Abstract
Objective
To study the adrenocortical response to an acute coronavirus disease-2019 (COVID-19) infection.
Methods
Morning plasma cortisol, adrenocorticotropic hormone (ACTH), and dehydroepiandrosterone sulfate levels were measured in 28 consecutive patients with COVID-19 (16 men, 12 women, median age 45.5 years, range 25-69 years) on day 1 to 2 of hospital admission. These tests were repeated twice in 20 patients and thrice in 15 patients on different days. The hormone levels were correlated with severity of the disease.
Results
The median morning cortisol level was 196 (31-587) nmol/L. It was <100 nmol/L in 8 patients (28.6%), <200 nmol/L in 14 patients (50%), and <300 nmol/L in 18 patients (64.3%). The corresponding ACTH values had a median of 18.5 ng/L (range 4-38 ng/L), and the ACTH level was <10 ng/L in 7 patients (26.9%), <20 ng/L in 17 patients (60.7%), and <30 ng/L in 23 patients (82.1%). The repeated testing on different days showed a similar pattern. Overall, if a cutoff level of <300 nmol/L is considered abnormal in the setting of acute disease, 9 patients (32%) had cortisol levels below this limit, regardless of whether the test was done only once (3 patients) or 3 times (6 patients). When the disease was more severe, the patients had lower cortisol and ACTH levels, suggesting a direct link between the COVID-19 infection and impaired glucocorticoid response.
Conclusion
Unexpectedly, the adrenocortical response in patients with COVID-19 infection was impaired, and a significant percentage of the patients had plasma cortisol and ACTH levels consistent with central adrenal insufficiency.
Key words: ACTH, cortisol, COVID-19, glucocorticoids, hypothalamic-pituitary-adrenal axis, SARS-CoV-2
Abbreviations: ACE2, angiotensin-converting enzyme 2; ACTH, adrenocorticotropic hormone; ARDS, acute respiratory distress syndrome; COVID-19, coronavirus disease-2019; CT, computed tomography; DHEAS, dehydroepiandrosterone sulfate; HPA, hypothalamic-pituitary-adrenal; ICU, intensive care unit; SARS, severe acute respiratory syndrome; WHO, World Health Organization
Introduction
An acute infection of the respiratory tract caused by a novel coronavirus (severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2]), officially named as coronavirus disease-2019 (COVID-19), was first reported in Wuhan, China, in December 2019 and spread throughout the world.1 On January 30, 2020, the World Health Organization (WHO) officially declared COVID-19 as a public health emergency of international concern.2 On March 13, 2020, WHO declared COVID-19 as a global pandemic. Worldwide, as of October 5, 2020, more than 35.5 million people have been infected, and more than 1 million have died secondary to COVID-19 infection.3 A major impact of COVID-19 is on the respiratory system, where severity ranges from being asymptomatic (detected with laboratory testing only) to showing flu-like symptoms, pneumonia, acute respiratory distress syndrome (ARDS), multiorgan failure, and death.4, 5, 6 A vast majority of patients have mild-to-moderate symptoms. However, about 10% to 15% have severe symptoms and require intensive care unit (ICU) and mechanical ventilation.4, 5, 6
Although the impact of COVID-19 is mainly on the respiratory system, many organs are affected, especially in patients with severe pneumonia and ARDS.4 , 5 , 7 These organs include the gastrointestinal tract, liver, kidneys, cardiovascular system, central nervous, and musculoskeletal systems.7, 8, 9, 10 Studies on the impact of COVID-19 on endocrine organs are limited.7 , 11 Angiotensin-converting enzyme 2 (ACE2) is the primary mechanism of entry of the virus and is richly expressed in many endocrine glands.12 Therefore, endocrine organs are likely to be involved, especially in patients with severe disease.7 However, this has not been adequately assessed in COVID-19 infection. Adrenocortical function has been reported to be affected in acute diseases13 , 14 but has not been assessed in acute COVID-19 disease.11 In this study, we report adrenocortical function in a series of patients with confirmed COVID-19 infection. Our findings suggest an impaired glucocorticoid response to this acute disease.
Methods
The study was approved by the institutional review board and ethics committee of the King Faisal Specialist Hospital & Research Centre, Riyadh, Saudi Arabia. After obtaining informed consents, blood was drawn for the measurement of hormone levels. We enrolled 42 consecutive patients who were admitted for COVID-19 infection during the period from May 7, 2020, to May 20, 2020. We measured plasma cortisol, adrenocorticotropic hormone (ACTH), and dehydroepiandrosterone sulfate (DHEAS) levels between 5:00 AM and 8:00 AM on the first or second day of admission. Repeated measurements of these hormone levels were done from 1 to 3 times on different days for majority of the patients. There is no consensus on the cutoff plasma cortisol value in acute disease below which adrenal insufficiency should be considered.15 Values ranging from 10 to 36 μg/dL have been proposed as cutoff values for “relative adrenal insufficiency” in critically ill patients.15 Some researchers have proposed 15 μg/dL,16 and others have proposed 18 μg/dL.17 We arbitrarily chose 300 nmol/L (10.8 μg/dL), which lies within this range, as a cutoff value under which the adrenocortical glucocorticoid response was considered abnormal and adrenal insufficiency possibly present. We enrolled 42 consecutive patients. We then excluded patients who had used glucocorticoids in the past (6 patients), including patients with bronchial asthma (2 patients) and connective tissue diseases (4 patients). Patients with renal impairment (3 patients) or liver diseases (2 patients) were also excluded. Finally, we excluded patients known to have adrenal insufficiency (2 patients) or Cushing syndrome (1 patient). After the exclusion of the 14 patients with any of the abovementioned conditions, 28 patients remained, and their data were analyzed.
All the patients tested positive for COVID-19. The severity of the disease was based on guidelines from WHO and the Center for Disease Control of North America, which are as follows:
-
1.
Asymptomatic (stage A): Patients with positive COVID-19 test result but no signs or symptoms of the infection.
-
2.
Mild infection (stage B): Patients with upper respiratory tract infection symptoms and other mild symptoms (including fever and gastrointestinal symptoms) without evidence of pneumonia.
-
3.
Moderate infection (stage C): Patients with hypoxia with oxygen saturation less than 93% at rest or presence of pneumonia not requiring ICU admission.
-
4.Severe infection (stage D): Patients with pneumonia requiring ICU admission or any of the following:
-
a)respiratory rate of 30 breaths/min or more
-
b)arterial oxygen partial pressure to fractional inspiratory oxygen ratio (PaO2/FiO2) less than 300
-
c)more than 50% lung involvement on imaging within 24 to 48 hours
-
d)critical respiratory failure requiring mechanical ventilation, septic shock, or multiorgan dysfunction.
-
a)
Laboratory Testing
COVID-19 Testing
For RNA extraction, we used Qiagen EZ1 Virus mini kit extraction (Qiagen). For real-time polymerase chain reaction amplification, we used Roche cobas severe acute respiratory syndrome coronavirus 2 qualitative assay on the 6800 system (Roche Diagnostics GmbH).
Measurement of Cortisol, ACTH, and DHEAS Levels
The cortisol, ACTH, and DHEAS levels were measured using electrochemiluminescence assays on the cobas e 801 immunoassay analyzer (Roche Diagnostics GmbH).
Statistical Analysis
Numerical values were expressed as medians and ranges, and categorical values as rates and percentages. One-way analysis of variance was used to compare the cortisol levels between different COVID-19 disease severity groups. SPSS version 21 (IBM) was used for the analysis.
Results
Patient Characteristics
A total of 28 patients were included in this study. Sixteen (57%) of these patients were completely healthy without any known medical illnesses before having contracted the COVID-19 infection. In contrast, the other 12 patients had medical conditions that are not known to interfere with adrenal function (Table 1 ). The median age was 45.5 years (range 25-69 years), and the male:female ratio was 16:12. Eleven patients (39.3%) were in stage A, 15 (53.6%) in stage B, and 2 (7.1%) in stage C. No patient was in stage D as we intentionally excluded patients admitted to the ICU to avoid the many confounding factors that may affect the adrenal function in the ICU setting. All the patients had positive COVID-19 real-time polymerase chain reaction test results. Computed tomography (CT) scans were done in 6 patients and chest X-rays in all the patients. A total of 7 patients had abnormal CT scan or chest X-ray results; 5 patients were found to have lung infiltrates on the chest CT scans, and 2 of 22 patients, who did not undergo CT scan of the lungs, were found to have lung infiltrates on the chest X-rays. The others had no evidence of lung infiltrates. All the patients received 500 mg of azithromycin daily for 3 days and 400 mg of hydroxychloroquine loading dose followed by 200 mg twice a day for 6 days. Two patients received tocilizumab, and 4 patients received ceftriaxone. All the patients received oral acetaminophen and some received intravenous acetaminophen for fever and pain, but only 2 patients received opioids, and this was on an as-needed basis and administered only after the testing for this study was completed. Only 1 patient needed to be transferred to the ICU after many days of admission, and she died later. All the patients were discharged to their homes, except the 1 patient who died secondary to the COVID-19 infection.
Table 1.
Clinical Characteristics and Hormone Levels in 28 Patients Admitted with Acute COVID-19 Infection
| Age (y) | Sex | Medical background | Severity of disease | Days 1-2 |
Days 3-5 |
Days 8-11 |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cortisol nmol/L | ACTH ng/L | DHEAS nmol/L | Cortisol nmol/L | ACTH ng/L | DHEAS nmol/L | Cortisol nmol/L | ACTH nmol/L | ||||
| 37 | F | Healthy | A | 181 | 19.1 | 6.45 | 445 | 30.5 | 6.75 | 329 | 16.9 |
| 31 | M | Healthy | B | 386 | 26.1 | 4.26 | 242 | 58.9 | 4.6 | 199 | 50.2 |
| 25 | M | Osteomyelitis of the jaw | B | 106 | 24.7 | 11.3 | 124 | 12.9 | 11.2 | 89.8 | 16.4 |
| 69 | F | HTN, osteoarthritis | B | 97 | 24 | 0.273 | 168 | 15.8 | 0.235 | 180 | 25.4 |
| 52 | M | DM 2, HTN, hypothyroidism | B | 149 | 9.4 | 1.96 | 313 | … | 1.78 | 229 | 5.6 |
| 35 | M | Healthy | A | 98.4 | 20.7 | 6.18 | 334 | 41.6 | 6.45 | 309 | 35.2 |
| 28 | F | Endometriosis | B | 30.6 | 24.2 | 2.36 | 26.7 | 27 | 2.22 | 57.4 | 7.3 |
| 34 | M | DM | A | 202 | 7.8 | 3.44 | 113 | 3.9 | 3.26 | 327 | 3.1 |
| 48 | M | Healthy | A | 96.9 | 17 | … | … | … | … | … | … |
| 46 | F | Healthy | A | 299 | 7.6 | 3.52 | 644 | 8.9 | 3.27 | 238 | 5.8 |
| 30 | F | Healthy | B | 162 | 19.1 | 2.73 | 499 | 32.6 | … | … | … |
| 56 | F | DM, dyslipidemia | B | 324 | 12.7 | 1.78 | … | 4.9 | … | … | … |
| 58 | M | Dyslipidemia | B | 190 | 18.9 | 5.52 | 518 | 39.1 | … | … | … |
| 31 | M | Healthy | A | 416 | 30 | … | … | … | … | 447 | … |
| 48 | M | HTN | B | 232 | 11.7 | 3.94 | 122 | 9.2 | 3.72 | 197 | … |
| 35 | F | HTN | B | 76.3 | 13.6 | 3.28 | 252 | 10.8 | 3.08 | 266 | 19.4 |
| 27 | M | Healthy | B | 133 | 19.9 | 8.65 | 361 | 48.6 | 10.1 | 77.1 | … |
| 49 | M | Healthy | A | 432 | 38.3 | 3.88 | 421 | 40.3 | 4.01 | … | … |
| 42 | F | Healthy | C | 482 | 4.0 | … | … | 1.8 | … | … | … |
| 26 | F | Healthy | A | 36.2 | 11.7 | … | … | … | … | … | … |
| 25 | F | Healthy | C | 37 | 8.4 | 3.1 | 52 | 8.6 | 3.8 | … | … |
| 54 | M | DM 2, HTN, IHD | A | 82 | 11 | 1.4 | 316 | 27 | 1.3 | … | … |
| 45 | F | Healthy | B | 330 | 6.7 | 6.6 | … | … | … | … | … |
| 54 | M | DM, HTN, dyslipidemia | A | 429 | 357 | 3.1 | … | … | 2.7 | … | … |
| 55 | M | Healthy | B | 366 | 17.9 | 2.35 | 380 | 22.6 | 1.8 | 328 | 28.7 |
| 61 | F | Healthy | B | 432 | 5.2 | 1.52 | 469 | 28 | 1.28 | 594 | 8.5 |
| 57 | M | Healthy | B | 291 | 31.9 | … | … | … | 1.84 | … | … |
| 50 | M | DM, HTN, dyslipidemia | A | 587 | 32.4 | 5.31 | 298 | 42.9 | … | … | … |
Abbreviations: ACTH = adrenocorticotropic hormone; COVID-19 = coronavirus disease-2019; DHEAS = dehydroepiandrosterone sulfate; DM 2 = diabetes mellitus type 2; F = female; HTN = hypertension; IHD = ischemic heart disease; M = male.
Adrenocortical Function
Initial Testing (1-2 Days After Admission)
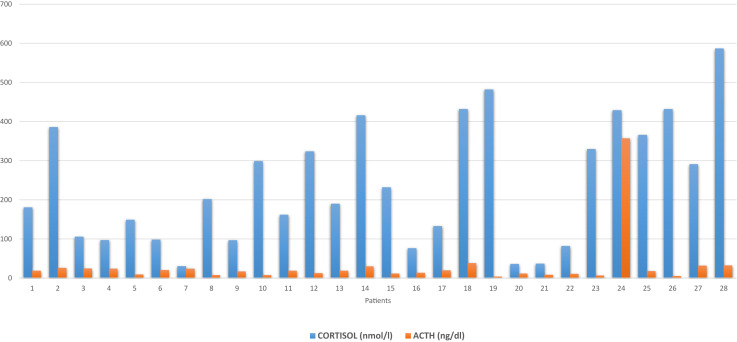
The median early morning cortisol level was 196 nmol/L (range 31-587 nmol/L). The AM cortisol level was <100 nmol/L in 8 patients (28.6%), <200 nmol/L in 14 patients (50%), and <300 nmol/L in 18 patients (64.3%) (Tables 1 and 2 and Fig. 1 ). The corresponding ACTH values had a median value of 18.5 ng/L (range 4-38 ng/L), with the ACTH levels <10 ng/L in 7 patients (26.9%), <20 ng/L in 17 patients (60.7%), and <30 ng/L in 23 patients (82.1%) (Table 2). All these values were within or below the normal range of the assay (5-60 ng/dL). Only 1 patient with a cortisol level of 429 nmol/L had a corresponding ACTH level above the normal range (357 ng/L).
Table 2.
Summary of Cortisol and ACTH Levels on Repeated Testing of 28 Patients Admitted to the Hospital with COVID-19 Infection
| Test 1 (n = 28) Days 1-2 |
Test 2 (n = 20) Days 3-5 |
Test 3 (n = 15) Days 8-11 |
|
|---|---|---|---|
| Cortisol (median, range), nmol/L | 196 (31-587) | 314 (27-644) | 238 (57-594) |
| Level < 100 | 8 (28.6%) | 2 (10%) | 3 (20%) |
| Level < 200 | 14 (50 %) | 6 (30%) | 6 (40%) |
| Level < 300 | 18 (64.3%) | 9 (45%) | 9 (60%) |
| ACTH (median, range) ng/dL | 18.5 (4-38) | 27 (2-59) | 16.5 (3.1-50.2) |
| Level < 10 | 7 (26.9%) | … | 5 (33.3%) |
| Level < 20 | 17 (60.7%) | … | 8 (53.3%) |
| Level < 30 | 23 (82.1 %) | … | 10 (66.6 %) |
Fig. 1.
Morning plasma cortisol and ACTH levels on days 1 to 2 after admission of 28 patients admitted with COVID-19 infection. ACTH = adrenocorticotropic hormone; COVID-19 = coronavirus disease-2019.
We measured the DHEAS levels in 24 cases. The median DHEAS level was 3.0 μmol/L (range 0.27-11 μmol/L), with the normal range for the assay being 1.81 to 8.3 μmol/L. Four patients (16.7%) had DHEAS levels below the normal range for the assay, and 2 patients (8.3%) had above the normal range for the assay; the other 18 patients (75%) had levels within the normal range.
Repeated Testing
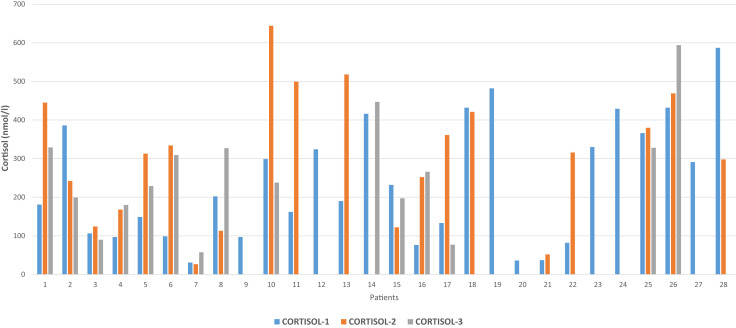
Twenty patients (71.4%) underwent repeated tests on another day after the initial testing, and 15 (53.6%) underwent repeated testing on 3 different days. The median morning plasma cortisol level in the second testing was 314.5 nmol/L (range 27-644 nmol/L), and the cortisol levels were <100 nmol/L in 2 patients (10%), <200 nmol/L in 6 patients (30%), and <300 nmol/L in 9 patients (45%) (Table 2, Fig. 2 ). Of the 20 patients who underwent repeated testing, 6 (30%) had a further drop while 14 (70%) had an increase in their cortisol levels compared to the initial testing (Fig. 2). Based on a cutoff limit of 300 nmol/L for the diagnosis of possible adrenal insufficiency in this setting, 7 patients qualified for such a diagnosis as their AM plasma cortisol levels remained below 300 nmol/L in the first and second testing (Fig. 2). Two of these patients had cortisol levels <100 nmol/L, 5 had <200 nmol/L, and 7 had <300 nmol/L.
Fig. 2.
Changes in morning plasma cortisol levels during admission for COVID-19 infection. The blue bar represents cortisol-1 done on days 1 to 2 of admission, the orange bar represents cortisol-2 done on days 3 to 5 after admission, and the gray bar represents cortisol-3 done on days 8 to 11 after admission. COVID-19 = coronavirus disease-2019.
The corresponding median ACTH level was 27 ng/L (range 2-59 ng/L). The ACTH level was below the normal range in only 2 patients (7.2%) and in the normal range in the rest of 19 retested patients. None had an ACTH value above the upper limit of the normal range of 60 ng/L.
Fifteen patients underwent repeated testing for the third time (Fig. 3 ). The median morning cortisol level was 238 nmol/L (range 57-594 nmol/L). The cortisol level was <100 nmol/L in 3 patients (20%), <200 nmol/L in 6 patients (40%), and <300 nmol/L in 9 patients (60%) (Table 2). The corresponding ACTH values had a median of 16.5 ng/L and was <10 ng/L in 5 patients, <20 ng/L in 8 patients, and <30 ng/L in 10 patients. Overall, if the morning plasma cortisol level below a cutoff level of 300 nmol/L is considered abnormal, 9 patients had cortisol levels below this limit, regardless of whether the test was done only once (3 patients) or 3 times (6 patients) (Fig. 2).
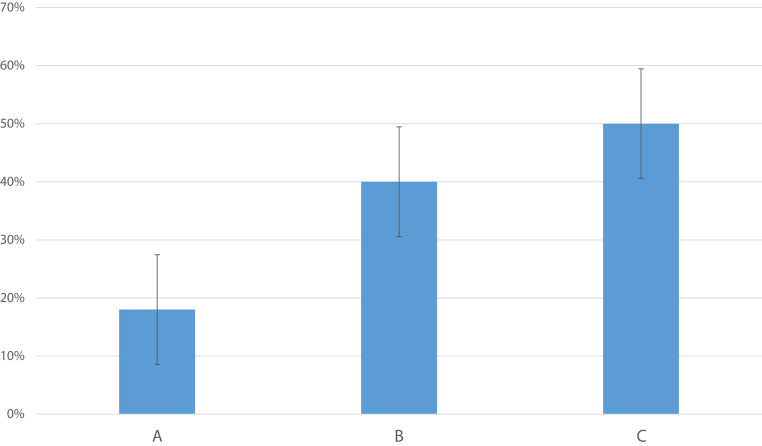
Fig. 3.
Bar chart showing the percentage of patients with AM cortisol levels consistently <300 nmol/L on one or more days with respect to severity of the disease. It shows a positive correlation between the severity of the disease and probability of having adrenal insufficiency (defined as AM cortisol level <300 nmol/L).
Correlation Between Cortisol Response and Disease Severity
Eleven cases (39.3%) had COVID-19 A severity, 15 (53.6%) had B severity, and 2 (7.1%) had C severity scores. The mean ± standard deviation of the morning plasma cortisol levels in these 3 groups was 259.9 ± 183, 220.3 ± 120, and 259.9 ± 314.7 nmol/L, respectively (P = .82). Of the 9 patients who were considered to have an inadequate cortisol response (<300 nmol/L), 2 of 11 (18%) had severity score A, 6 of 15 (40%) had severity score B, and 1 of 2 (50%) had severity score C (Fig. 3). Although hypotension and hypoglycemia are features of severe adrenal insufficiency, only 1 patient had 1 episode of hypoglycemia, with a glucose level of 2.6 mmol/L, and 2 patients had hypotension, with a blood pressure of <90/50 mm Hg; 1 of them died. This may reflect the relatively mild disease that most patients in this series had.
Discussion
COVID-19 is a new viral infection that has spread across the world in a relatively short time. Despite extensive research and several large studies describing the clinical, biochemical, and radiological features; management; and outcomes,2 , 4, 5, 6 , 9 , 10 the full spectrum of this new infectious disease has not been fully realized. Although the main effect of the disease involves the respiratory system, it has become clear that COVID-19 is a systemic disease that affects many organs in the body.4 , 7 , 18 , 19 However, limited data are available on these extrapulmonary manifestations and their pathophysiology.7 , 20 Data describing the effects of COVID-19 infection on the endocrine system are exceptionally scarce.7 , 11 , 20 Driven by the well-known changes in the acute disease and its critical role in hemodynamic adaptation and hemostasis, we sought to study the response of the hypothalamic-pituitary-adrenal (HPA) axis in COVID-19 infection. We expected a robust response of the HPA axis with a significant rise in the cortisol level during the acute phase of COVID-19 infection. To our surprise, we did not observe a robust response in the cortisol levels in any of the 28 patients included in this study. By contrast, the cortisol level was mostly in the lower end of the normal range or clearly low, frequently at levels that are diagnostic of adrenal insufficiency (Table 1 and Fig. 1). The corresponding ACTH levels measured in the same samples were also in the lower end of the normal range, suggesting that this was a form of secondary adrenal insufficiency. Whether this was due to a direct effect of the virus on the hypothalamus or pituitary glands or secondary to a humoral response and release of inhibitory cytokines is not clear. The DHEAS levels did not correlate well with the cortisol levels. However, a low DHEAS level reflects chronic ACTH deficiency,21 which is unlikely to be the case in our patients who presented with a few days’ history of COVID-19 infection. Although we did not perform the short cosyntropin test in any patient, partially due to infection control measures, the overall picture in a vast majority of the patients, with low plasma cortisol and consistently low cortisol levels in many patients who had the plasma cortisol levels measured on more than 1 occasion, strongly suggests that there is an inadequate glucocorticoid response to acute COVID-19 infection. However, only 1 patient developed acute systemic deterioration and needed ICU admission. She developed hypotension and multiorgan failure and died. Whether she developed acute adrenal crisis or not was unclear as she was systemically sick. An adrenal crisis is defined as “an acute deterioration in health status associated with absolute hypotension (systolic blood pressure < 100 mm Hg) or relative hypotension (systolic blood pressure ≥ 20 mm Hg lower than usual), with features that resolve within 1 to 2 hours after parenteral glucocorticoid administration (ie, a marked resolution of hypotension within 1 hour and improvement in clinical symptoms over a period of 2 hours).”22 With the exception of the above mentioned 1 patient, none of our patients had a full-blown adrenal crisis. Patients with adrenal crisis usually present with nonspecific symptoms and signs, including severe fatigue, weakness, myalgia, postural dizziness, nausea, vomiting, abdominal pain, and fever.22 These symptoms are also common in an acute COVID-19 infection, and it might be difficult to differentiate between the features of acute adrenal crisis and acute COVID-19 infection.
There are very limited data on the impact of COVID-19 viral infection on the HPA axis. To our knowledge, only 1 short report has been recently published as a correspondence.23 In that communication and in contrast to our findings, serum cortisol levels measured within 48 hours of admission were extremely high, and the cortisol level correlated with the outcome in patients with COVID-19 infection.23 Patients with a random cortisol level of >744 nmol/L had a worse outcome than patients with levels that were less than this value, suggesting a positive correlation between the cortisol level and severity of the disease.23 We did not observe this in our patients. However, in contrast to this study, all our patients had different grades of disease severity, but none needed ICU admission at presentation and during the days of the testing. One patient was transferred to the ICU after several days of being on the general floor and died secondary to the COVID-19 infection. She was a 42-year-old woman with no other morbidities. She presented with severe respiratory symptoms (grade C), and her morning serum cortisol level was 482 nmol/L on day 1 of admission. Unfortunately, she did not undergo additional cortisol measurements. Although there were no significant differences in the median cortisol levels between the 3 groups with different severities, there were more patients with “adrenal insufficiency” in the more severe disease group than in the milder disease group.
SARS viruses enter the body through ACE2.24, 25, 26 This enzyme is widely expressed on vascular epithelial cells, including those of the adrenal and pituitary glands and hypothalamus.12 , 26, 27, 28, 29, 30 In the previous outbreak of SARS in 2003, autopsy studies identified the virus in the adrenal cortical cells and showed that the cells undergo degeneration and necrosis, suggesting a direct cytopathic effect of the virus.29 , 30 Similar changes in the adrenal cortex in autopsy studies have recently been shown during the current epidemic of COVID-19.31 In a recent autopsy study, microscopic changes were observed in an examination of the adrenal glands in 12 of 28 patients studied (46%).31 Seven cases showed cortical necrosis, 4 showed cortical lipid degeneration, 2 showed hemorrhage, and 1 showed nonspecific focal adrenalitis. Vascular thrombosis was seen in 1 patient and focal inflammation in 3 patients. Interestingly, none of these patients had adrenal insufficiency.31 Another autopsy study of 10 patients with COVID-19 showed adrenal microinfarcations in 3 patients (33.3%).32 A recent report has been published on bilateral adrenal hemorrhage leading to acute adrenal insufficiency in a 66-year-old woman who presented with COVID-19 infection and was known to have antiphospholipid syndrome.33 SARS viruses may also affect the hypothalamus or pituitary gland. In a study by Leow et al,34 in 63 patients who recovered from the previous SARS infection, 40% had evidence of central adrenal insufficiency 3 months after recovery from their disease. Some of those patients also had low DHEAS levels, supporting chronic ACTH deficiency. Majority recovered, and the authors suggested that the virus induced hypophysitis or thalamic damage, which recovered over time.34 Edema and neuronal degeneration and evidence of the viral genome have been found in the hypothalamus in autopsies of patients who died of SARS viral infection.11 It is possible that the effect of the SARS viruses on the hypothalamus and pituitary gland is either directed by invading and damaging cells in these organs or mediated by their systemic inflammatory effects with the release of cytokines and other inflammatory mediators. It would be interesting to measure some cytokine levels and assess whether there is a correlation between their levels and cortisol plasma levels.
SARS viruses also have unique mechanisms to evade cortisol stress response by expressing some amino acids that mimic parts of ACTH. Certain amino acids in the 39-amino acid structure of ACTH are immunogenic. These include amino acids 26, 29, 31, 33, 37, and 39.11 , 35 SARS expresses amino acids homologous to these ACTH residues.11 , 35 The immune response to the virus may cross react with ACTH, sequestering it and inducing relative ACTH deficiency and secondary adrenal insufficiency.11
Glucocorticoids are widely used in the management of acute diseases, especially in an ICU setting.36 The concept of “relative adrenal insufficiency” or “critical illness-related corticosteroid insufficiency” in patients with acute stress and cortisol levels that are in the normal range but not matching the severity of the disease continues to be a controversial issue.14 , 36 Although this term is widely used in intensive care medicine, it is not as widely accepted in the field of endocrinology as it has little scientific evidence.37 Studies have suggested that cortisol levels increase secondary to stress rather than to combat the stress and are not necessary to survive the stress.15 , 37, 38, 39 This does not negate the fact that some critically ill patients may benefit from glucocorticoids for their general supportive actions but not necessarily for adrenal insufficiency.15 , 37 It also does not contradict the fact that some patients may develop real adrenal insufficiency for other reasons and need glucocorticoids. In the treatment of COVID-19 infection, the use of glucocorticoids was initially proposed as a general immunosuppressive measure to counteract the cytokine storm syndrome that was observed in a severe COVID-19 infection.40 , 41 This was not based on evidence but rather on personal experiences and common-sense recommendations derived from the known immunosuppressive effects of glucocorticoids.40 , 41 However, the recently announced RECOVERY trial has documented a favorable outcome of patients treated with 6 mg of dexamethasone daily for up to 10 days in addition to the usual care compared with patients treated with usual care alone.42 In this trial, 2104 patients who randomly received dexamethasone in addition to the usual care were compared with 4321 patients assigned to usual care alone. Overall, 454 patients (21.6%) assigned to the dexamethasone arm and 1065 patients (24.6%) assigned to the usual care arm died within 28 days, showing a clear survival benefit from dexamethasone (P < .001).42 Although the mechanisms by which dexamethasone improved the outcome were not studied, it is assumed that these are related to the generalized supportive benefits of glucocorticoids for critically ill patients and possible mitigation of the cytokine release syndrome and ARDS.40 However, our study suggests that a significant percentage of patients with COVID-19 has an inadequate glucocorticoid response, and it is possible that a part of this improvement of the outcome in the RECOVERY trial might be related to the treatment of undiagnosed adrenal insufficiency in those patients.
Apart from the short report that was mentioned earlier,23 our study is the first to report an adrenocortical response in patients with COVID-19 and show an unexpectedly low cortisol response during the acute infection phase, drawing attention to this important aspect of COVID-19 systemic effects and its potential therapeutic impact. However, our study has some shortcomings, including the relatively small sample size and non-utilization of the short cosyntropin test to assess the adequacy of the response to the stimulation. Nonetheless, the serum cortisol and ACTH level measurements were repeated at least once in most patients and showed consistent results. Most patients had moderate-severity disease (grade B), and only 2 had severe disease. However, most patients with COVID-19 tend to have mild-to-moderate disease, and thus, the sample represents the usual spectrum of patients seen with COVID-19 infection. We excluded patients in the ICU since they had several other confounding factors that may affect their adrenal function, including other superimposed infections, inotropic drugs, antibiotics, and others.
In summary, we have shown for the first time that the adrenocortical response to COVID-19 infection might be impaired in a significant number of patients, and if confirmed in larger studies, this may have significant therapeutic implications.
Acknowledgment
We would like to thank the nursing staff and laboratory personnel who assisted with this study despite the increased risk of infection.
Disclosure
The authors have no multiplicity of interest to disclose.
Footnotes
Note: This paper uses SI units for cortisol values. To convert cortisol in nmol/L to mcg/dL, divide by 27.6.
References
- 1.Guo Y.R., Cao Q.D., Hong Z.S. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res. 2020;7(1):11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . 2020. Coronavirus disease 2019 (COVID-19): situation report, 59. [Google Scholar]
- 3.Accessed October 5, 2020. https://www.worldometers.info/coronavirus/
- 4.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 7.Gavriatopoulou M., Korompoki E., Fotiou D. Organ-specific manifestations of COVID-19 infection. Clin Exp Med. 2020;20(4):493–506. doi: 10.1007/s10238-020-00648-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;5(7):831–840. doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 9.Wan S., Xiang Y., Fang W. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol. 2020;92(7):797–806. doi: 10.1002/jmv.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen J., Qi T., Liu L. Clinical progression of patients with COVID-19 in Shanghai, China. J Infect. 2020;80(5):e1–e6. doi: 10.1016/j.jinf.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pal R. COVID-19, hypothalamo-pituitary-adrenal axis and clinical implications. Endocrine. 2020;68(2):251–252. doi: 10.1007/s12020-020-02325-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamming I., Timens W., Bulthuis M.L.C., Lely A.T., Navis G.J., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boonen E., Van den Berghe G. Endocrine responses to critical illness: novel insights and therapeutic implications. J Clin Endocrinol Metab. 2014;99(5):1569–1582. doi: 10.1210/jc.2013-4115. [DOI] [PubMed] [Google Scholar]
- 14.Annane D., Pastores S.M., Arlt W. Critical illness-related corticosteroid insufficiency (CIRCI): a narrative review from a Multispecialty Task Force of the Society of Critical Care Medicine (SCCM) and the European Society of Intensive Care Medicine (ESICM) Intensive Care Med. 2017;43(12):1781–1792. doi: 10.1007/s00134-017-4914-x. [DOI] [PubMed] [Google Scholar]
- 15.Hamrahian A.H., Fleseriu M. Evaluation and management of adrenal insufficiency in critically ill patients: disease state review. Endocrine Practice. 2017;23(6):716–725. doi: 10.4158/EP161720.RA. [DOI] [PubMed] [Google Scholar]
- 16.Cooper M.S., Stewart P.M. Corticosteroid insufficiency in acutely ill patients. N Engl J Med. 2003;348(8):727–734. doi: 10.1056/NEJMra020529. [DOI] [PubMed] [Google Scholar]
- 17.Dellinger R.P., Levy M.M., Rhodes A. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39(2):165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cui Y., Tian M., Huang D. A 55-day-old female infant infected with 2019 novel coronavirus disease: presenting with pneumonia, liver injury, and heart damage. J Infect Dis. 2020;221(11):1775–1781. doi: 10.1093/infdis/jiaa113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rismanbaf A., Zarei S. Liver and kidney injuries in COVID-19 and their effects on drug therapy; a letter to editor. Arch Acad Emerg Med. 2020;8(1):e17. [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta A., Madhavan M.V., Sehgal K. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26(7):1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PubMed] [Google Scholar]
- 21.Al-Aridi R., Abdelmannan D., Arafah B.M. Biochemical diagnosis of adrenal insufficiency: the added value of dehydroepiandrosterone sulfate measurements. Endocr Pract. 2011;17(2):261–270. doi: 10.4158/EP10262.RA. [DOI] [PubMed] [Google Scholar]
- 22.Rushworth R.L., Torpy D.J., Falhammar H. Adrenal crisis. N Engl J Med. 2019;381(9):852–861. doi: 10.1056/NEJMra1807486. [DOI] [PubMed] [Google Scholar]
- 23.Tan T., Khoo B., Mills E.G. Association between high serum total cortisol concentrations and mortality from COVID-19. Lancet Diabetes Endocrinol. 2020;8(8):659–660. doi: 10.1016/S2213-8587(20)30216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X., Geng M., Peng Y., Meng L., Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal. 2020;10(2):102–108. doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu J., Yuan X., Wang B. Severe acute respiratory syndrome coronavirus 2: from gene structure to pathogenic mechanisms and potential therapy. Front Microbiol. 2020;11:1576. doi: 10.3389/fmicb.2020.01576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zemlin A.E., Wiese O.J. Coronavirus disease 2019 (COVID-19) and the renin-angiotensin system: a closer look at angiotensin-converting enzyme 2 (ACE2) Ann Clin Biochem. 2020;57(5):339–350. doi: 10.1177/0004563220928361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuba K., Imai Y., Penninger J.M. Multiple functions of angiotensin-converting enzyme 2 and its relevance in cardiovascular diseases. Circ J. 2013;77(2):301–308. doi: 10.1253/circj.cj-12-1544. [DOI] [PubMed] [Google Scholar]
- 28.Jia H. Pulmonary angiotensin-converting enzyme 2 (ACE2) and inflammatory lung disease. Shock. 2016;46(3):239–248. doi: 10.1097/SHK.0000000000000633. [DOI] [PubMed] [Google Scholar]
- 29.Ding Y., He L., Zhang Q. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol. 2004;203(2):622–630. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Q.L., Ding Y.Q., Hou J.L. Detection of severe acute respiratory syndrome (SARS)-associated coronavirus RNA in autopsy tissues with in situ hybridization. Di Yi Jun Yi Da Xue Xue Bao. 2003;23(11):1125–1127. [PubMed] [Google Scholar]
- 31.Freire Santana M., Borba M.G.S., Baía-da-Silva D.C. Case report: adrenal pathology findings in severe COVID-19: an autopsy study. Am J Trop Med Hyg. 2020;103(4):1604–1607. doi: 10.4269/ajtmh.20-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanley B., Naresh K.N., Roufosse C. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. Lancet Microbe. 2020;1(6):e245–e253. doi: 10.1016/S2666-5247(20)30115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frankel M., Feldman I., Levine M. Bilateral adrenal hemorrhage in coronavirus disease 2019 patient: a case report. J Clin Endocrinol Metab. 2020;105(12):dgaa487. doi: 10.1210/clinem/dgaa487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leow M.K.S., Kwek D.S.K., Ng A.W.K., Ong K.C., Kaw G.J.L., Lee L.S.U. Hypocortisolism in survivors of severe acute respiratory syndrome (SARS) Clin Endocrinol (Oxf) 2005;63(2):197–202. doi: 10.1111/j.1365-2265.2005.02325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wheatland R. Molecular mimicry of ACTH in SARS—implications for corticosteroid treatment and prophylaxis. Med Hypotheses. 2004;63(5):855–862. doi: 10.1016/j.mehy.2004.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pastores S.M., Annane D., Rochwerg B. Guidelines for the diagnosis and management of critical illness-related corticosteroid insufficiency (CIRCI) in critically ill patients (Part II): Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017. Intensive Care Med. 2018;44(4):474–477. doi: 10.1007/s00134-017-4951-5. [DOI] [PubMed] [Google Scholar]
- 37.Loriaux D.L., Fleseriu M. Relative adrenal insufficiency. Curr Opin Endocrinol Diabetes Obes. 2009;16(5):392–400. doi: 10.1097/MED.0b013e3283307d53. [DOI] [PubMed] [Google Scholar]
- 38.Udelsman R., Ramp J., Gallucci W.T. Adaptation during surgical stress. A reevaluation of the role of glucocorticoids. J Clin Invest. 1986;77(4):1377–1381. doi: 10.1172/JCI112443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kehlet H., Binder C. Adrenocortical function and clinical course during and after surgery in unsupplemented glucocorticoid-treated patients. Br J Anaesth. 1973;45(10):1043–1048. doi: 10.1093/bja/45.10.1043. [DOI] [PubMed] [Google Scholar]
- 40.Veronese N., Demurtas J., Yang L. Use of corticosteroids in coronavirus disease 2019 pneumonia: a systematic review of the literature. Front Med (Lausanne) 2020;7 doi: 10.3389/fmed.2020.00170. 170-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shang L., Zhao J., Hu Y., Du R., Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020;395(10225):683–684. doi: 10.1016/S0140-6736(20)30361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.RECOVERY Collaborative Group Dexamethasone in hospitalized patients with Covid-19—preliminary report. Published online July 17, 2020. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021436. NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]