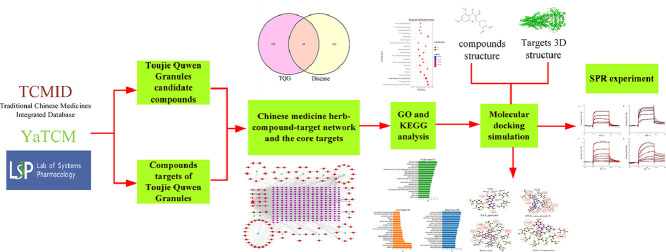
Abstract
Background
The Coronavirus disease 2019 pneumonia broke out in 2019 (COVID-19) and spread rapidly, which causes serious harm to the health of people and a huge economic burden around the world.
Purpose
In this study, the network pharmacology, molecular docking and surface plasmon resonance technology (SPR) were used to explore the potential compounds and interaction mechanism in the Toujie Quwen Granules (TQG) for the treatment of coronavirus pneumonia 2019.
Study design
The chemical constituents and compound targets of Lonicerae Japonicae Flos, Pseudostellariae Radix, Artemisia Annua L, Peucedani Radix, Forsythiae Fructus, Scutellariae Radix, Hedysarum Multijugum Maxim, Isatidis Folium, Radix Bupleuri, Fritiliariae Irrhosae Bulbus, Cicadae Periostracum, Poria Cocos Wolf, Pseudobulbus Cremastrae Seu Pleiones, Mume Fructus, Figwort Root and Fritillariae Thunbrgii Bulbus in TQG were searched. The target name was translated to gene name using the UniProt database and then the Chinese medicine-compound-target network was constructed. Protein-protein interaction network (PPI), Gene ontology (GO) function enrichment and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis of the core targets were performed in the Metascape to predict its mechanism. The top 34 compounds in the Chinese medicine-compound-target network were docked with SARS-CoV-2 3CL enzyme and SARS-CoV-2 RNA-dependent RNA polymerase (RdRp) and then the 13 compounds with lowest affinity score were docked with angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 Spike protein and interleukin 6 to explore its interaction mechanism. Lastly, SPR experiments were done using the quercetin, astragaloside IV, rutin and isoquercitrin, which were screened from the Chinese medicine-compound-target network and molecular docking.
Results
The Chinese medicine-compound-target network includes 16 medicinal materials, 111 compounds and 298 targets, in which the degree of PTGS2, TNF and IL6 is higher compared with other targets and which are the disease target exactly. The result of GO function enrichment analysis included the response to the molecule of bacterial origin, positive regulation of cell death, apoptotic signaling pathway, cytokine-mediated signaling pathway, cytokine receptor binding and so on. KEGG pathway analysis enrichment revealed two pathways: signaling pathway IL-17 and signaling pathway TNF. The result of molecular docking showed that the affinity score of compounds including quercetin, isoquercitrin, astragaloside IV and rutin is higher than other compounds. In addition, the SPR experiments revealed that the quercetin and isoquercitrin were combined with SARS-CoV-2 Spike protein rather than Angiotensin-converting enzyme 2, while astragaloside IV and rutin were combined with ACE2 rather than SARS-CoV-2 Spike protein.
Conclusion
TQG may have therapeutic effects on COVID-19 by regulating viral infection, immune and inflammation related targets and pathways, in the way of multi-component, multi-target and multi-pathway.
Keywords: Coronavirus disease 2019 pneumonia, Toujie Quwen Granules (TQG), Network pharmacology, Molecular docking, Quercetin
Abbreviations: ACE2, angiotensin-converting enzyme 2; ARDS, acute respiratory distress syndrome; COVID-19, Coronavirus disease 2019 pneumonia; DL, drug likeness; GO, gene ontology; HL, drug half-life; KEGG, Kyoto Encyclopedia of Genes and Genomes; OB, oral bioavailability; PPI, Protein protein interaction network; RBD, receptor binding domain; RdRp, RNA-dependent RNA polymerase; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SPR, surface plasmon resonance technology; TCMSP, Traditional Chinese Medicine Systems Pharmacology; TQG, Toujie Quwen Granules
Graphical abstract
Introduction
In December 2019, COVID-19 has broken out in Wuhan (Zhu et al., 2020), Hubei province, China and then it spreads rapidly and causes serious harm to the health of people and a huge economic burden all over the world. Now (September 28, 2020), World Health Organization reported that a total of 33 034 598 people have been infected around the world (WHO, 2020). COVID-19 is a highly contagious disease caused by a new Corona Virus. The incubation period of the COVID-19 is 7-14 days in generally, but in some cases, it may last for 24 days (Guan Wei-jie Ni, 2020). The clinical characteristics of COVID-19 are fever, cough and fatigue. But small number of patients had nasal congestion, runny nose, sore throat, myalgia, and diarrhea (The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team, 2020). In the worse cases scenario, severe patients usually had dyspnea and/or hypoxemia infection after one week, in which some of them can developed acute respiratory distress syndrome (ARDS), septic shock, refractory metabolic acidosis, bleeding and coagulation dysfunction, and multi-organ failure (Hemmati et al., 2020). This virus is easy to infect in the people and the incubation period could be long. Since the drugs for treating COVID-19 specifically are not available, and the vaccines of COVID-19 are being evaluated at present. China have got great achievement in the treatment of COVID-19 by integrating traditional Chinese medicine and Western medicine (Wang et al., 2020c), and the rate of cure have exceeded 94.12%.
Guangzhou eighth people's hospital, Guangzhou, Guangdong province, China, used Toujie Quwen Granules (TGQ) to treat the COVID-19 patient, which is the first case that used the traditional Chinese medicine to treat COVID-19 in China (Xiaoxia Fu et al., 2020). After evaluation and identification by Guangzhou Food and Drug Administration, it was determined that this method could effectively reduce the rate of severe illness in patients and also reduce the mortality caused by severe and critical COVID-19. This prescription mainly has the effect of detoxification and antipyretic. The lesions in the early stage of COVID-19 mainly occurred in the lungs. While Forsythiae Fructus is light, cold and moistening into the lung, removing heat of the lung, Pseudobulbus Cremastrae Seu Pleiones is used to remove the plague toxin, to remove phlegm, and to open the orifices, and Lonicerae Japonicae Flos, Scutellariae Radix and Isatidis Folium are used to remove the heat of the upper energizer. The lung is the reservoir of phlegm and the spleen is the source of phlegm. In order to prevent from consuming Qi, damp-heat and filth, Fritiliariae Irrhosae Bulbus and Fritillariae Thunbrgii Bulbus are used to remove heat and phlegm and to invigorate the spleen and transports dampness. Figwort Root, Mume Fructus are used to prevent infection of the uninfected part of the lung. In addition, in the early stage, Pseudostellariae Radix and Hedysarum Multijugum Maxim were given to protect the healthy Qi and remove the evil Qi, so as to prevent too much cold medicine and relieve the worries of deficiency of both Qi and Yin in the later stage (Wang et al., 2020b).
SARS-CoV-2 3CL hydrolase is one of the main proteases of coronavirus and plays an important role in the replication process of the virus and, therefore, it is a key target for drug design (Jin et al., 2020). RdRp is a kind of polymerases that use single stranded RNA as a template for the synthesis of complementary RNA strands and play an important role in viral genome replication and virus-induced host immune response (Elfiky, 2020). COVID-19 has been confirmed to cause infection by entering human cells through ACE2, which is one of the key targets for COVID-19 inhibition (Roshanravan et al., 2020). IL-6 have been associated with lung lesions in SARS-CoV-2 patients (Magro, 2020b). Coronavirus Spike protein is a very important surface protein of coronavirus, which is related to the infectious capacity of the virus. It includes two subunits, S1 and S2, in which S1 contains the receptor binding domain (RBD) (Wang et al., 2020a). It can bind to ACE2 protein on the surface of human cells and then infect it.
In this study, the active components and potential targets of TQG were searched in the database. The key compounds were further screened using the network pharmacology. And then, the core targets from the intersection of compounds’ target and COVID-19 disease target were used to PPI, GO and KEGG functional enrichment analysis. The interaction between four enzymes and key compounds was investigated by molecular docking. In addition, SPR experiments were used to verify the interaction between the selected compounds and proteins. This work provides theoretical and experimental basis for the treatment of COVID-19 with TQG.
Material and methods
Screening of active compound of TQG and prediction of corresponding targets
Sixteen traditional Chinese medicines (Yinhua, Taizishen, Qingsong, Qianhu, Lianqiao, Huangqin, Huangqi, Daqingye, Chaihu, Chuanbeimu,Chantui, Fuling, Shancigu, Wumei, Xuanshen and Zhebeimu) in TQG were respectively input into TCMSP database, which contains 499 herbs registered in Chinese pharmacopoeia (2010), related compound, target and so on (Ru et al., 2014). The active chemical constituents of sixteen traditional Chinese medicines were screened by the three conditions of oral bioavailability (OB) value ≥ 30 %, drug likeness (DL) value ≥ 0.18, drug half-life (HL) value ≥ 4 h. The targets of active compounds were collected through the TCMSP database and the target name was translated to gene name by UniProt database. In addition, the database of TCMID (Xue et al., 2012) and YaTCM (Li et al., 2018) were used to supplement the compounds and targets.
Construction of Chinese medicine-compound-target network
In order to illuminate the relationship between the active chemical component and the target protein, we constructed the Chinese medicine-compound-target network using the Cytoscape 3.7.1 (Paul Shannon et al., 2019), in which the important network topology parameters of the compounds and related targets, like the degree, betweenness centrality and closeness centrality were calculated. In addition, the edges representing intermolecular interactions is the links between nodes. The wider the edges, the stronger the interaction.
Screening targets of COVID-19 disease and Selection of core targets of TQG for COVID-19
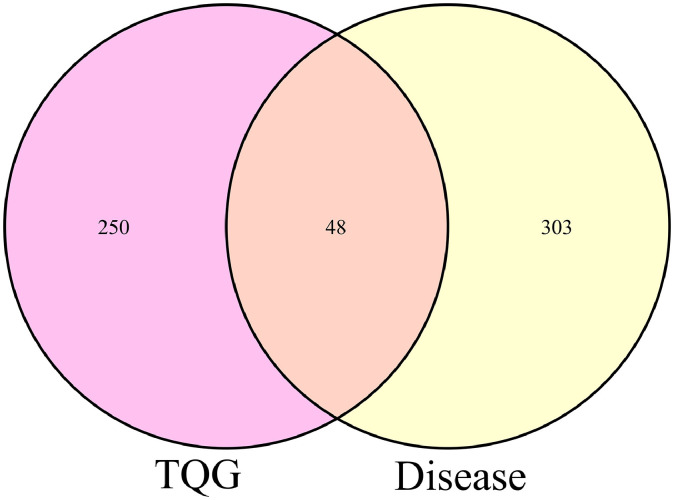
GeneCards database was used to search genes related to COVID-19 disease with “COVID-19” as the key word (Rappaport et al., 2017). The core targets of TQG in the treatment of COVID-19 were obtained by intersecting the targets of the active chemical components in TQG with the genes related to COVID-19 disease and the Venn diagram was then prepared.
Construct PPI of the core target of TQG for COVID-19
With the advent of molecular biology, it has been assumed that proteins have interactions with one another and also with other molecules. To study the interaction of proteins that are basic to perceive their role within the cell, protein-protein interaction (PPI) data can be used in a larger scale to map networks of interactions rely on their physical or functional association (Harel et al., 2009; Kibble, 2004; Safari-Alighiarloo et al., 2014). In order to explain the mechanisms of curative effect of the medicine more comprehensively, core targets of TQG for COVID-19 were input to STRING (von Mering et al., 2005) to conduct a PPI. In STRING databases, the confidence score thresholds including low confidence 0.15, medium confidence 0.4, high confidence 0.7 and highest confidence 0.9 represent the estimated likelihood that a given interaction is biologically meaningful, specific and reproducible given the supporting evidence (Szklarczyk et al., 2017). In general, the higher the confidence score, the more accurate it is. But the higher the confidence score, the less data is obtained. Therefore, we selected PPI data with a confidence score of > 0.7 to construct the protein interaction network, which satisfied the accuracy and quantity of PPI (Zheng et al., 2020).
GO function enrichment and KEGG pathway enrichment analysis
The Gene Ontology (GO) is a primary bioinformatics initiative to unify the representation of gene and gene product attributes across all species, which include biological process, molecular function and cellular component. Biological process involves with a biological objective to which the gene or gene product contributes. Molecular function is considered as the biochemical activity. Cellular component is defined as the place in the cell where a gene product is active (Kibble, 2004). KEGG is a knowledge base for biological interpretation of completely sequenced genomes by pathway mapping and the procedure to map genes in the genome to manually created pathway maps, which have been developed into a comprehensive knowledge base for both functional interpretation and practical application of genomic information (Kanehisa et al., 2017). The key target proteins of TQG for COVID-19 were submitted to Metascape to analyze GO function enrichment and KEGG pathway enrichment (Zhou et al., 2019). The operation is simply to copy and paste the genes in the Metascape's gene list and then select the species of H.salpaiens. In the Enrichment section of the analysis page, KEGG Pathways, GO Molecular Functions, GO Biological Processes and GO Cellular Components were selected for enrichment analysis of KEGG Pathways, GO Molecular Functions, GO Biological Processes and GO Cellular Components respectively.
Molecular docking
The three dimensional structure of SARS-CoV-2 3CL enzyme (ID: 6lu7) and SARS-CoV-2 RdRp (ID: 6m71), ACE2 (ID: 1r4l), SARS-CoV-2 Spike protein (ID: 6vsb) and interleukin 6 (ID: 1p9m) were obtained from the PDB database. Recently the “Novel Coronavirus Pneumonia Diagnosis and Treatment Scheme (Trial Edition 8)” issued by the National Health Commission (NHC) of China (National Health Commission) recommended ribavirin, lopinavir and ritonavir for clinical drugs. Here, ribavirin, lopinavir and ritonavir were selected as the positive control drug. The ligand and water in the protein were removed by AutoDock Tools and stored in PDBQT format. The mol2 format of 34 compounds screened from Chinese medicine-compound-target network and ribavirin, lopinavir and ritonavir were downloaded from the TCMSP database and PubChem database (Kim et al., 2019b) respectively, and then the software raccoon was used to convert the format of mol2 to PDBQT. The binding site of SARS-CoV-2 3CL is defined as the site of existing inhibitor in the protein. We removed the inhibitor and a grid box with the size 50 Å × 50 Å × 50 Å with coordinates (x, y, z) of -9.253, 16.459, 65.388 and having the grid spacing of 0.375 Å was used. The binding site of SARS-CoV-2 RNA-dependent RNA polymerase was consistent with the binding site of the inhibition of Remdesivir (Wanchao Yin et al., 2020). The grid box size of coordinates (x, y, z) was set to 128.447, 116.512, 102.160 and 60 Å × 60 Å × 60 Å, respectively, with grid spacing of 0.375 Å. The binding site of ACE2 was defined as a grid box centered at the center of mass of the co-crystallized inhibitor (x = 40.199, y = 6.024; z = 29.006), with the size 50 Å × 50 Å × 50 Å and grid spacing of 0.375 Å. The binding site of IL-6 was defined as the active site Phe229 and Phe279 reported in previous research (Blanchetot et al., 2016; Boulanger et al., 2003).
A grid box with the size 23.656 × 25.926 × 27.932 with coordinates (x, y, z) of -11.493, 15.366, 69.444 having the grid spacing of 0.375 Å was used. We used a grid spacing of 0.375 Å grid box with the size 50 Å × 50 Å × 50 Å and coordinates (x, y, z) of -37.192, 170.960, 47.861. The binding site of Spike protein is consistent with previous report by Ran Yu et al (Yu et al., 2020). Grid box (60 Å × 60 Å × 60 Å) centered at (214.246, 233.152, 240.842) with the grid spacing of 0.375Å. The Vina was used to perform molecular docking and the compounds were screened according to the binding energy (Trott and Olson, 2010). All docking simulations were run with default settings, generating a maximum of 10 binding modes at an exhaustiveness level of 8. The smaller the binding energy is, the better the ligand can bind to the protein.
SPR assay evaluated the combination of compound binding to SARS-CoV-2 Spike and ACE2 receptor protein
Surface Plasmon resonance (SPR) sensor is a powerful tool for real-time monitoring the interaction of binding kinetic and affinity. Due to its label-free and real-time monitoring, keen price and simple methods for sample preparation, the SPR technique has become one of the most important analytical tools for interrogation of bimolecular interactions, particularly in the study the binding between small molecules and target protein (Mohammadzadeh-Asl et al., 2020; Olaru et al., 2015; Singh, 2016).
Microarray conjugated ligand proteins
The SARS-CoV-2 Spike-RBD protein and ACE2 protein were purchased from Nanjing Kingsley Biotechnology Co., Ltd (Nanjing, China). Quercetin, astragaloside IV, rutin, and isoquercitrin were provided by Golden Health (Guangdong) Biotechnology Co (Foshan, China) and the purity of all compounds are higher than 98%.
The Spike-RBD protein and ACE2 protein were conjugated onto the surface of the esterified CM5 chip (Biacore™) via covalent bonds. In the experiments, the amino covalent coupling method was selected. First, the surface of the chip was esterified with the crosslinking agent EDC (E6383, Sigma-Aldrich) and NHS (130672, Sigma-Aldrich) at a pH of 4.5-5. Second, the Spike-RBD protein and ACE2 protein were coupled to the surface of the chip, which required that the purity of SARS-CoV-2 Spike-RBD protein and ACE2 protein are higher than 90%. Finally, the remaining reactive carboxyl on the matrix were blocked using 1 M β-Aminoethanol hydrochloride (E6133, Sigma-Aldrich), at pH 8.5.
Diluting compounds of traditional Chinese medicine
The compound was dissolved in DMSO (D8418, Sigma-Aldrich) and was diluted twice with 5% DMSO PBS-P buffer solution to 100 mM or 200 mM, which were diluted twice to 5 mM or 10 mM, and then diluted successively to 100 μM, 50 μM, 25 μM, 12.5 μM, 6.25 μM, 3.125 μM, 1.5625 μM (quercetin and isoquercitrin), 100 μM, 50 μM, 25 μM, 12.5 μM, 1.5625 μM (rutin), 25 μM, 12.5 μM, 6.25 μM, 3.125 μM, 1.5625 μM (astragaloside IV) using 5% DMSO PBS-P buffer. The sample was filtered by pumping through a 0.2 m membrane.
Inject sample to test
The SPR experiment was performed using the Biacore T200 SPR instrument (GE Healthcare Life Sciences, Uppsala, Sweden). Injection sample of time was 1.5 min and velocity was 30 μl/min. The time of protein dissociation was 2 min.
Result
Effective components and target prediction of TQG
A total of 111 compounds were selected from TCMSP, TCMID and YaTCM database based on the criteria about oral bioavailability, drug similarity and drug half-life mentioned in above. The basic information of these compounds in TQG was showed in Table S1.
Chinese medicine-compound-target network
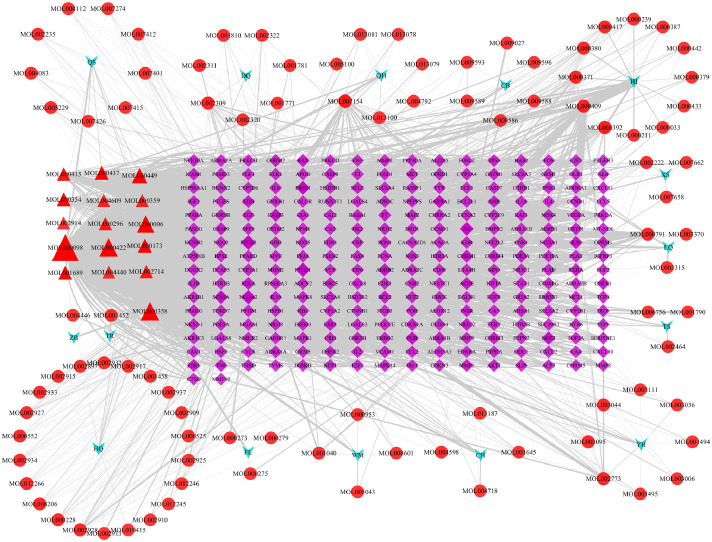
As showed in Fig. 1 , we can clearly find that Chinese medicine-compound-target network includes 425 nodes (16 of Chinese medicine, 111 of compound nodes and 298 of target) and 3018 edges. Among them, the blue arrow, red circle, and purple diamond represent the medicinal material, compound and target respectively. In addition, a compound interacts with multiple targets and different compounds also act on a target at the same time. According to topological analysis, with higher edge, the compounds with more than 2-fold of the median degree and betweenness centrality, of more than the median closeness centrality, were extracted from the network. So, we obtained 34 compounds by screening of the Chinese medicine-compound-target network. The topology parameter of Chinese medicine-compound-target network are shown in Table S4. According to the Chinese medicine-compound-target network, we know that different Chinese medicine may have the same active compounds (Fig. 1).
Fig. 1.
The Chinese medicine herb-compound-target network of TQG.
Core targets of TQG for COVID-19 and PPI
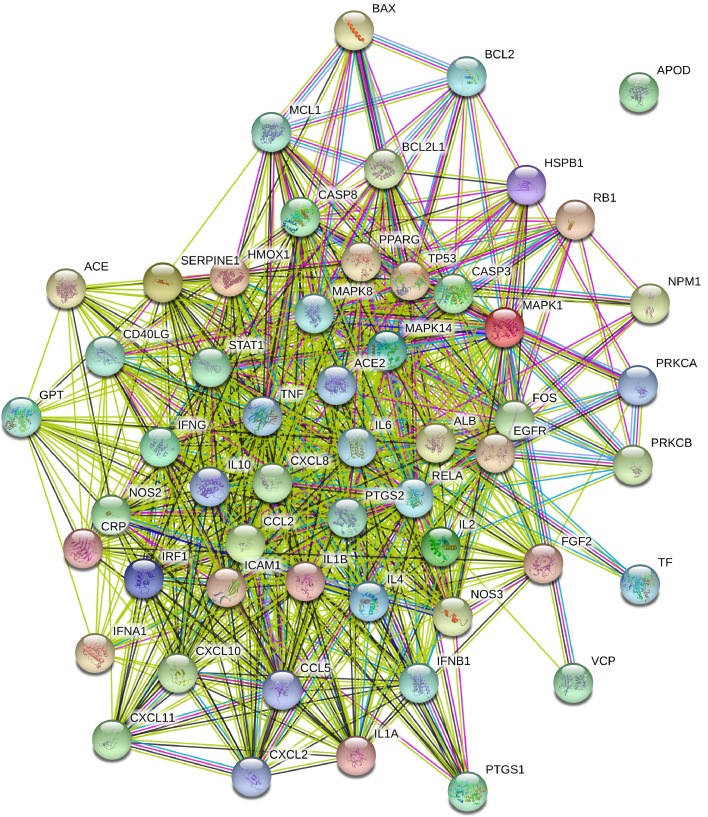
Three hundred and fifty-one genes associated with COVID-19 were collected. Among them, the minimum value of GIFtS and Score is 5 and 0.24, respectively. The GIFtS Score is a measure of the confidence in the known protein interactions for that gene, inferring an enhanced accuracy of known protein interactions for genes with a higher GIFtS score (Harel et al., 2009). The higher the score, the stronger the correlation between disease and genes. Therefore, we selected all the genes collected for further investigations. As showed in Fig. 2 , the intersection of the active compound targets and disease targets have 54 genes, which include PTGS2, PTGS1, IL6, IL10, TNF, ACE2 and so on. It was surprised that the degree of PTGS2 (117), PTGS1 (91), TNF (32) and IL6 (31) were higher in the Chinese medicine-compound-target network. The PPI of the above intersection targets was obtained using the String database. As showed in Fig. 3 , the proteins in the center of the PPI are PTGS2, IL6, TNF, MAPK14, and so on, which may play an important role in the treatment of COVID-19 with TQG. PTGS2, encoding COX2, plays an important role in SARS-CoV infections and SARS-CoV N protein causes lung inflammation by activating COX2 and stimulating multiple COX2 inflammatory cascades (Elkahloun and Saavedra, 2020; He et al., 2020). IL-6 plays an important role in the immunopathogenesis of COVID-19 and is supported by data from a range of studies reporting increased serum concentrations of this cytokine, foremost in the severe cases (Nasonov and Samsonov, 2020). MAPK signaling pathway involves a wide range of cellular functions like cell proliferation, differentiation and survival, which plays crucial roles in coronavirus propagation (Muthuramalingam et al., 2020). The TNF receptors are the crucial players involved in apoptosis and inflammation. The cross-talk between viral proteins and intracellular components of the TNF receptors confirmed the significant ability of viral replication machinery to evade the immunological responses (Favalli et al., 2020). IL-6 is the cytokine in COVID-19 correlated with severity, criticality, viral load, and prognosis of patients with COVID-19 (Saghazadeh and Rezaei, 2020). The biological activity of IL-6 depend on its potential to activate target genes that regulate cell differentiation, survival, proliferation, and apoptosis. IL-6 functions as an autocrine, paracrine, and “hormone-like” regulator of various normal and pathological biological processes associated with local and systemic inflammation (Nasonov and Samsonov, 2020).
Fig. 2.
The Venn diagram of TQG and COVID-19 disease.
Fig. 3.
PPI of TQG in treating COVID-19.
GO functional enrichment and KEGG signal pathway enrichment analysis of TQG for COVID-19
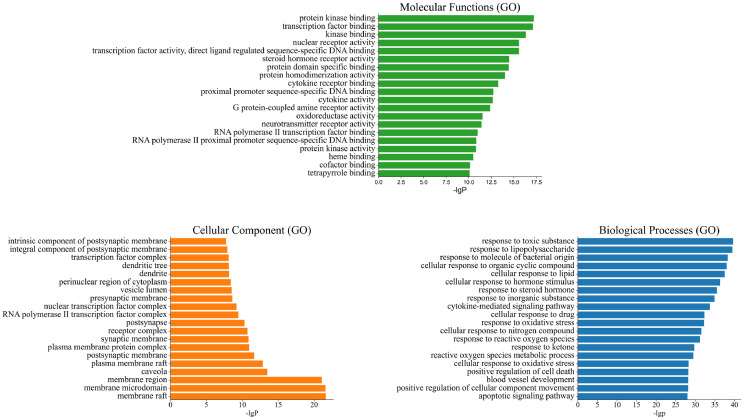
Relying on the Metascape database, GO functional enrichment analysis of key targets were performed, which include biological processes, cellular components and molecular functions. According to the p (p < 0.01) value, the terms were screened. As showed in Fig. 4 , the top 20 items of biological processes annotations included response to molecule of bacterial origin, response to lipopolysaccharide, cytokine-mediated signaling pathway and so on. For cellular components, the targets were enriched in membrane raft, RNA polymerase II transcription factor complex, membrane region, nuclear transcription factor complex, vesicle lumen, cytoplasmic vesicle lumen etc. Vesicles may play an important part in the spreading of COVID-19 virus as they transfer such receptors as CD9 and ACE2 (Hassanpour et al., 2020). Molecular Functions analysis revealed cytokine receptor binding, cytokine activity, receptor ligand activity, receptor regulator activity, chemokine receptor binding etc. The primary pathology of COVID-19 is viral pneumonia with pulmonary edema and patchy inflammatory cellular infiltration and the levels of many proinflammatory cytokines and chemokines is higher in COVID-19 patients (Dhama et al., 2020). The above biological processes or molecular functions may infer in the pathogenic of COVID-19.
Fig. 4.
The top 20 pathways for GO enrichment analysis of the targets of TQG.
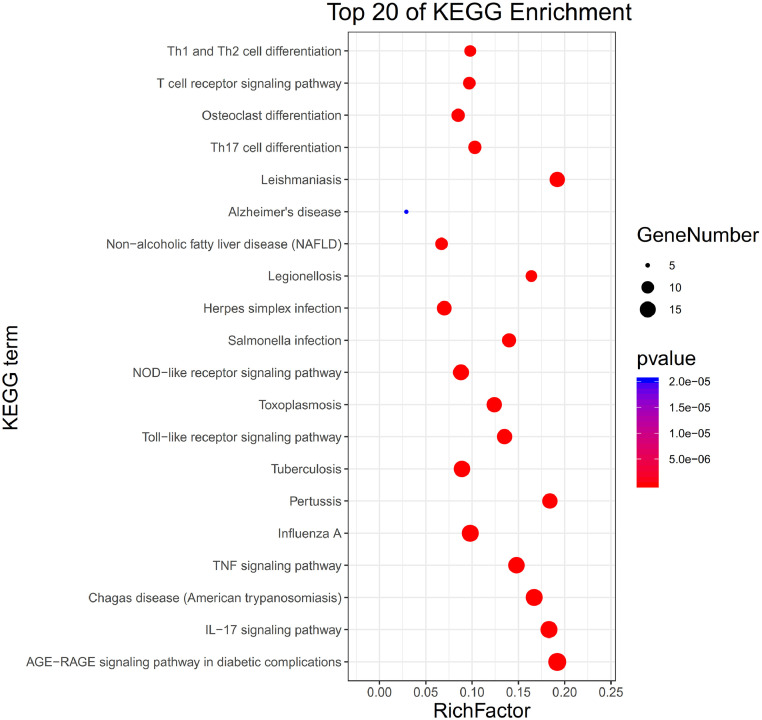
The KEGG signal pathway enrichment analysis contained 132 pathways. The top 20 pathways are showed in Fig. 5 , in which the ordinate represents KEGG pathways of the target genes, and the abscissa represents the rich factor, which indicated the ratio of the number of target genes belonging to a pathway to the number of all the annotated genes located in the pathway. The higher value for this ratio corresponds to a higher level of enrichment. In addition, the size of the dot indicates the number of target genes in the pathway. The gradually changing colors, from red to green, represent the highest to the lowest p values, respectively. It involved with IL-17 signaling pathway, TNF signaling pathway, T cell receptor signaling pathway and so on. On the pathogenesis of SARS-CoV-2 infection, ICU patients had significantly higher levels of interleukin IL-17 and TNF than the healthy controls (Gao et al., 2020). One study demonstrated that excessive non-effective host immune responses by pathogenic T cells may associate with severe lung pathology (Yonggang Zhou et al., 2020).
Fig. 5.
The top 20 pathways for KEGG enrichment analysis of the targets of TQG.
Molecular docking
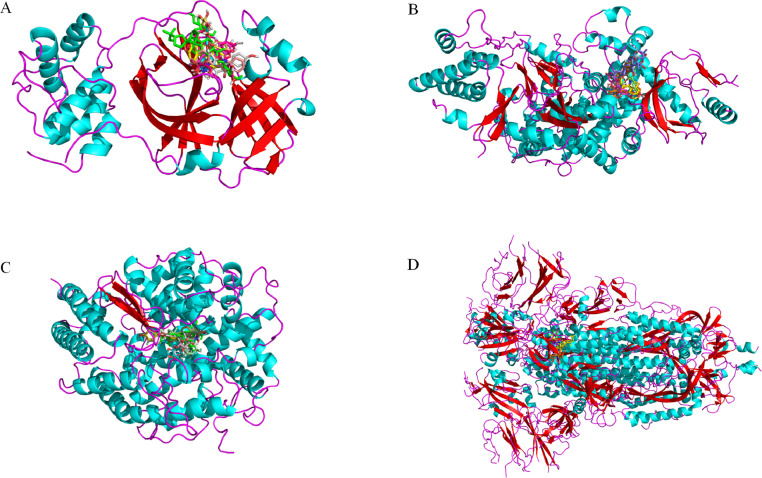
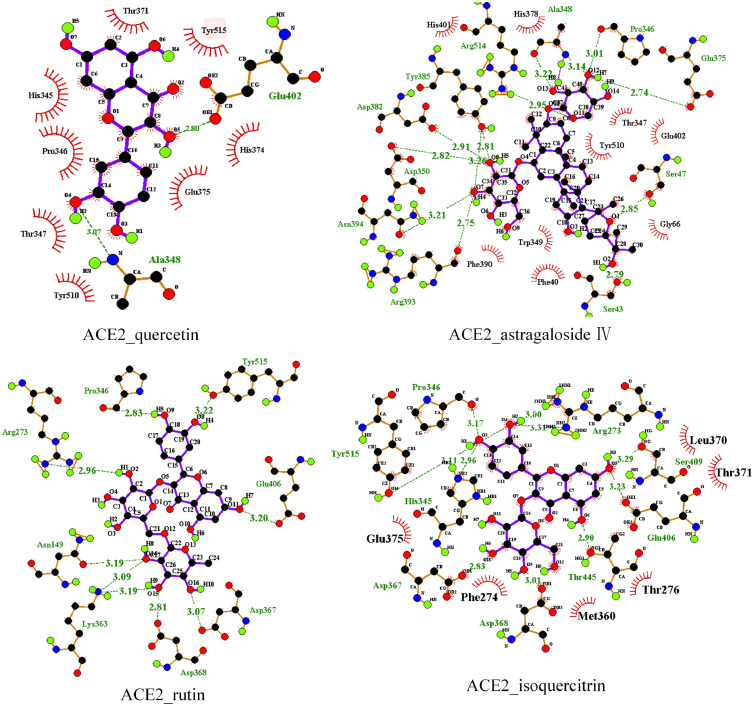
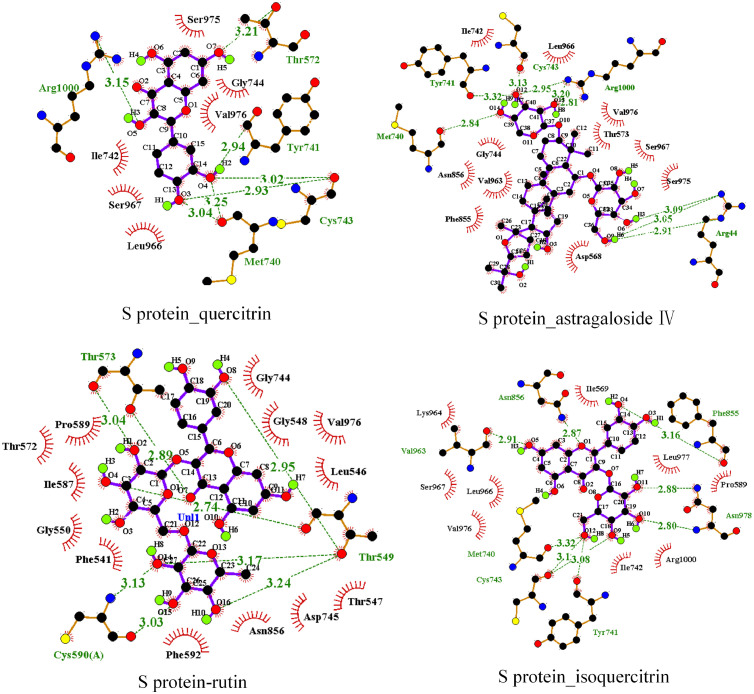
The 34 compounds obtained after the screening of Chinese medicine-compound-target network were docked with SARS-CoV-2 3CL enzyme and SARS-CoV-2 RdRp. It is believed that the lower the binding energy, the greater the protein binding affinity of the ligand. As showed in Table 1 , the binding energy of SARS-CoV-2 3CL enzyme and SARS-CoV-2 RdRp with compounds are low, which indicated that the conformation of protein binding of key compounds in the TQG are stable. So, for further study, we choose the 13 compounds with the lower affinity score in both SARS-CoV-2 3CL enzyme and SARS-CoV-2 RdRp, which were docked with ACE2, Spike protein and interleukin 6. The binding energy of these compounds was lower than the positive control drug, predicting that these components may affect the stability of the complex by binding with protein and play a role in the treatment of COVID-19. The binding energy of compounds including bicuculline, salvigenin, baicalein, quercetin, calycosin, astragaloside IV, rutin and isoquercitrin was found to be low. What’ more, the degree of quercetin (750), astragaloside IV (31), rutin (69) and isoquercitrin (44) is higher than that of bicuculline (19), salvigenin (12), baicalein (25) and calycosin (16) in network (Table S4). Numerous studies have demonstrated that quercetin acts as potent drug candidate towards inhibition of influenza virus infection through inferring the viral cellular immune system, inhibition of viral cellular targets, interfering the viral replication and inhibiting the viral growth phases and quercetin can be considered as a good candidate for further optimization and development, or repositioned for COVID-19 treatment (Abian et al., 2020; Sargiacomo et al., 2020; Enkhtaivan et al., 2017; Gansukh et al., 2021; Kim et al., 2019a). Astragaloside IV has been suggested to have anti-inflammatory, anti-oxidative and immunoregulatory activities in various diseases (Li et al., 2017; Zhi-guo Zhang et al., 2012). Many studies have confirmed that rutin can change the morphology of cells and reduce the apoptosis caused by toxic substances via eliminating ROS and inhibiting oxidative stress (Caglayan et al., 2019; Qian et al., 2020; Singh et al., 2019). Rutin also has been applied in the field of biotoxicity repair (Yang et al., 2008). Previous studies have suggested that isoquercitrin has biological activities of anti-inflammatory and antiapoptotic actions, which has used to be effective against cadmium-induced renal oxidative damage (Wang et al., 2017). Taking into account all these, we thus selected quercetin, astragaloside IV, rutin and isoquercitrin for experiment of SPR. In addition, the binding affinity score of these four compounds with ACE2 and Spike protein is higher than other protein. The GIFtS and Score of ACE2 which is also the compound target in the network, is the highest in the disease targets which obtained from the GeneCards. The SARS-CoV-2 could entry the host cells by viral Spike protein binding to the host cell receptor of ACE2, resulting in the virus invading the human body and causing disease (Hoffmann et al., 2020). Therefore, the ACE2 and S protein were selected for SPR investigation. The stable docking conformation pose of eight compounds with four enzymes are showed in Fig. 6 . The interaction between quercetin, astragaloside IV, rutin, isoquercitrin and ACE2 and Spike protein are showed in Fig. 7, Fig. 8 , which showed that the four enzymes have hydrogen bond interactions and hydrophobic interactions with quercetin, calycosin, astragaloside IV, rutin, isoquercitrin. (The amino acid of enzymes interaction with compounds are showed in Table S3). The interactions of SARS-CoV-2 3CL enzyme, SARSCoV2 RdRp, IL-6 with compounds are showed in Figs. S1, S2 and S3, respectively.
Table 1.
The result of core compounds docked with enzymes.
| Compound | molecular formula | CAS | 3CLaffinity score (kJ/mol) | RNA-dependent affinity score (KJ/mol) | ACE2 affinity score (KJ/mol) | IL-6 affinity score (KJ/mol) | S-protein (KJ/mol) |
|---|---|---|---|---|---|---|---|
| Ritonavir | C37H48N6O5S2 | 155213-67-5 | -7.1 | -7.3 | -8.0 | -6.0 | -5.9 |
| Ribavirin | C8H12N4O5 | 36791-04-5 | -6.2 | -7.2 | -7.2 | -5.9 | -5.9 |
| Lopinavir | C27H38N4O3 | 192725-17-0 | -7.2 | -7.3 | -8.1 | -6.0 | 6.0 |
| quercetin | C15H10O7 | 117-39-5 | -7.3 | -7.3 | -8.3 | -7.2 | -7.6 |
| astragaloside IV | C41H68O14 | 83207-58-3 | -7.3 | -7.7 | -9 | -6.6 | -7.5 |
| rutin | C27H30O16 | 153-18-4 | -8.8 | -8.1 | -9.9 | -6.0 | -8.2 |
| isoquercitrin | C21H20O12 | 21637-25-2 | -8.3 | -8.1 | -9.8 | -6.6 | -8.8 |
| kaempferol | C15H10O6 | 520-18-3 | -7.8 | -7.2 | -8.3 | -6.3 | -6.5 |
| bicuculline | C20H17NO6 | 485-49-4 | -7.8 | -8.9 | -10 | -7.2 | -7.2 |
| beta-carotene | C40H56 | 7235-40-7 | -7.7 | -7.3 | -9 | -6.3 | -6.9 |
| Salvigenin | C18H16O6 | 19103-54-9 | -7.6 | -8.1 | -11.1 | -7.6 | -6.0 |
| indirubin | C16H10N2O2 | 479-41-4 | -7.5 | -7.5 | -8.4 | -6.3 | -6.4 |
| baicalein | C15H10O5 | 491-67-8 | -7.5 | -7.6 | -9.1 | -7.6 | -6.6 |
| Calycosin | C16H12O5 | 20575-57-9 | -7.4 | -7.1 | -8.8 | -6.7 | -6.8 |
| Artemetin | C20H20O8 | 479-90-3 | -7.4 | -6.7 | -8.1 | -6 | -6.2 |
| (2R)-7-hydroxy-5-methoxy-2-phenylchroman-4-one | C16H14O4 | 1090-65-9 | -7.3 | -7 | -8.1 | -6.2 | -6.6 |
| Glycyrol | C21H18O6 | 23013-84-5 | -7.3 | -7.6 | |||
| 5-hydroxy-7-methoxy-2-(3,4,5-trimethoxyphenyl)chromone | C18H16O7 | 18103-41-8 | -7.3 | -7.4 | |||
| tanshinone iia | C19H18O3 | 568-72-9 | -7.3 | -7.5 | |||
| luteolin | C15H10O6 | 491-70-3 | -7.2 | -7.5 | |||
| beta-sitosterol | C29H50O | 83-46-5 | -7.0 | -7.1 | |||
| Skullcapflavone II | C19H18O8 | 55084-08-7 | -7.2 | -7.2 | |||
| 5,7,4′-Trihydroxy-8-methoxyflavone | C16H12O6 | 57096-02-3 | -7.2 | -7 | |||
| wogonin | C16H12O5 | 632-85-9 | -7.1 | -7.2 | |||
| isorhamnetin | C16H12O7 | 480-19-3 | -7.1 | -7.3 | |||
| (6aR,11aR)-9,10-dimethoxy-6a,11a-dihydro-6H-benzofurano[3,2-c]chromen-3-ol | C23H26O10 | 94367-42-7 | -7.1 | -7 | |||
| formononetin | C16H12O4 | 485-72-3 | -7.1 | -6.8 | |||
| Cirsiliol | C17H14O7 | 34334-69-5 | -7.1 | -7 | |||
| Moslosooflavone | C17H14O5 | 3570-62-5 | -7 | -7 | |||
| 5,2′-Dihydroxy-6,7,8-trimethoxyflavone | C18H16O7 | 86926-52-5 | -6.9 | -7.2 | |||
| Chryseriol | C16H12O6 | 491-71-4 | -6.9 | -7.1 | |||
| sitosterol | C29H50O | 68555-08-8 | -6.8 | -7.4 | |||
| oroxylin a | C16H12O5 | 480-11-5 | -6.8 | -7.1 | |||
| rivularin | C18H16O7 | 70028-59-0 | -6.8 | -7.3 | |||
| Stigmasterol | C29H48O | 83-48-7 | -6.7 | -7.1 | |||
| hederagenin | C30H48O4 | 465-99-6 | -6.6 | -7.5 | |||
| acacetin | C16H12O5 | 480-44-4 | -4.3 | -5.2 |
Fig. 6.
The docking pose of (A) SARS-CoV-2 3CL enzyme, (B) SARSCoV2 RdRp, (C) ACE2 and (D) Spike protein with bicuculline, salvigenin, baicalein, calycosin, quercetin, astragaloside IV, rutin and isoquercitrin.
Fig. 7.
The molecular interaction of ACE2 and quercetin, astragaloside IV, rutin and isoquercitrin.
Fig. 8.
The molecular interaction of Spike protein and quercetin, astragaloside IV, rutin and isoquercitrin.
SPR experiment
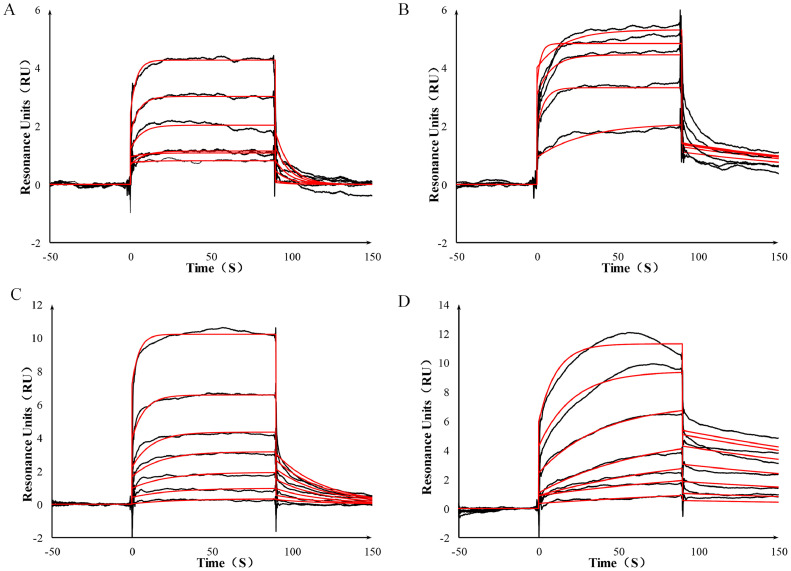
In the SPR experiments, quercetin, astragaloside IV, rutin and isoquercitrin were selected as an extracellular validation molecular model to conduct small-molecule and macromolecule interaction experiments with Spike protein and ACE2 protein, respectively. In the study, Spike protein and ACE2 protein were fixed in the chip through covalent bonds and the solutions of quercetin, astragaloside IV, rutin and isoquercitrin flowed through the chip to test the binding energy, respectively. Titration result is showed in Fig. 9 , which revealed that rutin and astragaloside IV bound to ACE2 rather than Spike protein, while quercetin and isoquercitrin bound to Spike protein rather than ACE2. The KD of quercetin and Spike protein is 1.69 × 10−5 M. The KD of astragaloside IV and ACE2 protein is 3.69 × 10−7 M. The KD of rutin and ACE2 protein is 6.68 × 10−5 M. The KD value of isoquercitrin and protein S is 4.54 × 10−6 M. Therefore, it could be speculated that quercetin and isoquercitrin could bind to the RBD region of Spike protein and inactivate it and then prevent Spike protein binding to ACE2 of epithelial cell surface. Astragaloside IV and rutin could bind to ACE2, which weakened the ability of SARS-Cov-2 infecting host to some extent.
Fig. 9.
The SPR titration curve of rutin (A) and astragaloside IV (B) interacted with ACE2 protein and quercetin (C) and isoquercitrin (D) interacted with Spike protein.
Discussion
Since the outbreak of COVID-19, it has spread all over the world, with the morbidity and mortality rate rising continuously. The global epidemic is extremely severe, but there is still lack of specific vaccine and therapeutic drugs. Currently, old drugs were used to treat the virus, but it could occur medication side effect (Di Lorenzo et al., 2020; Nakhleh and Shehadeh, 2020). COVID-19 patients not only showed respiratory symptoms, but also liver damage and inflammation (Wei et al., 2020). Chinese medicine plays an important role in the treatment of COVID-19.
Therefore, network pharmacology and molecular docking may be useful to excavate the potential compounds of TQG for COVID-19 treatment. According to the results of network pharmacology and molecular docking, the main active compounds with higher comprehensive scores of TQG included quercetin, astragaloside IV, rutin and isoquercitrin. Quercetin is a natural flavonoid with antioxidant, antiviral and anti-inflammatory effects, which can be used for the treatment of liver, heart, spleen, lung, kidney, orthopedic diseases, nervous system diseases and so on (Boots et al., 2008; Hatahet et al., 2016). Astragaloside IV is a saponin and serving as the predominant constituent of Astragalus membranaceus, which is used in Chinese medicine for the treatment of anti-inflammatory, anti-oxidative, immune disorders and liver cirrhosis with an excellent safety record (Li et al., 2017). Astragaloside IV inhibits HSC (the key effectors in hepatic fibrogenesis) activation by inhibiting generation of oxidative stress and associated p38 MAPK activation (Li et al., 2013). Increasing evidence supports that astragaloside IV play an important role in the treatment of pulmonary fibrosis and myocardial fibrosis (Shuang Ren et al., 2013). Rutin is a natural flavonoid derivative, which has been used to treat diseases including antiallergic, hypolipidemic, anti-inflammatory and vasoactive, antitumor, antibacterial, antiviral and antiprotozoal properties, cytoprotective, and antioxidant properties (Khan et al., 2012).
The results of network pharmacology and PPI analysis showed that the top targets involved PTGS2, PTGS1, TNF, IL-6, IL-2, and IL-10, which are the potential targets of the compounds including quercetin, astragaloside IV, rutin and isoquercitrin. Studies have shown that PTGS2 and PTGS1 play an important role in the treatment of inflammation and PTGS1 promotes osteogenic differentiation of adipogenic stem cells by inhibiting NF-kB signaling. IL-6 is an important inflammatory factor involved in the pathological process of inflammation (Magro, 2020). The result of KEGG functional enrichment analysis involved IL-17 signaling pathway, TNF signaling pathway and so on. Recent study reported that Anti-TNF therapy may have positive effect in COVID-19 patient (Ye et al., 2020). Therefore, we speculate that the multiple active compounds in TQG may play an important role in therapeutic of COVID-19 by multiple signal pathways including IL-17 signaling pathway, TNF signaling pathway, etc.
The KD of ACE2 protein with astragaloside IV and rutin is 3.69 × 10−7 M and 6.68 × 10−5 M respectively. While the affinity score of ACE2 between astragaloside IV and rutin is -9 KJ/mol and -9.9 KJ/mol respectively. It seems that the results of molecular docking were not consistent with the SPR assay. The difference of 0.9 in affinity score may not demonstrated the level of binding, which can be used as a reference only on the contrary.
By further analyzing the interaction mechanism, it could be found that the four enzymes have hydrophobic interaction and hydrogen bond interactions with the compounds shown in Table S3. Combined with the results of molecular docking and SPR experiment, quercetin, astragaloside IV, and isoquercitrin could be effective for the treatment of COVID-19. The interaction mechanism between four enzymes and the core compounds in TQG were analyzed and the results provided a theoretical basis for the study of drug therapy in COVID-19.
Conclusion
In summary, we explored the potential compounds of TQG for the treatment of COVID-19 using the network pharmacology and molecular docking. A number of compounds including quercetin, astragaloside IV, rutin and isoquercitrin were screened from TQG and were used to conduct SPR experiments. Our study provides both theoretical and experimental basis for the treatment of COVID-19 with TQG.
CRediT authorship contribution statement
Miaobo Ye: Investigation, Writing - original draft. Guiwen Luo: Investigation, Writing - original draft. Dexiao Ye: Investigation, Project administration. Mengting She: Investigation. Ning Sun: Data curation, Project administration. Yu-Jing Lu: Data curation, Project administration. Jie Zheng: Conceptualization, Methodology, Software, Validation, Resources.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (NOs. 81473082).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.phymed.2020.153401.
Appendix. Supplementary materials
References
- Abian O., Ortega-Alarcon D., Jimenez-Alesanco A., Ceballos-Laita L., Vega S., Reyburn H.T., Rizzuti B., Velazquez-Campoy A. Structural stability of SARS-CoV-2 3CLpro and identification of quercetin as an inhibitor by experimental screening. Int. J. Biol. Macromol. 2020;164:1693–1703. doi: 10.1016/j.ijbiomac.2020.07.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchetot C., De Jonge N., Desmyter A., Ongenae N., Hofman E., Klarenbeek A., Sadi A., Hultberg A., Kretz-Rommel A., Spinelli S., Loris R., Cambillau C., de Haard H. Structural mimicry of receptor interaction by antagonistic interleukin-6 (IL-6) antibodies. J. Biol. Chem. 2016;291:13846–13854. doi: 10.1074/jbc.M115.695528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boots A.W., Haenen G.R., Bast A. Health effects of quercetin: from antioxidant to nutraceutical. Eur. J. Pharmacol. 2008;585:325–337. doi: 10.1016/j.ejphar.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Boulanger M.J., Chow D.C., Brevnova E.E., Garcia K.C. Hexameric structure and assembly of the interleukin-6/IL-6 alpha receptor/gp130 complex. Science. 2003;300:2101–2104. doi: 10.1126/science.1083901. [DOI] [PubMed] [Google Scholar]
- Caglayan C., Kandemir F.M., Yildirim S., Kucukler S., Eser G. Rutin protects mercuric chloride-induced nephrotoxicity via targeting of aquaporin 1 level, oxidative stress, apoptosis and inflammation in rats. J. Trace Elem. Med. Biol. 2019;54:69–78. doi: 10.1016/j.jtemb.2019.04.007. [DOI] [PubMed] [Google Scholar]
- Dhama K., Patel S.K., Pathak M., Yatoo M.I., Tiwari R., Malik Y.S., Singh R., Sah R., Rabaan A.A., Bonilla-Aldana D.K., Rodriguez-Morales A.J. An update on SARS-CoV-2/COVID-19 with particular reference to its clinical pathology, pathogenesis, immunopathology and mitigation strategies. Travel Med. Infect. Dis. 2020;37 doi: 10.1016/j.tmaid.2020.101755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lorenzo G., Di Trolio R., Kozlakidis Z., Busto G., Ingenito C., Buonerba L., Ferrara C., Libroia A., Ragone G., Ioio C.d., Savastano B., Polverino M., De Falco F., Iaccarino S., Leo E. COVID 19 therapies and anti-cancer drugs: a systematic review of recent literature. Crit. Rev. Oncol./Hematol. 2020;152 doi: 10.1016/j.critrevonc.2020.102991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfiky A.A. Ribavirin, remdesivir, sofosbuvir, galidesivir, and tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): a molecular docking study. Life Sci. 2020;253 doi: 10.1016/j.lfs.2020.117592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkahloun A.G., Saavedra J.M. Candesartan could ameliorate the COVID-19 cytokine storm. Biomed. Pharmacother. 2020;131 doi: 10.1016/j.biopha.2020.110653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enkhtaivan G., Maria John K.M., Pandurangan M., Hur J.H., Leutou A.S., Kim D.H. Extreme effects of Seabuckthorn extracts on influenza viruses and human cancer cells and correlation between flavonol glycosides and biological activities of extracts. Saudi J. Biol. Sci. 2017;24:1646–1656. doi: 10.1016/j.sjbs.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favalli E.G., Ingegnoli F., Lucia O.D., Cincinelli G., Cimaz R., Caporali R. COVID-19 infection and rheumatoid arthritis: faraway, so close! Autoimmun. Rev. 2020;19(5):1–7. doi: 10.1016/j.autrev.2020.102523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gansukh E., Nile A., Kim D.H., Oh J.W., Nile S.H. New insights into antiviral and cytotoxic potential of quercetin and its derivatives–a biochemical perspective. Food Chem. 2021;334 doi: 10.1016/j.foodchem.2020.127508. [DOI] [PubMed] [Google Scholar]
- Gao Y., Li T., Han M., Li X., Wu D., Xu Y., Zhu Y., Liu Y., Wang X., Wang L. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J. Med. Virol. 2020;92:791–796. doi: 10.1002/jmv.25770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel A., Inger A., Stelzer G., Strichman-Almashanu L., Dalah I., Safran M., Lancet D. GIFtS: annotation landscape analysis with GeneCards. BMC Bioinform. 2009;10:348. doi: 10.1186/1471-2105-10-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassanpour M., Rezaie J., Nouri M., Panahi Y. The role of extracellular vesicles in COVID-19 virus infection. Infect. Genet. Evol. 2020;85 doi: 10.1016/j.meegid.2020.104422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatahet T., Morille M., Hommoss A., Devoisselle J.M., Muller R.H., Begu S. Quercetin topical application, from conventional dosage forms to nanodosage forms. Eur. J. Pharm. Biopharm. 2016;108:41–53. doi: 10.1016/j.ejpb.2016.08.011. [DOI] [PubMed] [Google Scholar]
- He T., Qu R., Qin C., Wang Z., Zhang Y., Shao X., Lu T. Potential mechanisms of Chinese herbal medicine that implicated in the treatment of COVID-19 related renal injury. Saudi Pharm. J. 2020;28:1138–1148. doi: 10.1016/j.jsps.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmati F., Saedi S., Hemmati-Dinarvand M., Zarei M., Seghatoleslam A. Mysterious virus: a review on behavior and treatment approaches of the novel coronavirus, 2019-nCoV. Arch. Med. Res. 2020;51(5):375–383. doi: 10.1016/j.arcmed.2020.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Muller M.A., Drosten C., Pohlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z., Du X., Xu Y., Deng Y., Liu M., Zhao Y., Zhang B., Li X., Zhang L., Peng C., Duan Y., Yu J., Wang L., Yang K., Liu F., Jiang R., Yang X., You T., Liu X., Yang X., Bai F., Liu H., Liu X., Guddat L.W., Xu W., Xiao G., Qin C., Shi Z., Jiang H., Rao Z., Yang H. Structure of M(pro) from SARS-CoV-2 and discovery of its inhibitors. Nature. 2020;582:289–293. doi: 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- Kanehisa M., Furumichi M., Tanabe M., Sato Y., Morishima K. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017;45:D353–D361. doi: 10.1093/nar/gkw1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M.M., Raza S.S., Javed H., Ahmad A., Khan A., Islam F., Safhi M.M., Islam F. Rutin protects dopaminergic neurons from oxidative stress in an animal model of Parkinson's disease. Neurotox. Res. 2012;22:1–15. doi: 10.1007/s12640-011-9295-2. [DOI] [PubMed] [Google Scholar]
- Kibble P.G.a.M. Review of uses of network and graph theory concepts within proteomics. Expert Rev. Proteomics. 2004;1:229–238. doi: 10.1586/14789450.1.2.229. [DOI] [PubMed] [Google Scholar]
- Kim D.H., Park G.S., Nile A.S., Kwon Y.D., Enkhtaivan G., Nile S.H. Utilization of Dianthus superbus L and its bioactive compounds for antioxidant, anti-influenza and toxicological effects. Food Chem. Toxicol. 2019;125:313–321. doi: 10.1016/j.fct.2019.01.013. [DOI] [PubMed] [Google Scholar]
- Kim S., Chen J., Cheng T., Gindulyte A., He J., He S., Li Q., Shoemaker B.A., Thiessen P.A., Yu B., Zaslavsky L., Zhang J., Bolton E.E. PubChem 2019 update: improved access to chemical data. Nucleic Acids Res. 2019;47:D1102–D1109. doi: 10.1093/nar/gky1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Ma C., Zhao X., Hu Z., Du T., Xu X., Wang Z., Lin J. YaTCM: yet another traditional Chinese medicine database for drug discovery. Comput. Struct. Biotechnol. J. 2018;16:600–610. doi: 10.1016/j.csbj.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Li H., Fang F., Deng X., Ma S. Astragaloside IV attenuates cognitive impairments induced by transient cerebral ischemia and reperfusion in mice via anti-inflammatory mechanisms. Neurosci. Lett. 2017;639:114–119. doi: 10.1016/j.neulet.2016.12.046. [DOI] [PubMed] [Google Scholar]
- Li X., Wang X., Han C., Wang X., Xing G., Zhou L., Li G., Niu Y. Astragaloside IV suppresses collagen production of activated hepatic stellate cells via oxidative stress-mediated p38 MAPK pathway. Free Radic. Biol. Med. 2013;60:168–176. doi: 10.1016/j.freeradbiomed.2013.02.027. [DOI] [PubMed] [Google Scholar]
- Magro Giuseppe. SARS-CoV-2 and COVID-19: is interleukin-6 (IL-6) the ‘culprit lesion’ of ARDS onset? What is there besides Tocilizumab? SGP130Fc. Cytokine: X. 2020;2 doi: 10.1016/j.cytox.2020.100029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magro G. SARS-CoV-2 and COVID-19: is interleukin-6 (IL-6) the ‘culprit lesion’ of ARDS onset? What is there besides Tocilizumab? SGP130Fc. Cytokine. 2020;2 doi: 10.1016/j.cytox.2020.100029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadzadeh-Asl S., Aghanejad A., Yekta R., de la Guardia M., Ezzati Nazhad Dolatabadi J., Keshtkar A. Kinetic and thermodynamic insights into interaction of erlotinib with epidermal growth factor receptor: surface plasmon resonance and molecular docking approaches. Int. J. Biol. Macromol. 2020;163:954–958. doi: 10.1016/j.ijbiomac.2020.07.048. [DOI] [PubMed] [Google Scholar]
- Muthuramalingam P., Jeyasri R., Valliammai A., Selvaraj A., Karthika C., Gowrishankar S., Pandian S.K., Ramesh M., Chen J.T. Global multi-omics and systems pharmacological strategy unravel the multi-targeted therapeutic potential of natural bioactive molecules against COVID-19: an in silico approach. Genomics. 2020;112(6):4486–4504. doi: 10.1016/j.ygeno.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhleh A., Shehadeh N. Interactions between antihyperglycemic drugs and the renin-angiotensin system: putative roles in COVID-19. A mini-review. Diabetes Metab. Syndr. 2020;14:509–512. doi: 10.1016/j.dsx.2020.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasonov E., Samsonov M. The role of Interleukin 6 inhibitors in therapy of severe COVID-19. Biomed. Pharmacother. 2020;131 doi: 10.1016/j.biopha.2020.110698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaru A., Bala C., Jaffrezic-Renault N., Aboul-Enein H.Y. Surface plasmon resonance (SPR) biosensors in pharmaceutical analysis. Crit. Rev. Anal. Chem. 2015;45:97–105. doi: 10.1080/10408347.2014.881250. [DOI] [PubMed] [Google Scholar]
- Paul Shannon D.R., Ozier O., Baliga N.S., Wang J.T., Markiel A. Cold Spring Harbor Laboratory Press; 2019. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y., Zhang Y., Zuh A.A., Qiao W. New application of rutin: repair the toxicity of emerging perfluoroalkyl substance to Pseudomonas stutzeri. Ecotoxicol. Environ. Saf. 2020;201 doi: 10.1016/j.ecoenv.2020.110879. [DOI] [PubMed] [Google Scholar]
- Rappaport N., Twik M., Plaschkes I., Nudel R., Iny Stein T., Levitt J., Gershoni M., Morrey C.P., Safran M., Lancet D. MalaCards: an amalgamated human disease compendium with diverse clinical and genetic annotation and structured search. Nucleic Acids Res. 2017;45:D877–D887. doi: 10.1093/nar/gkw1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roshanravan N., Ghaffari S., Hedayati M. Angiotensin converting enzyme-2 as therapeutic target in COVID-19. Diabetes Metab. Syndr. 2020;14:637–639. doi: 10.1016/j.dsx.2020.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ru J., Li P., Wang J., Zhou W., Li B., Huang C., Li P., Guo Z., Tao W., Yang Y., Xu X., Li Y., Wang Y., Yang L. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014;6:13. doi: 10.1186/1758-2946-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safari-Alighiarloo N., Taghizadeh M., Rezaei-Tavirani M., Goliaei B., Peyvandi A.A. Protein-Protein Interaction Networks (PPI) and Complex Diseases. Gastroenterol Hepatol Bed Bench. 2014;7(1):17–31. [PMC free article] [PubMed] [Google Scholar]
- Saghazadeh A., Rezaei N. Towards treatment planning of COVID-19: Rationale and hypothesis for the use of multiple immunosuppressive agents: anti-antibodies, immunoglobulins, and corticosteroids. Int. Immunopharmacol. 2020;84 doi: 10.1016/j.intimp.2020.106560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargiacomo Camillo, Sotgia Federica, P. Lisanti Michael. COVID-19 and chronological aging: senolytics and other anti-aging drugs for the treatment or prevention of corona virus infection? Aging. 2020;12(8):6511–6517. doi: 10.18632/aging.103001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuang Ren H.Z., Mu Y., Sun M., Liu P. Pharmacological effects of Astragaloside IV: a literature review. J. Traditi. Chin. Med. 2013;33:413–416. doi: 10.1016/s0254-6272(13)60189-2. [DOI] [PubMed] [Google Scholar]
- Singh P. SPR biosensors: historical perspectives and current challenges. Sens. Actuators B. 2016;229:110–130. [Google Scholar]
- Singh S., Singh D.K., Meena A., Dubey V., Masood N., Luqman S. Rutin protects tbutyl hydroperoxide-induced oxidative impairment via modulating the Nrf2 and iNOS activity. Phytomedicine. 2019;55:92–104. doi: 10.1016/j.phymed.2018.07.009. [DOI] [PubMed] [Google Scholar]
- Szklarczyk D., Morris J.H., Cook H., Kuhn M., Wyder S., Simonovic M., Santos A., Doncheva N.T., Roth A., Bork P., Jensen L.J., von Mering C. The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017;45:D362–D368. doi: 10.1093/nar/gkw937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19)–China, 2020. China CDC Wkly. 2020;2(8):113–122. [PMC free article] [PubMed] [Google Scholar]
- Trott O., Olson A.J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Mering Christian, J. Jensen Lars, Snel Berend, D. Hooper Sean, Krupp Markus, Foglierini Mathilde, Jouffre Nelly, A. Huynen Martijn, Bork Peer. STRING: known and predicted protein-protein associations, integrated and transferred across organisms. Nucleic Acids Res. 2005;33:D433–D437. doi: 10.1093/nar/gki005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanchao Yin C.M., Luan X., Shen D.-D., Shen Q., Su H., Wang X., Zhou F., Zhao W., Gao M., Chang S., Xie Y.-C., Tian G., Jiang H.-W., Tao S.-C., Shen J., Jiang Y., Jiang H., Xu Y., Zhang S., Yan Zhang2 H.E.X. Structural basis for inhibition of the RNA-dependent RNA polymerase from SARS-CoV-2 by remdesivir. Science. 2020;368(6498) doi: 10.1126/science.abc1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Cai-Ping, Shi Yun-Wei, Tang Miao, Zhang Xiao-Chuan, Liang Xin-Miao, Wang Zhi-Wei, Zhang X.-C., Ding Fei. Isoquercetin ameliorates cerebral impairment in focal ischemia through anti-oxidative, anti-inflammatory, and anti-apoptotic effects in primary culture of rat hippocampal neurons and hippocampal CA1 region of rats. Mol. Neurobiol. 2017;54:2126–2142. doi: 10.1007/s12035-016-9806-5. [DOI] [PubMed] [Google Scholar]
- Wang S.-x., Wang Y., Lu Y.-b., Li J.-y., Song Y.-j., Nyamgerelt M., Wang X.-x. Diagnosis and treatment of novel coronavirus pneumonia based on the theory of traditional Chinese medicine. J. Integr. Med. 2020;18(4):275–283. doi: 10.1016/j.joim.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.X., Wang Y., Lu Y.B., Li J.Y., Song Y.J., Nyamgerelt M., Wang X.X. Diagnosis and treatment of novel coronavirus pneumonia based on the theory of traditional Chinese medicine. J Integr Med. 2020;18(4):275–283. doi: 10.1016/j.joim.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Wang Y., Ye D., Liu Q. Review of the 2019 novel coronavirus (SARS-CoV-2) based on current evidence. Int. J. Antimicrob. Agents. 2020;55(6):105948. doi: 10.1016/j.ijantimicag.2020.105948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y., Wei L., Jiang Y., Shen S., Zhao Y., Hao Y., Du Z., Tang J., Zhang Z., Jiang Q., Li L., Chen F., Shen H. Implementation of clinical diagnostic criteria and universal symptom survey contributed to lower magnitude and faster resolution of the COVID-19 epidemic in Wuhan. Engineering. 2020 doi: 10.1016/j.eng.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, 2020. The confirmed cases data for Coronavirus disease (COVID-19), https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
- Xiaoxia Fu L.-p.L., Tan X. Clinical observation on effect of Toujie Quwen Granules in treatment of COVID-19. Chin. J. Exp. Tradit. Med. Formulae. 2020;26:44–48. [Google Scholar]
- Xue R., Fang Z., Zhang M., Yi Z., Wen C., Shi T. TCMID: traditional Chinese medicine integrative database for herb molecular mechanism analysis. Nucleic Acids Res. 2012;41:D1089–D1095. doi: 10.1093/nar/gks1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Guo J., Yuan J. In vitro antioxidant properties of rutin. LWT–Food Sci. Technol. 2008;41:1060–1066. [Google Scholar]
- Ye W., Lu S., Xue A. The potential role of TNFalpha in 2019 novel coronavirus pneumonia. Respir. Med. Case Rep. 2020;30 doi: 10.1016/j.rmcr.2020.101087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonggang Zhou B.F., Zheng X., Wang D., Zhao C., qi Y., Sun R., Tian Z., Xu X., Wei H. Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID-19 patients. Perspect. Immunol. 2020;7(6):998–1002. doi: 10.1093/nsr/nwaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R., Chen L., Lan R., Shen R., Li P. Computational screening of antagonists against the SARS-CoV-2 (COVID-19) coronavirus by molecular docking. Int. J. Antimicrob. Agents. 2020;56 doi: 10.1016/j.ijantimicag.2020.106012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S., Baak J.P., Li S., Xiao W., Ren H., Yan H., Gan Y., Wen C. Network pharmacology analysis of the therapeutic mechanisms of the traditional Chinese herbal formula Lian Hua Qing Wen in Corona virus Disease 2019 (COVID-19), gives fundamental support to the clinical use of LHQW. Phytomedicine. 2020;79 doi: 10.1016/j.phymed.2020.153336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhi-guo Zhang J.Z., Qin H.-z., Wu L., Zhang J., Wang J.-l., Li L.-h., Gao G.-d. Astragaloside IV prevents MPP+-induced SH-SY5Y cell death via the inhibition of Bax-mediated pathways and ROS production. Mol. Cell Biochem. 2012;364:209–216. doi: 10.1007/s11010-011-1219-1. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Zhou B., Pache L., Chang M.W., Khodabakhshi A.H., Tanaseichuk O., Benner C., Chanda S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019;10:1523. doi: 10.1038/s41467-019-09234-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020;382(8) doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.