Abstract
The coronavirus disease (COVID)-19 pandemic is a major challenge for the health systems worldwide. Acute respiratory distress syndrome (ARDS), is one of the most common complications of the COVID-19 infection. The activation of the coagulation system plays an important role in the pathogenesis of ARDS. The development of lung coagulopathy involves thrombin generation and fibrinolysis inhibition.
Unfractionated heparin and its recently introduced counterpart low molecular weight heparin (LMWH), are widely used anticoagulants with a variety of clinical indications allowing for limited and manageable physio-toxicologic side effects while the use of protamine sulfate, heparin's effective antidote, has made their use even safer. Tissue-type plasminogen activator (tPA) is approved as intravenous thrombolytic treatment. The present narrative review discusses the use of heparin and tPA in the treatment of COVID-19-induced ARDS and their related potential physio-toxicologic side effects. The article is a quick review of articles on anticoagulation in COVID infection and the potential toxicologic reactions associated with these drugs.
Keywords: Heparin, Tissue-type plasminogen activator, COVID-19, Acute respiratory distress syndrome, Toxicological side effects, Anticoagulant
1. Introduction
The recently emerged coronavirus disease (COVID-19) pandemic is a major challenge for health systems around the world, with almost all geographical areas affected by April 2020 (Goumenou et al., 2020), except for Antarctica and Polynesia. SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus 2) infection is difficult to prevent since there is no vaccine available to date (Calina et al., 2020a). The majority of the infected patients will develop only mild/moderate symptoms (Connors and Levy, 2020; Tsatsakis et al., 2020). However, some patients have aberrant inflammatory responses that lead to lung injury and hypoxemic respiratory failure, which is the most common cause of death in such patients (Goumenou et al., 2020).
Acute respiratory distress syndrome (ARDS) is one of the most frequent complications of the COVID-19; activation of the coagulation system plays a pivotal role in the pathogenesis of ARDS (Thachil et al., 2020).
ARDS is characterized by inflammation and the presence of anticoagulant factors in the lung, non-hydrostatic pulmonary edema, and disrupture of the alveolar-capillary barrier with increased permeability (Gonzales et al., 2015; Ware and Matthay, 2000), leading to activation of pulmonary macrophages, an increased influx of intravascular and extravascular neutrophils, platelets and fibrin, and epithelial and endothelial injury (Camprubí-Rimblas et al., 2018).
These result in the formation of fibrin-platelet microthrombi in the pulmonary vessels, with the rapid development of progressive respiratory dysfunction and right heart failure (Ware, 2006; Tian et al., 2020). Similar findings were observed in lung samples taken from COVID-19 infected patients (Matthay et al., 2012). Despite the supportive measures, the mortality and morbidity associated with ARDS remain high (35–40%) (Artigas et al., 2017) and there is a clear need for new therapeutic interventions that focus on the pathophysiology of ARDS (Force ARDSDTRanieri et al., 2012).
The pathophysiology of ARDS involves several pathways of the coagulation cascade: protein C, tissue factor, the regulation of fibrinolysis by the plasminogen activator (PA), and inhibitor pathway (Camprubí-Rimblas et al., 2018). As a result, therapies that target coagulation cascade and fibrinolysis may prove effective in the treatment and prevention of ARDS (Camprubí-Rimblas et al., 2018).
Although ARDS associated with COVID-19 infection can be defined according to the Berlin criteria (Gattinoni et al., 2020; Dolhnikoff et al., 2020), it represents a specific condition having as main feature the dissociation between the severity of hypoxemia and the maintenance of a relatively good respiratory mechanics (Laterre et al., 2003).
The discrepancy between gas exchange abnormalities, radiological changes, and respiratory mechanics indicates a vascular component of the disease, as demonstrated by autopsy studies showing the presence of thrombi in the microcirculation (Laterre et al., 2003; MacLaren and Stringer, 2007).
Targeting coagulation and fibrinolysis in the treatment of ARDS has been proposed since 2003 (Schultz et al., 2006; Ware et al., 2006; Liu et al., 2018; Hardaway et al., 2001a). Furthermore, using plasminogen activators to limit the progression of ARDS and mortality is supported by data from animal studies and the results of a phase I trial (Farsalinos et al., 2020a). Data published in 2001 showed that the administration of urokinase or streptokinase in patients with ARDS reduces the expected mortality from 100% to 70%, without increasing the risk of bleeding (Aime et al., 2015). Of note, the cause of mortality for patients who eventually died was kidney or hepatic failure, and not lung failure (Aime et al., 2015).
Based on the link between thrombosis and inflammation, there is an intuitive relationship between COVID-19 and coagulation abnormalities, characterized by increased levels of procoagulants (fibrinogen) and D-dimers (Zhou et al., 2020); these changes have also been associated with increased mortality in the presence of the COVID-19 (Wang et al., 2020; Levi and van der Poll, 2017; Schmitt et al., 2019).
In this context, endothelial dysfunction, induced by COVID-19, results in excess thrombin generation and inhibition of fibrinolysis, leading to a hypercoagulant (Fig. 1 ) status in COVID-19 patients (Idell, 2003). Furthermore, hypoxia occurring in severe forms of COVID-19 stimulates thrombosis, not only by increasing blood viscosity but also by occlusion and formation of microthrombi in the small lung vessels (Ware, 2006; Tian et al., 2020). Acute pulmonary injury leads to a pro-inflammatory status, secondary to cytokine storm and macrophage and endothelial activation, with increased levels of interleukin (IL)-1, IL-6, IL-8, tumor necrosis factor (TNF)-alpha, ferritin, C-reactive protein (CRP), D-dimers, and fibrinogen (Farsalinos et al., 2020b). Data show that this is not a primary thrombotic process, but thrombosis occurs secondary to inflammation and hypoxia.
Fig. 1.
Coagulability induced by COVID-19 with consequences on lung.
Indeed, the increase in D-dimer levels appears to be due to intense inflammation that stimulates intrinsic fibrinolysis in the lungs, with subsequent release into the circulation (Farsalinos et al., 2020b). Of note, pre-existing pro-inflammatory diseases, such as atherosclerotic disease, diabetes, and obesity, increase the risk of COVID-19 pneumonia (Farsalinos et al., 2020b; Mycroft-West et al., 2020).
Heparin is a well-known anticoagulant and tissue-type plasminogen activator (tPA) is approved as intravenous thrombolytic treatment. Since COVID-19 infected patients are prone to thrombotic complications, the present narrative review discusses the use of heparin and tPA in the setting of the COVID-19 infection.
2. Heparin use in COVID-19
Heparin, a polydispersed, heterogeneous, natural product, is a well-tolerated anticoagulant drug that has been used successfully for over 80 years, with limited and manageable side effects. (Gaertner and Massberg, 2016). Besides, heparin belongs to a unique class of pharmaceuticals, which has effective antidotes, thus making its use safe in daily practice.
Based on the bidirectional relationship between the immune system and thrombin generation, the blockade of thrombin by heparin should decrease the inflammatory response (Young, 2009). Indeed, several studies have demonstrated the anti-inflammatory properties of heparin (Esmon, 2014; Mousavi et al., 2015; Xu et al., 2009). The underlying mechanisms involve binding of inflammatory cytokines, inhibition of neutrophil chemotaxis and leukocyte migration, neutralization of the C5a fraction of the complement, and sequestration of acute-phase proteins (Esmon, 2014; Mousavi et al., 2015; Xu et al., 2009).
Endothelial dysfunction plays a key role in these processes, leading to organ failure. Histones, released as a result of cell injury, aggravate endothelial injury (Iba et al., 2015), whereas heparin antagonizes histones and therefore protects the endothelium (Zhu et al., 2019; Liu et al., 2019). This protective function can be extended to the endothelial junctions, as demonstrated by experimental models, in which unfractionated heparin decreases pulmonary edema and vascular permeability (Ma and Bai, 2015). Another protective mechanism involves the impact of heparin on histone methylation, as well as on the mitogen-activated protein kinase (MAPK) and nuclear factor kappa-light-chain enhancer of activated B cells (NF-κB) signaling pathways, leading to improvements in the microcirculatory dysfunction and protection from organ injury (Shanghai Clinical Treatment Expert Group for COVID-19, 2020).
Based on the above data, early administration of anticoagulant therapy (Fig. 2 ) has been proposed in severe forms of the COVID-19 since it was associated with improved prognosis (https://www.clinicaltrial, 2020).
Fig. 2.
Anticoagulant therapy benefits in Covid-19 infection.
To address the question of heparin use in COVID-19 infection, more than 20 clinical trials, most of which are still recruiting, have been registered with the National Institutes of Health as of November 15, 2020). (Chen et al., 2020).
2.1. Heparin's physio-toxicologic side effects and safety margins
One of the most interesting side effects of heparin administration is “heparin-induced thrombocytopenia”. This side effect is especially difficult to recognize it in septic patients where at first platelets are increased and then they may decrease. Moreover in cases of propagated DIC, platelets also sharply decrease so you may need to be careful in patients with already low platelet counts under low-molecular weight heparin (LMWH) who are likely to become septic. In most cases, death is related to disseminated intravascular coagulation since 71.4% of patients who die from COVID-19 have diffuse intravascular coagulation (DIC), whereas only 0.6% of the surviving patients meet the DIC criteria (Huang et al., 2020; Tang et al., 2020a).
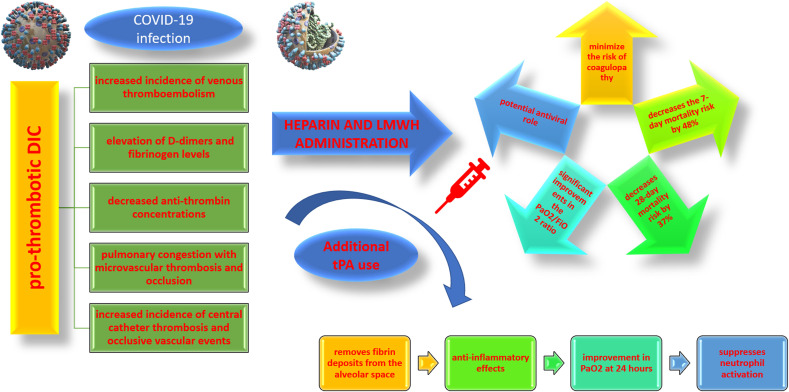
In addition, a pro-thrombotic DIC, leading to an increased incidence of venous thromboembolism, the elevation of D-dimers and fibrinogen levels, decreased anti-thrombin concentrations, as well as to pulmonary congestion with microvascular thrombosis and occlusion, increased incidence of central catheter thrombosis, and occlusive vascular events (e.g. myocardial ischemia, stroke) is associated with COVID-19 (Tang et al., 2020a; Han et al., 2020a).
Coagulopathy secondary to COVID-19 is characterized by features of coagulation that mimic DIC (but differs from DIC related with sepsis by less prominent thrombocytopenia, lower consumption of coagulation factors); clinical and pathological signs of thrombotic microangiopathy; and elevated D-dimer levels which are predictive of unfavorable prognosis (Levi and van der Poll, 2017; Schmitt et al., 2019). Severe COVID-19 results in a pro-hemostatic stage, leading to an increased incidence of venous thromboembolism (Schmitt et al., 2019).
Several COVID-19 infected patients meet the criteria of the Third International Consensus Definitions for Sepsis (Sepsis‐3) (Singer et al., 2016). In addition, immobilization of the patient during severe COVID-19 increases the risk of venous thromboembolism, and thus the prophylactic use of LMWH in such patients is currently recommended to minimize the risk of coagulopathy (Tang et al., 2020a; Li et al., 2018). In this context, a meta-analysis (9 trials; n = 465 patients) reported that the initiation of LMWH therapy in the first 7 days of the onset of ARDS decreases the 7-day mortality risk by 48% and the 28-day mortality risk by 37%, related to significant improvements in the PaO2/FiO2 ratio, especially in the subgroup of patients receiving high doses of LMWH (i.e. ≥ 5000 units/day) (Hanify et al., 2017). The need for higher doses of heparin was also observed in critically ill septic patients who did not respond to thromboprophylaxis (Camprubí-Rimblas et al., 2020).
Of note, since coagulopathy during ARDS is initiated in the lungs and in many cases is limited to the lungs, a reasonable strategy seems to be the administration of anticoagulation by nebulization (Shukla and Spear, 2001).
An interesting concept studied in experimental models is related to the potential antiviral role of heparin. The polyanionic nature of heparin allows it to bind to several proteins and thus serve as an effective inhibitor of viral attachment (Pantea Stoian et al., 2020). For example, in the case of herpes simplex virus infection, heparin competes with the virus for the binding of cell surface glycoproteins, thus limiting the infection (Vicenzi et al., 2004). An Italian study showed that heparin administration at concentrations of 100 μg/mL inhibited the infection in culture cells injected with sputum from a patient with SARS-CoV strain HSR1 (Heat Shock Response 1) pneumonia (Ghezzi et al., 2017). A recent publication showed that the Spike S1 binding domain of SARS-CoV-2 interacts with heparin, but the clinical implications of this interaction in viral infections remain to be established (Gaertner and Massberg, 2016).
Unfractionated heparin and LMWH are approved as anticoagulants with an excellent profile of safety, stability, bioavailability, and pharmacokinetics. Still, heparin and its derivatives are not properly exploited as antiviral drugs, despite a wide range of activity against several viruses, including coronaviruses (SARS-CoV/HSR1) (Ghezzi et al., 2017), flaviviruses (Vicenzi et al., 2018; Wu Dunn and Spear, 1989), herpesviruses (Skidmore et al., 2015), influenza [457], and HIV (human immunodeficiency virus) (Harrop and Rider, 1998; https://www.coronavirusto).
On March 30, 2020, a new clinical trial protocol (COVID-19 HOPE Trial – A safety and efficacy clinical trial of nebulized Heparin-N-acetylcysteine in COVID-19 Patients by Evaluation of pulmonary function) was announced, evaluating the effects of the combination of nebulized heparin with N-acetylcysteine (NAC), called H-NAC, on lung function and mechanical ventilation in patients with COVID-19 infection (Geiler et al., 2010). These two drugs are already approved by the Food and Drug Administration (FDA) for other conditions. Experimental data have shown that both drugs (i.e. heparin and NAC) can interfere with infection from coronaviruses in general, and SARS-CoV-2 in particular (Calina et al., 2020b). In this context, 12 clinical trials including over 780 patients reported that nebulized heparin, alone or in combination with NAC, was effective in the treatment of burn-induced lung injury and patients with ARDS, improving lung function and reducing the necessity for mechanical ventilation, with an excellent safety profile and minor side effects (Geiler et al., 2010). However, since the pharmacologic profile of various drugs hasn't been properly tested in the context of COVID-19 (Vivarelli et al., 2020; Kostoff et al., 2020) and the fact that in most cases of severe COVID-19 there is a constellation of contributing factors ranging from chronic environmental toxicity (Moore et al., 2020a)to chronic inflammatory diseases, clinicians ought to follow local guidelines maintaining experimental data only for last resort cases.
3. tPA use in COVID-19
An essential feature of ARDS is the deposition of fibrin in the alveoli and the lung parenchyma (Whyte et al., 2020). Fibrin deposits in the lung parenchyma are developed due to abnormalities of the coagulation and fibrinolytic systems (https://www.coronavirusto). Tissue factor (TF) is exposed to damaged alveolar endothelial cells and on the surface of leukocytes, while elevated levels of plasminogen inhibitor factor 1 (PAI-1) in the lung epithelium and endothelial cells creates a hypofibrinolytic status (Hardaway, 2006). Prophylactic treatment with LMWH in COVID-19 infected patients is important in limiting coagulopathy, but for the degradation of fibrin deposits in the lungs, it is essential to promote local fibrinolysis by the use of fibrinolytic drugs (i.e. tPA). tPA is approved as an intravenous thrombolytic treatment, whereas its nebulized form is effective in plastic bronchitis and is currently being evaluated in phase II clinical trials (Hardaway, 2006). Overall, nebulizing plasminogen activators may be used in patients with COVID-19 infection to target fibrin degradation and improve oxygenation in critically ill patients (Hardaway, 2006).
Anticoagulant therapy is essential in minimizing fibrin deposition and microthrombus formation in the ARDS and the treatment of pro-thrombotic complications (Hardaway et al., 2001b). However, LMWH is ineffective in removing fibrin deposits from the alveolar space. As a result, to restore the balance of fibrinolysis in the lungs, it is necessary either to stimulate the activation of plasminogen or to inhibit fibrinolysis inhibitors. In this context, a phase I clinical trial showed a significant improvement in PaO2 at 24 h, in 19 of 20 patients with severe ARDS secondary to trauma or sepsis, after administration of urokinase or streptokinase, without increasing the risk of bleeding (Hardaway et al., 2001b; Tang et al., 2020b).
The use of tPA in the treatment of ARDS in COVID-19 infected patients has recently been proposed (Harrop and Rider, 1998). In addition to rebalancing the fibrinolytic balance, administration of tPA in ARDS patients could also provide anti-inflammatory effects, suppressing neutrophil activation, as shown in experimental mice models (Harrop and Rider, 1998).
Evidence shows that lethal COVID-19 associated ARDS is a result of disseminated intravascular fibrin deposition (Han et al., 2020b; Moore et al., 2020b) and there is a growing interest in the role of tPA in reducing the mortality of COVID-19 associated ARDS. A USA multicenter group has proposed that tPA could be a severity reducing for COVID-19 associated ARDS and may act as a salvage technique to rescue patients when mechanical ventilation is not available (Choudhury et al., 2020). Since “Extraordinary times call for extraordinary measures”, it is unlikely to carry out a randomized clinical trial, because, under the pressure exerted by the pandemic, time is the enemy. Even with lack of high-quality evidence emerging from randomized clinical trials, protocols for salvage use of systemic tPA are in planning at several centers in the USA (Bodier-Montagutelli et al., 2018).
3.1. Physio-toxicologic side effects of tPA
A major side effect of anticoagulant or thrombolytic therapy is bleeding; the administration of drugs in the form of aerosols limits their diffusion from the lungs into the bloodstream (Abdelaal Ahmed Mahmoud et al., 2020). It would be worthy to note that in cases of bleeding ROTEM (rotational thromboelastometry) may prove useful as a fast and trustful way to differentiate between insufficiency of the intrinsic or the extrinsic coagulation pathway. Experimental data show that anticoagulant therapy given in the form of nebulization reduces lung injury without increasing the risk of bleeding (Practical guidance for th, 2020). Heparin prevents fibrin deposition, but cannot remove pre-existing fibrin. A recent publication comparing the efficacy of the nebulized form of plasminogen activator (streptokinase) and heparin in the treatment of ARDS reported that the improvement in the PaO2/FIO2 ratio was significantly greater and the ICU (Intensive Care Unit) mortality was significantly lower in the streptokinase group (Llitjos et al., 2020). A phase II clinical trial using tPA in nebulization (PLATyPuS; alteplase, NCT02315898) in the treatment of plastic bronchitis is currently on going (https://clinicaltrials.gov/ct2/show/NCT02315898). However, intravenous administration of tPA is required to remove large thrombi from the circulation. Furthermore, a potential problem with nebulization is that nebulized proteins are susceptible to degradation (Bikdeli et al., 2020).
The doubling of doses used for prophylaxis in the ICU should be considered due to the high risk of thrombosis. However, these recommendations are based on general thromboprophylaxis and are not specific to COVID-19 (Bikdeli et al., 2020; Suceveanu et al., 2020). A small study of 26 patients admitted to the ICU showed a higher incidence of venous thrombotic events (VTE) in patients receiving prophylactic anticoagulation comparing with therapeutic anticoagulation (100% versus 56%) (Firdous et al., 2020, Stoian et al., 2020).
4. Conclusion
Thromboprophylaxis is indicated in all patients with COVID-19 infection. Heparin and tPA may be useful in these patients minimizing the risk for ARDS complications and/or reducing the pressure on the ventilator support in the ICU. Both anticoagulation and fibrinolysis are clinically important in COVID-19 infected patients. However, further research is needed to unveil the potential pharmacologic and toxicologic side-effects induced by the co-administration of classical and novel antiviral therapies aiming against SARS-CoV-2.
CRediT authorship contribution statement
Laura Mazilu: Conceptualization, Writing – original draft, made the graphical figures prepare the draft. Niki Katsiki: Writing – original draft, preparation, made revisions. Taxiarchis Konstantinos Nikolouzakis: Data curation, search for data, made revisions. Minas I. Aslanidis: Data curation, search for data, Writing – original draft, preparation. George Lazopoulos: Writing – original draft, preparation. Dimitrios Kouretas: Data curation, search for data, Writing – review & editing. Aristidis Tsatsakis: Conceptualization, Data curation, search for data. Andra-Iulia Suceveanu: Writing – original draft, preparation, made revisions. Anca-Pantea Stoian: Conceptualization, made the graphical figures, made revisions, Writing – original draft, prepare the draft. Irinel-Raluca Parepa: Supervision, Writing – review & editing, speeling editing. Felix Voinea: Writing – review & editing, made revisions. Adrian Paul Suceveanu: Writing – review & editing. Andreea Letiția Arsene: Writing – review & editing. Bruno Ștefan Velescu: Writing – original draft, preparation. Cosmin Vesa: Writing – review & editing, made the graphical figures. Cornelia Nitipir: Data curation, collect data, Writing – original draft, preparation.
Declaration of competing interest
NK has given talks, attended conferences, and participated in trials sponsored by Astra Zeneca, Bausch Health, Boehringer Ingelheim, Elpen, Mylan, Novo Nordisk, Sanofi, and Servier.
LM has given talks, attended conferences, and participated in trials sponsored by Accord, AstraZeneca, Astellas, Bayer, BMS, Eli Lilly, Ipsen, Johnson & Johnson Romania, Merck, MSD, Novartis, Pfizer, Roche, Sandoz.
APS has given talks, attended conferences, and participated in trials sponsored by Astra Zeneca, Boehringer Ingelheim, Coca-Cola, Johnson and Johnson, Lilly, Medtronic, Merck, Novo Nordisk, and Sanofi and is currently Vice-President, National Diabetes Commission, Ministry of Health, Romania.
Handling editor: Dr Michael Aschner
References
- Abdelaal Ahmed Mahmoud A., et al. Streptokinase versus unfractionated heparin nebulization in patients with severe acute respiratory distress syndrome (ARDS): a randomized controlled trial with observational controls. J. Cardiothorac. Vasc. Anesth. 2020;34:436–443. doi: 10.1053/j.jvca.2019.05.035. [DOI] [PubMed] [Google Scholar]
- Aime T., et al. Blood. 2015;126(5):582–588. doi: 10.1182/blood-2014-08-531582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artigas A., et al. Inhalation therapies in acute respiratory distress syndrome. Ann. Transl. Med. 2017;5(14):293. doi: 10.21037/atm.2017.07.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikdeli B., Madhavan M.V., Jimenez D., et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J. Am. Coll. Cardiol. 2020;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodier-Montagutelli E., Mayor A., Vecellio L., Respaud R., Heuze-Vourc'h N. Designing inhaled protein therapeutics for topical lung delivery: what are the next steps? Expet Opin. Drug Deliv. 2018;15:729–736. doi: 10.1080/17425247.2018.1503251. [DOI] [PubMed] [Google Scholar]
- Calina D., et al. Towards effective COVID 19 vaccines: updates, perspectives and challenges (Review) Int. J. Mol. Med. 2020;46:1–14. doi: 10.3892/ijmm.2020.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calina D., Hartung T., Docea A.O., et al. COVID-19 vaccines: ethical framework concerning human challenge studies. DARU J Pharm Sci. 2020;28(8) doi: 10.1007/s40199-020-00371-8. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camprubí-Rimblas M., et al. Anticoagulant therapy in acute respiratory distress syndrome. Ann. Transl. Med. 2018;6(2):36. doi: 10.21037/atm.2018.01.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camprubí-Rimblas M., et al. Effects of nebulized antithrombin and heparin on inflammatory and coagulation alterations in an acute lung injury model in rats. J. Thromb. Haemostasis. 2020;18(3):571–583. doi: 10.1111/jth.14685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. 10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury R., Barrett C.D., Moore H.B., et al. Salvage use of tissue plasminogen activator (tPA) in the setting of acute respiratory distress syndrome (ARDS) due to COVID-19 in the USA: a Markov decision analysis. World J. Emerg. Surg. 2020;15:29. doi: 10.1186/s13017-020-00305-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors J.M., Levy J.H. Thromboinflammation and the hypercoagulability of COVID‐19. J.Thromb.Haemost. 2020;18:1559–1561. doi: 10.1111/jth.14849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolhnikoff M., et al. Pathological evidence of pulmonary thrombotic phenomena in severe COVID-19. J. Thromb. Haemostasis. 2020;18(6):1517–1519. doi: 10.1111/jth.14844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmon C.T. Targeting factor Xa and thrombin: impact on coagulation and beyond. Thromb. Haemostasis. 2014;111(4):625–633. doi: 10.1160/TH13-09-0730. [DOI] [PubMed] [Google Scholar]
- Farsalinos K., et al. Editorial: nicotine and SARS-CoV-2: COVID-19 may be a disease of the nicotinic cholinergic system. Toxic Rep. 2020;20:7500. doi: 10.1016/j.toxrep.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos K., et al. Editorial: nicotine and SARS-CoV-2: COVID-19 may be a disease of the nicotinic cholinergic system. Tox. Rep. 2020;20:7500. doi: 10.1016/j.toxrep.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firdous S.M., Varnita K., Zainab I. Hydroxychloroquine and azithromycin combination could be lethal to covid-19 patients. FARMACIA. 2020;68(3):384–389. doi: 10.31925/farmacia.2020.3.2. [DOI] [Google Scholar]
- Force Ardsdt, Ranieri V.M., et al. Acute respiratory distress syndrome: the Berlin definition. J. Am. Med. Assoc. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- Gaertner F., Massberg S. Blood coagulation in immunothrombosis-At the frontline of intravascular immunity. Semin. Immunol. 2016;28(6):561–569. doi: 10.1016/j.smim.2016.10.010. [DOI] [PubMed] [Google Scholar]
- Gattinoni L., et al. COVID-19 pneumonia: ARDS or not? Crit. Care. 2020;24:154. doi: 10.1186/s13054-020-02880-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiler J., et al. N-acetyl-L-cysteine (NAC) inhibits virus replication and expression of pro-inflammatory molecules in A549 cells infected with highly pathogenic H5N1 influenza A virus. Biochem. Pharmacol. 2010;79(3):413–420. doi: 10.1016/j.bcp.2009.08.025. [DOI] [PubMed] [Google Scholar]
- Ghezzi S., et al. Heparin prevents Zika virus induced-cytopathic effects in human neural progenitor cells. Antivir. Res. 2017;140:13–17. doi: 10.1016/j.antiviral.2016.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales J.N., Lucas R., Verin A.D. The acute respiratory distress syndrome: mechanisms and perspective therapeutic approaches. Austin J Vasc Med. 2015;2(1):1009. [PMC free article] [PubMed] [Google Scholar]
- Goumenou M., Sarigiannis D., Tsatsakis A., Anesti O., Docea, et al. COVID 19 in Northern Italy: an integrative overview of factors possibly influencing the sharp increase of the outbreak (Review) Mol. Med. Rep. 2020;20:20–32. doi: 10.3892/mmr.2020.11079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H., et al. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin. Chem. Lab. Med. 2020;58(7):1116–1120. doi: 10.1515/cclm-2020-0188. [DOI] [PubMed] [Google Scholar]
- Han H., et al. Prominent changes in blood coagulation of patients with SARSCoV-2 infection. Clin. Chem. Lab. Med. 2020;58(7):1116–1120. doi: 10.1515/cclm-2020-0188. [DOI] [PubMed] [Google Scholar]
- Hanify J.M., Dupree L.H., Johnson D.W., Ferreira J.A. Failure of chemical thromboprophylaxis in critically ill medical and surgical patients with sepsis. J. Crit. Care. 2017;37:206–210. doi: 10.1016/j.jcrc.2016.10.002. [DOI] [PubMed] [Google Scholar]
- Hardaway R.M. A brief overview of acute respiratory distress syndrome. World J. Surg. 2006;30:1829–1834. doi: 10.1007/s00268-006-0030-8. discussion 35. [DOI] [PubMed] [Google Scholar]
- Hardaway R.M., et al. Treatment of severe acute respiratory distress syndrome: a final report on a phase I study. Am. Surg. 2001;67(4):377–382. [PubMed] [Google Scholar]
- Hardaway R.M., et al. Treatment of severe acute respiratory distress syndrome: a final report on a phase I study. Am. Surg. 2001;67:377–382. [PubMed] [Google Scholar]
- Harrop H., Rider C. Heparin and its derivatives bind to HIV-1 recombinant envelope glycoproteins, rather than to recombinant HIV-1 receptor, CD4. Glycobiology. 1998;8(2):131–137. doi: 10.1093/glycob/8.2.131. [DOI] [PubMed] [Google Scholar]
- https://www.clinicaltrials.gov/https://clinicaltrials.gov/ct2/show/NCT04394377 Acessed in 20 May 2020.
- https://www.coronavirustoday.com/covid-19-hope-trial-open-use-medical-community
- Huang C., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. 10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iba T., et al. Heparins attenuated histone-mediated cytotoxicity in vitro and improved the survival in a rat model of histone-induced organ dysfunction. Intensive Care Med Exp. 2015;3(1):36. doi: 10.1186/s40635-015-0072-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idell S. Coagulation, fibrinolysis, and fibrin deposition in acute lung injury. Crit. Care Med. 2003;31(4 Suppl. l):S213–S220. doi: 10.1097/01.CCM.0000057846.21303.AB. [DOI] [PubMed] [Google Scholar]
- Kostoff R.N., et al. The under-reported role of toxic substance exposures in the COVID-19 pandemic. Food Chem. Toxicol. 2020;145:111687. doi: 10.1016/j.fct.2020.111687. [published online ahead of print, 2020 Aug 14] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laterre P.F., Wittebole X., Dhainaut J.F. Anticoagulant therapy in acute lung injury. Crit. Care Med. 2003;31(Suppl. 4):S329–S336. doi: 10.1097/01.CCM.0000057912.71499.A5. [DOI] [PubMed] [Google Scholar]
- Levi M., van der Poll T. Coagulation and sepsis. Thromb. Res. 2017;149:38–44. doi: 10.1016/j.thromres.2016.11.007. [DOI] [PubMed] [Google Scholar]
- Li J., Li Y., Yang B., Wang H., Li L. Low-molecular-weight heparin treatment for acute lung injury/acute respiratory distress syndrome: a meta-analysis of randomized controlled trials. Int. J. Clin. Exp. Med. 2018;11(2):414–422. [Google Scholar]
- Liu C., et al. Meta-analysis of preclinical studies of fibrinolytic therapy for acute lung injury. Front. Immunol. 2018;9:1898. doi: 10.3389/fimmu.2018.01898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Mu S., Li X., Liang Y., Wang L., Ma X. Unfractionated heparin alleviates sepsis-induced acute lung injury by protecting tight junctions. J. Surg. Res. 2019;238:175–185. doi: 10.1016/j.jss.2019.01.020. [DOI] [PubMed] [Google Scholar]
- Llitjos J.F., et al. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J. Thromb. Haemostasis. 2020;18:1743–1746. doi: 10.1111/jth.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Bai J. Protective effects of heparin on endothelial cells in sepsis. Int. J. Clin. Exp. Med. 2015;8(4):5547–5552. [PMC free article] [PubMed] [Google Scholar]
- MacLaren R., Stringer K.A. Emerging role of anticoagulants and fibrinolytics in the treatment of acute respiratory distress syndrome. Pharmacotherapy. 2007;27(6):860–873. doi: 10.1592/phco.27.6.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthay M.A., et al. The acute respiratory distress syndrome. J. Clin. Invest. 2012;122(8):2731–2740. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore H.B., et al. Is there a role for tissue plasminogen activator as a novel treatment for refractory COVID-19 associated acute respiratory distress syndrome? J Trauma Acute Care Surg. 2020;88(6):1–2. doi: 10.1097/TA.0000000000002694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore H.B., Barett C.D., et al. Is there a role for tissue plasminogen activator (tPA) as a novel treatment for refractory COVID-19 associated acute respiratory distress syndrome (ARDS)? J Trauma Acute Care Surg. 2020;88(6):713–714. doi: 10.1097/TA.0000000000002694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavi S., Moradi M., Khorshidahmad T., Motamedi M. Antiinflammatory effects of heparin and its derivatives: a systematic review. Adv Pharmacol Sci. 2015:507151. doi: 10.1155/2015/507151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mycroft-West C., et al. The 2019 coronavirus (SARS-CoV-2) surface protein (Spike) S1 Receptor Binding Domain undergoes conformational change upon heparin binding. 2020. https://www.biorxiv.org/content/10.1101/2020.02.29.97109.3v1.full
- Pantea Stoian A., Banerjee Y., Rizvi A.A., Rizzo M. Diabetes and the COVID-19 pandemic: how insights from recent experience might guide future management. Metab. Syndr. Relat. Disord. 2020;18(4):173–175. doi: 10.1089/met.2020.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Practical guidance for the prevention of thrombosis and management of coagulopathy and disseminated intravascular coagulation of patients infected with COVID-19, Thrombosis UK. 2020. https://thrombosisuk.org/covid-19-thrombosis.php Accessed on May 11, 2020.
- Schmitt F.C.F., Manolov V., Morgenstern J., et al. Acute fibrinolysis shutdown occurs early in septic shock and is associated with increased morbidity and mortality: results of an observational pilot study. Ann. Intensive Care. 2019;9(1):19. doi: 10.1186/s13613-019-0499-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz M.J., Haitsma J.J., Zhang H., Slutsky A.S. Pulmonary coagulopathy as a new target in therapeutic studies of acute lung injury or pneumonia – a review. Crit. Care Med. 2006;34(3):871–877. [PubMed] [Google Scholar]
- Shanghai Clinical Treatment Expert Group for Covid-19 Comprehensive treatment and management of coronavirus disease 2019: expert consensus statement from Shanghai (in Chinese) Chin J Infect Dis. 2020;38 doi: 10.3760/cma.j.issn.1000-6680.2020.0016. Epub ahead of print. [DOI] [Google Scholar]
- Shukla D., Spear P.G. Herpesviruses and heparan sulfate: an intimate relationship in aid of viral entry. J. Clin. Invest. 2001;108(4):503–510. doi: 10.1172/JCI13799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M., et al. The third international consensus definitions for sepsis and septic shock (Sepsis‐3) J. Am. Med. Assoc. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skidmore M., et al. Inhibition of influenza H5N1 invasion by modified heparin derivatives. MedChemComm. 2015;6(4):640–646. [Google Scholar]
- Stoian A.P., Catrinoiu D., Rizzo M., Ceriello A. Hydroxychloroquine, COVID‐19 and diabetes. Why it is a different story. Diabetes Metab Res Rev. 2020 doi: 10.1002/dmrr.3379. [published online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suceveanu A.I., et al. Assertion for montelukast in the covid-19 pandemics? FARMACIA. 2020;68(4):579–585. doi: 10.31925/farmacia.2020.4.1. [DOI] [Google Scholar]
- Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemostasis. 2020 doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N., et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemostasis. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thachil J., et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J. Thromb. Haemostasis. 2020;18:1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian S., et al. Pulmonary pathology of earlyphase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J. Thorac. Oncol. 2020;S1556–0864(20):30132–30135. doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsatsakis A., et al. COVID-19, an opportunity to reevaluate the correlation between long-term effects of anthropogenic pollutants on viral epidemic/pandemic events and prevalence. Food Chem. Toxicol. 2020;141:111418. doi: 10.1016/j.fct.2020.111418. https://doi: 10.1016/j.fct.2020.111418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicenzi E., et al. Coronaviridae and SARS-associated coronavirus strain HSR1. Emerg. Infect. Dis. 2004;10(3):413–418. doi: 10.3201/eid1003.030683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicenzi E., et al. Subverting the mechanisms of cell death: flavivirus manipulation of host cell responses to infection. Biochem. Soc. Trans. 2018;46(3):609–617. doi: 10.1042/BST20170399. [DOI] [PubMed] [Google Scholar]
- Vivarelli S., et al. Cancer management during COVID-19 pandemic: is immune checkpoint inhibitors-based immunotherapy harmful or beneficial? Cancers. 2020;12(8):2237. doi: 10.3390/cancers12082237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in wuhan, China. J. Am. Med. Assoc. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. http://doi:10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware L.B. Pathophysiology of acute lung injury and the acute respiratory distress syndrome. Semin. Respir. Crit. Care Med. 2006;27(4):337–349. doi: 10.1055/s-2006-948288. [DOI] [PubMed] [Google Scholar]
- Ware L.B., Matthay M.A. The acute respiratory distress syndrome. N. Engl. J. Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- Ware L.B., et al. Bench to bedside: targeting coagulation and fibrinolysis in acute lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2006;291(3):L307–L311. doi: 10.1152/ajplung.00157.2006. [DOI] [PubMed] [Google Scholar]
- Whyte C.S., et al. Fibrinolytic abnormalities in acute respiratory distress syndrome (ARDS) and versatility of thrombolytic drugs to treat COVID‐19. JThromb Haemost. 2020 doi: 10.1111/jth.14872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Dunn D., Spear P. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J. Virol. 1989;63(1):52–58. doi: 10.1128/jvi.63.1.52-58.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., et al. Extracellular histones are major mediators of death in sepsis. Nat. Med. 2009;15(11):1318–1321. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young E. The anti-inflammatory effects of heparin and related compounds. Thromb. Res. 2009;122(6):743–752. doi: 10.1016/j.thromres.2006.10.026. 2008. Li JP, Vlodavsky I. Heparin, heparan sulfate and heparanase in inflammatory reactions. Thromb Haemost. 102(5), 823-828. [DOI] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C., Liang Y., Li X., Chen N., Ma X. Unfractionated heparin attenuates histone-mediated cytotoxicity in vitro and prevents intestinal microcirculatory dysfunction in histone-infused rats. J Trauma Acute Care Surg. 2019;87(3):614–622. doi: 10.1097/TA.0000000000002387. [DOI] [PubMed] [Google Scholar]