. The SARS-CoV-2 spread via respiratory droplets attempts to develop antiviral probiotic nasal spray may be protective (Su et al., 2020).
5.1. Antiviral role played by lung and gut probiotics
The probiotics present in the gut or respiratory system produce an anti-microbial peptide called bacteriocin. L. acidophilus bacteriocin contains different subsets, such as lactocin B, lactocin F, and acidocin A/B, which prevent harmful microbes in the lungs and gut and reduces discomfort in lungs due to viral infection (Nanis et al., 2019). The engineered Lactobacillus acidophilus ATCC 4356 stably expressed the HIV-1 receptor CD4 to capture and neutralize HIV-1 in a humanized mouse model (Wei et al., 2019). The COVID-19 induced intestinal dysbiosis got recovered by nutritional support with L. acidophilus (Kageyama et al., 2020). Colorado State University is developing a novel oral COVID-19 vaccine candidate using recombinant Lactobacillus acidophilus expressing the viral S protein. This platform has been shown to induce Th1 and Th17 responses against HIV-1 epitopes in mice after oral administration (Dwyer, 2020). L. acidophilus is also tried as a potential vaccine vector, and a genetically modified L. acidophilus NCFM strain is identified as a new vaccine carrier to target the corona virus (Coronavirus, 2020). L. brevis, with the immunity-enhancing role through natural killer cells reduced the incidence of influenza viral infection (Waki et al., 2014). The oral bacteriotherapy approach for COVID-19 using L. brevis DSM27961 reduced respiratory failure risk, eight times lower than the controlled group, and the remission of diarrheal symptoms within 72 h (d'Ettorre et al., 2020). The GABA production by L. brevis BGZLS-10-17 showed a strong immune regulatory effect on mesenteric lymph node cells and induced autophagy in CD48, T-cells, NK, NKT and antigen-presenting cells (Bajić et al., 2020). L. delbrueckii Sub sp. bulgarius boosts systemic immunity by inducing inflammatory cytokines, macrophage activation, Natural Killer cell’s functions, and increased interleukin production slowing down the aging of T-cell subspecies, increasing immature T-cells subspecies (Oyeniran et al., 2020). The respiratory passage is the major route for the invasive pathogens and NK cells in the airway plays a major role in the airways to regulate chronic infection and inflammation by secreting cytokine IFN-γ to induce immune response. After the clearance of pathogens the NK cells response is down-regulated by homeostasis mechanism. Other than IFN-γ production, NK cells recognises and kill the collagen-secreting stellate cells responsible for pulmonary fibrosis (Culley, 2009).
In the digestive system NK cells interact with epithelial cells, fibroblasts, macrophages, dendritic cells, and T lymphocytes, to maintain the immune homeostasis and vibrant immune responses through the production of IFNγ, which in turn reduce inflammatory bowel diseases. In the gut NK cells participates in both physiological and pathogenic responses Poggi et al., 2019). The SARS-CoV-2 virus damages the gut epithelial layers. L. plantarum is found to strengthen the viral damaged mucus epithelial barrier and enhance probiotics' transport to lungs through the gut –lung axis and execute innate and adaptive immunity in the gut associated lymphoid tissue, secondary lymphoid organs (Zhang et al., 2020, Lundstrom, 2020).
Ligillactobacillus salavarius in the gut also enhances immunity against virus that induce inflammatory cytokines, IL-10 (Zhai et al., 2020). L. reuteri secretions like bacteriocins, reutericin and reutericyclin show a rich antibacterial, antiviral, antifungal, and anti-protozoan activity. Tryptophan[trp] derived indole derivatives AhR produced by L. reuteri down-regulate Thpok production leading to reprogramming of CD4+ IELs into DPIELs that enhance the production of antimicrobial peptides (Reg3-lectins) to arouse the innate immune response against inflammation-inducing intestinal pathogens (Ang et al., 2016, Chen et al., 2018). Angiotensin-converting enzyme (ACE) and dipeptidyl peptidase 4 (DPP-4) produced by L. lactis have immunoregulatory functions. So a recombinant Lactobacillus vaccines are planned against COVID-infection (Jiang et al., 2016). L. casei based oral vaccine against the transmissible gastroenteritis coronavirus (TGEV) stimulated Th 17 pathway. It inhibited TGEV. Many research work is being carried out globally to use different Lactobacillus species as a new platform for COVID-19 vaccine rectors (ISAPP, 2020, Frederiksen et al., 2020). For the COVID-19 problem, research studies indicated the need to enhance immunity, particularly T-cell action against the virus (Olaimat et al., 2020).
6. Immunity boosting palm dates and COVID-19 impacts in Saudi Arabia
From the existing literature and practical knowledge, it is well known that nutritional therapy promotes immunity against viral infections. Ripe and dried dates are good sources of vitamin C, A1, B1, B2, B3 and B5, essential nutrients to boost immunity and respiratory problems. It enhances antioxidant potential. The dates have bioactive compounds like vitamin E, carotenoids, polyphenols especially phenolicacids isoflavones, lignans, and flavonoids, tannins, and sterols that influences immunity. This is well seen in the high recovery rate and less than 0.7% mortality among COVID-19 infected persons in the Kingdom of Saudi Arabia [KSA]. The country KSA is one of the top twenty countries globally and ranks first among the Arab world. Having a second position in the global natural fuel resource, this energy super nation with 35 million population, including 30% repatriates from multicultural nationalities, was less affected than other countries in COVID-19 mortality rate. Further, most of the death reported during COVID-19 period was because of comorbidities and viral attack. The KSA administration’s previous experience with MERS-CoV and effective provision of a hygienic environment to more than 2 million Hajj pilgrims from the whole world, the COVID-19 did not give much panic among the population. Our inquiry with around a hundred Indian repatriates in KSA, among whom few got COVID-19 infection, did not panic over the problem, unlike their counterpart in India. From our personal observation, the immunity against the viral or other infection is good in people living in KSA. When the reasons for elevated resistance were analyzed, the food habits, particularly the regular intake of palm date fruits and its derivatives, make their difference with people living in other countries. Further, the Nation's COVID-19 management strategies developed from their 2012 MERS-CoV episode, helped them to make effective situation handling after the report of the first case of COVID-19 on March, 2nd 2020. In the hundred year history of the holy city Mecca, this is the first time the country stopped pilgrims. Further, the WHO social vaccine-like hand washing, mask-wearing, [all women are covering their face], and personal hygiene are already in practice as per the holy Quran. The regular intake of probiotic fiber-rich dates fruits and dates kernel and other food strengthened their innate immunity and antioxidant status (Guardole et al., 2016). Several studies have proved that the dates fruit and its extracts stimulate probiotic lactobacilli (Al-Thubiani and Ahmed Khan, 2017, Mustafa et al., 2020). The consumption of date palm fruits enhances intestinal microflora and increases bowel movements. After the MERS-CoV episode of the bat's origin, it is advised to wash the fruits of dates with boiled water before use (Eid et al., 2015).
7. Conclusion
As per literature review of COVID-19 cases, it is evident that people with good natural immunity overcomes the virus load. As we know, the gold standard for healthy and robust immunity lies in the gut and diet functions. The nutritional and immunity enhancing probiotics operating homeostasis in the gut must be paid research attention. A regular physical exercise, a healthy lifestyle, and probiotics supplementation can be prominent players to induce immunity. The specific role of probiotics to enhance natural killer cells function, stimulation of IgA antibodies, and mucosal barrier inflammation control promoted an interest in new generation probiotics to strengthen immunity to treat COVID-19 viruses.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
“This Research Project Was Supported By a Grant From The Research Center For The Humanities, Deanship of Scientific Research, King Saud University, as part of Initiative (4.4): Promoting Interdisciplinary Research Among Social Sciences, Humanities, and Other Sciences (PIR-1-19-06).
Conflicts of Interest.
NIL.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ahlawat S., Asha, Sharma K.K. Immunological co-ordination between gut and lungs in SARS-CoV-2 infection. Virus Res. 2020;286:198103. doi: 10.1016/j.virusres.2020.198103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed-Hassan H., Sisson B., Shukla R.K., Wijewantha Y., Funderburg N.T., Li Z., Hayes D., Jr., Demberg T., Liyanage N. Innate immune responses to highly pathogenic coronaviruses and other significant respiratory viral infections. Front. Immunol. 2020;11:1979. doi: 10.3389/fimmu.2020.01979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Thubiani A.S., Ahmed Khan M.S. The probiotic properties of date palm (Phoena declycifera L) seeds in stimulating probiotic Lactobacillus. JPAM. 2017;11(4):1675–1686. doi: 10.22207/JPAM.11.4.05. [DOI] [Google Scholar]
- Akour A. Probiotics and COVID‐19: is there any link? Lett. Appl. Microbiol. 2020;71(3):229–234. doi: 10.1111/lam.13334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang L.Y., Too H.K., Tan E.L., Chow T.V., Shek L.P., Tham E.H., Alonso S. Erratum to: antiviral activity of Lactobacillus reuteri Protectis against Coxsackievirus A and Enterovirus 71 infection in human skeletal muscle and colon cell lines. Virol. J. 2016;13(1):186. doi: 10.1186/s12985-016-0633-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes A.E.C., Vinderola G., Xavier-Santos D., Sivieri K. Potential contribution of beneficial microbes to face the COVID-19 pandemic. Food Res. Int. 2020;136 doi: 10.1016/j.foodres.2020.10957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwar F., Altayb H.N., Al-Abbasi F.A., Al-Malki A.L., Kamal M.A., Kumar V. Antiviral effects of probiotic metabolites on COVID-19. J. Biomol. Struct. Dyn. 2020:1–10. doi: 10.1080/07391102.2020.1775123. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarti C., Martina C., Khusro A. Antimycobacterium, anticancer, and antiviral properties of probiotics: an overview. Microbes Inf. Dis. 2020;1 doi: 10.21608/mid.2020.34124.1029. [DOI] [Google Scholar]
- Baud D., Dimopoulou A.V., Gibson G.R., Reid G., Giannoni E. Using probiotics to flatten the curve of coronavirus disease COVID-2019 pandemic. Front. Public Health. 2020;8:186. doi: 10.3389/fpubh.2020.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahadur Gurung A., Ajmal Ali M., Lee J., Abul Farah M., Mashay, Al-Anazi, Hide K. Structure-based virtual screening of phytochemicals and repurposing of FDA approved antiviral drugs unravels lead molecules as potential inhibitors of coronavirus 3C-like protease enzyme. J. King Saud Univ. – Sci. 2020;32(6,):2845–2853. doi: 10.1016/j.jksus.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajić S.S., Đokić J., Dinić M., Tomić S., Popović N., Brdarić E., Golić N., Tolinački M. GABA potentiate the immunoregulatory effects of Lactobacillus brevis BGZLS10-17 via ATG5-dependent autophagy in vitro. Sci. Rep. 2020;10(1):1347. doi: 10.1038/s41598-020-58177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhimraj, A., Morgan, R. L., Shumaker, A. H., Lavergne, V., Baden, L., Cheng, V. C., Edwards, K. M., Gandhi, R., Muller, W. J., O'Horo, J. C., Shoham, S., Murad, M. H., Mustafa, R. A., Sultan, S., Falck-Ytter, Y., 2020. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, ciaa478. Advance online publication. https://doi.org/10.1093/cid/ciaa478. [DOI] [PMC free article] [PubMed]
- Blanco-Melo D., Nilsson-Payant B.E., Liu W.C., Uhl S., Hoagland D., Møller R., Jordan T.X., Oishi K., Panis M., Sachs D., Wang T.T., Schwartz R.E., Lim J.K., Albrecht R.A., tenOever B.R. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell. 2020;181(5):1036–1045.e9. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell D., Roberton S., Hunter P.R. Animal origins of SARS coronavirus: possible links with the international trade in small carnivores. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2004;359:1107–1114. doi: 10.1098/rstb.2004.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan P.K., Chan M.C. Tracing the SARS-coronavirus. J. Thorac. Dis. 2013;2:S118–S121. doi: 10.3978/j.issn.2072-1439.2013.06.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B., Ni X., Sun R., Zeng B., Wei H., Tian Z., Wei H. Commensal Bacteria-Dependent CD8αβ+ T cells in the intestinal epithelium produce antimicrobial peptides. Front. Immunol. 2018;9:1065. doi: 10.3389/fimmu.2018.01065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culley F.J. Natural killer cells in infection and inflammation of the lung. Immunology. 2009;128(2):151–163. doi: 10.1111/j.1365-2567.2009.03167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Ettorre G., Ceccarelli G., Marazzato M., Campagna G., Pinacchio C., Alessandri F., Ruberto F., Rossi G., Celani L., Scagnolari C., Mastropietro C., Trinchieri V., Recchia G.E., Mauro V., Antonelli G., Pugliese F., Mastroianni C.M. Challenges in the management of SARS-CoV2 infection: the role of oral bacteriotherapy as complementary therapeutic strategy to avoid the progression of COVID-19. Front. Med. 2020;7:389. doi: 10.3389/fmed.2020.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosten C., Gunther S., Preiser W. Identification of a novel coronavirus in patients with the severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- Dwyer A. A Researchers explore. Lacto bacillus acidophilus for use in corona virus vaccine. Gen. Health. 2020;21 April 2020. [Google Scholar]
- Eid N., Osmanova H., Natchez C., Walton G., Costabile A., Gibson G., Rowland I., Spencer J.P. Impact of palm date consumption on microbiota growth and large intestinal health: a randomised, controlled, cross-over, human intervention study. Br. J. Nutr. 2015;114(8):1226–1236. doi: 10.1017/S0007114515002780. [DOI] [PubMed] [Google Scholar]
- Fanos V., Pintus M.G., Pintus R., Marcialis M.A. Lung microbiota in the acute respiratory disease: from coronavirus to metabolomics. J. Pediatr. Neonatal. Individ. Med. 2020;9 doi: 10.7363/090139. [DOI] [Google Scholar]
- Frederiksen L.S.F., Zhang Y., Foged C., Thakur A. The Long Road Toward COVID-19 herd immunity: vaccine platform technologies and mass immunization strategies. Front. Immunol. 2020;11:1817. doi: 10.3389/fimmu.2020.01817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu C., Peng P., Loschko J., Feng L., Pham P., Cui W., Lee K.P., Krug A.B., Jiang A. Plasmacytoid dendritic cells cross-prime naive CD8 T cells by transferring antigen to conventional dendritic cells through exosomes. Proc. Natl. Acad. Sci. U.S.A. 2020;117(38):23730–23741. doi: 10.1073/pnas.2002345117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Yan L., Huang Y., Liu F., Zhao Y., Cao L., Wang T., Sun Q., Ming Z., Zhang L., Ge J., Zheng L., Zhang Y., Wang H., Zhu Y., Zhu C., Hu T., Hua T., Zhang B., Yang X., Rao Z. Structure of the RNA-dependent RNA polymerase from COVID-19 virus. Science. 2020;368(6492):779–782. doi: 10.1126/science:abb7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardole F.A., Porcinoc C., Cerezuela R., Cuesta A., Faggio C., Esteban M.A. Impact of date palm fruit extracts and probiotic enriched diet on antioxidant status, innate immune response and immune related gene expression of European Sea bass (Dicentrachus labrax) Fish Shell Fish Immunol. 2016;52:298–308. doi: 10.1016/j.fsi.2016.03.152. [DOI] [PubMed] [Google Scholar]
- Gupta A., Madhavan M.V., Sehgal K., Nair N., Mahajan S., Sehrawat T.S., Bikdeli B., Ahluwalia N., Ausiello J.C., et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020;26(7):1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PubMed] [Google Scholar]
- Hanifi G., Samadi Kafil H., Tayebi Khosroshahi H., Shapouri R., Asgharzadeh M. Lactobacilli species diversity in gut microbiota of renal failure patients. J. King Saud Univ. – Sci. 2020;32(4):2365–2369. doi: 10.1016/j.jksus.2020.03.015. [DOI] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hon E., Li A., Nelson E.A.S., Leung C.W., Cherry D. In: Textbook of Paediatric Infectious Diseases. 5th ed. Feigin R.D., Cherry J.D., Demmler G.J., Kaplan S., editors. WB Saunders Co; Philadelphia, PA: 2003. Severe acute respiratory syndrome (SARS) pp. 2389–2393. [Google Scholar]
- Hulswit R.J., de Haan C.A., Bosch B.J. Coronavirus spike protein and tropism changes. Adv. Virus Res. 2016;96:29–57. doi: 10.1016/bs.aivir.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England) 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Scientific Association Probiotics and Prebiotics (ISAPP) Science Blog. News August, 26, 2020). Colorado University Blog – Coleman cornellius 2 April 2020.
- Jancovich, J.K., Chinchar, V.G., Hyatt, A., Miyazaki, T., Williams, T. & Zhang, Q.Y. 2012. In: King, A.M.Q., Adams, M.J., Carstens, E.B., Lefkowitz. E.J. (eds.), Virus Taxonomy, Ninth Report of the International Committee on Taxonomy of Viruses. pp. 193-210. ISBN 978-0-12-384684-6.
- Jiang X., Hou X., Tang L., Jiang Y., Ma G., Li Y. A phase trial of the oral Lactobacillus casei vaccine polarizes Th2 cell immunity against transmissible gastroenteritis coronavirus infection. Appl. Microbiol. Biotechnol. 2016;100(17):7457–7469. doi: 10.1007/s00253-016-7424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X., Lian J.S., Hu J.H., Gao J., Zheng L., Zhang Y.M., Hao S.R., Jia H.Y., Cai H., Zhang X.L., Yu G.D., Xu K.J., Wang X.Y., Gu J.Q., Zhang S.Y., Ye C.Y., Jin C.L., Lu Y.F., Yu X., Yu X.P., Yang Y. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69(6):1002–1009. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama Y., Akiyama T., Nakamura T. Intestinal dysbiosis and probiotics in COVID-19. J. Clin. Trials. 2020;10:421. doi: 10.35248/2167-0870.20.10.421. [DOI] [Google Scholar]
- Kayode T.A. Coronavirus and probiotics: past, present and future. J. Prob. Health. 2020;8 doi: 10.35248/2329-8901.20.8.e124. [DOI] [Google Scholar]
- Kwok K.O., Tang A., Wei V.W.I., Park W.H., Yeoh E.K., Riley S. Epidemic models of contact tracing: systematic review of transmission studies of severe acute respiratory syndrome and middle east respiratory syndrome. Comput. Struct. Biotechnol. J. 2019;17:186–194. doi: 10.1016/j.csbj.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Geng M., Peng Y., Meng L., Lu S. Review paper-Molecular immune pathogenesis and diagnosis of COVID-19. J. Pharm. Anal. 2020 doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L., Jiang X., Zhang Z., Huang S., Zhang Z., Fang Z., Gu Z., Gao L., Shi H., Mai L., Liu Y., Lin X., Lai R., Yan Z., Li X., Shan H. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020;69(6):997–1001. doi: 10.1136/gutjnl-2020-321013. [DOI] [PubMed] [Google Scholar]
- Liisa L., Sinikka L., Markus J.L. Role of probiotics in stimulating the immune system in viral respiratory tract infections: a narrative review. Nutrients. 2020;12:3163. doi: 10.3390/nu12103163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundstrom K. Coronavirus pandemic-therapy and vaccines. Biomedicines. 2020;8(5):109. doi: 10.3390/biomedicines8050109. PMID: 32375268; PMCID: PMC7277397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak J., Chan F., Ng S.C. Probiotics and COVID-19: one size does not fit all. The Lancet. Gastroenterol. Hepatol. 2020;5(7):644–645. doi: 10.1016/S2468-1253(20)30122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marra M.A., Jones S.J.M., Astell C.R. The genome sequence of the SARS-associated coronavirus. Science. 2003;300:1399–1404. doi: 10.1126/science.1085953. [DOI] [PubMed] [Google Scholar]
- McKechnie J.L., Blish C.A. The innate immune system: fighting on the front lines or fanning the flames of COVID-19? Cell Host Microbe. 2020;27(6):863–869. doi: 10.1016/j.chom.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meo S.A., Zaidi S.Z.A., Shang T., Zhang J.Y., Al-Khlaiwi T., Bukhari I.A., Akram J., Klonoff D.C. Biological, molecular and pharmacological characteristics of chloroquine, hydroxychloroquine, convalescent plasma, and remdesivir for COVID-19 pandemic: a comparative analysis. J. King Saud Univ.– Sci. 2020;32(7):3159–3166. doi: 10.1016/j.jksus.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metchnikoff I., Mitchd P.C. Kessinger publications; 1910, White fish, MT, USA: 1910. Nature of Man or Studies in Optimistic Philosophy. [Google Scholar]
- Morais A.H.A., Passos T.S., Maciel B.L.L., da Silva-Maia J.K. Can probiotics and diet promote beneficial immune modulation and purine control in coronavirus infection? Nutrients. 2020;12(6):1737. doi: 10.3390/nu12061737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa H.S., Marwa Rashad A.L., Mohamed R.M. Production of a novel probiotic date juice with anti-proliferative activity against Hep-2 cancer cells. Food Sci. Technol. 2020 doi: 10.1590/fst.09920. [DOI] [Google Scholar]
- Nanis G.A., Mohamed L.S., Hassan E., Maii M.N. Lactobacillus acidophilus and Bifidobacteria spp having antibacterial and antiviral effects on chronic HCV infection. Afr. J. Microbiol. Res. 2019;13(5):77–90. doi: 10.5897/AJMR2018.9028. [DOI] [Google Scholar]
- Olaimat A.N., Aolymat I., Al-Holy M., Ayyash M., Ghoush A.B., Al-Nabulsi A.A., Osaili T. The potential application of probiotics and prebiotics for the prevention and treatment of COVID-19. npj Sci Food. 2020;4:17. doi: 10.1038/s41538-020-00078-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyeniran A., Gyawali R., Aljaloud S.O., Krastanov A., Ibrahim S.A. Probiotic Characteristics and Health Benefits of the Yogurt Bacterium Lactobacillus delbrueckii sp. Bulgaricus. IntechOpen Limited; London, SW7 2QJ: 2020. Open access peer-reviewed chapter. [DOI] [PubMed] [Google Scholar]
- Poggi A., Benelli R., Venè R., Costa D., Ferrari N., Tosetti F., Zocchi M.R. Human gut-associated natural killer cells in health and disease. Front. Immunol. 2019;10:961. doi: 10.3389/fimmu.2019.00961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourhossein, M., Moravejolahkami, A R., 2020. Probiotics in viral infections, with a focus on COVID-19: A Systematic Review. 10.22541/au.158938616.61042433. [DOI]
- Research and Market com/reports/4858168/Saudi Arabia – probiotics – market – Forecost from 2020-2025 October, 2020.
- Sarah Lebeer, University of Antwerp, Belgium: Relevance of the airway microbiome profile to COVID-19 respiratory infection and using certain lactobacilli to enhance delivery or efficacy of vaccines May 4, 2020/in ISAPP Science Blog, News.
- Senapati S., Dash J., Sethi M., Chakraborty S. Bioengineered probiotics to control SARS-CoV-2 infection. Res. Ideas Outcomes. 2020;6 doi: 10.3897/rio.6.e54802. [DOI] [Google Scholar]
- Sundararaman A., Ray M., Ravindra P.V., Halami P.M. Role of probiotics to combat viral infections with emphasis on COVID-19. Appl. Microbiol. Biotechnol. 2020;104(19):8089–8104. doi: 10.1007/s00253-020-10832-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su M., Jia Y., Li Y., Zhou D., Jia J. Probiotics for the prevention of ventilator-associated pneumonia: a meta-analysis of randomized controlled trials. Respir. Care. 2020;65:5. doi: 10.4187/respcare.07097. [DOI] [PubMed] [Google Scholar]
- Taghizadeh-Hesary F., Akbari H. The powerful immune system against powerful COVID-19: A hypothesis. Med. Hypotheses. 2020;140 doi: 10.1016/j.mehy.2020.109762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari S.K., Dicks L., Popov I.V., Karaseva A., Ermakov A.M., Suvorov A., Tagg J.R., Weeks R., Chikindas M.L. Probiotics at war against viruses: what is missing from the picture? Front. Microbiol. 2020;11:1877. doi: 10.3389/fmicb.2020.01877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trottein F., Sokol H. Potential causes and consequences of gastrointestinal disorders during a SARS-CoV-2 infection. Cell Rep. 2020;32(3) doi: 10.1016/j.celrep.2020.107915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana S.D., Nunes S., Reis F. ACE2 imbalance as a key player for the poor outcomes in COVID-19 patients with age-related comorbidities – role of gut microbiota dysbiosis. Ageing Res. Rev. 2020;62 doi: 10.1016/j.arr.2020.101123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlasak R., Luytjes W., Spaan W., Palese P. Human and bovine coronaviruses recognize sialic acid-containing receptors similar to those of influenza C viruses. Proc. Natl. Acad. Sci. U.S.A. 1988;85(12):4526–4529. doi: 10.1073/pnas.85.12.4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waki N., Matsumoto M., Fukui Y., Suganuma H. Effects of probiotic Lactobacillus brevis KB290 on incidence of influenza infection among schoolchildren: an open-label pilot study. Lett. Appl. Microbiol. 2014;59:565–571. doi: 10.1111/lam.12340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W., Wiggins J., Hu D., Vrbanac V., Bowder D., Mellon M., Tager A., Sodroski J., Xiang S.H. Blocking HIV-1 Infection by Chromosomal Integrative Expression of Human CD4 on the Surface of Lactobacillus acidophilus ATCC 4356. J. Virol. 2019;93(8):e01830–e1918. doi: 10.1128/JVI.01830-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilk A.J., Rustagi A., Zhao N.Q., Roque J., Martínez-Colón G.J., McKechnie J.L., Ivison G.T., Ranganath T., Vergara R., Hollis T., Simpson L.J., Grant P., Subramanian A., Rogers A.J., Blish C.A. A single-cell atlas of the peripheral immune response in patients with severe COVID-19. Nat Med. 2020;26:1070–1076. doi: 10.1038/s41591-020-0944-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Infection prevention and control during health care when COVID-19 is suspected <https://www.who.int/publications-detail/infection-prevention-and-control-during-health-care-when-novel-coronavirus-(ncov)-infection-is-suspected-2020-0125>.
- WHO Coronavirus Disease (COVID-19) Dashboard 2020 -Data last updated: 2020/11/20, 3:59pm CET.
- Xiong Y., Liu Y., Cao L., Wang D., Guo M., Jiang A., Guo D., Hu W., Yang J., Tang Z., Wu H., Lin Y., Zhang M., Zhang Q., Shi M., Liu Y., Zhou Y., Lan K., Chen Y. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerging Microbes Infect. 2020;9(1):761–770. doi: 10.1080/22221751.2020.1747363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., Tai Y., Bai C., Gao T., Song J., Xia P., Dong J., Zhao J., Wang F.S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. The Lancet. Respir. Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan F., Cao H., Cover T.L., Washington M.K., Shi Y., Liu L., Chaturvedi R., Peek R.M., Wilson K.T., Polk D.B. Colon-specific delivery of a probiotic-derived soluble protein ameliorates intestinal inflammation in mice through an EGFR-dependent mechanism. J. Clin. Invest. 2011;21(6):2242–2253. doi: 10.1172/JCI44031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D., Chu H., Hou Y., Chai Y., Shuai H., Lee A.C., Zhang X., Wang Y., Hu B., Huang X., Yuen T.T., Cai J.P., Zhou J., Yuan S., Zhang A.J., Chan J.F., Yuen K.Y. Attenuated interferon and proinflammatory response in SARS-CoV-2-infected human dendritic cells is associated with viral antagonism of STAT1 phosphorylation. J. Infect. Dis. 2020;222(5):734–745. doi: 10.1093/infdis/jiaa356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeager C.L., Ashmun R.A., Williams R.K., Cardellichio C.B., Shapiro L.H., Look A.T., Holmes K.V. Human aminopeptidase N is a receptor for human coronavirus 229E. Nature. 1992;357(6377):420–422. doi: 10.1038/357420a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N., Li C., Niu Z., Kang H., Wang M. Colonization and immunoregulation of Lactobacillus plantarum BF_15, a novel probiotic strain from the faces of breast-fed infants. Food Funct. 2020;11:3156–3166. doi: 10.1039/C9FO02745A. [DOI] [PubMed] [Google Scholar]
- Zhai O., Shen X., Cen S., Zhang C., Tian F., Zhao J., Zhang H., Xue Y., Chen W. Screening of Lactobacillus salivarius strains from the feces of Chinese populations and the evaluation of their effects against intestinal inflammation in mice. Food Funct. 2020;11(1):221–235. doi: 10.1039/c9fo02116g. [DOI] [PubMed] [Google Scholar]
- Zhou R., To K.K., Wong Y.C., Liu L., Zhou B., Li X., Huang H., Mo Y., Luk T.Y., Lau T.T., Yeung P., Chan W.M., Wu A.K., Lung K.C., Tsang O.T., Leung W.S., Hung I.F., Yuen K.Y., Chen Z. Acute SARS-CoV-2 infection impairs dendritic cell and T cell responses. Immunity. 2020 doi: 10.1016/j.immuni.2020.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]