Graphical abstract
Abbreviation: SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; COVID-19: Coronavirus Disease 2019.
Keywords: SARS-CoV-2, COVID-19, Traditional Chinese medicine, Direct evidence, Efficacy advantage, Mechanism
Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the third coronavirus causing serious human disease to spread across the world in the past 20 years, after SARS and Middle East respiratory syndrome. As of mid-September 2020, more than 200 countries and territories have reported 30 million cases of coronavirus disease 2019 (COVID-19) caused by SARS-CoV-2, including 950,000 deaths. Supportive treatment remains the mainstay of therapy for COVID-19. The World Health Organization reported that four candidate drugs, including remdesivir, are ineffective or have little effect on COVID-19. According to China News, 90 % of Chinese patients with COVID-19 use traditional Chinese medicine (TCM), with an effectiveness rate of 80 %, and no deterioration in patient condition. We have compiled the direct evidence of TCM treatment for COVID-19 as of December 31, 2020. We describe the advantages of TCM in the treatment of COVID-19 based on clinical evidence and the required methods for its clinical use. TCM can inhibit virus replication and transcription, prevent the combination of SARS-CoV-2 and the host, and attenuate the cytokine storm and immune deficiency caused by the virus infection. The cooperation of many countries is required to establish international guidelines regarding the use of TCM in patients with severe COVID-19 from other regions and of different ethnicities. Studies on the psychological abnormalities in patients with COVID-19, and medical staff, is lacking; it is necessary to provide a complete chain of evidence to determine the efficacy of TCM in the related prevention, treatment, and recovery. This study aims to provide a reference for the rational use of TCM in the treatment of COVID-19.
1. Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) may have infected humans from the host for the first time between October 6 and December 11, 2019 [1], and a large-scale outbreak of coronavirus disease 2019 (COVID-19) first occurred in 2019 in Wuhan, China. The World Health Organization (WHO) declared the outbreak a public health emergency of international concern on January 30, 2020. SARS-CoV-2 is the third coronavirus causing serious human disease to have spread globally in the past 20 years after severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) [2]. As of mid-September 2020, more than 200 countries and territories have reported a total of 30 million confirmed cases and 950,000 deaths [3], placing a huge social burden and economic pressure on the world. Traditional Chinese medicine (TCM) has been used in China for more than 2,000 years. During the SARS epidemic, TCM played an important role in reducing mortality and alleviating patients' symptoms [4]. In the absence of specific drugs to treat COVID-19, China and South Korea have widely promoted the use of TCM. According to China News, 90 % of Chinese patients with COVID-19 use TCM, with a reported effectiveness rate of over 80 % and no cases of deterioration in patient condition [5].
At present, many reviews have described the potential efficacy of TCM as an intervention method for COVID-19, based on the evidence of the use of TCM for the treatment of influenza A (H1N1) and SARS. Consequently, we have compiled the current direct evidence regarding the use of TCM for the treatment of COVID-19. We searched Pubmed, EMBASE, Cochrane library, Clinical Trials (https://clinicaltrials.gov), Chinese Clinical Trial Registry (http://www.chictr.org.cn/searchproj.aspx), China National Knowledge Internet, Wanfang Database, and China Biomedical Database for clinical and basic research on TCM intervention related to COVID-19 before December 31, 2020. For clinical trials, we included exclusively randomized controlled and cohort trials. Basic research included animal experiments, cell experiments, and research based on network pharmacology.
We describe the historical evolution of TCM in the treatment of acute and chronic diseases, its therapeutic advantages in the treatment of COVID-19 based on clinical research evidence, and the required methods for its clinical use. At the mechanism level, TCM can inhibit the replication and transcription of SARS-CoV-2, prevent the combination of SARS-CoV-2 and the host, and attenuate the cytokine storm and immune deficiency caused by the virus infecting the human body. This review, thus, aims to provide a reference for the rational use of TCM in the clinical treatment of COVID-19.
2. COVID-19
COVID-19 is caused by SARS-CoV-2, which has a diameter of 60–140 nm and obvious spike proteins ranging from 9 to 12 nm, giving the virion the appearance of a solar corona [6]. Preliminary analysis of 103 SARS-CoV-2 genomes in Wuhan, China, revealed two main subtypes (respectively, named L and S) [7]; the L-form is found in approximately 70 % of cases and is more aggressive and infectious compared to the S-form [7]. Clinical data reveals that SARS-CoV-2 has several main characteristics, such as a strong contagion potential, evoking a weak immune response, and causing severe illness when complicated by comorbid diseases.
2.1. SARS-CoV-2 with strong infectious potential
Compared to previous coronavirus epidemics, such as MERS and SARS, more than 50 % of COVID-19 infections can be attributed to asymptomatic and presymptomatic transmission, thereby accelerating its transmission capacity [8]. As SARS-CoV-2 can use the furin enzyme in the host to cut the S protein, mutations and recombination events occur rather frequently [9,10]. The mutated SARS-CoV-2 is constantly adapting to the human host [1], causing a lung invasion rate of 100 to 1,000 times that of SARS [11]. Patients with coronary heart disease are more likely to be infected by SARS-CoV-2 than individuals who are healthy. This may be related to the increased expression of angiotensin-converting enzyme 2 (ACE2) in the lungs and other tissues [12,13], or it may be related to the upregulation of ACE2 expression caused by hypoglycemic agents, such as thiazolidinedione, glucagon-like peptide-1, ACE inhibitors, angiotensin receptor blockers, antihypertensive drugs, and statins [[14], [15], [16], [17], [18]].
2.2. SARS-CoV-2 could weak patients with COVID-19 immune response
For patients with COVID-19, long-term infection with SARS-CoV-2 increases the numbers of monocytes, neutrophils, NK cells, and CD4 + T cells but at the same time reduces the total numbers of lymphocytes and CD8 + T cells [19,20] while producing low levels of neutralizing antibodies [21]. Compared to SARS, SARS-CoV-2 may induce a weak immune response in patients with COVID-19 through "immune stealth", prolonging the recovery process [22,23]. This is related to the low level of cytosine-phosphate-guanosine in the genome, glycosylation to shield essential elements including the receptor binding domain, RNA shielding, and active blocking of antiviral interferon responses related to viral proteins [[24], [25], [26]].
2.3. Patients with COVID-19 and comorbid disease present with severe illness
A large study of 72,314 patients with COVID-19 in China revealed that the mortality rate of patients with diabetes was three times higher than that of patients without diabetes (7.3 % vs. 2.3 %) [27]. Similarly, there is a higher all-cause mortality rate in patients with coronary heart disease [12] and cancer [28]. A possible explanation is that approximately 7–10 days after acquiring COVID-19, there may be a hyperinflammatory response (cytokine storm) that may cause damage to the microcirculation of different vascular beds. The clinical sequelae include thromboembolic events, renal failure, shock, and multiple organ failure [[29], [30], [31]]. COVID-19 can also lead to increased myocardial oxygen consumption, and energy consumption caused by fever, sympathetic nerve activation, and tachycardia [32]. Hypoxic brain damage is aggravated, which may lead to neuronal swelling and cerebral edema, and ultimately nervous system damage [33]. SARS-CoV binds to ACE2 in the pancreatic islet cells, destroys them, and causes acute hyperglycemia; it may cause excessive mortality even in patients without diabetes [34].
2.4. In the absence of specific drugs, supportive treatment remains the primary treatment for COVID-19
Regarding the treatment regime of COVID-19, the WHO initiated a clinical trial called "Solidarity", evaluating the effect of drugs including remdesivir, chloroquine or hydroxychloroquine, and lopinavir/ritonavir [35]. Recently, interim results revealed that all four candidate drugs, including remdesivir, are ineffective or have little effect on COVID-19 [36]. The vaccines currently under study mainly include inactivated vaccines (Vero cells), adenovirus vector vaccines, nucleic acid vaccines, recombinant protein vaccines, and attenuated influenza virus vector vaccines [37]. The governments of China, the United States, and the United Kingdom have gradually approved vaccines for official use [[38], [39], [40]]. Supportive treatment for patients with COVID-19, including supplemental oxygen, is currently the main treatment used in most cases. There is currently no human vaccine available against SARS-CoV-2, although approximately 120 candidate vaccines are under development. During the next few months, other prevention methods may emerge, including monoclonal antibodies, hyperimmune globulin, and moderate titers [41].
2.5. TCM has shown significant advantages in treating acute and chronic diseases
TCM is a treasure of ancient Chinese medicine with a history of more than 2,000 years, unique theories, and rich experience [42]. Evidence from randomized controlled trials shows that TCM can effectively treat chronic diseases such as hypertension and diabetes [43]. For example, Gegen Qinlian decoction can significantly reduce blood sugar in patients with type 2 diabetes; the mechanism of action is related to structural changes of the intestinal flora that is rich in beneficial bacteria [44]. Literature shows that oseltamivir and maxingshigan-yinqiaosan, separately and in combination, can reduce the duration of fever in patients with H1N1 influenza virus infection, indicating that maxingshigan-yinqiaosan can be used as an alternative treatment in patients with H1N1 influenza infection [45]. During the current COVID-19 pandemic, in the absence of specific vaccines and medicines to prevent and treat COVID-19, China and South Korea have widely promoted the use of TCM. According to China News, 90 % of Chinese patients with COVID-19 use TCM, with a reported effectiveness rate of over 80 % and no deterioration in patient condition [5].
3. Obvious advantages of TCM for the treatment of COVID-19: direct evidence based on clinical studies
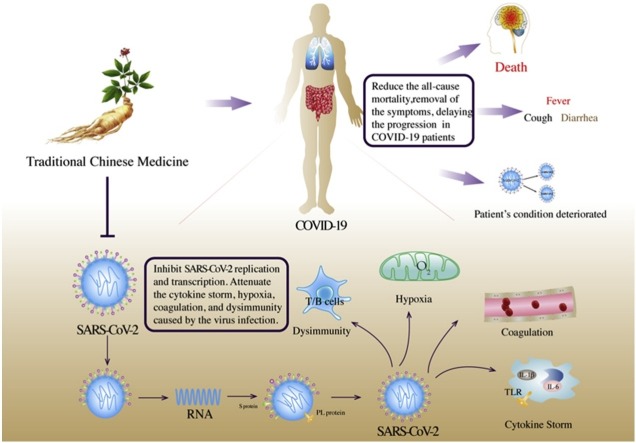
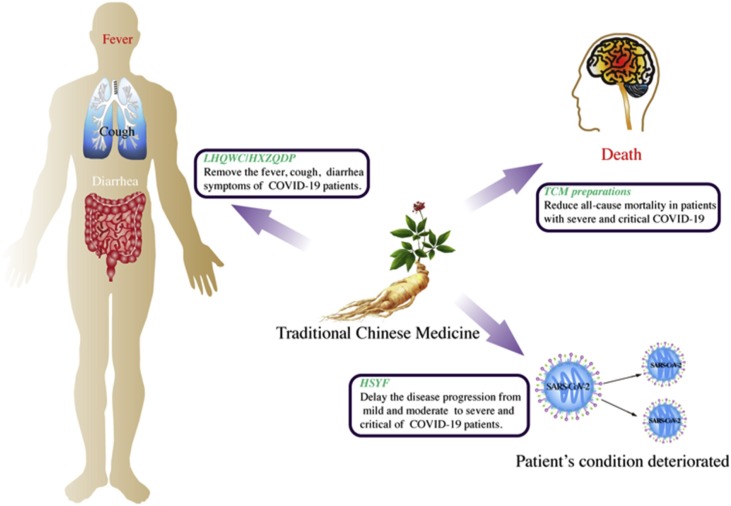
We retrieved clinical studies that met the inclusion criteria from the relevant databases, including three randomized controlled and seven cohort studies. The advantages of TCM for the treatment of COVID-19 are mainly reflected in the following three aspects: the effective alleviation of symptoms in patients suspected of and confirmed as having COVID-19, delaying the disease progression from mild and moderate to severe and critical, and reducing the all-cause mortality of patients with a severe and critical disease status. (Table 1 , Fig. 1 ). Of course, TCM can show its multi-target advantages for COVID-19patients with multiple organ systems. For example, MXSGD can not only improve fever by regulating the immune system, but also improve patients' cough symptoms.
Table 1.
Studies of direct evidence of TCM in the treatment of COVID-19.
| Author, year | Method | Inclusion criteria | Number of patients | Basic treatment | Traditional Chinese Medicine | Outcome | Gender (Male/Female) |
Age (years) | Comorbidity | Initial symptoms |
|---|---|---|---|---|---|---|---|---|---|---|
| Jie Zhao, 2020 [58] | Randomized controlled trial | Patients with severe COVID-19 | 39 (WM group 24, TCM and WM group 15) | Bed rest and adjuvant therapy; ensure adequate calorie and water intake; maintain water and electrolyte balance and homeostasis and strengthen psychological treatment for older children when necessary. | Yidu-toxicity blocking lung decoction (Bitter almonds, raw gypsum, trichosanthes, raw rhubarb, raw ephedra, roasted ephedra, Tinglizi, peach kernel, grass fruit, betel nut, atractylodes), 2 weeks | White blood cell count, neutrophil count, lymphocyte count, IL-6, IL-8, IL-2R, TNF-α, C-reactive protein, immune function, cure rate, length of hospital stays | 22/17 | – | – | Fever (94.87 %); dry cough (79.49 %) |
| Jiaxing Tian, 2020 [60] | Retrospective cohort trial | Mild, moderate COVID-19, HSYF in the exposed group used for more than 2 days | 721 (exposed group 430, non-exposed group 291) | Treatment Guideline for COVID-19 released by the National Health Commission of the People’s Republic of China | HSYF (Ephedra, Gypsum, Almond, Qianghuo, Tinglizi, Guanzhong, Earth Dragon, Xu Changqing, Huoxiang, Peilan, Cangzhu, Yunling, Raw Atractylodes, Jiao Hawthorn, Jiao Shenqu, Jiao Malt, Magnolia, Jiao Betel Nut, Simmering Grass Fruit, ginger) | Proportion of mild and moderate COVID-19 patients who progressed to severe disease | 347/374 | 48 | Hypertension (16.9 %), fatty liver (7.9 %), diabetes (7.1 %) | Fever (51.2 %), cough (27.6 %), diarrhea (20.8 %), fatigue (19.0%) |
| Mingzhong Xiao, 2020 [56] | Randomized controlled trial | Suspicious and confirmed COVID-19 cases; 18−85 years old, regardless of gender; provide informed consent | 283 (LHQWC 94, HXZQG + LHQC 95, WM 94) | Oral oseltamivir (75 mg per tablet) once a day, Arbidol (100 mg per tablet), twice a day, three tablets; Ribavirin (100 mg per tablet) orally, 3 times a day, Half piece | HXZQG (Atractylodes, Tangerine Peel, Jiang Magnolia, Angelica dahurica, Poria cocos, Big belly peel, Raw Pinellia, Licorice extract, Patchouli oil, Perilla leaf oil); LHQWC (Forsythia suspensa, Lonicera japonica, Ephedra sinica, Isatis indigotica, Pogostemon cablin, Rheum pal- matum, Glycyrrhiza uralensis, Dryopteris crassirhizoma, Rhodiola crenu- lata, Houttuynia cordata, Prunus sibirica, gypsum and 1-menthol) | The rate of improvement and disappearance of clinical symptoms after 14 days of treatment, the proportion of patients who progressed to a severe state despite the same treatment time | LHQWC 58/36;HXZQG + LHQWC 47/48;WM 50/44 | LHQWC 54.58 ± 13.76;HXZQG + LHQWC 54.31 ± 11.63;WM 54.06 ± 13.90 | Bronchial asthma1.06 %;Chronic obstructive pulmonary disease1.41 %, Coronary artery disease3.18 %;High blood pressure19.08 %;Diabetes7.42 %, | Fever (73.14 %), Cough (38.87 %), Diarrhea (3.53 %), Lack of strength (32.86 %) |
| Hu K,2002 [52] | Randomized controlled trial | Suspicious and confirmed COVID-19 cases; 18−85 years old, regardless of gender; provide informed consent | 284(T 142, C 142) | supportive treatment such as oxygen therapy, antiviral medications and symptomatic therapies | LHQWC (Forsythia suspensa, Lonicera japonica, Ephedra sinica, Isatis indigotica, Pogostemon cablin, Rheum pal- matum, Glycyrrhiza uralensis, Dryopteris crassirhizoma, Rhodiola crenu- lata, Houttuynia cordata, Prunus sibirica, gypsum and 1-menthol) | The improvement and remission rate of clinical symptoms and the rate of patients who develop a severe disease state | 52.82 %/47.18 % |
T 50.4 ± 15.2; C 51.8 ± 14.8 | – | Fever52.11 %, Fatigue50.70 %, Cough88.03 % |
| Chen Ling,2002 [50] | Retrospective cohort trial | Be at least 18 years old, meet the diagnostic criteria of the new coronavirus pneumonia diagnosis and treatment plan (trial version seven) for common COVID-19, and be hospitalized for more than 7 days | 68 (T 34, C 34) | Routine treatment is bed rest and supportive treatment. When oxygen saturation is low, catheterization is given to inhale oxygen; low fever is given physical cooling, high fever (≥ 38.3 °C), oral ibuprofen suspension is used to help reduce fever; those with severe sputum expectoration Ambroxol hydrochloride tablets were given oral expectorant, 0.6 g/time, 3 times/d); patients with infection were given oral anti-infective moxifloxacin hydrochloride tablets (0.4 g/time, 1 time/d); viral drug hydrochloric acid was also given Arbidol capsules are taken orally (0.2 g/time, 3 times/d). | Shufeng Jiedu Capsules (Polygonum cuspidatum, Forsythia suspensa, Verbena, Patriniaceae, Geshanxiao, Licorice), 2.08 g/time, 3 times/d, treatment course ≥7 d | The main symptoms (fever, cough, expectoration, fatigue) disappeared in 3, 5, and 7 days respectively, the number of days the main symptoms disappeared and the disappearance rate of other symptoms and signs, the effective rate of the main symptoms, the laboratory indicators of the patient's treatment for 7 days, chest CT improvement rate, clinical conversion rate and hospital stay comparison | T 14/20, C 15/19 | T 65.06 ± 10.63;C 64.35 ± 10.34 | Hypertension 30.88 %, coronary heart disease 8.82%, diabetes 13.24%, hyperlipidemia 2.94%, cerebral infarction 2.94%, gout 2.94% | Fever 54.41 %, cough 69.12%, sputum 22.06%, fatigue 51.47% |
| Lv RuiBing, 2020 [55] | Retrospective cohort trial | Meet the diagnostic criteria for suspected cases, and are over 18 years old; inpatients with imaging features of new coronary pneumonia | 101 (T 63, C 38) | Nutritional support treatment, symptomatic treatment, antiviral and antibiotic drug treatment | LHQWC (Forsythia suspensa, Lonicera japonica, Ephedra sinica, Isatis indigotica, Pogostemon cablin, Rheum pal- matum, Glycyrrhiza uralensis, Dryopteris crassirhizoma, Rhodiola crenu- lata, Houttuynia cordata, Prunus sibirica, gypsum and 1-menthol) | Symptoms and signs disappeared, common type changed to severe, critical illness aggravated during treatment, blood routine, urine routine, stool routine, liver function, kidney function was checked before and after treatment | – | T 59.12 ± 16.56, C 60.20 ± 17.01 | Hypertension 31.68 %, coronary heart disease 10.89 %, diabetes 10.89 %, cerebral infarction 14.85 % | Fever 93.07 %, fatigue 68.32 %, cough 89.11 % |
| Huo XiangKun, 2020. [159] | Retrospective cohort trial | Confirmed COVID-19 (positive throat swab nucleic acid); no medication contraindications; no malignant tumor; good compliance; voluntary participation | 70 (40 in combination group, 30 in Abidol group) | General treatment: bed rest, strengthen supportive treatment to ensure sufficient calories; pay attention to water and electrolyte balance, and maintain a stable internal environment; symptomatic treatment: in a resting state, oxygen saturation is less than 93%, and oxygen is given by nasal cannula. Fever is mainly caused by physical cooling. If the body temperature is higher than 38.5 °C, temporarily take 0.2 g ibuprofen orally to reduce fever. If cough or expectoration is given, ambroxol is used to eliminate phlegm; if there is an infection basis, moxifloxacin 0.4 g/time, once/ d. | Shufeng Jiedu Capsules (Polygonum cuspidatum, Forsythia suspensa, Verbena, Patriniaceae, Geshanxiao, Licorice), 2.08 g/time, 3 times/d, treatment course 10 d | The clinical manifestations such as fever, dry cough, nasal congestion, runny nose, sore throat, fatigue, and diarrhea were observed and counted on the 2, 3, 5, 7, and 10 days of treatment, as well as the negative status of the throat swab virus. | combination group 25/15, Abidol group 16/14 | combination group 40.65 ± 8.23),Abidol group 39.82 ± 6.40 | – | – |
| Shi Jia, 2020 [160] | Retrospective cohort trial | Diagnosed with COVID-19; the diagnosis types were mild, normal, and severe. | 67 (WM 18, TCM and WM 49) | Antiviral drugs, antibiotic medications | Chinese medicine decoction is based on the Chinese medicine treatment plan in the "New Coronavirus Pneumonia Diagnosis and Treatment Plan (Trial Fifth Edition)", combined with the characteristics of patients with new coronary pneumonia in Shanghai. | Clinical symptoms, critical conversion rate, length of hospital stay, total duration of illness | 53.73 %/46.27 % | 47.61 ± 15.18 | 31.34% | – |
| Xia WenGuang, 2020 [161] | Retrospective cohort trial | COVID-19 | 52 (WM 18, TCM and WM 34) | Antiviral drugs (arbidol, ribavirin, alpha interferon, lopinavir/ritonavir, oseltamivir), anti-infective drugs (moxifloxacin, levofloxacin, azithromycin, cephalosporins) are given And penicillin drugs), auxiliary support drugs (gamma globulin, methylprednisolone) | Chinese medicine decoction, Chinese patent medicine and Chinese Medicine injections, including Shidu Yufei Decoction, Yudu Biaofei Decoction, Ganlu Xiaodu Dan, Maxing Shigan Decoction, Lianhua Qingwen Granules, Huoxiang Zhengqi Water, In Vitro Cultured Bezoar, Xuebijing Injection, Phlegm Heat Qing injection, Shengmai injection, Shenfu injection | Clinical recovery time, time for body temperature to return to normal, incidence of disappearance of symptoms such as cough, fatigue, dyspnea, and diarrhea | 23/29 | 54. 00 ± 12. 83 | Tuberculosis 9.6 %, chronic obstructive pulmonary disease 11.5 %, hypertension 48.1 %, diabetes 50.0 %, chronic kidney disease 7.7 %, chronic liver disease 15.4%, stroke 5.8% | Fever 75.0 %, cough 50.0 %, fatigue 61.5 %, diarrhea 5.8 % |
| Guohua Chen, 2020 [62] | Retrospective cohort trial | severe/critical COVID-19 | 662 (exposed group 484, non-exposed group 178) | Patients were treated with oxygen, antivirals (such as interferon or ribavirin), antibiotics (such as moxifloxacin, cefoperazone sodium, and sulbactam sodium), and Chinese medicine based on the Diagnosis and Treatment Guideline for COVID-19 (Trial Version). | The TCM mainly consisted of Fuling (Poria cocos (Schw.) Wolf.), Huangqi (Astragalus membranaceus (Fisch.) Bge. var. mongholicus (Bge.) Hsiao.), Huoxiang (Pogostemon cablin (Blanco) Benth.), Kuxingren (Prunus armeniaca L. var. ansu Maxim.), Baizhu (Atractylodes macrocephala Koidz.), Banxia (Pinellia ternata (Thunb.) Breit.), Gancao (Glycyrrhiza uralensis Fisch.), Houpo (Magnolia officinalis Rehd. et Wils.), Mahuang (Ephedra sinica Stapf), Guizhi (Cinnamomum cassia Presl), Huangqin (Scutellaria baicalensis Georgi.), Sharen (Amomum villosum Lour.), Jiegeng (Platycodon grandiflorum (Jacq.) A.DC.), Peilan (Eupatorium fortunei Turcz.), and Dangsheng (Codonopsis pilosula (Franch.) Nannf.) purchased from Hubei Tianji TCM Decoction Pieces Co., Ltd. orally 200 mL each time, twice a day in hospital days. | all-cause mortality | 296/336 | 60 (47, 70) | Chronic obstructive lung disease 19 (2.9 %), Hypertension 208 (31.4 %), Cardiovascular disease 53 (8.0 %), Diabetes 94 (14.2 %), | Fever 450 (68.0 %), Dry cough 408 (61.6 %), Expectoration 184 (27.8 %), Fatigue 298 (45.0 %), Shortness of breath 221 (33.4%), |
Abbreviation: TCM: traditional Chinese medicine; WM: Western medicine; COVID-19: Corona Virus Disease 2019; IL-6: Interleukin 6; IL-8: Interleukin 8;, IL-2R: Interleukin 2R;, TNF-α: Tumor necrosis factor α; HSYF: HanShiYi formula; LHQWC: Lianhua Qingwen Capsules; HXZQG: Huoxiang Zhengqi Granules; T: Treat group; C: Control group.
Fig. 1.
Advantages of TCM in the treatment of COVID-19 based on clinical evidence.
Abbreviation: LHQWC: Lianhua Qingwen capsules; HXZQDP: Huoxiang Zhengqi dropping pill; HSYF: Hanshiyi formula.
The advantages of TCM in the treatment of COVID-19 are mainly reflected in three aspects, including effective removal of the symptoms of suspected and confirmed COVID-19 patients, including fever, cough, shortness of breath, fatigue, myalgia, nausea/vomiting or diarrhea, fatigue and rhinorrhea; delaying the progression from mild and common to severe and critical; and reducing all-cause mortality of severe and critical patients.
3.1. TCM can effectively alleviate symptoms, reduce pulmonary exudation, control inflammatory overreaction, and prevent deterioration of the condition of patients with suspected and confirmed COVID-19
According to a nationwide study of 1,099 patients with COVID-19 from 552 hospitals in China, the most common initial symptoms upon admission were fever (88.7 %); cough (67.8 %) [46]; shortness of breath (53–80 %); fatigue (38 %); myalgia (15–44 %); nausea, vomiting, or diarrhea (15–39 %); fatigue (25 %); and rhinorrhea (7 %) [47]. The appearance of these symptoms, such as fever, can increase emotional disorders such as stress and anxiety in patients with SARS [48] as well as COVID-19 [49]. At the same time, prolonged coughing can aggravate lung tissue damage; therefore, these symptoms also require attention. Presently, there is evidence that TCM can improve the clinical symptoms of patients with COVID-19 and may, thus, be potentially effective as a treatment [50,51].
TCM has been shown to increase the overall cure rate. In a multicenter, prospective, randomized controlled trial, Lianhua Qingwen capsules effectively increased the recovery rate of patients with COVID-19 (91.5 % vs. 82.4 %) compared to the pure application of Western medicine. Similar positive results were also found regarding chest computed tomography manifestations (83.8 % vs. 64.1 %) and the clinical cure rate (78.9 % vs. 66.2 %) [52]. Furthermore, TCM can significantly improve the clinical symptoms, such as fever, cough, fatigue, and loss of appetite, of patients with COVID-19 [53,54]. In a retrospective cohort study, the suspected cases were treated with Lianhua Qingwen granules (6 g, three times per day) on the basis of nutritional support. Within 10 days of treatment, the rate of disappearance of fever, cough, and fatigue symptoms in the treatment group was 86.7 %, 55.6 %, and 82.5 %, respectively, which is significantly higher than that in patients who were not treated with Lianhua Qingwen (67.7 %, 30.6 %, and 58.6 %, respectively) [55]. In another randomized controlled trial, 283 patients were enrolled and randomly divided into one of three treatment groups: 1) a Western medicine group; 2) a Western medicine, Huoxiang Zhengqi dropping pill, and Lianhua Qingwen granule group; and 3) a Western medicine and Lianhua Qingwen granule group. After 14 days of treatment, the Huoxiang Zhengqi dropping pills and Lianhua Qingwen granules showed advantages in the treatment of nausea, vomiting, and sore limbs, and the proportion of patients who became severely ill was the lowest in these groups. This indicates that integrating TCM and Western medicine may have the potential to improve the prognosis of patients with COVID-19 [56]. TCM may also reduce inflammation in the lungs and other organs of patients with COVID-19, as revealed by a pilot randomized clinical trial. Compared to the conventional therapy group, patients with COVID-19 who were treated with Xuanfei Baidu decoction and conventional medication showed a significant increase in the number of white blood cells and lymphocytes (P < 0.05) [53]. Along with Pneumonia No. 1 formula [54], Shufeng Jiedu capsule [50], Qingfei Paidu decoction [57], and Yidu-toxicity blocking lung decoction [58] can reduce the levels of C-reactive protein and other inflammation indicators such as erythrocyte sedimentation rate and significantly improve lung imaging indications. This shows that TCM can promote the attenuation of lung inflammation.
Indeed, there is another significant advantage of TCM that cannot be ignored, i.e., it can involve multiple targets. The most typical was during the H1N1 and COVID-19 epidemics. MXSGD, when applied alone or in combination with other TCMs, such as LHQWC, XFPDD [53], and Pneumonia No. 1 [54], can eliminate fever, cough, fatigue, and other symptoms [55], increase the total numbers of white blood cells and lymphocytes, and reduce C-reactive protein levels. Therefore, during the COVID-19 pandemic, China has promoted many common prescriptions, including QFPDD and HSYF, which are used not only to remove SARS-CoV-2 from the body, but also to relieve the clinical symptoms of COVID-19.
3.2. TCM can delay the disease progression of patients with confirmed mild and moderate COVID-19 to severe and critical
On January 29, 2020, in the previously mentioned study of 1,099 laboratory-confirmed COVID-19 cases, 926 patients were classified as having nonsevere disease, while 173 had severe disease. Of the patients with severe disease, a composite endpoint event occurred in 43, including admission to the intensive care unit, use of mechanical ventilation, or death [46]. This shows that patients with severe COVID-19 have a worse prognosis and may incur more medical expenditure. Existing evidence shows that integrating TCM into the treatment of COVID-19 can significantly delay the progression of patients with mild and moderate disease to severe and critical [59]. Accordingly, it may indirectly assist in suppressing the progress of the epidemic and reducing the medical burden.
A cohort study with a total of 721 patients with COVID-19 included 430 in the Hanshiyi formula (HSYF) group and 291 in the control group. The main outcome was the COVID-19 rate of exacerbation, where it was found that 0 (0.0 %) and 19 (6.5 %) cases in the HSYF and control group, respectively, progressed to severe disease. The difference was statistically significant. After considering gender and age using stratified analysis and propensity score matching, the difference in the aggravation rate between the HSYF and control group remained statistically significant [60].
3.3. TCM can reduce all-cause mortality in patients with severe and critical COVID-19
As of mid-September 2020, there were 950,000 deaths in more than 200 countries and territories due to COVID-19 [3]. According to a retrospective study on 1,305 COVID19 cases treated at Tongren Hospital of Wuhan University, the risk of death in patients with severe or critical disease was 74.364 times higher than that of patients with moderate disease, while the risk of death in patients using TCM preparations was lower than that in patients not using TCM [61]. This shows that TCM can be used as a potential treatment method to reduce the mortality of patients with severe and critical disease. Another study confirmed the efficacy of TCM, where a total of 484 patients using TCM and 178 who did not use TCM were included. Propensity score matching resulted in 156 users of TCM being matched with 156 non-users. There was no significant difference between the two groups regarding over 40 baseline variables. Among them, 13 patients in the TCM group died compared to 36 patients in the non-TCM group. After multi-factor adjustment, the use of TCM resulted in an 82.2 % reduction in the risk of death compared to not using TCM (odds ratio 0.178, 95 % Cl 0.076–0.418), indicating that the use of TCM by patients with severe or critical disease reduces their risk of mortality [62].
4. Adhering to scientific methods is the key to the successful use of TCM
4.1. “One TCM prescription for all patients with COVID-19” can be implemented in emergency situations, although the principle of treating patients according to syndrome differentiation is critical
Due to the extensive transmission of COVID-19 and the subsequent shortage of medical resources and implementation of emergency measures, the Chinese guidelines specify that all patients with COVID-19 can use general treatments such as Qingfei Paidu decoction and HSYF; however, this does not mean that treatment according to syndrome differentiation should be ignored, which is the principle of the rational use of TCM. The guide also recommends choosing the most suitable prescription according to the patient's physique in different stages; this guideline was followed in the cohort study mentioned above [62], where different TCM treatments were used according to the clinical differentiation of each patient, effectively reducing the risk of mortality in patients with severe and critical disease. In the 7th edition of the guidelines, it is also clear that the corresponding selection should be made according to the patient’s condition and physique. For example, during the medical observation period, it is recommended to use Huoxiang Zhengqi capsules for fatigue and gastrointestinal discomfort; Jinhua Qinggan granules, Lianhua Qingwen capsules, or Shufeng Jiedu capsules for fatigue and fever; and Qingfei Paidu decoction for patients with mild, moderate, severe, and critical disease. In addition, different stages of disease severity require the prescription of different TCM recipes, as per the essence of TCM where the appropriate recipe is chosen according to the patient's condition. Regarding COVID-19, this includes mild disease (including cold-damp lung syndrome and damp-heat accumulation lung syndrome), moderate disease (damp toxin stagnation syndrome and cold-dampness blocking lung syndrome), severe disease (epidemic virus closed lung syndrome and Qi Ying two burnt syndrome), critical disease (internal closure and external loss syndrome), and the recovery period (spleen lung Qi deficiency syndrome and Qi and Yin deficiency syndrome).
4.2. The current advantage of TCM is based on supportive treatment
The abovementioned clinical evidence shows that TCM may effectively improve the symptoms of patients with COVID-19, delay the progression to severe disease, and reduce all-cause mortality. However, due to the critical situation of the epidemic, there is no direct evidence that the application of TCM alone can be an effective treatment, as all studies are performed on the basis of TCM as a supportive treatment (complementing bed rest, supplementation with water and electrolytes, timely effective oxygen therapy measures, antiviral treatment, antibacterial treatment, and renal replacement therapy). Additionally, TCM may reduce the adverse reactions caused by the use of Western medicine, such as antiviral drugs and corticosteroids, particularly regarding the gastrointestinal and liver-related systems [59]. Therefore, evidence exists that, in the treatment of COVID-19, TCM can be supplemented as a supportive treatment, rendering the effect more significant.
5. The mechanism of TCM in the treatment of COVID-19
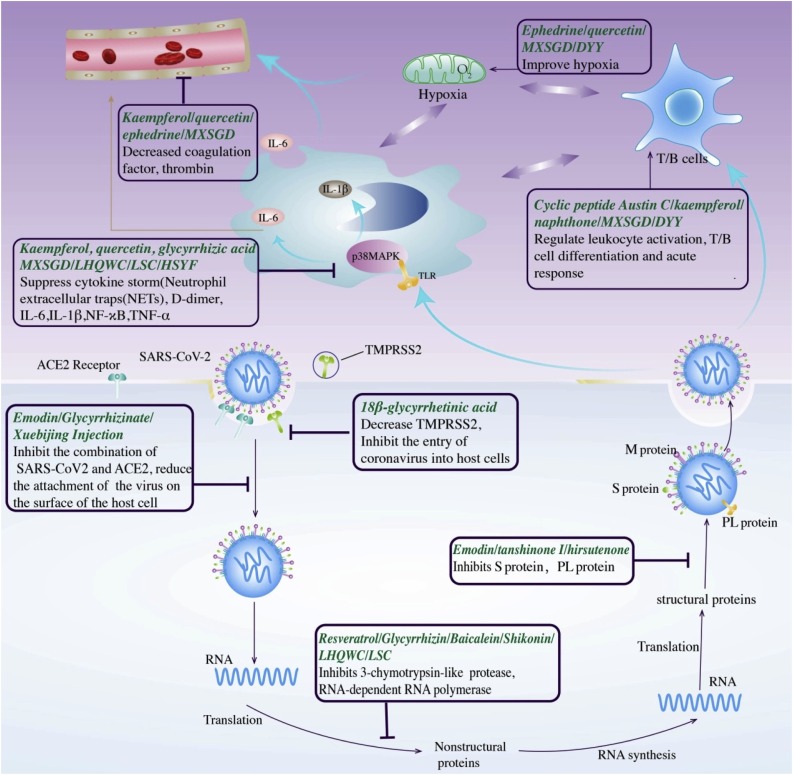
TCM can inhibit SARS-CoV-2 replication and transcription, interfering with the normal physiological functions of the virus, and attenuate a series of infection-related processes, including the cytokine storm, immune abnormalities, and coagulation abnormalities (Table 2 , Fig. 2 ).
Table 2.
Mechanism of TCM treatment of COVID-19.
| TCM | Method | Composition | Active ingredients | Pharmacological effects | Related cytokines |
|---|---|---|---|---|---|
| Maxing Shigan Decoction [75] | network pharmacological |
Ephedra equisetina Bge., Prunus armeniaca L., Gypsum Fibrosum, Glycyrrhiza uralensis Fisch. |
Quercetin, Kaempferol, Herbacetin, Delphinidin, Resivit, Estrone, Stigmasterol, CLR;Sitosterol, Isotrifoliol, Inflacoumarin A, Kanzonol F, Quercetin, Formononetin, CaSO4, CaSO4. 2H2O, Fe, Mn, Zn | Inflammation, immune response, hypoxia, apoptosis | TNF, IL-1β, IL-2, MAPK14, HSP90AB1, MAPK1, JUN, VEGFA, IL-10, IL6 |
| Respiratory Detox Shot [124] | Network pharmacological | Schizonepeta tenuifolia Briq., Lonicera japonica Thunb., Forsythia suspensa (Thunb.) Vahl, Polistes olivaceous(DeGeer), Scrophularia ningpoensis Hemsl., Cleditsia sinensis Lam., Ziziphus jujuba Mill. var. spinosa (Bunge) Hu ex H.F.Chou, Glycyrrhiza uralensis Fisch., Panax ginseng C. A. Mey. | Luteolin, Licoisoflavone B, Fisetin, Quercetin, Glyasperin F, Isolicoflavonol, Semilicoisoflavone-B | Leukocyte migration, inflammation, anti-virus | VCAM-1, IKKA, ELP1, NFKBIA, ESR1, HSP90AA1, AR, PPARG, GSK3B |
| Qingfei Paidu Decoction, Maxing Shigan Decoction [123] | Network pharmacology, vitro experiments |
Ephedra equisetina Bge., Prunus armeniaca L., Gypsum Fibrosum, Glycyrrhiza uralensis Fisch., Cinnamomum cassia Presl, Notopterygium incisum Ting ex H.T.Chang, Alisma orientalis (Sam.)Juzep., Polyporus umbellatus (Pers.)Fries, Atractylodes macrocephala Koidz., Poria cocos(Schw.)Wolf, Isatis indigotica Fort., Bupleurum chinense DC., Scutellaria baicalensis Georgi, Pinellia ternata (Thunb.)Breit., Zingiber officinale Rosc., Aster tataricus.L.f., Lonicera japonica Thunb., Tussilago farfara L., Belamcanda chinensis (L.)DC., Asarum heterotropoides Fr.Schmidt var.mandshuricum(Maxim.)Kitag., Dioscorea opposita Thunb., Citrus aurantium` L., Citrus reticulata Blanco, Pogostemon cablin (Blanco) Benth. |
Amygdalin, Baicalin, Ephedrine, Glycyrrhizic acid, Hesperidin, Narirutin, Neohesperidin | Anticoagulant, inflammation | TLR signal pathway |
| Gan cao [82] | Molecular docking simulation, molecular dynamics simulation | Glycyrrhiza uralensis Fisch. | Glycyrrhetinic Acid, Glycyrrhizin A | Inhibit virus replication and interfere with the combination of virus and host | ACE-2 |
| 96,606 classic prescriptions [73] | Data mining and web pharmacology | Glycyrrhiza uralensis Fisch., Scutellaria baicalensis Georgi, Rheum palmatum L., Bupleurum chinense DC. | Quercetin, kaempferol, 4'-hydroxy vitellogenin, glycosides, glycyrrhizin, norvitelloxanthin | Immune, inflammatory, prevent binding to host cells | ACE2, 3CL |
| Lianhua Qingwen capsule [162] | Cytopathic effect (CPE) and plaque reduction test | Forsythia suspensa (Thunb.) Vahl, Lonicera japonica Thunb., Ephedra equisetina Bge., Prunus armeniaca L., Gypsum Fibrosum, Glycyrrhiza uralensis Fisch., Isatis indigotica Fort., Rhizoma Dryopteridis, Houttuynia cordata Thunb., Pogostemon cablin (Blanco) Benth., Rheum palmatum L., Rhodiola crenulata(Hook.f.et Thoms.)H. Ohba, Mentha haplocalyx Briq. | NR | Antivirus | TNF-α, IL-6, CCL-2/MCP-1, CXCL-10 / IP-10 |
| Liushen Capsule [125] | Cytopathic effect (CPE) and plaque reduction test | Calculus bovis, Muskmelon Base Pedicellus Melo, TokayGecko, Bottle Brush Herb Herba Equiseti Arvensis, Pteria martensii (Dunker), Realgar | Gamabufotalin, arenobufagin, telocinobufagin, desacetylcinobufotalin, bufotalin, cinobufotalin | Antivirus, inflammation, protection of host cells | TNF-α, IL-6, IL-1β, IL-8, CCL-2/MCP-1, CXCL-10/IP-10, NF-κB/MAPK, p-NF-κBp65, p-IκBα, p-p38 MAPK, IκBα |
| Da Yuan yin [133] | Network pharmacology | Areca catechu L., Magnolia officinalis Rehd. et Wils., Amomum tsao-ko Crevost et Lemaire, Anemarrhena asphodeloides Bge., Paeonia lactiflora Pall., Scutellaria baicalensis Georgi, Glycyrrhiza uralensis Fisch. | kaempferol, quercetin, 7-Methoxy-2-methyl isoflavone, naringenin, formononetin | Inflammatory, immune | IL6, MAPK3, MAPK8, CASP3, IL10, IL1B, CXCL8, MAPK1, CCL2, IFNG, IL4 |
| Two CHM formulas were obtained from the Hubei Province Diagnosis and Treatment Protocol for COVID-19 [163] |
Network pharmacology | Formula A: Rhizoma Atractylodis, Flos Lonicerae, Pericarpium Citri Reticulatae, Rhizoma Phragmitis, Folium Mori, Radix Astragali seu Hedysari; Formula B: Radix Astragali seu Hedysari, Rhizoma Atractylodis Macrocephalae, Radix Saposhnikoviae, Cyrtomium fortunei J. Sm., Flos Lonicerae, Eupatorium fortunei Turcz., Pericarpium Citri Reticulatae. |
Astragalus polysaccharide, Mairin, Oxysanguinarine, Stigmasterol, Dammaradienyl acetate, Stigmasterol, Hederagenin | Antiviral | P13 K/Akt signal pathway |
| Qingfei Paidu Decoction [164] | Network pharmacology | Ephedra equisetina Bge., Prunus armeniaca L., Gypsum Fibrosum, Glycyrrhiza uralensis Fisch., Cinnamomum cassia Presl, Notopterygium incisum Ting ex H.T.Chang, Alisma orientalis (Sam.)Juzep., Polyporus umbellatus (Pers.)Fries, Atractylodes macrocephala Koidz., Poria cocos(Schw.)Wolf, Isatis indigotica Fort., Bupleurum chinense DC., Scutellaria baicalensis Georgi, Pinellia ternata (Thunb.)Breit., Zingiber officinale Rosc., Aster tataricus.L.f., Lonicera japonica Thunb., Tussilago farfara L., Belamcanda chinensis (L.)DC., Asarum heterotropoides Fr.Schmidt var.mandshuricum(Maxim.)Kitag., Dioscorea opposita Thunb., Citrus aurantium` L., Citrus reticulata Blanco, Pogostemon cablin (Blanco) Benth. | 3-O-Methylviolanone, Cianidanol, (+)-Epicatechin, ZINC13130930, (2S)-dihydrobaicalein, naringenin, SR-01,000,767,148, cyclo(L-Tyr-l-Phe), (-)-taxifolin, Eriodyctiol (flavanone) | Inflammation, antiviral, lipid metabolism | ACE2, CD147, JAK-STAT signal pathway |
Abbreviation: NR: Not reported; TNF: Tumor Necrosis Factor; IL-1β: Interleukin -1β; IL-2: Interleukin -2; MAPK14: mitogen-activated protein kinase 14; HSP90AB1: Heat shock protein HSP 90-beta; VEGFA: Vascular endothelial growth factor A; IL-10: Interleukin -10; IL-6: Interleukin -6; VCAM-1: Vascular cell adhesion protein 1; IKKA: Inhibitor of nuclear factor kappa-B kinase subunit alpha; ELP1: Elongator complex protein 1; NFKBIA: NF-kappa-B inhibitor alpha; ESR1: Estrogen receptor1; AR: Androgen receptor; PPARG; Peroxisome proliferator-activated receptor; GSK3B: Glycogen synthase kinase-3 beta; TLR: Toll-like receptor; ACE2: angiotensin converting enzyme 2; CCL-2: C-C motif chemokine 2; MCP-1: Monocyte chemoattractant protein 1; CXCL-10: C-X-C motif chemokine 10; IκBα: I-kappa-B-alpha; CASP3: Caspase-3; IFNG: Immune interferon; P13K: Phosphatidylinositol 3; JAK: Janus kinase; STAT: Signal transducer and activator of transcription.
Fig. 2.
The mechanism of TCM in the treatment of COVID-19.
Emodin [63], xuebijing injection [64,65] can reduce the binding of SARS-CoV2 to ACE2 and reduce the attachment of the virus to the host cell. 18β-Glycyrrhetinic acid [66] can reduce the combination of SARS-CoV2 and type 2 transmembrane serine protease (TMPRSS2) and inhibit the virus from entering the host cell. Resveratrol [67], LHQWC [68,69] can inhibit the functions of 3-chymotrypsin-like protease (3CLpro) and RNA-dependent RNA polymerase, and inhibit SARS-CoV2 replication and transcription. Emodin [63] can interfere with viral glycosylation spike (S) protein, (M) protein, papain and other proteins to affect the normal biological functions of SARS-CoV2. After the virus infects the human body, immune abnormalities, cytokine storms, hypoxia, and abnormal blood coagulation would affect each other. Kaempferol [70] and MXSGD [[71], [72], [73], [74]] can inhibit the cytokine storm and IL-6, IL-1β, and TNF-α released in the process after the virus binds to TLR and activates p38MAPK. The cyclic peptide Austin C [75] can regulate immune function, leukocyte activation, T cell differentiation, and acute response. Ephedrine and pseudoephedrine [76] can improve hypoxia in the body; Kaempferol [70] can inhibit coagulation factors, thrombin, and reduce the occurrence of intravascular coagulation.
5.1. TCM can interfere with SARS-CoV-2 replication and transcription by inhibiting RNA-dependent RNA polymerase and 3-chymotrypsin-like protease
SARS-CoV-2 has 4 structural proteins, including the envelope (E), spike (S), nucleocapsid (N), and membrane (M) protein. These are composed of functional subunits S1 and S2. SARS-CoV-2 replication and transcription and the realization of biological functions are carried out with the support of multiple active enzymes; among them, RNA-dependent RNA polymerase, also known as nonstructural protein 12 (Nsp12), is essential [77]. Helicase (Nsp13) is a superfamily 1 helicase, a multifunctional protein with an N-terminal metal-binding domain and a helicase domain. Studies have shown that the disassembly of Nsp13 is an important process in SARS-CoV-2 replication, transcription, and translation [78]. 3-Chymotrypsin-like protease (3CLpro) mediates the maturation of the nonstructural proteins, which is essential for the life cycle of SARS-CoV-2. First, 3CLpro is cleaved from the polyprotein to produce a mature enzyme, and then further cleaves downstream nonstructural proteins at 11 sites to produce Nsp4–Nsp16 [79]. In-depth research on the structure and catalytic mechanism of 3CLpro makes it an interesting target for the development of anti-COVID-19 drugs.
Studies have confirmed that resveratrol can not only inhibit MERS-CoV infection in vitro and prolong cell survival but also inhibit the expression of the MERS-CoV N protein necessary for virus replication and MERS-CoV-induced host cell apoptosis [67], indicating that resveratrol may also be effective against SARS-CoV-2 infection. Glycyrrhizin, an active ingredient in Glycyrrhiza uralensis Fisch. (licorice), can inhibit the replication of SARS-CoV in vitro [80]. The newly developed glycyrrhizic acid derivatives are more capable of inhibiting the replication of SARS-CoV [81]. Glyasperin A entering the host cell inhibits the virus replication process [82]. TCM can also affect the physiological cycle of the virus by inhibiting 3CLpro [83]. Baicalein is an inhibitor of the main SARS-CoV-2 protease, 3CLpro, that can effectively inhibit virus proliferation in Vero cells. Therefore, in addition to the attachment and entry of viruses, essential drugs, such as Shuanghuanglian preparations, may also inhibit virus uncoating, genome release, protein maturation, and the germination and diffusion assembly of new virions, resulting in reduced infectivity [84]. Shikonin is derived from Lithospermum erythrorhizon Sieb. et Zucc. (Lithospermum erythrorhizon) as an active ingredient, which has an inhibitory activity on the 3CLpro of SARS-CoV-2, with a half maximal inhibitory concentration (IC50) of 15.75 ± 8.22 μmol/L [85]. The effects of different components of TCM are not mutually exclusive; they often work through multiple pathways and have multiple targets. For example, Lianhua Qingwen capsules can inhibit the replication of SARS-CoV-2 and at the same time reduce the cytokines (TNF-α, IL-6, IL-1β) released by host cells to exert antiviral activity, thereby supporting the clinical application in COVID-19 [68,69].
5.2. TCM can inhibit glycosylated spike protein and papain proteins to affect the normal biological functions of SARS-CoV-2
Coronaviruses, including SARS-CoV-2, are enveloped viruses with a nucleocapsid mainly composed of phosphorylated N protein embedded in a phospholipid double-layer envelope. This envelope plays an important role in virus assembly and release, and it is the key to viral disease [86,87]. Glycosylated S protein is the main cause of the host immune response [88]. Since the S protein plays a vital role in the interaction between the virus and cell receptors, it is an important potential target for antiviral agents [89]. The E protein is the smallest transmembrane structural protein of coronaviruses [90] and has important biological functions regarding the structural integrity and virulence of the virus [91]. Additionally, the main function of the M protein is to maintain the shape of the virus envelope. It performs this activity by interacting with other coronavirus proteins, incorporating the Golgi complex into the new virion and stabilizing the N protein [92]. Papain protease (PLpro) is present in all coronaviruses [93]. The PLpro of SARS-CoV has been shown to have deubiquitination and interferon antagonistic activity [94]. Therefore, inhibiting the activity of these proteases can prevent certain coronaviruses from entering their host cells and is regarded as the target of anti-COVID-19 drug development.
Emodin, the main component of Rheum palmatum L (rhubarb), has been proven to have antiviral effects against SARS-CoV, mainly through targeting the S protein and blocking the binding of the S protein to ACE2; this is achieved in a dose-dependent manner [63]. Moreover, tanshinone I (isolated from Salviae Miltiorrhizae Radix et Rhizoma (Salvia miltiorrhiza Bge)) [95] and hirsutenone (isolated from Alnus japonica (Thunb.) Steud. (Alnus japonica)) [96] exhibited dose-dependent inhibitory effects against SARS-CoV through targeting PLpro, with IC50 values of 0.7 and 4.1 μmol/L, respectively.
5.3. TCM can act on ACE2 and type 2 transmembrane serine protease to interfere with the binding of SARS-CoV-2 to host cells
5.3.1. TCM can interfere with the binding of SARS-CoV-2 to ACE2
The infection of human cells by SARS-CoV-2 involves two consecutive steps: 1) attachment of the virus to the surface receptor of the target cell, and 2) fusion of the viral membrane with the host membrane. The former requires at least one receptor-binding domain on the SARS-CoV-2 S protein that can interact with cell surface receptors expressed on human cells. The latter requires at least one or more host proteases to mediate the proteolytic cleavage of the SARS-CoV-2 S protein into the S1 and S2 subunits, thereby promoting the fusion of the viral membrane and the host membrane [97]. SARS-CoV-2 has four structural proteins, including the E, S, N, and M proteins. They are composed of functional subunits S1 and S2. The S1 subunit forms a receptor domain [98] that can bind to ACE2 to enter the host cells [99,100]. ACE2 is a type of polypeptide that is highly expressed on the surface of epithelial cells, arteriovenous endothelial cells, arterial smooth muscle cells, and small intestinal epithelial cells [98,[101], [102], [103]]. The decrease of the ACE2 function after viral infection may lead to renin-angiotensin system dysfunction, which affects blood pressure and fluid/electrolyte balance and enhances respiratory tract inflammation and vascular permeability [104].
Emodin is an anthraquinone compound that is found in various TCM recipes, and it has been shown to inhibit the interaction between the SARS-CoV S protein and its receptor, ACE2, in a dose-dependent manner [63]. Glycyrrhizic acid can also interfere with the binding of the virus to the ACE2 receptor [105]. With network pharmacology and molecular docking methods, the compounds in Huoxiang Zhengqi oral liquid can also inhibit the interaction between the SARS-CoV S protein and ACE2, thereby exerting a preventive or therapeutic effect on COVID-19 [106], and also for NRICM101 [107] and MXSGD [74]. Network pharmacology has shown that the possible mechanism of Xuebijing injection may be related to the 70 proteins that regulate the interaction with ACE2 and inhibit virus replication [64,65].
5.3.2. TCM can reduce the expression of type 2 transmembrane serine protease
The type 2 transmembrane serine protease (TMPRSS2) present in host cells promotes virus uptake by cleaving ACE2 and activating the SARS-CoV-2 S protein (mediating the entry of SARS-CoV-2 into the host cell) [103], triggering SARS-COV and MERS-COV infection [108]. TMPRSS2 is expressed in host target cells, especially type II alveolar epithelial cells [109,110]. Maxing Shigan decoction, HSYF, and Qingfei Paidu decoction contain licorice. Early related studies have shown that 18β-glycyrrhetinic acid, an active ingredient in Glycyrrhiza uralensis Fisch. (licorice), can inhibit the expression of TMPRSS2 [66].
5.4. TCM can attenuate the cytokine storm, weakened immunity, and abnormal blood coagulation post infection with SARS-CoV-2
5.4.1. Attenuating the cytokine storm
In the infected airway epithelium, SARS-CoV-2 can activate the mitogen-activated protein kinases (MAPK) pathway by binding to Toll-like receptors (TLRs), in particular the p38 MAPK pathway. This can cause the immune system to overreact and produce a variety of inflammatory mediators, called a "cytokine storm" [111], characterized by the release of high levels of IL-6, IL-1β, and TNF-α [112,113]. The presence of C-reactive protein, D-dimer, ferritin, and IL-6 is associated with an increase in major complications and mortality [114]. The autopsy of patients who died from COVID-19 showed that lymphocytic endotheliitis with apoptotic bodies and viral inclusion structures exists in multiple organs, including the lungs, heart, kidneys, and intestines [115,116]. Obvious inflammation with endotheliitis can also lead to diffuse intravascular coagulation with thrombosis and infarction of the small or large vessels [117]. Overproduction of IL-6 may aggravate inflammation and tissue damage [118]. Increasing evidence indicates that an excessive inflammatory response to SARS-CoV-2 is an important factor in the severity of COVID-19-related disease and death [119,120]. These data suggest that the cytokine storm is the basis of the immunopathology of severe COVID-19 [121]. Recently, it has been proposed that monoclonal antibodies, Tocilizumab, that block the IL-1β pathway may help treat COVID-19 [122].
Maxing Ganshi decoction is composed of Ephedra sinica Stapf (ephedra), Semen Armeniacae Amarum. (almonds), Gypsum (gypsum), and Glycyrrhiza uralensis Fisch. (licorice). It is predicted that its active ingredients are quercetin, kaempferol, herbacetin, delphinidin, and resivit [75], of which kaempferol and quercetin may inhibit the coagulation pathway triggered by IL-6. It can inhibit diffuse intravascular coagulation and ultimately reduce the fatal cytokine storm [70] as well as reduce the level of TNF-α and IL-1β by blocking the MAPK pathway [[71], [72], [73], [74]]. Glycyrrhizic acid can significantly reduce the IL-6 release of macrophages through the TLR pathway [123]. Respiratory Detox Shot mainly includes luteolin, licoisoflavone B, fisetin, quercetin, glyasperin F, isolicoflavonol, and semilicoisoflavone-B, which can reduce leukocyte migration and inflammation, including nuclear factor kappa B [124]. Liushen capsules are mainly composed of gamabufotalin, arenobufagin, telocinobufagin, desacetylcinobufotalin, bufotalin, and cinobufotalin. Cytopathic effect inhibition and plaque reduction assays reveal that it can reduce the expression of mRNA of TNF-α, IL-6, IL1β, IL-8 [125]. The active ingredients and candidate targets of HSYF were obtained through database mining, and a "HSYF–herbal-components–targets–pathways–COVID-19" network was constructed. In the central target analysis, the highest central target IL-6 and ACE2 were selected for molecular docking analysis, which revealed good binding activity [126].
5.4.2. TCM can improve the immunity of patients with COVID-19
Patients with COVID-19 often show specific immune function abnormalities, including activation of immune invisibility, paralysis, and memory. Compared to SARS-CoV, SARS-CoV-2 may cause a weak immune response or "immune stealth", which prolongs the patient's recovery process [22]. In the context of cytokine release syndrome, the production of the anti-inflammatory cytokine IL-10 is usually related to the downregulation of neutrophil and monocyte function, which is termed immune paralysis [127] and is crucial for survival [128]. While IL-1β drives macrophage activation syndrome, IL-6 drives immune disorders [129]. SARS-CoV-2 and the destruction of lung cells trigger a local immune response, recruiting macrophages and monocytes that respond to the infection, releasing cytokines and triggering adaptive T and B cell immune responses. In most cases, this process will resolve the infection. However, in some cases, a dysfunctional immune response can occur, which can lead to severe lung disease and even systemic pathology [104]. If the patient is infected with SARS-CoV-2, the immune memory due to the previous infection with the low pathogenic coronavirus will be activated. Memory B cells rapidly produce cross-reactive antibodies. Fc receptors mediate viral antibody complexes into monocytes/macrophages, resulting in antibody-dependent enhancement. The virus replicates after immune escape, resulting in a rapid increase of its offspring, the release of a variety of pro-inflammatory cytokines, a reduction in lymphocytes, immune disorders, and resultant severe and critical illness in patients with COVID-19 [130]. Metabolic-related diseases such as hypertension, coronary heart disease, type 2 diabetes, metabolic syndrome, and obesity can downregulate key mediators of the host's innate immune response to pathogenesis, thereby affecting the function of the innate and humoral immune system [131,132].
For patients with COVID-19 and immune abnormalities, TCM can directly regulate the immune system and indirectly regulate the immune response by regulating cytokines. The cyclopeptide astin C, isolated from Aster tataricus L. f. (Aster tataricus), can specifically inhibit the immune-related adaptor protein STING (stimulator of interferon genes), thereby regulating the immune response. The rich triterpenoids in Glycyrrhiza uralensis Fisch. (licorice), Dioscorea oppositifolia L. (yam), Poria cocos(Schw.)Wolf (Poria), and Polyporus (Polyporus) can be used as steroid hormones to regulate the activity of the mammalian immune system. Kaempferol and methanone can also directly aid in treating COVID-19 through the immune response [75]. Maxing Shigan decoction regulates immune processes, such as white blood cell activation, T cell differentiation, and acute response, and tends to limit pro-inflammatory cytokines and control myeloid cells through the above-mentioned cell differentiation molecules. Glycyrrhiza uralensis Fisch. (Licorice) and Scutellaria baicalensis Georgi (Scutellaria) show potential anti-SARS-CoV-2 activity by binding to ACE2 and 3CL hydrolase and regulating the immune system [73]. Da Yuan Yin can regulate the activation of T and B cells in IL-6 and C-C motif chemokine 2 to transmit information and exert an immune response [133].
5.4.3. TCM can reduce the release of coagulation factors and thrombin
Chinese cardiologists reported that patients who died of COVID-19 presented with diffuse microvascular thrombosis in various organs, especially in the lungs. In severe COVID-19, severe coagulation activation and consumption of coagulation factors occur [134,135], often resulting in thrombotic complications [136]. Considering this diffuse thrombosis, Chinese physicians recommend that patients are treated with anticoagulant therapy [137]. First, the body's anti-disease process, including neutrophil extracellular traps (NETs) and increased D-dimer levels, leads to abnormal blood coagulation. As part of the immune response, white blood cells release NETs to capture and kill pathogens and help fight COVID-19 [138]. In severe infections, NETs can block blood vessels and cause inflammatory tissue damage [139,140]. In addition to pulmonary embolism, COVID-19 can also cause sepsis-related disseminated intravascular coagulation, defined as "septicemia-induced coagulopathy" [141]. The blood of patients with underlying diseases tends to coagulate. Diabetes is associated with elevated plasminogen levels, which is thought to increase the virulence of SARS CoV-2 [142]. The presence of these inflammatory and thrombotic factors has been confirmed in 174 hospitalized patients with COVID19 in Wuhan, China; the serum D-dimer level in patients with diabetes was significantly higher than in patients without diabetes [143]. The cytokine storm and hypoxia can also aggravate the outcome of blood coagulation. For example, hypercoagulability caused by inflammation may lead to plaque rupture, which can lead to thrombosis and myocardial damage [144]. As mentioned earlier, COVID-19 infection may cause endothelial dysfunction and a hypercoagulable state. Hypoxia can exacerbate this condition as well as increase thrombosis by increasing the blood viscosity and hypoxia-induced transcription factor-dependent signaling pathways [145]. Therefore, the prior introduction can reduce the harm caused by the cytokine storm and indirectly attenuate coagulation abnormalities.
Kaempferol and quercetin can inhibit endothelial cell dysfunction and the diffuse intravascular coagulation caused by IL-6 [70]. Maxing Shigan decoction increases the dissolution of ephedrine, which has an anti-platelet aggregation effect, from ephedra [123,146]. Maxing Shigan also shows a therapeutic effect in the rat model of lipopolysaccharide-induced pneumonia. The effect of Maxing Shigan treatment is related to the regulation of proteins such as coagulation factor XII (F12), F13b, and antithrombin III (AT3) reducing the production of thrombin [123].
5.4.4. TCM can improve hypoxia
Severe pneumonia can cause severe gas exchange disorders and lead to hypoxemia. Acidosis and the oxygen free radicals that accumulate in cells destroy the phospholipid layer of the cell membrane. As hypoxia continues, the intracellular calcium ion concentration increases significantly, leading to a series of cell damage processes, including cell apoptosis [147]. At the same time, hypoxia can also induce inflammatory responses, such as the infiltration of inflammatory cells and the release of cytokines, leading to further tissue ischemia and possibly even myocardial infarction [148].
Maxing Shigan decoction may reduce inflammatory fluid exudation and promote the absorption of edema fluid, protecting the alveolar endothelium and epithelium to maintain the integrity of the barrier connection between cells, and ultimately improve the lung ventilation function. Ephedrine and pseudoephedrine are the main active components of Ephedra sinica Stapf (ephedra), and they are clinically proven analgesics and bronchodilators. Quercetin can reduce apoptosis caused by hypoxia and rescue adenosine monophosphate-activated protein kinase phosphorylation [76]. It can be speculated that these genes play a key role in the mechanism of Da Yuan Yin treatment of COVID-19. IL-Iβ represents a wide range of inflammatory mediators and pathways. During the treatment of COVID-19, Da Yuan Yin can regulate IL-Iβ to exert its anti-hypoxia function [133].
6. Outlook
As of mid-September 2020, more than 200 countries and territories around the world had reported a total of 30 million COVID-19 cases and 950,000 deaths [3]. Although the current measures include bed rest, supportive treatment, supplementation of water and electrolytes, timely effective oxygen therapy, antiviral therapy, antibacterial therapy, and renal replacement therapy, the important role of TCM during this period is evident and numerous high-quality evidences regarding its curative effect exists. However, currently, only China and South Korea have formulated relevant guidelines that include TCM as a therapy. This is far from enough. In order to formulate international guidelines similar to the "International traditional Chinese medicine guideline for diagnostic and treatment principles of diabetes" [149], the international cooperation of many countries is required to develop a more convenient TCM prescription, adapted to patients with COVID-19 from various regions and of different ethnicities.
At present, there is good evidence that TCM can improve the symptoms of patients with COVID-19, delay the progression of the disease, and reduce the mortality rate. However, many patients may develop "Post-coronavirus Stress Syndrome" (PCSS), which could pose a noteworthy global challenge [150]. For example, some patients who are recovering from the virus may have a negative nucleic acid test, although symptoms such as fatigue, cough, poor mental status, and especially lung-related changes, such as unabsorbed inflammation, remain. A metabolomics study was conducted in 25 survivors of SARS; their lipid metabolism remained disrupted 12 years after clinical recovery [151]. In the observation of eight patients with avian influenza A (H7N9), cardiac abnormalities returned to normal after one year of follow-up [152]. Current drugs used to treat COVID-19, including hydroxychloroquine, prolong the QT interval and cause torsades de pointes [153]. In the psychological evaluation of 89 patients with COVID-19, 52 % had no psychological symptoms, 35 % had mild symptoms, and 13 % had moderate to severe symptoms [154]. In a survey of 230 medical staff, the incidence of anxiety was 23 %, while the incidence of severe, moderate, and mild anxiety were 2.17 %, 4.78 %, and 16.09 %, respectively [155]. Therefore, in response to the COVID-19 epidemic, mental illness and psychological problems are important issues for patients and medical staff. Currently, there are ongoing studies regarding the use of Sancai granules for the recovery of liver and kidney function [156], Shengmaiyin for lung and heart function [157], and Bufeihuoxue capsule for, the recovery of patients with lung and spleen Qi syndrome, respiratory function, and fatigue [158]. However, interventional studies on the psychological abnormalities of patients, including medical staff, are lacking. The follow-up needs to be expanded to provide a complete chain of evidence related to the efficacy of TCM in the prevention, treatment, and recovery period of PCSS.
7. Conclusion
At present, the COVID-19 epidemic has lasted for nearly 12 months and the number of globally confirmed cases and deaths continue to rise. Current literature shows that four candidate drugs, including remdesivir, are ineffective or have little effect on COVID-19. Supportive treatments, such as oxygen inhalation, antibiotics, and antiviral drugs, remain the mainstay. Presently, there is good evidence that TCM can effectively alleviate the symptoms of patients with suspected and confirmed COVID-19, delay the progression from mild and moderate to severe and critical disease, and reduce severe and critical all-cause mortality. The role of TCM is related to affecting the transcription, replication, and binding of SARS-CoV-2 to the host and attenuating the cytokine storm and weak immunity in patients with COVID-19. However, considering the deteriorating global epidemic situation, we must also consider formulating international guidelines for patients with COVID-19 from other countries, territories, and ethnicities, in order for TCM to serve patients around the world. At the same time, we must pay attention to PCSS and provide a complete chain of evidence for the prevention, treatment, and recovery using TCM.
Author contributions
FML and XLT designed the protocol. LYD, DJ, RRZ, and YYD searched related literature. FML, XDA, and YHZ drafted the manuscript and figures. HYZ, and SHZ drafted the tables. All authors approved the final version of the manuscript.
Funding
This work was supported by National Key R&D Program (2020YFC0845000), State Administration of Traditional Chinese Medicine Special Project of Chinese Medicine Emergency Response to New Coronavirus Pneumonia (2020ZYLCYJ04- 1)The funders had no role in the study design, data collection, data analysis, interpretation, or writing of the report.
Transparency document
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgement
Not applicable.
References
- 1.van Dorp L., Acman M., Richard D., Shaw L.P., Ford C.E., Ormond L., et al. Emergence of genomic diversity and recurrent mutations in SARS-CoV-2. Infect. Genet. Evol. 2020;83 doi: 10.1016/j.meegid.2020.104351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coronavirus Worldometer . Worldometer; 2020. Cases; pp. 1–22. [DOI] [Google Scholar]
- 4.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.http://www.gov.cn/index.htm.
- 6.Goldsmith C.S., Tatti K.M., Ksiazek T.G., Rollin P.E., Comer J.A., Lee W.W., et al. Ultrastructural characterization of SARS coronavirus. Emerg. Infect Dis. 2004;10(2):320–326. doi: 10.3201/eid1002.030913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang X., Wu C., Li X., Song Y., Yao X., Wu X., et al. On the origin and continuing evolution of SARS-CoV-2. Natl. Sci. Rev. 2020;7(6):1012–1023. doi: 10.1093/nsr/nwaa036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moghadas S.M., Fitzpatrick M.C., Sah P., Pandey A., Shoukat A., Singer B.H., et al. The implications of silent transmission for the control of COVID-19 outbreaks. Proc. Natl. Acad. Sci. U. S. A. 2020;117(30):17513–17515. doi: 10.1073/pnas.2008373117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kautz T.F., Forrester N.L. RNA virus fidelity mutants: a useful tool for evolutionary biology or a complex challenge? Viruses. 2018;10(11) doi: 10.3390/v10110600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith E.C. The not-so-infinite malleability of RNA viruses: viral and cellular determinants of RNA virus mutation rates. PLoS Pathog. 2017;13(4) doi: 10.1371/journal.ppat.1006254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cyranoski D. Profile of a killer: the complex biology powering the coronavirus pandemic. Nature. 2020;581(7806):22–26. doi: 10.1038/d41586-020-01315-7. [DOI] [PubMed] [Google Scholar]
- 12.Aggarwal G., Cheruiyot I., Aggarwal S., Wong J., Lippi G., Lavie C.J., et al. Association of cardiovascular disease with coronavirus disease 2019 (COVID-19) severity: a meta-analysis. Curr. Probl. Cardiol. 2020 doi: 10.1016/j.cpcardiol.2020.100617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cai G., Bossé Y., Xiao F., Kheradmand F., Amos C.I. Tobacco smoking increases the lung gene expression of ACE2, the receptor of SARS-CoV-2. Am. J. Respir. Crit. Care Med. 2020;201(12):1557–1559. doi: 10.1164/rccm.202003-0693LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romaní-Pérez M., Outeiriño-Iglesias V., Moya C.M., Santisteban P., González-Matías L.C., Vigo E., et al. Activation of the GLP-1 receptor by liraglutide increases ACE2 expression, reversing right ventricle hypertrophy, and improving the production of SP-A and SP-B in the lungs of type 1 diabetes rats. Endocrinology. 2015;156(10):3559–3569. doi: 10.1210/en.2014-1685. [DOI] [PubMed] [Google Scholar]
- 15.Ferrario C.M., Jessup J., Chappell M.C., Averill D.B., Brosnihan K.B., Tallant E.A., et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111(20):2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 16.Tikoo K., Patel G., Kumar S., Karpe P.A., Sanghavi M., Malek V., et al. Tissue specific up regulation of ACE2 in rabbit model of atherosclerosis by atorvastatin: role of epigenetic histone modifications. Biochem. Pharmacol. 2015;93(3):343–351. doi: 10.1016/j.bcp.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 17.Wösten-van Asperen R.M., Lutter R., Specht P.A., Moll G.N., van Woensel J.B., van der Loos C.M., et al. Acute respiratory distress syndrome leads to reduced ratio of ACE/ACE2 activities and is prevented by angiotensin-(1-7) or an angiotensin II receptor antagonist. J. Pathol. 2011;225(4):618–627. doi: 10.1002/path.2987. [DOI] [PubMed] [Google Scholar]
- 18.Zhang W., Xu Y.Z., Liu B., Wu R., Yang Y.Y., Xiao X.Q., et al. Pioglitazone upregulates angiotensin converting enzyme 2 expression in insulin-sensitive tissues in rats with high-fat diet-induced nonalcoholic steatohepatitis. Sci. World J. 2014;2014 doi: 10.1155/2014/603409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin L., Luo S., Qin R., Yang M., Wang X., Yang Q., et al. Long-term infection of SARS-CoV-2 changed the body’s immune status. Clin. Immunol. 2020;218 doi: 10.1016/j.clim.2020.108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., et al. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020;71(15):762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu F., Wang A., Liu M., Wang Q., Chen J., Xia S., et al. Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications. medRxiv. 2020;2020 03.30.20047365. [Google Scholar]
- 22.Shang J., Wan Y., Luo C., Ye G., Geng Q., Auerbach A., et al. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. U. S. A. 2020;117(21):11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hackbart M., Deng X., Baker S.C. Coronavirus endoribonuclease targets viral polyuridine sequences to evade activating host sensors. Proc. Natl. Acad. Sci. U. S. A. 2020;117(14):8094–8103. doi: 10.1073/pnas.1921485117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Volk A., Hackbart M., Deng X., Cruz-Pulido Y., O’Brien A., Baker S.C. Coronavirus endoribonuclease and deubiquitinating interferon antagonists differentially modulate the host response during replication in macrophages. J. Virol. 2020;94(11) doi: 10.1128/JVI.00178-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amor S., Fernandez Blanco L., Baker D. Innate immunity during SARS-CoV-2: evasion strategies and activation trigger hypoxia and vascular damage. Clin. Exp. Immunol. 2020;202(2):193–209. doi: 10.1111/cei.13523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323(17):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 28.Kuderer N.M., Choueiri T.K., Shah D.P., Shyr Y., Rubinstein S.M., Rivera D.R., et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395(10241):1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Markousis-Mavrogenis G., Tromp J., Ouwerkerk W., Devalaraja M., Anker S.D., Cleland J.G., et al. The clinical significance of interleukin-6 in heart failure: results from the BIOSTAT-CHF study. Eur. J. Heart Fail. 2019;21(8):965–973. doi: 10.1002/ejhf.1482. [DOI] [PubMed] [Google Scholar]
- 30.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Black J.R.M., Bailey C., Przewrocka J., Dijkstra K.K., Swanton C. COVID-19: the case for health-care worker screening to prevent hospital transmission. Lancet. 2020;395(10234):1418–1420. doi: 10.1016/S0140-6736(20)30917-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Violi F., Cangemi R., Falcone M., Taliani G., Pieralli F., Vannucchi V., et al. Cardiovascular complications and short-term mortality risk in community-acquired pneumonia. Clin. Infect. Dis. 2017;64(11):1486–1493. doi: 10.1093/cid/cix164. [DOI] [PubMed] [Google Scholar]
- 33.Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A., et al. Compassionate use of remdesivir for patients with severe Covid-19. N. Engl. J. Med. 2020;382(24):2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang J.K., Lin S.S., Ji X.J., Guo L.M. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47(3):193–199. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;323(18):1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 36.https://www.medrxiv.org/content/10.1101/2020.10.15.20209817v1.full.pdf.
- 37.Chung Y.H., Beiss V., Fiering S.N., Steinmetz N.F. COVID-19 vaccine frontrunners and their nanotechnology design. ACS Nano. 2020;14(10):12522–12537. doi: 10.1021/acsnano.0c07197. [DOI] [PubMed] [Google Scholar]
- 38.Mahase E. Covid-19: UK approves Oxford vaccine as cases of new variant surge. BMJ. 2020;371:m4968. doi: 10.1136/bmj.m4968. [DOI] [PubMed] [Google Scholar]
- 39.Oliver S.E., Gargano J.W., Marin M., Wallace M., Curran K.G., Chamberland M., et al. The advisory committee on immunization practices’ interim recommendation for use of moderna COVID-19 vaccine - United States, December 2020. MMWR Morb. Mortal. Wkly. Rep. 2021;69(5152):1653–1656. doi: 10.15585/mmwr.mm695152e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.https://baijiahao.baidu.com/s?id=1687632982727196598&wfr=spider&for=pc.
- 41.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 42.Hao P.P., Jiang F., Chen Y.G., Yang J., Zhang K., Zhang M.X., et al. Traditional Chinese medication for cardiovascular disease. Nat. Rev. Cardiol. 2015;12(6):318. doi: 10.1038/nrcardio.2015.60. [DOI] [PubMed] [Google Scholar]
- 43.Hao P., Jiang F., Cheng J., Ma L., Zhang Y., Zhao Y. Traditional Chinese medicine for cardiovascular disease: evidence and potential mechanisms. J. Am. Coll. Cardiol. 2017;69(24):2952–2966. doi: 10.1016/j.jacc.2017.04.041. [DOI] [PubMed] [Google Scholar]
- 44.Xu J., Lian F., Zhao L., Zhao Y., Chen X., Zhang X., et al. Structural modulation of gut microbiota during alleviation of type 2 diabetes with a Chinese herbal formula. ISME J. 2015;9(3):552–562. doi: 10.1038/ismej.2014.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang C., Cao B., Liu Q.Q., Zou Z.Q., Liang Z.A., Gu L., et al. Oseltamivir compared with the Chinese traditional therapy maxingshigan-yinqiaosan in the treatment of H1N1 influenza: a randomized trial. Ann. Intern. Med. 2011;155(4):217–225. doi: 10.7326/0003-4819-155-4-201108160-00005. [DOI] [PubMed] [Google Scholar]
- 46.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mao R., Qiu Y., He J.S., Tan J.Y., Li X.H., Liang J., et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2020;5(7):667–678. doi: 10.1016/S2468-1253(20)30126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu Q., Qang M. Psychological intervention of anxiety in patients with fever during SARS epidemic. J. Nurs. 2004;2004(5):25–26. [Google Scholar]
- 49.Wang Q., Cao X., Wu X., Liu J., Xie J., Hou D. Investigation on the anxiety and depression status of hospital emergency patients during the COVID-19 epidemic. J. Southern Med. Univ. 2020;40(09):1369–1372. doi: 10.12122/j.issn.1673-4254.2020.09.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen L., Liu F., Wu J., Song H., Xia J., Sheng B., et al. Retrospective analysis of the clinical efficacy of Shufeng Jiedu Capsules combined with Western medicine in the treatment of patients with common new coronavirus pneumonia. Chin. J. Exp. Formulas. 2020;26(16):14–20. [Google Scholar]
- 51.Zhang H.T., Huang M.X., Liu X., Zheng X.C., Li X.H., Chen G.Q., et al. Evaluation of the adjuvant efficacy of natural herbal medicine on COVID-19: a retrospective matched case-control study. Am. J. Chin. Med. 2020;48(4):779–792. doi: 10.1142/S0192415X20500391. [DOI] [PubMed] [Google Scholar]
- 52.Hu K., Guan W.J., Bi Y., Zhang W., Li L., Zhang B., et al. Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: a multicenter, prospective, randomized controlled trial. Phytomedicine. 2020 doi: 10.1016/j.phymed.2020.153242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xiong W.Z., Wang G., Du J., Ai W. Efficacy of herbal medicine (Xuanfei Baidu decoction) combined with conventional drug in treating COVID-19:a pilot randomized clinical trial. Integr. Med. Res. 2020;9(3) doi: 10.1016/j.imr.2020.100489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ba Y., Wang L., Li W., Li M., Tao R., Zuo X., et al. “Pneumonia No. 1” in the treatment of 451 cases of novel coronavirus pneumonia. World Trad. Chin. Med. 2020;15(13):1962–1966. [Google Scholar]
- 55.Lv R., Wang W., Li X. Clinical observation on the treatment of 63 suspected cases of novel coronavirus pneumonia with Lianhua Qingwen granules combined with Western medicine conventional therapy. Chin. Med. J. 2020;61(08):655–659. [Google Scholar]
- 56.Xiao M., Tian J., Zhou Y., Xu X., Min X., Lv Y., et al. Efficacy of Huoxiang Zhengqi dropping pills and Lianhua Qingwen granules in treatment of COVID-19: a randomized controlled trial. Pharmacol. Res. 2020;161 doi: 10.1016/j.phrs.2020.105126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xin S., Cheng X., Zhu B., Liao X., Yang F., Song L., et al. Clinical retrospective study on the efficacy of Qingfei Paidu decoction combined with Western medicine for COVID-19 treatment. Biomed. Pharmacother. 2020;129 doi: 10.1016/j.biopha.2020.110500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao J., Yang X., Wang C., Song S., Cao K., Wei T., et al. Yidu-toxicity blocking lung decoction ameliorates inflammation in severe pneumonia of SARS-COV-2 patients with Yidu-toxicity blocking lung syndrome by eliminating IL-6 and TNF-a. Biomed. Pharmacother. 2020;129 doi: 10.1016/j.biopha.2020.110436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang W.Y., Xie Y., Zhou H., Liu L. Contribution of traditional Chinese medicine to the treatment of COVID-19. Phytomedicine. 2020 doi: 10.1016/j.phymed.2020.153279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tian J., Yan S., Wang H., Zhang Y., Zheng Y., Wu H., et al. Hanshiyi Formula, a medicine for Sars-CoV2 infection in China, reduced the proportion of mild and moderate COVID-19 patients turning to severe status: a cohort study. Pharmacol. Res. 2020;161 doi: 10.1016/j.phrs.2020.105127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luo M., Jiang B., Xu H.J., Yang Q., Zhou X.Q., Lv K. Analysis of influencing factors of death in patients with COVID-19. Chin. Trad. Herb. Drugs. 2020;6:1450–1456. [Google Scholar]
- 62.Chen G., Su W., Yang J., Luo D., Xia P., Jia W., et al. Chinese herbal medicine reduces mortality in patients with severe and critical Coronavirus disease 2019: a retrospective cohort study. Front Med. 2020;14(6):752–759. doi: 10.1007/s11684-020-0813-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ho T.Y., Wu S.L., Chen J.C., Li C.C., Hsiang C.Y. Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction. Antiviral Res. 2007;74(2):92–101. doi: 10.1016/j.antiviral.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shi X., Wei J., Liu M., Jin X., Zhou H., Zhu W., et al. Study on the overall regulation of Xuebijing injection in treating COVID-19. Shanghai J. Trad. Chin. Med. 2020;54:46–52. [Google Scholar]
- 65.Xing Y., Hua Y.R., Shang J., Ge W.H., Liao J. Traditional Chinese medicine network pharmacology study on exploring the mechanism of Xuebijing Injection in the treatment of coronavirus disease 2019. Chin. J. Nat. Med. 2020;18(12):941–951. doi: 10.1016/S1875-5364(20)60038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sun Y., Jiang M., Park P.H., Song K. Transcriptional suppression of androgen receptor by 18beta-glycyrrhetinic acid in LNCaP human prostate cancer cells. Arch. Pharm. Res. 2020;43(4):433–448. doi: 10.1007/s12272-020-01228-z. [DOI] [PubMed] [Google Scholar]
- 67.Lin S.C., Ho C.T., Chuo W.H., Li S., Wang T.T., Lin S.C. Effective inhibition of MERS-CoV infection by resveratrol. BMC Infect. Dis. 2017;17(1):144. doi: 10.1186/s12879-017-2253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ma Q., Pan W., Li R., Liu B., Li C., Xie Y., et al. Liu Shen capsule shows antiviral and anti-inflammatory abilities against novel coronavirus SARS-CoV-2 via suppression of NF-kappaB signaling pathway. Pharmacol. Res. 2020;158 doi: 10.1016/j.phrs.2020.104850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Runfeng L., Yunlong H., Jicheng H., Weiqi P., Qinhai M., Yongxia S., et al. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2) Pharmacol. Res. 2020;156 doi: 10.1016/j.phrs.2020.104761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tanaka T., Narazaki M., Kishimoto T. Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy. 2016;8(8):959–970. doi: 10.2217/imt-2016-0020. [DOI] [PubMed] [Google Scholar]
- 71.Chen X., Yang X., Liu T., Guan M., Feng X., Dong W., et al. Kaempferol regulates MAPKs and NF-κB signaling pathways to attenuate LPS-induced acute lung injury in mice. Int. Immunopharmacol. 2012;14(2):209–216. doi: 10.1016/j.intimp.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 72.Yahfoufi N., Alsadi N., Jambi M., Matar C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients. 2018;10(11) doi: 10.3390/nu10111618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ren X., Shao X.X., Li X.X., Jia X.H., Song T., Zhou W.Y., et al. Identifying potential treatments of COVID-19 from Traditional Chinese Medicine (TCM) by using a data-driven approach. J. Ethnopharmacol. 2020;258 doi: 10.1016/j.jep.2020.112932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li Q., Bai C., Yang R., Xing W., Pang X., Wu S., et al. Deciphering the pharmacological mechanisms of Ma Xing Shi Gan decoction against COVID-19 through integrating network pharmacology and experimental exploration. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.581691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang Y.X., Ma J.R., Wang S.Q., Zeng Y.Q., Zhou C.Y., Ru Y.H., et al. Utilizing integrating network pharmacological approaches to investigate the potential mechanism of Ma Xing Shi Gan Decoction in treating COVID-19. Eur. Rev. Med. Pharmacol. Sci. 2020;24(6):3360–3384. doi: 10.26355/eurrev_202003_20704. [DOI] [PubMed] [Google Scholar]
- 76.Guo G., Gong L., Sun L., Xu H. Quercetin supports cell viability and inhibits apoptosis in cardiocytes by down-regulating miR-199a. Artif. Cells Nanomed. Biotechnol. 2019;47(1):2909–2916. doi: 10.1080/21691401.2019.1640711. [DOI] [PubMed] [Google Scholar]
- 77.Subissi L., Imbert I., Ferron F., Collet A., Coutard B., Decroly E., et al. SARS-CoV ORF1b-encoded nonstructural proteins 12-16: replicative enzymes as antiviral targets. Antiviral Res. 2014;101:122–130. doi: 10.1016/j.antiviral.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Frick D.N., Lam A.M. Understanding helicases as a means of virus control. Curr. Pharm. Des. 2006;12(11):1315–1338. doi: 10.2174/138161206776361147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang H., Xie W., Xue X., Yang K., Ma J., Liang W., et al. Design of wide-spectrum inhibitors targeting coronavirus main proteases. PLoS Biol. 2005;3(10):e324. doi: 10.1371/journal.pbio.0030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Michaelis M., Geiler J., Naczk P., Sithisarn P., Ogbomo H., Altenbrandt B., et al. Glycyrrhizin inhibits highly pathogenic H5N1 influenza A virus-induced pro-inflammatory cytokine and chemokine expression in human macrophages. Med. Microbiol. Immunol. 2010;199(4):291–297. doi: 10.1007/s00430-010-0155-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hoever G., Baltina L., Michaelis M., Kondratenko R., Baltina L., Tolstikov G.A., et al. Antiviral activity of glycyrrhizic acid derivatives against SARS-coronavirus. J. Med. Chem. 2005;48(4):1256–1259. doi: 10.1021/jm0493008. [DOI] [PubMed] [Google Scholar]
- 82.Sinha S.K., Prasad S.K., Islam M.A., Gurav S.S., Patil R.B., AlFaris N.A., et al. Identification of bioactive compounds from Glycyrrhiza glabra as possible inhibitor of SARS-CoV-2 spike glycoprotein and non-structural protein-15: a pharmacoinformatics study. J. Biomol. Struct. Dyn. 2020:1–15. doi: 10.1080/07391102.2020.1779132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ye C., Gao M., Lin W., Yu K., Li P., Chen G. Theoretical study of the anti-NCP molecular mechanism of traditional Chinese medicine Lianhua-Qingwen formula (LQF) ChemRxiV. 2020 [Google Scholar]
- 84.Su H.X., Yao S., Zhao W.F., Li M.J., Liu J., Shang W.J., et al. Anti-SARS-CoV-2 activities in vitro of Shuanghuanglian preparations and bioactive ingredients. Acta Pharmacol. Sin. 2020;41(9):1167–1177. doi: 10.1038/s41401-020-0483-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jin Z., Du X., Xu Y., Deng Y., Liu M., Zhao Y., et al. Structure of M(pro) from SARS-CoV-2 and discovery of its inhibitors. Nature. 2020;582(7811):289–293. doi: 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- 86.Lundin A., Dijkman R., Bergstrom T., Kann N., Adamiak B., Hannoun C., et al. Targeting membrane-bound viral RNA synthesis reveals potent inhibition of diverse coronaviruses including the middle East respiratory syndrome virus. PLoS Pathog. 2014;10(5) doi: 10.1371/journal.ppat.1004166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Johnson B.A., Graham R.L., Menachery V.D. Viral metagenomics, protein structure, and reverse genetics: key strategies for investigating coronaviruses. Virology. 2018;517:30–37. doi: 10.1016/j.virol.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Du L., He Y., Zhou Y., Liu S., Zheng B.J., Jiang S. The spike protein of SARS-CoV--a target for vaccine and therapeutic development. Nat. Rev. Microbiol. 2009;7(3):226–236. doi: 10.1038/nrmicro2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O., et al. bioRxiv; 2020. Cryo-EM Structure of the 2019-nCoV Spike in the Prefusion Conformation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kuo L., Hurst K.R., Masters P.S. Exceptional flexibility in the sequence requirements for coronavirus small envelope protein function. J. Virol. 2007;81(5):2249–2262. doi: 10.1128/JVI.01577-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Venkatagopalan P., Daskalova S.M., Lopez L.A., Dolezal K.A., Hogue B.G. Coronavirus envelope (E) protein remains at the site of assembly. Virology. 2015;478:75–85. doi: 10.1016/j.virol.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schoeman D., Fielding B.C. Coronavirus envelope protein: current knowledge. Virol. J. 2019;16(1):69. doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen X., Yang X., Zheng Y., Yang Y., Xing Y., Chen Z. SARS coronavirus papain-like protease inhibits the type I interferon signaling pathway through interaction with the STING-TRAF3-TBK1 complex. Protein Cell. 2014;5(5):369–381. doi: 10.1007/s13238-014-0026-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lindner H.A., Fotouhi-Ardakani N., Lytvyn V., Lachance P., Sulea T., Menard R. The papain-like protease from the severe acute respiratory syndrome coronavirus is a deubiquitinating enzyme. J. Virol. 2005;79(24):15199–15208. doi: 10.1128/JVI.79.24.15199-15208.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Park J.Y., Kim J.H., Kim Y.M., Jeong H.J., Kim D.W., Park K.H., et al. Tanshinones as selective and slow-binding inhibitors for SARS-CoV cysteine proteases. Bioorg. Med. Chem. 2012;20(19):5928–5935. doi: 10.1016/j.bmc.2012.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Park J.Y., Jeong H.J., Kim J.H., Kim Y.M., Park S.J., Kim D., et al. Diarylheptanoids from Alnus japonica inhibit papain-like protease of severe acute respiratory syndrome coronavirus. Biol. Pharm. Bull. 2012;35(11):2036–2042. doi: 10.1248/bpb.b12-00623. [DOI] [PubMed] [Google Scholar]
- 97.Saghazadeh A., Rezaei N. Towards treatment planning of COVID-19: rationale and hypothesis for the use of multiple immunosuppressive agents: anti-antibodies, immunoglobulins, and corticosteroids. Int. Immunopharmacol. 2020;84 doi: 10.1016/j.intimp.2020.106560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367(6485):1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Letko M., Marzi A., Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020;5(4):562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zheng Y.Y., Ma Y.T., Zhang J.Y., Xie X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020;17(5):259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N. Engl. J. Med. 2020;382(17):1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020;20(6):363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cinatl J., Morgenstern B., Bauer G., Chandra P., Rabenau H., Doerr H.W. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet. 2003;361(9374):2045–2046. doi: 10.1016/S0140-6736(03)13615-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Deng Y., Liu B., He Z., Liu T., Zheng R., Yang A., et al. Research on active compounds of Huoxiangzhengqi oral liquid to prevent new coronavirus pneumonia (COVID-19) based on network pharmacology and molecular docking. Chin. Herb. Med. 2020;51(05):1113–1122. [Google Scholar]
- 107.Tsai K.C., Huang Y.C., Liaw C.C., Tsai C.I., Chiou C.T., Lin C.J., et al. A traditional Chinese medicine formula NRICM101 to target COVID-19 through multiple pathways: a bedside-to-bench study. Biomed. Pharmacother. 2021;133 doi: 10.1016/j.biopha.2020.111037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B., et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436(7047):112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sungnak W., Huang N., Becavin C., Berg M., Queen R., Litvinukova M., et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020;26(5):681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zou X., Chen K., Zou J., Han P., Hao J., Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14(2):185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hassan S.M., Jawad M.J., Ahjel S.W., Singh R.B., Singh J., Awad S.M., et al. The Nrf2 Activator (DMF) and Covid-19: Is there a Possible Role? Med. Arch. 2020;74(2):134–138. doi: 10.5455/medarh.2020.74.134-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Conti P., Gallenga C.E., Tetè G., Caraffa A., Ronconi G., Younes A., et al. How to reduce the likelihood of coronavirus-19 (CoV-19 or SARS-CoV-2) infection and lung inflammation mediated by IL-1. J. Biol. Regul. Homeost. Agents. 2020;34(2) doi: 10.23812/Editorial-Conti-2. [DOI] [PubMed] [Google Scholar]
- 114.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F., et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hendren N.S., Drazner M.H., Bozkurt B., Cooper L.T., Jr. Description and proposed management of the acute COVID-19 cardiovascular syndrome. Circulation. 2020;141(23):1903–1914. doi: 10.1161/CIRCULATIONAHA.120.047349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hunter C.A., Jones S.A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 2015;16(5):448–457. doi: 10.1038/ni.3153. [DOI] [PubMed] [Google Scholar]
- 119.Dinh Q.N., Drummond G.R., Sobey C.G., Chrissobolis S. Roles of inflammation, oxidative stress, and vascular dysfunction in hypertension. Biomed Res. Int. 2014;2014 doi: 10.1155/2014/406960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu J., Li S., Liu J., Liang B., Wang X., Wang H., et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55 doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gustine J.N., Jones D. Immunopathology of hyperinflammation in COVID-19. Am. J. Pathol. 2020;191(1):4–17. doi: 10.1016/j.ajpath.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ceribelli A., Motta F., De Santis M., Ansari A.A., Ridgway W.M., Gershwin M.E., et al. Recommendations for coronavirus infection in rheumatic diseases treated with biologic therapy. J. Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yang R., Liu H., Bai C., Wang Y., Zhang X., Guo R., et al. Chemical composition and pharmacological mechanism of Qingfei Paidu Decoction and Ma Xing Shi Gan Decoction against Coronavirus Disease 2019 (COVID-19): in silico and experimental study. Pharmacol. Res. 2020;157 doi: 10.1016/j.phrs.2020.104820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang Z.J., Wu W.Y., Hou J.J., Zhang L.L., Li F.F., Gao L., et al. Active constituents and mechanisms of Respiratory Detox Shot, a traditional Chinese medicine prescription, for COVID-19 control and prevention: network-molecular docking-LC-MS(E) analysis. J. Integr. Med. 2020;18(3):229–241. doi: 10.1016/j.joim.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ma Q., Pan W., Li R., Liu B., Li C., Xie Y., et al. Liu Shen capsule shows antiviral and anti-inflammatory abilities against novel coronavirus SARS-CoV-2 via suppression of NF-κB signaling pathway. Pharmacol. Res. 2020;158 doi: 10.1016/j.phrs.2020.104850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Han L., Wei X.X., Zheng Y.J., Zhang L.L., Wang X.M., Yang H.Y., et al. Potential mechanism prediction of Cold-Damp Plague Formula against COVID-19 via network pharmacology analysis and molecular docking. Chin. Med. 2020;15:78. doi: 10.1186/s13020-020-00360-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Frazier W.J., Hall M.W. Immunoparalysis and adverse outcomes from critical illness. Pediatr. Clin. North Am. 2008;55(3):647–668. doi: 10.1016/j.pcl.2008.02.009. xi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Monneret G., Lepape A., Voirin N., Bohé J., Venet F., Debard A.L., et al. Persisting low monocyte human leukocyte antigen-DR expression predicts mortality in septic shock. Intensive Care Med. 2006;32(8):1175–1183. doi: 10.1007/s00134-006-0204-8. [DOI] [PubMed] [Google Scholar]
- 129.Giamarellos-Bourboulis E.J., Netea M.G., Rovina N., Akinosoglou K., Antoniadou A., Antonakos N., et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27(6):992–1000. doi: 10.1016/j.chom.2020.04.009. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zheng Y., Li R., Liu S. Immunoregulation with mTOR inhibitors to prevent COVID-19 severity: a novel intervention strategy beyond vaccines and specific antiviral medicines. J. Med. Virol. 2020;92(9):1495–1500. doi: 10.1002/jmv.26009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Huttunen R., Syrjänen J. Obesity and the risk and outcome of infection. Int. J. Obes. (Lond.) 2013;37(3):333–340. doi: 10.1038/ijo.2012.62. [DOI] [PubMed] [Google Scholar]
- 132.Odegaard J.I., Chawla A. Connecting type 1 and type 2 diabetes through innate immunity. Cold Spring Harb. Perspect. Med. 2012;2(3) doi: 10.1101/cshperspect.a007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ruan X., Du P., Zhao K., Huang J., Xia H., Dai D., et al. Mechanism of Dayuanyin in the treatment of coronavirus disease 2019 based on network pharmacology and molecular docking. Chin. Med. 2020;(15):62. doi: 10.1186/s13020-020-00346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Thachil J., Tang N., Gando S., Falanga A., Cattaneo M., Levi M., et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J. Thromb. Haemost. 2020;18(5):1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Davoodi L., Jafarpour H., Taghavi M. Razavi A. COVID-19 presented with deep vein thrombosis: an unusual presenting. J. Investig. Med. High Impact Case Rep. 2020;8 doi: 10.1177/2324709620931239. 2324709620931239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Campbell C.M., Kahwash R. Will complement inhibition be the new target in treating COVID-19-related systemic thrombosis? Circulation. 2020;141(22):1739–1741. doi: 10.1161/CIRCULATIONAHA.120.047419. [DOI] [PubMed] [Google Scholar]
- 138.Brinkmann V., Zychlinsky A. Beneficial suicide: why neutrophils die to make NETs. Nat. Rev. Microbiol. 2007;5(8):577–582. doi: 10.1038/nrmicro1710. [DOI] [PubMed] [Google Scholar]
- 139.Meng H., Yalavarthi S., Kanthi Y., Mazza L.F., Elfline M.A., Luke C.E., et al. In vivo role of neutrophil extracellular traps in antiphospholipid antibody-mediated venous thrombosis . 2017;69(3):655–667. doi: 10.1002/art.39938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Döring Y., Soehnlein O., Weber C. Neutrophil extracellular traps in atherosclerosis and atherothrombosis. Circ. Res. 2017;120(4):736–743. doi: 10.1161/CIRCRESAHA.116.309692. [DOI] [PubMed] [Google Scholar]
- 141.Iba T., Levy J.H., Warkentin T.E., Thachil J., van der Poll T., Levi M. Diagnosis and management of sepsis-induced coagulopathy and disseminated intravascular coagulation. J. Thromb. Haemost. 2019;17(11):1989–1994. doi: 10.1111/jth.14578. [DOI] [PubMed] [Google Scholar]
- 142.Ji H.L., Zhao R., Matalon S., Matthay M.A. Elevated plasmin(ogen) as a common risk factor for COVID-19 susceptibility. Physiol. Rev. 2020;100(3):1065–1075. doi: 10.1152/physrev.00013.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Guo W., Li M., Dong Y., Zhou H., Zhang Z., Tian C., et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab. Res. Rev. 2020 doi: 10.1002/dmrr.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Górka J., Polok K., Iwaniec T., Górka K., Wludarczyk A., Fronczek J., et al. Altered preoperative coagulation and fibrinolysis are associated with myocardial injury after non-cardiac surgery. Br. J. Anaesth. 2017;118(5):713–719. doi: 10.1093/bja/aex081. [DOI] [PubMed] [Google Scholar]
- 145.Gupta N., Zhao Y.Y., Evans C.E. The stimulation of thrombosis by hypoxia. Thromb. Res. 2019;181:77–83. doi: 10.1016/j.thromres.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 146.Flordal P.A., Svensson J. Hemostatic effects of ephedrine. Thromb. Res. 1992;68(3):295–302. doi: 10.1016/0049-3848(92)90086-p. [DOI] [PubMed] [Google Scholar]
- 147.Villee C.A., Hagerman D.D. Effect of oxygen deprivation on the metabolism of fetal and adult tissues. Am. J. Physiol. 1958;194(3):457–464. doi: 10.1152/ajplegacy.1958.194.3.457. [DOI] [PubMed] [Google Scholar]
- 148.Tamis-Holland J.E., Jneid H., Reynolds H.R., Agewall S., Brilakis E.S., Brown T.M., et al. Contemporary diagnosis and management of patients with myocardial infarction in the absence of obstructive coronary artery disease: a scientific statement from the American Heart Association. Circulation. 2019;139(18):e891–e908. doi: 10.1161/CIR.0000000000000670. [DOI] [PubMed] [Google Scholar]
- 149.Lian F., Ni Q., Shen Y., Yang S., Piao C., Wang J., et al. International traditional Chinese medicine guideline for diagnostic and treatment principles of diabetes. Ann. Palliat. Med. 2020;9(4):2237–2250. doi: 10.21037/apm-19-271. [DOI] [PubMed] [Google Scholar]
- 150.Masic I., Naser N., Zildzic M. Public health aspects of COVID-19 infection with focus on cardiovascular diseases. Mater. Sociomed. 2020;32(1):71–76. doi: 10.5455/msm.2020.32.71-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wu Q., Zhou L., Sun X., Yan Z., Hu C., Wu J., et al. Altered lipid metabolism in recovered SARS patients twelve years after infection. Sci. Rep. 2017;7(1):9110. doi: 10.1038/s41598-017-09536-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang J., Xu H., Yang X., Zhao D., Liu S., Sun X., et al. Cardiac complications associated with the influenza viruses A subtype H7N9 or pandemic H1N1 in critically ill patients under intensive care. Braz. J. Infect. Dis. 2017;21(1):12–18. doi: 10.1016/j.bjid.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.de Olano J., Howland M.A., Su M.K., Hoffman R.S., Biary R. Toxicokinetics of hydroxychloroquine following a massive overdose. Am. J. Emerg. Med. 2019;37(12):2264. doi: 10.1016/j.ajem.2019.158387. e5-.e8. [DOI] [PubMed] [Google Scholar]
- 154.Xu K., Cai H., Shen Y., Ni Q., Chen Y., Hu S., et al. [Management of corona virus disease-19 (COVID-19): the Zhejiang experience] Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020;49(1):0. doi: 10.3785/j.issn.1008-9292.2020.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Huang J.Z., Han M.F., Luo T.D., Ren A.K., Zhou X.P. [Mental health survey of medical staff in a tertiary infectious disease hospital for COVID-19] Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2020;38(3):192–195. doi: 10.3760/cma.j.cn121094-20200219-00063. [DOI] [PubMed] [Google Scholar]
- 156.http://www.chictr.org.cn/showproj.aspx?proj=56771.
- 157.http://www.chictr.org.cn/showproj.aspx?proj=52662.
- 158.http://www.chictr.org.cn/showproj.aspx?proj=53225.
- 159.Huo X., He S., Ma J., Wei G., Song K., Tang C., et al. Retrospective study of Shufeng Jiedu Capsules combined with Arbidol in the treatment of new coronavirus pneumonia. Chin. Herb. Med. 2020;51(05):1167–1170. [Google Scholar]
- 160.Shi J., Yang Z., Ye C., Chen S., Lu Y., Lv Y., et al. Clinical observation on the treatment of 49 cases of non-critical novel coronavirus pneumonia in Shanghai. Shanghai J. of Trad. Chin. Med. 2020;54(04):30–35. [Google Scholar]
- 161.Xia W., An C., Zheng C., Zhang J., Huang M., Wang Y., et al. Clinical study on 34 cases of new coronavirus pneumonia treated by integrated traditional Chinese and Western medicine. Chin. Med. J. 2020;61(05):375–382. [Google Scholar]
- 162.Runfeng L., Yunlong H., Jicheng H., Weiqi P., Qinhai M., Yongxia S., et al. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2) Pharmacol. Res. 2020 doi: 10.1016/j.phrs.2020.104761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yu S., Wang J., Shen H. Network pharmacology-based analysis of the role of traditional Chinese herbal medicines in the treatment of COVID-19. Ann. Palliat. Med. 2020;9(2):437–446. doi: 10.21037/apm.2020.03.27. [DOI] [PubMed] [Google Scholar]
- 164.Chen J., Wang Y.K., Gao Y., Hu L.S., Yang J.W., Wang J.R., et al. Protection against COVID-19 injury by qingfei paidu decoction via anti-viral, anti-inflammatory activity and metabolic programming. Biomed. Pharmacother. 2020;129 doi: 10.1016/j.biopha.2020.110281. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.