Abstract
Background
Limited literature exists on Cerebrospinal fluid (CSF) findings in COVID-19 patients with neurological symptoms. In this review, we conducted a descriptive analysis of CSF findings in patients with COVID-19 to understand prognosis and explore therapeutic options.
Methods
We searched PubMed, Google Scholar, and Scopus databases using the keywords “SARS-CoV-2 in cerebrospinal fluid” and “SARS-CoV-2 and CNS Complications”" for reports of CSF findings in COVID-19 related neurological manifestations. Descriptive analyses were conducted to observe the CSF protein and cell counts based on age, gender, severity, fatality of COVID-19, and whether central (CNS) or peripheral nervous system (PNS) was associated.
Results
A total of 113 patients were identified from 67 studies. Of these, 7 patients (6.2%) were fatal COVID-19 cases and 35 patients (31%) were considered severe COVID-19 cases. CSF protein was elevated in 100% (7/7) of the fatal cases with an average of 61.28 mg/dl and in 65.0% (52/80) in non-fatal cases with an average 56.73 mg/dl. CSF protein levels were elevated in 74.5% (38/51) patients with non-severe COVID-19 and 68.6% (24/35) in those with a severe COVID-19 infection. CSF cell count was increased in 43% of fatal cases, 25.7% severe cases, and 29.4% of non-severe cases.
Conclusion
Our analysis showed that the most common CSF findings situation in COVID-19 infection is elevated protein with, very occasionally, mild lymphocyte predominant pleocytosis. Further studies to elucidate the pathophysiology of neurological complications in COVID-19 are recommended.
Abbreviations: nCov, NovelCoronavirus; COVID-19, Coronavirus infectious disease-2019; MERS, MiddleEast Respiratory Syndrome; SARS-CoV-2, Severe Acute Respiratory Distress Syndrome coronavirus 2; CSF, Cerebrospinal fluid; CNS, Central Nervous System; PNS, Peripheral Nervous System; NFL, Neurofilament Light Chain; RT-PCR, Reverse Transcription Polymerase Chain Reaction; GBS, Guillain-Barre Syndrome; IDSA/ATS, Infectious Diseases Society of America/American Thoracic Society
Keywords: COVID-19, SARS-CoV-2, Oligoclonal bands, CSF Protein, CSF cell count
Introduction
The novel Coronavirus disease 2019 (COVID-19) pandemic continues to present clinical challenges worldwide. Since the beginning of the pandemic, neurological manifestations have been vastly described in patients with COVID-19 (Li et al., 2020, Huang et al., 2020a, Munhoz et al., 2020).
In addition to specific central nervous system (CNS) and peripheral nervous system (PNS) symptoms, nonspecific neurological manifestations such as headache, myalgia, anosmia, hypogeusia, and myopathy have been reported in patients with COVID-19 (Iadecola et al., 2020). The spectrum of neurologic complications ranges from CNS involvement like meningitis/encephalitis, encephalopathy, and stroke to PNS involvement like Guillian-Barre Syndrome (GBS) (Moriguchi et al., 2020, Virhammar et al., 2020, Koralnik and Tyler, 2020, Helms et al., 2020a, Al Saiegh et al., 2020, Berger, 2020, de Melo Espíndola et al., 2020, Pfefferkorn et al., 2020, Toscano et al., 2020, Mozhdehipanah et al., 2020, Lascano et al., 2020). The severity of the disease varies based on the virus nervous tissue tropism. Positive nasal swab test for the virus has not been correlated with the presence or absence of the virus in the CSF in patients with neurologic manifestations. Therefore, CSF analysis via lumbar puncture (LP) should be considered in patients with confirmed COVID-19 in order to further understand the virus pathology (Moriguchi et al., 2020, de Melo Espíndola et al., 2020, Khodamoradi et al., 2020, Lu et al., 2020) In general, neurologists tend to avoid doing an invasive and expensive procedure such as LP in patients with non-specific COVID-19 neurological symptoms.
It is currently not clear whether the mechanism of injury is due to direct viral invasion into the CNS or inflammatory cytokine release induced by the virus leading to a cascade of immune cells within the CNS (Li et al., 2020, Wang et al., 2020, Xu et al., 2005, Baig et al., 2021, Chu et al., 2020). The virus is potentially neurotropic, spreading via the systemic circulation or across the cribriform plate (Baig et al., 2021, Wu et al., 2020). ACE-2 receptors on the capillary endothelium may be the target for the SARS-CoV-2 spike protein facilitating viral entry into the nervous system (Baig et al., 2021). Infection of the CNS can cause manifestations such as anosmia, dysgeusia, and, in more severe cases, encephalitis (Baig et al., 2021, Mao et al., 2020). In addition to direct viral invasion, the brain may be affected secondarily due to extensive endothelial dysfunction from cytokine storm that predisposes to arterial/venous thrombosis or hemorrhage and resultant stroke (Wu et al., 2020, Umapathi et al., 2004). Parainfectious complications secondary to dysregulated immunity may lead to antibody-mediated damage to the CNS or PNS leading to Bickerstaff’s encephalitis and GBS that was described in MERS-CoV. Prolonged ICU stay and mechanical ventilation may lead to critical care neuropathy/myopathy (Kim et al., 2017, Peeri et al., 2020).
Other coronaviruses that have been studied demonstrated a combination of invasion of the CNS, damage to the neurons, and a cascade of inflammatory cycle (Xu et al., 2005, Lau et al., 2004, Hung et al., 2003) SARS-CoV-1 in mice showed expression of human ACE2 which was also found in human brain autopsies of infected patients (Xu et al., 2005) The same receptor specifically found in neurons has also been implicated in the pathogenesis of SARS-CoV-2, emphasizing a possible invasion of the CNS (Baig et al., 2021)
A review by Espíndola, Otávio de Melo et al. found a total of seven cases of autoimmune encephalitis and meningoencephalitis which underwent CSF analysis. Interestingly, only one case had positive CSF RT-PCR for COVID-19, while the rest had negative CSF RT-PCR. Elevated CSF protein, lactate, or WBCs with a negative COVID-19 PCR may indicate inflammatory changes seen in autoimmune encephalitis (de Melo Espíndola et al., 2020). A recent correspondence by Lucchese G. highlights that many recent systematic reviews of CSF analysis in patients with COVID-19 resulted in negative CSF RT-PCR, most likely due to autoimmunity (Lucchese, 2020). The high frequency of anti-neuronal and anti-glial antibodies is remarkable. However, larger cohorts are needed to establish an understanding of these connections.
CSF analysis can be of utmost importance in patients with confirmed COVID-19 for further understanding virus pathology and for ruling out other infectious or inflammatory pathologies. We performed a literature review of CSF analysis in COVID-19 patients with neurological manifestations to understand if there is a common pattern of CSF findings. We tried to elucidate if there is a difference in pattern of CSF findings when looking at CNS versus PNS manifestations. We also explored the differences in CSF findings based on demographic characteristics and severity of COVID-19 to prognosticate patients with neurological complications.
Methods
Study Design
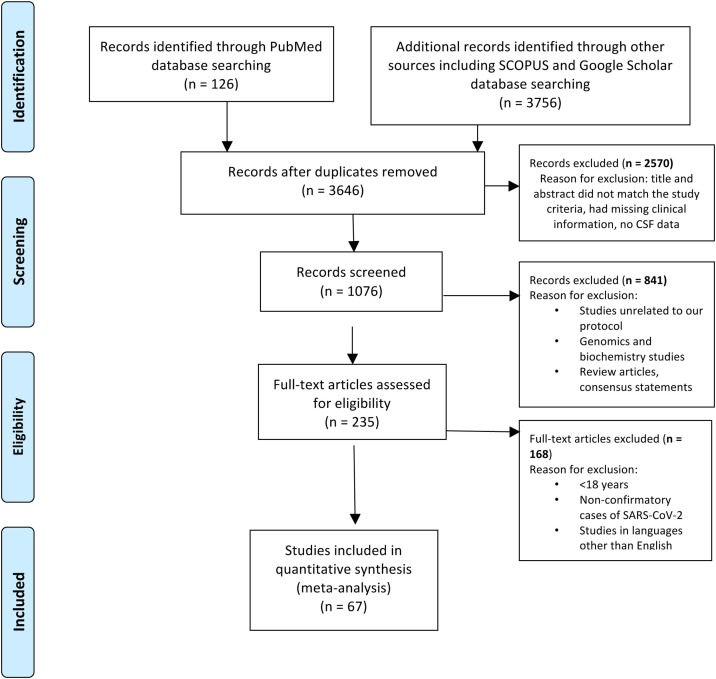
We conducted a thorough literature review in August 2020 using the terms “SARS-CoV-2 in cerebrospinal fluid” and “SARS-CoV-2 and CNS Complication”. We searched PubMed, Google Scholar, and Scopus databases for identifying case series and case reports published between December 01, 2019, to September 15, 2020 (Figure 1 ). Review articles and consensus statements were excluded from the analysis. We used the preferred reporting items for systematic reviews and meta-analyses (PRISMA) for relevant inclusions and exclusions (Moher et al., 2021). Based on our search criteria, we found 126 articles from PubMed, 3646 articles from Google Scholar, and 110 articles from Scopus. Of the final pool of studies, 236 were identified as duplicates and were thus excluded. Finally, we screened 1076 articles for title and abstracts and reviewed full-text literatures in accordance with our study objective after removing 2570 articles which were either missing clinical information, CSF data, or did not meet our study objective. We included 67 articles for review for observational analysis (Figure 1) based on data availability.
Figure 1.
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram showing criteria for article selection.
n = number of articles.
Inclusion criteria
The inclusion criteria for the published studies comprised 1) Patient age ≥18 years; 2) COVID-19 diagnosis confirmed by RT-PCR nasopharyngeal or serum IgG antibody test; and 3) CSF study findings available in COVID-19.
Exclusion criteria
The exclusion criteria for the studies comprised 1) Duplicate studies which involved repetition of cases; 2) Articles in languages other than English; 3) Studies that had no available individual patient’s data; and 4) Editorials.
Quality assessment
The critical appraisal checklist for case reports provided by the Joanna Briggs Institute (JBI) was used to perform assessment of overall quality of case series and case reports (Joanna Briggs, 2019)
Data acquisition
From the selected articles, we extracted the following data for our analysis: study type, date of publication, age, gender, clinical presentation of COVID-19, diagnostic tests for SARS-CoV-2 infection including RT-PCR nasopharyngeal and serum antibodies, and CSF markers including cell count, lymphocytes percentage, protein, IL-6, and severity of COVID-19 (based on IDSA/ATS criteria) (Metlay et al., 2019)
Data Analysis
We reported demographics including age and gender, severity of COVID-19 cases, and fatality of the cases. Pooled descriptive analyses were conducted to assess differences in CSF protein and cell count markers based on demographic characteristics, COVID-19 severity, fatality, and whether CNS or PNS.
Results
A total of 67 articles describing 113 patients were examined. There were 57 males and 29 females; sex was not reported for 27 patients. The mean age for the patients was 57.0 (SD = 14.3). The baseline characteristics for the patients are shown in Table 1 . Serum COVID-19 IgG Antibody test was reported for all patients except eight and was positive for all except one. CSF RT-PCR for IgM and IgG antibodies were reported for 78 patients and was positive for only 12 patients. One patient with a negative CSF-RT-PCR and negative nasopharyngeal swab RT-PCR showed positive IgM and IgG CSF COVID-19 antibodies. Aside from this, two other patients showed positive IgG antibodies against COVID-19 in their CSF. The following inflammatory markers for CSF were considered—CSF cell count, CSF proteins, and elevated CSF lymphocytes percentage. Cell count >5 cells/μL and CSF proteins >45 mg/dl were considered elevated. We considered patients only with elevated cell counts for analysis of lymphocytes percentage.
Table 1.
General characteristics of SARS-CoV-2 (n = 113) patients with CSF study and neurological manifestation.
| Characteristics | N (%) |
|---|---|
| Mean Age in years, SD | 57 (SD = 14.3) |
| *Gender | |
| Male | 57 (50.40) |
| Female | 29 (25.66) |
| Neurological manifestation | |
| GBS and its variants | 55 (48.67) |
| Encephalopathy | 14 (12.39) |
| Meningoencephalitis | 11 (9.73) |
| Seizure | 11 (9.73) |
| Ischemic stroke | 5 (4.42) |
| Hemorrhagic stroke | 3 (2.65) |
| CNS demyelinating disorder | 3 (2.65) |
| Cranial nerve enhancement | 3 (2.65) |
| **Others | 8 (7.08) |
| •Severity of COVID-19 | |
| Severe | 35 (30.97) |
| Non-Severe | 78 (69.03) |
| Outcomes | |
| Fatal | 7 (6.19) |
| Non-fatal | 106 (93.81) |
* 27 cases gender not available
** Other includes neuropsychiatric manifestations and delirium
• Severity based on Infectious Disease Society of America/American Thoracic Society
IDSA/ATS criteria
Based on IDSA/ATS criteria of either requiring vasopressor for septic shock or mechanical ventilation, 35 patients were considered to have a severe COVID infection. In our observational study, there were seven fatal cases. Elevated cell counts (>5 cells/μL) were found in 43% (3/7) of the fatal cases, 25.7% (9/35) of severe cases, and 29.4% (15/51) of non-severe cases. Of the patients who had a CNS neurological symptom, 43.2% (19/44) had elevated cell counts while 16.7% (9/54) patients with PNS symptoms had elevated cell counts. CSF protein was elevated in 100% (7/7) of the fatal cases, with an average of 61.28 mg/dl, and in 65.0% (52/80) in non-fatal cases, with an average 56.73 mg/dl. CSF protein levels were elevated in 74.5% (38/51) patients with non-severe COVID-19 and 68.6% (24/35) of those with a severe COVID-19 infection. In addition, 59.1% (26/44) of the patients with CNS complications and 77.8% (42/54) of the patients with a PNS manifestation showed elevated protein levels (refer Table 2 ).
Table 2.
Demographic characteristics of 104 patients for CSF observational study on COVID-19-associated neurological complications∗.
| CSF high protein** | CSF normal protein | CSF Elevated cell count *** | CSF normal cell count | |
|---|---|---|---|---|
| Age(year)mean ± SD | 56.9 ± 15.0 | 57.3 ± 12.6 | 54.6 ± 17.9 | 58.0 ± 12.6 |
| Gender (%)Female | 21 (33.9) | 8 (33.3) | 10 (41.7) | 19 (30.7) |
| Male | 41 (66.1) | 16 (66.7) | 14 (58.3) | 43 (69.4) |
| COVID-19 Severity | ||||
| Non-Severe | 38 (74.5) | 13 (25.5) | 15 (29.4) | 36 (70.6) |
|
Severe Fatality |
24 (68.6) | 11 (31.4) | 9 (25.7) | 26 (74.3) |
| Non-fatal | 52 (65.0) | 28 (35.0) | 23 (28.7) | 57 (71.3) |
| Fatal | 7 (100.0) | 0 (0.0) | 3 (42.9) | 4 (57.1) |
| Outcome | ||||
| CNS Manifestationa | 26 (59.1) | 18 (40.9) | 19 (43.2) | 25 (56.8) |
| PNS Manifestationb | 42 (77.8) | 12 (22.2) | 9 (16.7) | 45 (83.3) |
∗Severity was not reported in 27 cases, fatality was not reported in 26 cases, CNS status or PNS status was not reported in 15 cases
**Cerebrospinal fluid
**Cerebrospinal fluid cell count
Central nervous system
Peripheral nervous system
Cell count in CSF analysis was reported in 97 patients. We only considered the lymphocyte percentage in patients who had a cell count of >5 cells/μL. The value was reported in nine patients. Only one patient reported lymphocyte percentage of 10%, whereas the rest of the eight patients had lymphocyte percentage >80%. Out of these eight patients, six had CNS manifestations, one had PNS manifestations, and one patient did not report any manifestation. All except for one case were non-severe, and none of the eight cases where the outcome was reported were fatal.
The other CSF marker that we studied for the purpose of this study was IL-6 level. This was reported in five patients. It was found to be elevated in two non-severe cases and three severe cases. Only one out of five fatal cases showed elevated IL-6 levels, and three out of five non-fatal cases reported this finding. None of the cases with PNS manifestation had elevated IL-6 in their CSF, while all five cases reported elevated IL-6 with CNS manifestation.
Discussion
The novel coronavirus has been associated with predominant respiratory symptoms which have proved to be fatal in nature. Several studies describing the neurological manifestations of the disease have been published recently showing its potential to invade the PNS as well as the CNS and manifest as isolated syndromes (Helms et al., 2020a, Berger, 2020) Moriguchi et al. reported a first case of meningitis associated with SARS-CoV-2. This case put forward the neuroinvasive nature of the SARS-CoV-2 as the RT-PCR test using the nasopharyngeal swab was negative but the disease was detected in the CSF (Moriguchi et al., 2020). However, there are only limited data available that can help us point to a specific biomarker for the CNS manifestations of this virus. This may be due to the risk of cross-infection from invasive procedure and the risk of complications including bleeding as many patients are on anticoagulant therapy. The technique could be also technically challenging due to the severity of underlying illness of COVID-19 patients. However, CSF analysis can be a very useful indicator of neurological involvement. CSF analysis can be considered as a venue to look for biomarkers and inflammatory markers which may be of diagnostic or prognostic use.
Of the limited available studies that include CSF analysis as an investigation for patients with neurological manifestation, most CSF analyses showed no presence of SARS-CoV-2 RNA (Helms et al., 2020a, Pfefferkorn et al., 2020, Scheidl et al., 2020, Yin et al., 2020). However, there was a small fraction of case reports and studies that showed its presence in CSF RT-PCR (Moriguchi et al., 2020, Virhammar et al., 2020, Khodamoradi et al., 2020, Lau et al., 2004, Domingues et al., 2020, Helms et al., 2020b, Fadakar et al., 2020, Sun and Guan, 2020, Huang et al., 2020b, Westhoff et al., 2020, Mardani et al., 2020, Cebrián et al., 2020) but were either absent or not available in nasal swab RT-PCR (Moriguchi et al., 2020, Khodamoradi et al., 2020, Lau et al., 2004, Domingues et al., 2020, Sun and Guan, 2020). Only a few studies showed the concurrent presence of the virus in nasal swabs as well as CSF RT-PCR (Virhammar et al., 2020, Helms et al., 2020b, Fadakar et al., 2020, Huang et al., 2020b, Westhoff et al., 2020, Mardani et al., 2020, Cebrián et al., 2020)
In our study, we reported four cases in which CSF IGM was positive (Wang et al., 2020, Benameur et al., 2020) whereas Wang et al. reported a case where both IgG and IgM antibodies against COVID -19 were positive (Wang et al., 2020). In addition, CSF IGG was positive for COVID-19 in 2 cases (Virhammar et al., 2020, Lu et al., 2020) A study by Karima et al. reported the presence of IgM in CSF analysis of these patients with elevated cell count and protein content in only one patient. They concluded that a negative CSF RT-PCR does not rule out CSF neuroinvasion by the virus in these patients and recommended CSF analysis in such patients (Benameur et al., 2020). However, the antibody COVID tests performed were performed solely for research purposes and were not available for commercial use at this time. Guilmot et al. reported cases of limbic encephalitis and a patient with parainfectious polyradiculitis. Six cases had equal numbers of oligoclonal bands in CSF and serum, whereas one patient had a CSF-specific antibody. One case had positive Anti-caspr2 positive in CSF (Guilmot et al., 2020). Franke et al. reported the presence of several antibodies such as anti-neuronal antibodies, anti Yo antibodies, anti-myelin antibodies, and anti-NMDAR antibodies. The presence of these antibodies in the CSF hints at the multi-organ involvement of those infected with the novel coronavirus and may prove to play a significant role in creating immunotherapeutic guidelines for these patients in the future (Franke et al., 2020) (refer Table 3).
Table 3.
CSF Findings of COVID-19 and related Neurological manifestation (for published case reports and case series).
| Author/Country All studies from year/year published 2020 | No. of patients included in the study | Neurological manifestation CNS = 1, PNS = 2 | CSF findings | Severity of COVID-19∗ (Non-severe = 1, Severe = 2) | Outcome (Non-fatal = 1, fatal = 2) |
|---|---|---|---|---|---|
| Toscano et al. Italy 2020 | 5 | Patients 1 to 5 = 2 | Patient 1 to 3: High protein, Albuminocytological dissociation Patient 4 to 5: Normal CSF findings | Patient 1 = 2 Patient 2 = 1 Patient 3 = 2 Patient 4 = 1 Patient 5 = 2 | Patient 1 = 1 Patient 2 = 1 Patient 3 = 2 Patient 4 = 1 Patient 5 = 1 |
| Paybast, Sepideh Iran 2020 | 3 | Patients 1 to 3 = 2 | Patient 1 to 3: High protein, Albuminocytological dissociation | Patient 1 = N/A Patient 2 = 2 Patient 3 = 1 | Patient 1 = 1Patient 2 = 2 Patient 3 = N/A |
| Agustina M. Lascano Switzerland 2020 | 3 | Patients 1 to 3 = 2 | Patient 1 and 3: High protein, Albuminocytological dissociation Patient 2: Normal CSF findings | Patient 1 = 1 Patient 2 = 1 Patient 3 = 2 | Patient 1 = 1Patient 2 = 1Patient 3 = 1 |
| Otávio de Melo Espíndola Brazil 2020 | 8 | Patient 1 = 1 Patient 2 = 1Patient 3 = 1Patient 4 = 2Patient 5 = N/A Patient 6 = N/A Patient 7 = 1Patient 8 = 2 | Patient 1=High Protein, Low Sugar, Cell = 18 (100% Lymphocytes) Patient 2 = Normal Patient 3 = Normal Patient 4 = Normal Patient 5=High Protein, Cells = 3 (100% Lymphocytes) Patient 6= High Protein, Cells = 3 (100% Lymphocytes) Patient 7 = Normal Patient 8 = Normal | Patient 1 = 1 Patient 2 = 1Patient 3 = 2Patient 4 = 1Patient 5 = 1 Patient 6 = 2Patient 7 = 1Patient 8 = 1 | Patient 1 = 1 Patient 2 = 1 Patient 3 = 2 Patient 4 = 1 Patient 5 = 1 Patient 6 = 2 Patient 7 = 1 Patient 8 = 1 |
| Karima Benameur USA 2020 | 3 | Patient 1 to 3 = 1 | Patient 1= High opening pressure; High Protein; Low Sugar; Cells = 115; Lympho = 10%, Macrophages = 39%, Neutro = 51%; IL-6,8,10: elevated; Anti-S1 IgM, Anti-E IgM Positive Patient 2= High opening pressure; IL-8, 10: elevated; Anti-S1 IgM Positive Patient 3= IL-8, 10: elevated; Anti-S1 IgM Positive | Patient 1 = 2Patient 2 = 2 Patient 3 = 2 | Patient 1 = 2 Patient 2 = N/A Patient 3 = N/A |
| Antoine Guilmot Belgium 2020 | 15 | Patient 1 = 2Patient 2 = 2Patient 3 = 1Patient 4 = 1Patient 5 = N/A Patient 6 = N/A Patient 7 = N/A Patient 8 = N/A Patient 9 = N/A Patient 10 = N/A Patient 11 = N/A Patient 12 = N/A Patient 13 = 1 Patient 14 = 1 Patient 15 = 1 | Patient 1= High Protein; Cells = 101; Lympho = 95%; anti-GD1b IgG Positive Patient 2 = Normal Patient 3=elevated Qalb Patient 4=elevated Qalb Patient 5= High Protein; Cells = 9; Lympho = 100%; Anti-Caspr2, anti-GD1b IgG Positive Patient 6= High Protein; elevated Qalb Patient 7= Normal Patient 8= Normal Patient 9= elevated Qalb Patient 10= Normal Patient 11= Normal Patient 12= Normal Patient 13= High Protein Patient 14= High Protein Patient 15= Normal | Patient 1 = 1Patient 2 = 1Patient 3 = 2 Patient 4 = 2Patient 5 = 1Patient 6 = 2Patient 7 = 1 Patient 8 = 1Patient 9 = 2 Patient 10 = 2 Patient 11 = 2 Patient 12 = 2 Patient 13 = 2 Patient 14 = 1 Patient 15 = 2 | Patient 1 = 1Patient 2 = 1Patient 3 = 1Patient 4 = 1Patient 5 = 1 Patient 6 = 1 Patient 7 = 1 Patient 8 = 1 Patient 9 = 1 Patient 10 = 1 Patient 11 = 2 Patient 12 = 1 Patient 13 = N/APatient 14 = N/A Patient 15 = N/A |
| Christiana Franke, MD Germany 2020 | 11 | Patient 1 = 1Patient 2 = 1Patient 3 = 2 Patient 4 = N/A Patient 5 = 1Patient 6 = 1 Patient 7 = 1 Patient 8 = 1 Patient 9 = 1 Patient 10 = N/A Patient 11 = 1 | Patient 1= High Protein; Cells = 8; OCBs; High NfL; High lactate Patient 2= OCBs; High NfL; High lactate Patient 3= High lactate; OCBs Patient 4= High lactate; OCBs Patient 5= High Protein; Cells = 117; High lactate; OCBs; High NfL Patient 6= High lactate Patient 7= High lactate Patient 8= High Protein; High lactate; OCBs; High NfL Patient 9= High Protein; High lactate Patient 10= High lactate Patient 11= High lactate | N/A | Patient 1 to 11 = 1 |
| Duong L, Xu P, Liu A USA April 2020 | 1 | 1 | Cells high 100% lymphocytes, Protein high, Glucose normal | 1 | 1 |
| Moriguchi T, Harii N, Goto J, et al Japan 2020 | 1 | 1 | Cells high 90% mono. | 2 | 1 |
| Xiang P. Et al China 2020 | 1 | 1 | N/A | N/A | N/A |
| Huang YH, Et al USA 2020 | 1 | 1 | SARS Positive | N/A | 1 |
| Färber K Et al Germany 2020 | 1 | 1 | SARS-CoV-2 positive | N/A | 1 |
| Kremer S France 2020 | 37 31 HAS LP | 1 | Only one positive for SARS-COV-2 21= HIGH PTN, CELLS, | N/A | N/A |
| Domingues RB BRAZIL 2020 | 1 | 2 | SARS COV-2 POSITIVE Normal protein, cells, and glucose | 1 | 1 |
| Westhoff TH, et al Germany 2020 | 1 | 1 | Cells normal, protein high normal glucose high oligoclonal IgG SARS‐CoV‐2 RNA positive | 1 | 1 |
| Mardani M Et al Iran 2020 | 1 | 1 | Cells high 90% polymorphs, glucose low, High protein, Negative PCR | 2 | N/A |
| Fadakar N Et al Iran 2020 | 1 | 1 | Cells high mainly lymphocytic High protein SARS-CoV-2 RNA positive | 1 | 1 |
| Helms J, Kremer S, France 2020 | 58 | 1 | Cells high mainly lymphocytic High protein SARS-CoV-2 RNA positive | 1 | 1 |
| Cebrián J Et al spain 2020 | 1 | 1 | OCB = 2 Positive High protein = 1 Low albumin = 4Negative SARS = 7 | N/A | N/A |
| Cebrián J Et al spain 2020 | 1 | 1 | Normal csf | 1 | 1 |
| Virhammar J Et al Sweden 2020 | 1 | 1 | the cell normal, IgG high, protein normal negative autoantibodies negative PCR for HSV, VZV, and SARS-CoV-2 | 2 | 1 |
| Khodamoradi Z, Iran 2020 | 1 | 1 | Cells high, mononuclear 100% Protein normal Glucose normal COVID 19 positive | 1 | 1 |
| Yin R, Feng W, China 2020 | 1 | 1 | Opening pressure high Cells high Protein high Glucose normal | 1 | 1 |
| Destras G, Bal A, Escuret France 2020 | 578 | N/A | Only 2 COVID-19 positive | N/A | N/A |
| Espíndola OM, Siqueira M Brazil 2020 | 8 | Patient 1 = 1 Patient 2 = 1 Patient 3 = 1 Patient 4 = 1 Patient 5 = 2Patient 6 = 2Patient 7 = 1 Patient 8 = 1 | Patient 1= Cells high, protein high Patient 2= normal Patient 3= normal Patient 4= normal Patient 5= normal Patient 6= Only protein high Patient 7= Normal Patient 8= Normal | N/A | N/A |
| Wang M, Li T, Qiao F China 2020 | 1 | 1 | Cells no, protein high, IgM and IgG high PCR negative | 1 | 1 |
| Lu S, Wei N, Jiang J, et al. China 2020 | 1 | 1 | Cells high Protein normal IgG POSITIVE PCR negative | 1 | 1 |
CNS manifestation includes -stroke (ischemic, hemorrhagic, dural sinus venous thrombosis, meningoencephalitis, encephalitis, encephalopathy, seizure, ADEM, acute hemorrhagic necrotizing, CNS vasculitis, transverse myelitis.
PNS manifestation includes-GBS, polyneuritis cranialis, facial nerve palsy
∗Severity based on Infectious Diseases Society of America/ American Thoracic Society
(IDSA/ATS) guidelines
Legend: CNS, Central Nervous system, CSF; CSF, Cerebrospinal fluid; PNS, Peripheral Nervous System; GBS, Guillain-Barre Syndrome; ADEM, Acute Disseminated Encephalomyelitis
In this systematic review, we describe findings of CSF analysis in COVID-19 patients with neurological manifestations. Our analysis reveals that the most common CSF situation in COVID-19 infection is elevated protein with very occasionally mild lymphocyte predominant pleocytosis. Interestingly, based on our analysis, all fatal cases had elevations of CSF protein. However, elevated CSF protein was also noted in almost three-quarters of patients with both severe and non-severe presentations. Similar findings were also noted in cell counts, and one-fourth of patients with both severe and non-severe presentation had elevation in cell count. We found that 55 patients (55/113, 48.6%) (please refer Table 1) included in our review had GBS associated with COVID-19 infection, and all of them had elevated protein which helps to understand the etiology behind the observed elevated protein levels due to albumino-cytologic disproportion secondary to demyelination. The AIDP variant of GBS is one of the most common PNS manifestation of COVID-19 according to recent studies and can thus point toward the observed elevated protein level in our study. A recent study by Sriwastava et al. highlighted that the most common PNS manifestation associated with COVID-19 is GBS and its variants (Sriwastava et al., 2020).
Antoine Guilmot et al. reported CSF findings of 15 COVID-19 cases with neurological manifestations, among which, 6 had equal number of oligoclonal bands in CSF and serum, while one case had a specific elevated oligoclonal band with neuropsychiatric manifestation with COVID-19. Among the 15 cases, 5 had elevated CSF protein, out of which 2 had neuropsychiatric manifestation, 2 had cranial neuropathy, and 1 had acute cerebrovascular disease (Guilmot et al., 2020). Virhammar J. et al. reported a case of acute necrotizing encephalopathy that who had elevated protein and elevated oligoclonal bands (Virhammar et al., 2020). Christian Franke et al. found that CSF protein was elevated in 4 of their 11 reported cases and oligoclonal bands were detected in 6 out of 9 patients. The diagnosis of these patient varied with neurological manifestation ranging from down beating nystagmus to myoclonus (Franke et al., 2020). IgG Index details were not available in any of these cases (Refer Table 3).
As the elevated protein is noted in all spectra of neurological manifestations including fatal, it cannot be used as a marker of prognosis. However, the similarity of the CSF situation indicates that there might be only one common neuro-inflammatory pathophysiology responsible for the neurological manifestations of COVID-19 especially when the CSF findings are similar between CNS and PNS manifestations. Because of limited therapeutic options in COVID-19, CSF markers and protein levels could be used as a future guiding marker for treatment initiation with a higher dose of steroids or another immunotherapy.
A recent study of 5 patients with persistent encephalopathy showed significant improvement with high dose steroids, but their CSF findings did not show elevated protein or cell count (Pugin et al., 2020). The trends noted in our observational study suggest that presence of SARS-CoV-2 in the CSF is highly variable and may depend on the turnover of the virus and the level of neuroinvasion. CSF IgM testing has shown variable results and cannot be relied upon as the sole marker for SARS-CoV-2 presence.
In our entire cohort, we found a total of 17 cases in which MRI imaging of brain and cranial nerves was reported. Among them, 6 (35%) had abnormal findings that were inclusive of cranial nerve CN III, CN VI, and CN VII enhancement (Toscano et al., 2020, Bigaut et al., 2020, Chan et al., 2020, Lantos et al., 2020, Dinkin et al., 2020, Hutchins et al., 2020). Apart from these, one case of leptomeningeal enhancement of brainstem and cervical spine was noted (Bigaut et al., 2020). Moreover, MRI of the lumbar spine was also performed in 36% of the cases (18/50), out of which 5 (27%) were found to have abnormal lumbar spine nerve root enhancement and the remaining 73% (13/18) had normal spine imaging (Pfefferkorn et al., 2020, Toscano et al., 2020, Lascano et al., 2020, Bigaut et al., 2020).
Our study had several limitations. First, the CSF analysis reported in the studies can be highly variable due to different timing of lumbar puncture from timing of infection or positive serum test. As this is a systematic review of all case reports and case review studies, there is likely a presence of differences in demographics data, treatment methods, and management protocols of patients from different studies. Second, in order to account for smaller sample size and avoid spurious associations with a limited literature on the emerging topic of COVID-19, we decided to avoid reporting p-values. Given the heterogeneity of samples and the cases coming from different sources, reporting a p-value would be erroneous as there would be a lack on internal validity in the merged group of subjects from different sources. Third, we only included studies reported in English language which might have led to interesting CSF findings having been missed. Our study might also suffer from a final limitation of lack of search from EMBASE and other databases. Despite these limitations, we believe the data considered in our study are truly representative of CSF findings in COVID-19 patients and help provide a more comprehensive picture of this understudied topic.
Conclusion
More severe and fatal cases with neurological complications were associated with elevated protein as compared to non-severe and nonfatal cases, which can be a marker for CNS involvement. In patients with COVID-19 with neurological manifestations, a combination of SARS-CoV-2 serum and CSF RT-PCR, CSF IgM testing, and specific CNS targeting antibodies can be used together to guide diagnosis, management, and outcomes. The most common finding of CSF analysis was elevated CSF protein with occasionally elevated, predominantly lymphocytic cell count. CSF protein was elevated across all severities of neurological illness. This may be indicative of only one common pathophysiological mechanism of neurological illness. However, SARS-CoV-2 CSF RT-PCR, CSF IgM testing, and CNS-targeting antibodies should be further studied to understand the pathophysiology of neurological complications in COVID-19.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships. The authors have no conflict of interest.
Funding
None.
Ethics approval and consent to participate
Not applicable
Author contribution statement
Drafting the manuscript: Medha Tandon, Saurabh Kataria, Jenil Patel, Tejas R Mehta, Maha Daimee, Shitiz Sriwastava
Data abstraction and data analysis: Medha Tandon, Saurabh Kataria, Jenil Patel, Viral Patel, Anisa Anila Chowdhary, Shruti Jaiswal
Editing and Final Draft: Shitiz Sriwastava
CRediT authorship contribution statement
Medha Tandon: Conceptualization. Saurabh Kataria: Conceptualization. Jenil Patel: Conceptualization. Tejas R Mehta: Conceptualization. Maha Daimee: . Viral Patel: . Apoorv Prasad: Conceptualization. Anisa Anila Chowdhary: . Shruti Jaiswal: . Shitiz Sriwastava: Conceptualization.
Acknowledgments
We would like to thank Riddhi Patel MBBS, MPH, PhD(c) (UTHealth School of Public Health, Houston, TX) for providing assistance in the study.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ijid.2021.01.002.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Al Saiegh F. Status of SARS-CoV-2 in cerebrospinal fluid of patients with COVID-19 and stroke. J Neurol Neurosurg Psychiatry. 2020 doi: 10.1136/jnnp-2020-323522. [DOI] [PubMed] [Google Scholar]
- Baig A.A.-O.X. 2020. Evidence of the COVID-19 Virus Targeting the CNS: Tissue Distribution, Host-Virus Interaction, and Proposed Neurotropic Mechanisms. 1948-7193 (Electronic) [DOI] [PubMed] [Google Scholar]
- Benameur K. 2020. Early Release-Encephalopathy and Encephalitis Associated with Cerebrospinal Fluid Cytokine Alterations and Coronavirus Disease, Atlanta, Georgia, USA, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger J.R. COVID-19 and the nervous system. J Neurovirol. 2020:1. doi: 10.1007/s13365-020-00840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigaut K. Guillain-Barré syndrome related to SARS-CoV-2 infection. Neurol Neuroimmunol Neuroinflamm. 2020;7(5) doi: 10.1212/NXI.0000000000000785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebrián J. Headache and impaired consciousness level associated with SARS-CoV-2 in CSF: a case report. Neurology. 2020;95(6):266–268. doi: 10.1212/WNL.0000000000010213. [DOI] [PubMed] [Google Scholar]
- Chan J.L., Ebadi H., Sarna J.R. Guillain-Barré syndrome with facial diplegia related to SARS-CoV-2 infection. Can J Neurolog Sci. 2020:1–3. doi: 10.1017/cjn.2020.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu H. Comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19: an observational study. Lancet Microbe. 2020 doi: 10.1016/S2666-5247(20)30004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Melo Espíndola O. Patients with COVID-19 and neurological manifestations show undetectable SARS-CoV-2 RNA levels in the cerebrospinal fluid. Int J Infecti Dis. 2020 doi: 10.1016/j.ijid.2020.05.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkin M. COVID-19 presenting with ophthalmoparesis from cranial nerve palsy. Neurology. 2020 doi: 10.1212/WNL.0000000000009700. [DOI] [PubMed] [Google Scholar]
- Domingues R.B. First case of SARS-COV-2 sequencing in cerebrospinal fluid of a patient with suspected demyelinating disease. J Neurol. 2020:1–3. doi: 10.1007/s00415-020-09996-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadakar N. A First Case of Acute Cerebellitis Associated with Coronavirus Disease (COVID-19): a Case Report and Literature Review. Cerebellum. 2020:1–4. doi: 10.1007/s12311-020-01177-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke C. High frequency of cerebrospinal fluid autoantibodies in COVID-19 patients with neurological symptoms. medRxiv. 2020 doi: 10.1016/j.bbi.2020.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilmot A. Immune-mediated neurological syndromes in SARS-CoV-2-infected patients. J Neurol. 2020:1–7. doi: 10.1007/s00415-020-10108-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms J. Neurologic Features in Severe SARS-CoV-2 Infection. N Engl J Med. 2020 doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms J. Delirium and encephalopathy in severe COVID-19: a cohort analysis of ICU patients. Crit Care. 2020;24(1):1–11. doi: 10.1186/s13054-020-03200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. 2020. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.H., Jiang D., Huang J.T. A Case of COVID-19 Encephalitis. Brain Behav Immun. 2020 doi: 10.1016/j.bbi.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung E.C.W. Detection of SARS coronavirus RNA in the cerebrospinal fluid of a patient with severe acute respiratory syndrome. Clin Chem. 2003;49(12):2108. doi: 10.1373/clinchem.2003.025437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchins K.L. COVID-19–Associated Bifacial Weakness with Paresthesia Subtype of Guillain-Barré Syndrome. Am J Neuroradiol. 2020 doi: 10.3174/ajnr.A6654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C., Anrather J., Kamel H. Effects of COVID-19 on the nervous system. Cell. 2020 doi: 10.1016/j.cell.2020.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joanna Briggs I. 2019. The Joanna Briggs Institute critical appraisal tools for use in JBI systematic review: checklists for case reports. [Google Scholar]
- Khodamoradi Z. COVID‐19 meningitis without pulmonary involvement with positive cerebrospinal fluid PCR. Eur J Neurol. 2020 doi: 10.1111/ene.14536. [DOI] [PubMed] [Google Scholar]
- Kim J.E. Neurological Complications during Treatment of Middle East Respiratory Syndrome. J Clin Neurol. 2017;13(3):227–233. doi: 10.3988/jcn.2017.13.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koralnik I.J., Tyler K.L. COVID‐19: a global threat to the nervous system. Ann Neurol. 2020 doi: 10.1002/ana.25807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantos J.E., Strauss S.B., Lin E. COVID-19–Associated Miller Fisher Syndrome: MRI Findings. Am J Neuroradiol. 2020 doi: 10.3174/ajnr.A6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lascano A.M. SARS‐CoV‐2 and Guillain‐Barré syndrome: AIDP variant with favorable outcome. Eur J Neurol. 2020 doi: 10.1111/ene.14368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau K.-K. Possible central nervous system infection by SARS coronavirus. Emerg Infect Dis. 2004;10(2):342. doi: 10.3201/eid1002.030638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.C., Bai W.Z., Hashikawa T. The neuroinvasive potential of SARS‐CoV2 may play a role in the respiratory failure of COVID‐19 patients. J Med Virol. 2020;92(6):552–555. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S. First report of manic-like symptoms in a COVID-19 patient with no previous history of a psychiatric disorder. J Affect Disord. 2020;277:337–340. doi: 10.1016/j.jad.2020.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchese G. 2020. Cerebrospinal fluid findings in COVID-19 indicate autoimmunity. 2666-5247 (Electronic) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L., L Wang M., Chen S. Neurological manifestations of hospitalised patients with COVID-19 in Wuhan, China: a retrospective case series study. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardani M. COVID-19 infection recurrence presenting with meningoencephalitis. New Microbes New Infect. 2020;37:100732. doi: 10.1016/j.nmni.2020.100732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metlay J.P. Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45–e67. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D. The, PRISMAG (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. PLoS Med. 2020;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi T. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozhdehipanah H., Paybast S., Gorji R. Guillain–Barré Syndrome as a Neurological Complication of COVID-19 Infection: A Case Series and Review of the Literature. Inter Clin Neurosci J. 2020;7(3):156–161. doi: 10.1097/NRL.0000000000000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munhoz R.P. Neurological complications in patients with SARS-CoV-2 infection: a systematic review. Arquivos de Neuro-Psiquiatria. 2020;78(5):290–300. doi: 10.1590/0004-282x20200051. [DOI] [PubMed] [Google Scholar]
- Peeri N.C. The SARS, MERS and novel coronavirus (COVID-19) epidemics, the newest and biggest global health threats: what lessons have we learned? Int J Epidemiol. 2020 doi: 10.1093/ije/dyaa033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferkorn T. Acute polyradiculoneuritis with locked-in syndrome in a patient with Covid-19. J Neurol. 2020:1. doi: 10.1007/s00415-020-09897-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugin D. COVID-19–related encephalopathy responsive to high-dose glucocorticoids. Neurology. 2020;95(12):543–546. doi: 10.1212/WNL.0000000000010354. [DOI] [PubMed] [Google Scholar]
- Scheidl E. Guillain‐Barre syndrome during SARS‐CoV‐2 pandemic: a case report and review of recent literature. J Peripheral Nervous System. 2020 doi: 10.1111/jns.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriwastava S. Guillain Barré Syndrome and its variants as a manifestation of COVID-19: A systematic review of case reports and case series. J Neurolog Sci. 2020:117263. doi: 10.1016/j.jns.2020.117263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T., Guan J. Novel coronavirus and central nervous system. Eur J Neurol. 2020 doi: 10.1111/ene.14227. [DOI] [PubMed] [Google Scholar]
- Toscano G. Guillain-Barre Syndrome Associated with SARS-CoV-2. N Engl J Med. 2020 doi: 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umapathi T. Large artery ischaemic stroke in severe acute respiratory syndrome (SARS) J Neurol. 2004;251(10):1227–1231. doi: 10.1007/s00415-004-0519-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virhammar J. Acute necrotizing encephalopathy with SARS-CoV-2 RNA confirmed in cerebrospinal fluid. Neurology. 2020;95(10):445–449. doi: 10.1212/WNL.0000000000010250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M. Coronavirus disease 2019 associated with aggressive neurological and mental abnormalities confirmed based on cerebrospinal fluid antibodies: a case report. Medicine. 2020;99(36) doi: 10.1097/MD.0000000000021428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhoff T.H. Allograft infiltration and meningoencephalitis by SARS‐CoV‐2 in a pancreas‐kidney transplant recipient. Am J Transplant. 2020 doi: 10.1111/ajt.16223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020 doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J. Detection of severe acute respiratory syndrome coronavirus in the brain: potential role of the chemokine mig in pathogenesis. Clin Infect Dis. 2005;41(8):1089–1096. doi: 10.1086/444461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin R. Concomitant neurological symptoms observed in a patient diagnosed with coronavirus disease 2019. J Med Virol. 2020 doi: 10.1002/jmv.25888. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.