Abstract
Rationale & Objective
Patients receiving maintenance hemodialysis (MHD) are highly vulnerable to infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The current study was designed to evaluate the prevalence of SARS-CoV-2 infection based on both nucleic acid testing (NAT) and antibody testing in Chinese patients receiving MHD.
Study Design
Cross-sectional study.
Setting & Participants
From December 1, 2019, to March 31, 2020, a total of 1,027 MHD patients in 5 large hemodialysis centers in Wuhan, China, were enrolled. Patients were screened for SARS-CoV-2 infection by symptoms and initial computed tomography (CT) of the chest. If patients developed symptoms after the initial screening was negative, repeat CT was performed. Patients suspected of being infected with SARS-CoV-2 were tested with 2 consecutive throat swabs for viral RNA. In mid-March 2020, antibody testing for SARS-CoV-2 was obtained for all MHD patients.
Exposure
NAT and antibody testing results for SARS-CoV-2.
Outcomes
Morbidity, clinical features, and laboratory and radiologic findings.
Analytical Approach
Differences between groups were examined using t test or Mann-Whitney U test, comparing those not infected with those infected and comparing those with infection detected using NAT with those with infection detected by positive serology test results.
Results
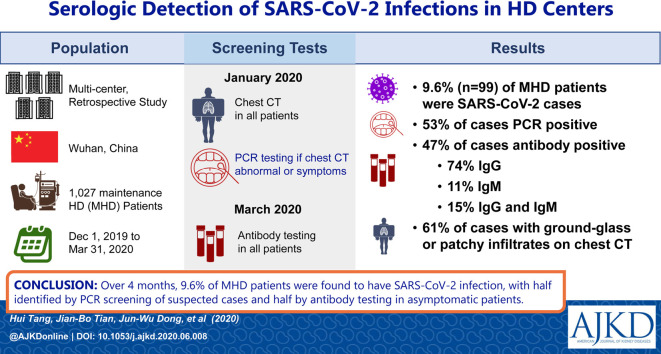
Among 1,027 patients receiving MHD, 99 were identified as having SARS-CoV-2 infection, for a prevalence of 9.6%. Among the 99 cases, 52 (53%) were initially diagnosed with SARS-CoV-2 infection by positive NAT; 47 (47%) were identified later by positive immunoglobulin G (IgG) or IgM antibodies against SARS-CoV-2. There was a spectrum of antibody profiles in these 47 patients: IgM antibodies in 5 (11%), IgG antibodies in 35 (74%), and both IgM and IgG antibodies in 7 (15%). Of the 99 cases, 51% were asymptomatic during the epidemic; 61% had ground-glass or patchy opacities on CT of the chest compared with 11.6% among uninfected patients (P < 0.001). Patients with hypertensive kidney disease were more often found to have SARS-CoV-2 infection and were more likely to be symptomatic than patients with another primary cause of kidney failure.
Limitations
Possible false-positive and false-negative results for both NAT and antibody testing; possible lack of generalizability to other dialysis populations.
Conclusions
Half the SARS-CoV-2 infections in patients receiving MHD were subclinical and were not identified by universal CT of the chest and selective NAT. Serologic testing may help evaluate the overall prevalence and understand the diversity of clinical courses among patients receiving MHD who are infected with SARS-CoV-2.
Index Words: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), SARS-CoV-2 infection, coronavirus disease 2019 (COVID-19), hemodialysis, end-stage kidney disease (ESKD), dialysis unit, nucleic acid testing (NAT), serology, antibody testing, chest computed tomography, infection detection, subclinical infection, asymptomatic infection, chronic kidney disease (CKD), disease surveillance, pandemic
Graphical abstract
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is a global health threat, especially to the elderly or those with underlying diseases.1, 2, 3 SARS-CoV-2 was identified as the causative agent of COVID-19 on January 7, 2020, through deep sequencing analysis of patients’ respiratory tract samples.4 , 5 Since then, nucleic acid testing (NAT) against SARS-CoV-2 was developed and used to identify patients infected with the virus. However, due to variable virus shedding,6 sample collection issues, lack of symptoms, and limited availability of NAT, some current or previous infections may not be detected. Screening with computed tomography (CT) of the chest may identify cases7 , 8 but requires confirmation by using NAT. The difficulties associated with NAT make the timely and accurate diagnosis of SARS-CoV-2 infection a potential hurdle for minimizing the damage of the current outbreak.
NAT or CT of the chest of the whole population is not practical and is costly. Therefore, initial surveillance was focused primarily on patients with obvious symptoms or close contact history with an infected individual. However, the full spectrum of the disease, including mild or asymptomatic infections that do not require medical attention, is not clear. Whether those who are reported as asymptomatic may be able to transmit the virus to other individuals also needs further investigation.9 The latest (7th edition) of the New Coronavirus Pneumonia Prevention and Control Program, published by the National Health Commission of China, added serologic evidence to the diagnostic criteria for COVID-19. Surveillance of antibody seropositivity in a population may help clarify the prevalence of infection.
Maintenance hemodialysis (MHD) patients are a population with increased exposure risk during the pandemic because they must routinely visit the hospital (often using public transit) and are treated in a group setting in dialysis units. Limited information is available regarding the prevalence and serologic characteristics of SARS-CoV-2 infection in MHD patients. To clarify the full spectrum of SARS-CoV-2 infection in MHD patients, we retrospectively collected and analyzed detailed data from all MHD patients in 5 medical institutions in Wuhan, China, who were screened for SARS-CoV-2 infection.
Methods
Study Design and Participants
For this multicenter retrospective observational study, we reviewed data from December 1, 2019, to March 31, 2020, of the 1,048 patients who represent all MHD patients in 5 large dialysis centers in Wuhan, China. Since late January 2020, these 5 centers have been performing regular strict entrance screening of body temperature and respiratory or digestive tract symptoms at the time of every dialysis treatment. All MHD patients also underwent initial screening using CT of the chest. In addition, all patients underwent laboratory tests (C-reactive protein [CRP] concentration and complete blood cell count) at the time of initial CT. If patients developed symptoms after the initial screening was negative, repeat CT and laboratory testing were performed.10 Patients having an abnormal result for CT of the chest suggestive of viral pneumonia were regularly monitored to determine whether there were progressive changes on CT of the chest consistent with the classic evolution of COVID-19. Around the middle of March 2020, antibody testing for SARS-CoV-2 was performed in all MHD patients in the 5 centers, except for those who had died during the pandemic or who declined. Patients who were negative during the previous screening but had a positive serologic result underwent NAT 2 consecutive times after the serology results were known.
This study was approved by the Ethics Committee of Wuhan Union Hospital. The requirement for informed patient consent was waived by the ethics committee for this retrospective study.
SARS-CoV-2 Nucleic Acid and Serum Antibody Measurement
NAT for SARS-CoV-2 was done using a quantitative real-time reverse transcriptase–polymerase chain reaction (RT-PCR) assay of throat swab specimens as previously described.11 Serum immunoglobulin M (IgM) and IgG antibodies against SARS-CoV-2 were detected using Colloidal Gold Immunochromatography Assay kits supplied by Tangshan Yingnuote Biotechnology Enterprise Co, Ltd, according to the manufacturer’s instructions.12 The assay is primarily directed against the SARS-CoV-2 nucleocapsid (N) protein, with some reactivity to the spike (S) protein.
Briefly, serum samples were diluted 10-fold and added onto the sample pad of the test strip; antibodies present in serum form a complex with virus antigen that had been labeled with colloidal gold nanoparticles. This complex migrates by capillary action until it is captured by a strip of coating antibody (mouse anti-human IgG or IgM monoclonal antibody), which generates a band of visible color that appears in less than 10 minutes, with intensity determined by the amount of the antibody. Sensitivity of the assay was 87.3% for both IgM and IgG antibodies, determined by testing 126 RT-PCR–confirmed SARS-CoV-2 infections. The specificity of the assays was 100% for both IgM and IgG antibodies, determined by testing 62 samples from clinically excluded cases. No cross-reaction was found to coronaviruses (including HKU1, OC43, NL63, and 229E) and also to other viruses (including influenza H1N1, H3N2, H5N1, H7N9; influenza B virus; respiratory syncytial virus; adenovirus; rhinovirus; and parainfluenza virus). There was also no cross-reaction with rheumatoid factors, antinuclear antibodies, and anti-mitochondrial antibody.
Data Collection
We collected exposure history, demographics, clinical features, laboratory findings, and images from CT of the chest for all enrolled patients from December 1, 2019, to March 31, 2020. Direct communication with patients or their families or attending physicians occurred if important data were missing from the records or clarification was needed. The cutoff temperature used in this study to define fever was 37.3°C. Laboratory testing (including a complete blood cell count, assessment of liver function, and measurements of serum albumin, electrolytes, CRP, lactate dehydrogenase, and coagulation testing) was performed according to the clinical care needs of the patient. For NAT-positive patients, the laboratory results used were the results on admission or those nearest to disease onset. For antibody-positive patients, the laboratory results around the time of antibody testing were used. The presence of a radiologic abnormality was determined based on descriptions in medical charts or associated documentation; if imaging scans were available, they were reviewed by attending physicians in respiratory medicine or an imaging specialist. All data were carefully reviewed and cross-checked by 2 physicians (H.T. and J.H.).
Statistical Analysis
Continuous variables are presented as mean ± standard deviation for normally distributed data or median and interquartile range for skewed data. Categorical variables were expressed as number with percent. Normally distributed continuous variables were analyzed using t test for between-group comparisons and analysis of variance for comparison among multiple groups. Variables displaying skewed distribution were compared using Mann-Whitney U test (between groups) and Kruskal-Wallis test (among multiple groups). Categorical variables were compared using Pearson χ2 test, continuity correction χ2 test, or Fisher exact test according to the following situations: (1) if all expected counts T ≥ 5 and total sample size n ≥ 40, Pearson χ2 tests were used; (2) if the expected counts 5 > T ≥ 1 and n ≥ 40, continuity correction χ2 tests were used; and (3) if the expected counts T < 1 or n ≥ 40, Fisher tests were used.13 A 2-sided P < 0.05 was considered statistically significant. All statistical analyses were performed using SAS (version 9.4, SAS institute) or SPSS (version 22.0, IBM Corp.).
Results
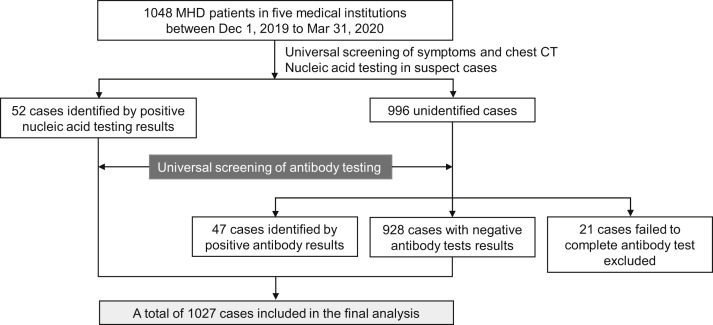
After excluding 21 patients for whom serologic testing could not be performed before March 31, 2020, a total of 1,027 MHD patients were included in the final analysis (Fig 1 ). Demographic, epidemiologic, and clinical data of these patients were retrospectively collected and analyzed.
Figure 1.
Flow chart of the study population selection. Abbreviations: CT, computed tomography; MHD, maintenance hemodialysis.
Comparison of MHD Patients With and Without SARS-CoV-2 Infection
Patients with positive results for either NAT or antibody testing were categorized as having SARS-CoV-2 infection, all others were categorized as being without SARS-CoV-2 infection (Table 1 ). In the overall cohort, mean age of patients was 60.3 ± 13.4 years, with a male preponderance (60.3%). No significant differences in age distribution (P = 0.5) or sex (P = 0.3) were identified between patients with and without infection. Close contact history with patients with COVID-19 was more common in infected patients (P < 0.001). No significant differences in body mass index and current smoking status were identified between patients with and without infection.
Table 1.
Demographics, Baseline and Clinical Characteristics of MHD Patients With or Without SARS-CoV-2 Infection
| Characteristics | Total Patients (N = 1,027) | Patients Without Infection (n = 928) | Patients With Infection (n = 99) | P |
|---|---|---|---|---|
| Patient origin | ||||
| Hospital No. 1 | 158 (15.4%) | 136 (14.7%) | 22 (22%) | |
| Hospital No. 2 | 314 (30.6%) | 299 (32.2%) | 15 (15%) | |
| Hospital No. 3 | 183 (17.8%) | 168 (18.1%) | 15 (15%) | |
| Hospital No. 4 | 233 (22.7%) | 217 (23.3%) | 16 (16%) | |
| Hospital No. 5 | 139 (13.5%) | 108 (11.6%) | 31 (31%) | |
| Age, y | 60.3 ± 13.4 | 60.2 ± 13.4 | 61.3 ± 13.8 | 0.4a |
| Sex | ||||
| Male | 619 (60.3%) | 564 (60.8%) | 55 (56%) | 0.3b |
| Female | 408 (39.7%) | 364 (39.2%) | 44 (44%) | 0.3b |
| BMI, kg/m2 | 22.0 [20.1-24.5] | 22.1[20.1-25.0] | 21.8 [20.4-24.2] | 0.8c |
| Current smoker | 374 (36.4%) | 342 (36.9%) | 32 (32%) | 0.3b |
| Contact with COVID-19 patient(s) | 45 (4.4%) | 28 (3.0%) | 17 (17%) | <0.001d,e |
| COVID-19 patient(s) in the family | 13 (1.3%) | 7 (0.8%) | 6 (6%) | <0.001d,e |
| Primary cause of kidney failure | ||||
| Diabetic nephropathy | 278 (27.1%) | 257 (27.7%) | 21 (21%) | 0.1b |
| Hypertensive kidney disease | 117 (11.4%) | 93 (10.0%) | 24 (24%) | <0.001b,d |
| Glomerulonephritis | 460 (44.8%) | 423 (45.6%) | 37 (37%) | 0.1b |
| Polycystic kidney disease | 24 (2.3%) | 23 (2.5%) | 1 (1%) | 0.6e |
| Lupus nephritis | 7 (0.7%) | 5 (0.5%) | 2 (2%) | 0.1f |
| Others | 152 (14.8%) | 136 (14.7%) | 16 (16%) | 0.6b |
| Comorbid conditions | ||||
| Cardiovascular disease | 701 (68.3%) | 623 (67.1%) | 78 (79%) | 0.01b,d |
| Diabetes mellitus | 184 (17.9%) | 160 (17.2%) | 24 (24%) | 0.08b |
| COPD | 8 (0.8%) | 6 (0.6%) | 2 (2%) | 0.2f |
| Cancer | 19 (1.9%) | 19 (2.0%) | 0 (0%) | 0.3e |
| Use of ACEi/ARB | 178/474 (37.6%) | 151/412 (36.7%) | 27/62 (44%) | 0.1b |
| Hemodialysis modality | ||||
| Hemodialysis | 1,020 (99.3%) | 926 (99.8%) | 94 (95%) | <0.001b,d |
| Hemodiafiltration | 768 (74.8%) | 721 (77.7%) | 47 (47%) | <0.001b,d |
| Hemoperfusion | 284 (27.7%) | 273 (29.4%) | 11 (11%) | <0.001b,d |
| Hemodialysis access | 0.04d,f | |||
| AVF | 727/1,001 (72.6%) | 665/906 (73.4%) | 62/95 (65%) | |
| CVC | 267/1,001 (26.7%) | 236/906 (26.0%) | 31/95 (33%) | |
| CVC/AVF | 1/1,001 (0.1%) | 0/906 (0%) | 1/95 (1%) | |
| AVG | 3/1,001 (0.3%) | 2/906 (0.2%) | 1/95 (1%) | |
| Others | 3/1,001 (0.3%) | 3/906 (0.3%) | 0/95 (0%) | |
| Hemodialysis frequency | 0.4c | |||
| <3×/wk | 442/999 (44.2%) | 403/903 (44.6%) | 39/96 (41%) | |
| 3×/wk | 557/999 (55.8%) | 500/903 (55.4%) | 57/96 (59%) |
Note: Values for categorical variables given as count (percentage) or n/N (percentage); for continuous variables, as mean ± standard deviation or median [interquartile range].
Abbreviations: ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; AVF, arteriovenous fistula; AVG, arteriovenous graft; BMI, body mass index; COPD, chronic obstructive pulmonary disease; CVC, central venous catheter; MHD, maintenance hemodialysis.
P values were calculated using at test or cMann-Whitney U test for continuous variables and bPearson χ2 test, econtinuity correction χ2 test, or fFisher exact test for categorical variables; denoted dstatistically significant if P < 0.05.
The primary causes of kidney failure were similar in the 2 groups, but hypertensive kidney disease was more common in the group with infection (P < 0.001). Among the overall cohort, cardiovascular disease (including hypertension) was the most common comorbid condition (68.3%) and was more common in patients with infection than without (79% vs 67.1%; P = 0.01). Chronic obstructive pulmonary disease and cancer were not significantly different between the 2 groups.
During the study period, hemodialysis modalities used in the outpatient setting by MHD patients in our cohort included hemodialysis (99.3%), hemodiafiltration (74.8%), and hemoperfusion (27.7%); patients may have received more than 1 modality during the period of observation. Overall, most (72.6%) patients were using an arteriovenous fistula for hemodialysis access and more than half (55.6%) received dialysis 3 times per week during the study period. There was a statistically significant difference in type of dialysis access between patients with and without SARS-CoV-2 infection (P = 0.04; Table 1).
As shown in Table 2 , the most common radiologic finding in infected patients was ground-glass or patchy opacity, and the lesions often involved both lungs. We also noted that 12% of patients had ground-glass opacities on CT of the chest accompanied with or without obvious symptoms but with negative NAT and antibody testing results. The CT images of these patients showed no progressive worsening in contrast to the classic radiologic evolution of COVID-19. Data from laboratory tests showed that in patients with SARS-CoV-2 infection, median white blood cell and lymphocyte counts were lower, serum calcium concentrations were lower, and CRP levels were higher than values in patients without infection.
Table 2.
Radiologic and Laboratory Findings of MHD Patients With or Without SARS-CoV-2 Infection
| Findings | Total (N = 1,027) | Patients Without Infection (n = 928) | Patients With Infection (n = 99) | P |
|---|---|---|---|---|
| Radiologic Findings | ||||
| CT image features | ||||
| Ground-glass/patchy opacity | 131/720 (18.2%) | 72/623 (11.6%) | 59/97 (61%) | <0.001a,b |
| Fibrosis | 104/720 (14.4%) | 88/623 (14.1%) | 16/97 (16%) | 0.5b |
| Consolidation | 23/720 (3.2%) | 21/623 (3.4%) | 2/97 (2%) | 0.7c |
| Others | 104/720 (14.4%) | 94/623 (15.1%) | 10/97 (10%) | 0.2b |
| Normal | 360/720 (50.0%) | 348/623 (55.9%) | 12/97 (12%) | <0.001a,b |
| Lesion region | 0.07c | |||
| Bilateral lung | 230/351 (65.5%) | 169/271 (62.4%) | 61/80 (76%) | |
| Left lung | 59/351 (16.8%) | 49/271 (18.1%) | 10/80 (13%) | |
| Right lung | 62/351 (17.7%) | 53/271 (19.6%) | 9/80 (11%) | |
| Laboratory Findings | ||||
| Hemoglobin, g/dL | 10.3 [8.7-11.5] | 10.3 [8.7-11.5] | 10.2 [8.5-11.3] | 0.3d |
| Platelets, ×103/μL | 160 [125-203] | 160 [126-203] | 161 [117-200] | 0.8d |
| Leukocytes, /μL | 5,500 [4,500-6,990] | 5,570 [4,535-7,030] | 4,900 [4,040-6,510] | 0.002a,d |
| Lymphocytes, /μL | 970 [730-1,260] | 970 [740-1,260] | 860 [660-1,150] | 0.01a,d |
| Absolute lymphocyte count < 1,000/μL | 476/896 (53.1%) | 421/809 (52.0%) | 55/87 (63%) | 0.03a,b |
| Neutrophils, /μL | 3,870 [3,040-5,110] | 3,920 [3,050-5,110] | 3,450 [2,870-4,520] | 0.05d |
| Albumin, g/dL | 3.8 [2.2-4.1] | 3.8 [1.8-4.1] | 3.8 [3.4-4.0] | 0.4d |
| AST, U/L | 11.6 [9.0-16.5] | 11.0 [8.8-16.0] | 13.5 [10.5-20.0] | 0.001a,d |
| ALT, U/L | 8.4 [6.0-13.6] | 8.0 [6.0-13.4] | 10.8 [8.0-14.7] | 0.001a,d |
| Serum phosphorus, mmol/L | 2.2 [1.7-434.6] | 2.2 [1.7-540.9] | 1.9 [1.6-2.7] | 0.1d |
| Serum calcium, mmol/L | 2.3 [2.1-15.1] | 2.3 [2.1-15.9] | 2.2 [2.0-2.4] | 0.02a,d |
| CRP, mg/L | 1.4 [0.5-164.3] | 1.4 [0.5-168.2] | 2.0 [1.0-5.4] | 0.3d |
| D-Dimer, mg/L | 2.1 [1.5-4.6] | 2.1 [1.4-3.5] | 5.6 [2.2-30.1] | <0.001a,d |
Note: Values for categorical variables given as n/N (percentage); for continuous variables, as median [interquartile range]. Reference range for CRP, 0 to 4 mg/L; for D-dimer, 0 to 0.5 mg/L.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRP, C-reactive protein; CT, computed tomography; MHD, maintenance hemodialysis.
P values were calculated using dMann-Whitney U test for continuous variables and bPearson χ2 test, ccontinuity correction χ2 test for categorical variables; denoted astatistically significant if P < 0.05.
Comparison of Patients With SARS-CoV-2 Infection Detected by NAT Versus Antibody Testing
Although intensive monitoring of symptoms, universal radiologic screening, and selective RNA testing had been carried out since late January 2020, about half (47 [47%]) of the SARS-CoV-2–infected patients were retrospectively identified by antibody testing. No significant differences in age distribution (P = 0.7) or sex (P = 0.7) were identified between the 2 groups, but current smokers were more common in patients identified by positive antibody responses than in those identified by NAT (43% vs 23%; P = 0.03; Table 3 ). Twelve percent of patients in the NAT-identified group had 1 or more than 1 family member with COVID-19 diagnosed compared with none in the antibody testing–identified group (P = 0.04). The primary causes of kidney failure in the NAT-identified group were similar to those of the antibody testing–identified group, except for a higher percentage of hypertensive kidney disease (33% vs 15%; P = 0.03). There was no significant difference identified in comorbid conditions between the 2 groups, but dialysis modality patterns and dialysis frequency were different. In the group identified by antibody testing, more patients received hemodiafiltration (66% vs 31%; P < 0.001) and hemoperfusion (19% vs 4%; P = 0.01).
Table 3.
Characteristics of MHD Patients Infected With SARS-CoV-2 Identified by NAT or Antibody Testing
| Characteristic | Patients Identified by NAT (n = 52) | Patients Identified by Antibody Testing (n = 47) | P |
|---|---|---|---|
| Age, y | 61.65 ± 14.65 | 60.79 ± 12.87 | 0.7a |
| Male sex | 28 (54%) | 27 (57%) | 0.7b |
| BMI, kg/m2 | 21.90 [20.57-24.97] | 21.45 [19.92-23.84] | 0.3c |
| Current smoker | 12 (23%) | 20 (43%) | 0.03b,d |
| COVID-19 patient(s) in the family | 6 (12%) | 0 (0%) | 0.04d,e |
| Primary cause of kidney failure | |||
| Diabetic nephropathy | 11 (21%) | 10 (21%) | 0.9b |
| Hypertensive kidney disease | 17 (33%) | 7 (15%) | 0.03b,d |
| Glomerulonephritis | 16 (31%) | 21 (45%) | 0.1b |
| Polycystic kidney disease | 1 (2%) | 0 (0%) | 0.9f |
| Lupus nephritis | 1 (2%) | 1 (2%) | 0.9f |
| Others | 6 (12%) | 10 (21%) | 0.1b |
| Comorbid condition | |||
| Cardiovascular disease | 41 (79%) | 37 (79%) | 0.9b |
| Diabetes mellitus | 13 (25%) | 11 (23%) | 0.8b |
| COPD | 2 (4%) | 0 | 0.5e |
| Hemodialysis modality | |||
| Hemodialysis | 47 (90%) | 47 (100%) | 0.08b |
| Hemodiafiltration | 16 (31%) | 31 (66%) | <0.001b,d |
| Hemoperfusion | 2 (4%) | 9 (19%) | 0.01b |
| Hemodialysis access | 0.8f | ||
| AVF | 33 (63%) | 29/43 (67%) | |
| CVC | 18 (35%) | 13/43 (30%) | |
| CVC/AVF | 1 (2%) | 0/43 (0%) | |
| AVG | 0 (0%) | 1/43 (2%) | |
| Others | 0 (0%) | 0/43 (0%) | |
| Symptoms | |||
| Fever | 25 (48%) | 2 (4%) | <0.001b,d |
| Cough | 18 (35%) | 9 (19%) | 0.08b |
| Sputum production | 13 (25%) | 3 (6%) | 0.01b,d |
| Dyspnea | 11 (21%) | 3 (6%) | 0.06c |
| Nausea/Vomiting | 7 (13%) | 0 (0%) | 0.02e,d |
| Diarrhea | 4 (8%) | 0 (0%) | 0.1e |
| Sore throat | 4 (8%) | 0 (0%) | 0.1e |
| Asymptomatic | 13 (25%) | 37 (79%) | <0.001b,d |
| Radiologic findings | |||
| CT image features | |||
| Ground-glass/patchy opacity | 42/51 (82%) | 17/46 (37%) | <0.001b,d |
| Fibrosis | 3/51 (6%) | 13/46 (28%) | 0.003b,d |
| Consolidation | 1/51 (2%) | 1/46 (2%) | 0.9f |
| Others | 5/51 (10%) | 5/46 (11%) | 0.9e |
| Normal | 0/51 (0%) | 12/46 (26%) | <0.001b,d |
| Lesion region | |||
| Bilateral lung | 40/47 (85%) | 21/33 (64%) | 0.08f |
| Left lung | 4/47 (9%) | 6/33 (18%) | 0.3b |
| Right lung | 3/47 (6%) | 6/33 (18%) | 0.1b |
| Laboratory findings | |||
| Hemoglobin, g/dL | 10.3 ± 1.9 | 9.9 ± 2.2 | 0.6a |
| Platelets, ×103/μL | 167 ± 69 | 165 ± 65 | 0.9a |
| Leukocytes, /μL | 4,760 [3,800-6,740] | 5,000 [4,330-6,200] | 0.5c |
| Lymphocytes, /μL | 760 [440-930] | 990 [760-1,310] | <0.001c,d |
| Neutrophils, /μL | 3,420 [2,800-5,810] | 3,470 [2,970-4,190] | 0.4c |
| Albumin, g/dL | 3.8 [3.5-4.0] | 3.7 [3.3-4.0] | 0.2c |
| CRP, mg/L | 16.6 [5.2-88.8] | 2.4 [1.2-7.4] | <0.001c,d |
Abbreviations: AVF, arteriovenous fistula; AVG, arteriovenous graft; BMI, body mass index. COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; CT, computed tomography; CVC, central venous catheter; MHD, maintenance hemodialysis; NAT, nucleic acid testing.
P values were calculated using at test or cMann-Whitney U test for continuous variables and bPearson χ2 test, econtinuity correction χ2 test, or fFisher exact test for categorical variables; denoted dstatistically significant if P < 0.05.
More than half (51%) the patients infected with SARS-CoV-2 were asymptomatic during the study period. The most common symptoms at onset in the NAT-identified group were fever (48%) and dry cough (35%). Other symptoms included sputum production, dyspnea, nausea or vomiting, diarrhea, and sore throat. All the symptoms were less common in the group identified by positive antibody test results (Table 3). Compared with the NAT-identified group, the antibody testing–identified group presented less frequently with ground-glass or patchy opacity abnormalities (37% vs 82%; P < 0.001) but more frequently with fibrosis (28% vs 6%; P = 0.003) in the images from CT of the chest. The lesions often involved both lungs in the NAT-identified group (85%), which was nominally more frequent than in the antibody testing–identified group (64%), though this was not statistically significant (P = 0.08). Compared with the NAT-identified group, patients in the antibody testing–identified group had significantly higher lymphocyte counts (P < 0.001) and lower CRP levels (P < 0.001). Other laboratory findings did not significantly differ between the 2 groups.
Serologic Profile of Patients With SARS-CoV-2 Infection
The 47 patients whose infection was detected by positive antibody testing underwent NAT 2 consecutive times when serology results were known; all had negative NAT results. Five of these 47 patients presented with IgM antibodies only; 35 patients, with IgG antibodies only; and 7, with both IgM and IgG antibodies. All these patients continued routine dialysis in the outpatient department, and none were hospitalized during the study period.
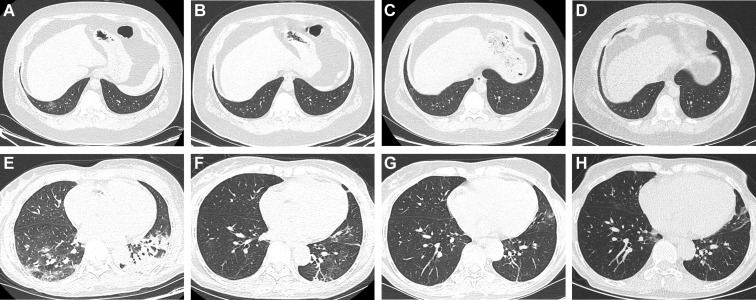
Figure 2 shows the evolution of manifestations on CT of the chest over time in 2 patients with SARS-CoV-2 infection retrospectively identified by positive test results for IgG antibody. The first patient was asymptomatic and the second patient had a mild cough for a week during the study period. Two consecutive quantitative RT-PCR tests had been performed in each of these 2 patients after the first abnormal CT result, and results were consistently negative.
Figure 2.
Representative images from computed tomography (CT) of the chest of 2 patients with SARS-CoV-2 infections identified by antibody testing. (A-D) Transverse images from CT of the chest from a 39-year-old man who was asymptomatic but with a transient decrease in white blood cell and lymphocyte counts and elevated C-reactive protein level. The images were obtained (A) 42, (B) 35, (C) 21, and (D) 7 days before the first positive antibody (immunoglobulin G [IgG]) response was found in this patient, showing a time-dependent resolution of a unilateral ground-glass opacity in the right lower lobe. (E-H) Transverse images from CT of the chest from a 63-year-old woman with mild cough for a week and elevated C-reactive protein level. Images were obtained (E) 39, (F) 36, and (G) 0 days before and (H) 20 days after the first positive antibody (IgG) response against SARS-CoV-2 was found in this patient. Bilateral patchy opacity and consolidation in the earliest images were gradually absorbed and disappeared over time.
Of the 52 patients with positive NAT results, 13 had no serologic test results because they died, 31 were found to be positive for IgG but negative for IgM, 5 were positive for both IgG and IgM, and 3 were negative for IgG and IgM. The interval between initial NAT diagnosis and antibody testing ranged from 14 to 55 days.
Discussion
In this multicenter study focused on MHD patients in Wuhan, we found that almost half the SARS-CoV-2 infections were subclinical and not identified by NAT and CT of the chest screening. Serologic tests detected asymptomatic SARS-CoV-2 infection in a large minority of infected patients and provided further information regarding the full spectrum of the disease in MHD patients. Our observations suggest that patients who have hypertensive kidney disease and with accompanying cardiovascular disease may be more susceptible to SARS-CoV-2 infection and tend to develop obvious symptoms.
MHD patients may be vulnerable to virus infection due to the uremic state and high prevalence of coexisting disorders such as cardiovascular disease, diabetes, and cerebrovascular disease.14, 15, 16 Additional risk factors in MHD patients include the need to routinely visit the hospital (often using public transportation) and the potential for close contact between patients in the dialysis unit. Therefore, extra efforts must be made to prevent infection spread within dialysis units. Since late January, health workers in hemodialysis centers in Wuhan have been performing strict entrance screening using body temperature and the presence of respiratory or digestive symptoms, as well as a universal screen by CT of the chest. When symptoms or an abnormal result from CT of the chest suggestive of viral pneumonia were present, patients were immediately isolated and asked to complete NAT 2 consecutive times.10 Universal screening of symptoms and pulmonary CT combined with selective NAT were undoubtedly important measures for helping identify and isolate SARS-CoV-2–infected patients, especially in the early phase of the epidemic. However, some infected patients who were asymptomatic, lacked typical radiology features, or had a low level of virus load may be missed. Additionally, the symptoms and manifestations on CT of the chest of COVID-19 were difficult to distinguish from those due to the uremic state or other common respiratory tract pathogens in MHD patients, which added an extra level of difficulty identifying SARS-CoV-2–infected patients in the MHD population.
For many known pathogenic viruses, antibody testing is suggested for the confirmation of infection. Recent studies have found that a serologic response is detectable 7 to 10 days or later after the onset of symptoms.6 , 17 Even in the early stages of the illness, when some patients with a low virus load in the upper respiratory tract may have negative NAT results, the infections could be identified through antibody testing.18 Normally, the IgG antibody response in serum lasts longer and indicates past infection whereas IgM may represent recent infection. Compared with NAT, serologic testing has the advantages of high throughput and less workload. With further understanding of host antibody responses during infection, serologic testing may have an important role in diagnosing acute and past SARS-CoV-2 infections.
To gain a better understanding of the antibody response and clarify the full extent of the infection, we screened the patients in these 5 large dialysis centers with antibody testing against SARS-CoV-2. In addition to 52 COVID-19–infected patients who previously had been diagnosed through positive NAT results, 47 patients were newly identified by antibody seropositivity, which indicates an overall SARS-CoV-2 infection rate of 9.6% in MHD patients in these dialysis centers. These newly identified patients were all in good condition with favorable prognoses to date, indicating a mild clinical course in these patients.
When comparing the 99 SARS-CoV-2–infected MHD patients with noninfected MHD patients, our findings are similar to those of previous studies in the general population1 , 19 and our earlier investigation of the MHD population.10 Hypertensive kidney disease as the primary cause of kidney and cardiovascular disease as a comorbid condition were more common in infected patients. The most common symptoms were fever and cough, with ground-glass opacity or patchy opacity involving both lungs as the typical radiologic findings. Of the SARS-CoV-2–infected patients, 25% who had been identified with NAT and 79% of those identified by serologic testing were asymptomatic during the whole clinical course. Decreased white blood cell and lymphocyte counts, lower serum calcium concentration, and elevated CRP levels were found in patients with versus without infection. These findings suggested an immune response in the infected MHD patients that is similar to that of the general population.11
Within SARS-CoV-2–infected patients, we compared those identified by NAT versus antibody testing. The antibody group had twice the proportion of current smokers compared with the NAT group. Recent studies suggest that smoking is linked to higher expression of ACE2 (the receptor for SARS-CoV-2).20 Nonetheless, according to the current literature, there is no strong evidence supporting an association between smoking and the prevalence or severity of COVID-19.21 However, respiratory symptoms caused by smoking may mask those caused by the virus, making patients and health workers unaware of the infection.
In the NAT-identified group, 12% of patients had 1 or more family members with COVID-19 diagnosed compared with none in the antibody-identified group, which may indicate that lower viral loads were present in patients identified by antibody testing. Examining the timeline of infections in patients and their families may provide further clarification. Also, patients in the antibody-identified group were mostly asymptomatic, which indicates the possible limited utility of case identification by screening CT of the chest and NAT in asymptomatic patients.
It is unknown whether dialysis itself has an impact on SARS-CoV-2 infection or disease severity. Previous studies have demonstrated that continuous kidney replacement therapy has been successfully applied in the treatment of SARS, MERS, and sepsis infections22 , 23 by helping to remove potentially damaging toxins and stabilizing the metabolic and hemodynamic status of patients. Plasma exchange and adsorption were shown to have a potential effect in managing the cytokine storm and pathogenic antibodies in 3 critically ill patients with COVID-19.24 The possible benefits of other dialysis modalities in COVID-19 are still unknown. In our study, we found that infected patients receiving hemodiafiltration or hemoperfusion accompanied by hemodialysis tended to develop less obvious symptoms and may have a milder clinical course and lower virus load. Hemodiafiltration and hemoperfusion are adjuvant therapies for sepsis and acute respiratory distress syndrome in that they remove circulating endotoxin and inflammatory cytokines.25 , 26 The tissue damage in patients with COVID-19 may be caused by an unchecked cytokine storm,27 , 28 which could potentially be alleviated by hemodiafiltration or hemoperfusion. However, the specific mechanism needs to be further investigated.
There were several limitations of our study. Some patients may have been falsely characterized as infected with SARS-CoV-2 because of false-positive NAT or antibody testing results, whereas some infected patients may be unrecognized because of false-negative test results. Also, the data relating to patients identified by antibody seropositivity were retrospectively collected, medical information at the onset of infection was difficult to obtain, and complete laboratory testing was not always performed. To gain a better understanding of the serologic response profile and related mechanisms of SARS-CoV-2 infection in MHD patients, long-term follow-up studies are needed. The population studied was Chinese with kidney failure primarily due to glomerulonephritis, so results may not be generalizable to other populations.
By performing widespread serologic testing in MHD patients, we obtained a better understanding of the full spectrum of SARS-CoV-2 infection in these patients and demonstrated the important role of antibody testing to identify patients with asymptomatic infections. The large proportion of subclinical cases increases the difficulty identifying SARS-CoV-2–infected MHD patients and controlling outbreaks in the dialysis centers. The combination of radiologic evidence, NAT, and antibody testing was critical for the optimal screening of SARS-CoV-2 infection and for providing timely and accurate diagnosis and appropriate isolation of infected patients.
Article Information
Authors’ Full Names and Academic Degrees
Hui Tang, MD, PHD, Jian-Bo Tian, PhD, Jun-Wu Dong, MD, Xiao-Tie Tang, MD, Zhen-Yuan Yan, MD, Yuan-Yuan Zhao, MD, Fei Xiong, MD, Xin Sun, BSN, Cai-Xia Song, MD, Chang-Gang Xiang, MD, Can Tu, MD, Chun-Tao Lei, MD, PhD, Jing Liu, MD, PhD, Hua Su, MD, PhD, Jing Huang, MD, Yang Qiu, MD, Xiao-Ping Miao, PhD, and Chun Zhang, MD, PhD.
Authors’ Contributions
Conceived and designed the study: CZ, X-PM; analyzed data: J-BT, HT; collected data: HT, C-TL, JL, JH, YQ, Y-YZ, C-XS, J-WD, Z-YY, X-TT, FX, XS, C-GX, CT; additional guidance for data collection and analysis: HS. HT, J-BT, J-WD, X-TT, Z-YY, and Y-YZ contributed equally to this work. Each author contributed important intellectual content during manuscript drafting or revision and agrees to be personally accountable for the individual’s own contributions and to ensure that questions pertaining to the accuracy or integrity of any portion of the work, even one in which the author was not directly involved, are appropriately investigated and resolved, including with documentation in the literature if appropriate.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Support
This work was financially supported by the National Natural Science Foundation of China (81700603, 81961138007, 81974096, 81770711, 81873602), the National Key Research and Development Program (2020YFC0845800, 2020YFC0844700, 2018YFC1314000), and the Program for HUST Academic Frontier Youth Team (2017QYTD20). The funders did not have a role in study design; data collection, analysis, or reporting; or the decision to submit for publication.
Acknowledgements
We acknowledge all health care workers involved in the diagnosis and treatment of MHD patients in Wuhan.
Peer Review
Received April 14, 2020. Evaluated by 2 external peer reviewers and a radiologist, with direct editorial input from a Statistics/Methods Editor, an Associate Editor, and the Editor-in-Chief. Accepted in revised form June 20, 2020.
Footnotes
Complete author and article information provided before references.
References
- 1.Guan W.J., Ni Z.Y., Hu Y. clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.No author listed COVID-19, a pandemic or not? Lancet Infect Dis. 2020;20(4):383. doi: 10.1016/S1473-3099(20)30180-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dong Y., Mo X., Hu Y. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145(6):e20200702. doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 4.Zhou P., Yang X.L., Wang X.G. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu N., Zhang D., Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.To K.K.-W., Tsang O.T.-Y., Leung W.-S. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20(5):565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao X., Liu B., Yu Y. The characteristics and clinical value of chest CT images of novel coronavirus pneumonia. Clin Radiol. 2020;75(5):335–340. doi: 10.1016/j.crad.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi H., Han X., Jiang N. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20(4):425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bai Y., Yao L., Wei T. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323(14):1406–1407. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiong F., Tang H., Liu L. Clinical characteristics of and medical interventions for COVID-19 in hemodialysis patients in Wuhan, China [published online ahead of print May 8, 2020] J Am Soc Nephrol. 2020;31(7):1387–1397. doi: 10.1681/ASN.2020030354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen B., Zheng Y., Zhang X. Clinical evaluation of a rapid colloidal gold immunochromatography assay for SARS-Cov-2 IgM/IgG. Am J Transl Res. 2020;12(4):1348–1354. [PMC free article] [PubMed] [Google Scholar]
- 13.Hazra A., Gogtay N. Biostatistics series module 4: comparing groups - categorical variables. Indian J Dermatol. 2016;61(4):385–392. doi: 10.4103/0019-5154.185700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saran R., Robinson B., Abbott K.C. US Renal Data System 2017 Annual Data Report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2018;71(3 suppl 1):A7–A8. doi: 10.1053/j.ajkd.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang L., Zhao M.H., Zuo L. China Kidney Disease Network (CK-NET) 2015 Annual Data Report. Kidney Int Suppl. 2019;9(1):e1–e81. doi: 10.1016/j.kisu.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaziri N.D., Pahl M.V., Crum A., Norris K. Effect of uremia on structure and function of immune system. J Ren Nutr. 2012;22(1):149–156. doi: 10.1053/j.jrn.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haveri A., Smura T., Kuivanen S. Serological and molecular findings during SARS-CoV-2 infection: the first case study in Finland, January to February 2020. Euro Surveill. 2020;25(11) doi: 10.2807/1560-7917.ES.2020.25.11.2000266. 2000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao J., Yuan Q., Wang H. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019 [published online ahead of print March 28, 2020]. Clin Infect Dis. https://doi.org/10.1093/cid/ciaa344 [DOI] [PMC free article] [PubMed]
- 19.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brake S.J., Barnsley K., Lu W., McAlinden K.D., Eapen M.S., Sohal S.S. Smoking upregulates angiotensin-converting enzyme-2 receptor: a potential adhesion site for novel coronavirus SARS-CoV-2 (Covid-19) J Clin Med. 2020;9(3) doi: 10.3390/jcm9030841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cai H. Sex difference and smoking predisposition in patients with COVID-19. Lancet Respir Med. 2020;8(4):e20. doi: 10.1016/S2213-2600(20)30117-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chu K.H., Tsang W.K., Tang C.S. Acute renal impairment in coronavirus-associated severe acute respiratory syndrome. Kidney Int. 2005;67(2):698–705. doi: 10.1111/j.1523-1755.2005.67130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arabi Y.M., Arifi A.A., Balkhy H.H. Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Ann Intern Med. 2014;160(6):389–397. doi: 10.7326/M13-2486. [DOI] [PubMed] [Google Scholar]
- 24.Ma J., Xia P., Zhou Y. Potential effect of blood purification therapy in reducing cytokine storm as a late complication of critically ill COVID-19. Clin Immunol. 2020;214:108408. doi: 10.1016/j.clim.2020.108408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsuda K., Moriguchi T., Oda S., Hirasawa H. Efficacy of continuous hemodiafiltration with a cytokine-adsorbing hemofilter in the treatment of acute respiratory distress syndrome. Contrib Nephrol. 2010;166:83–92. doi: 10.1159/000314856. [DOI] [PubMed] [Google Scholar]
- 26.Nakata H., Yamakawa K., Kabata D. Identifying septic shock populations benefitting from polymyxin b hemoperfusion: a prospective cohort study incorporating a restricted cubic spline regression model [published online ahead of print March 17, 2020]. Shock. https://doi.org/10.1097/SHK.0000000000001533 [DOI] [PubMed]
- 27.Pedersen S.F., Ho Y.C. SARS-CoV-2: a storm is raging. J Clin Invest. 2020;130(5):2202–2205. doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]