Abstract
The recent emergence of novel coronavirus disease (COVID-19) triggered by Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) has resulted in substantial mortality worldwide. Presently, there is no approved treatment for COVID-19. Consequently, the clinical, scientific, and regulatory authorities have joint efforts to reduce the severe impact of COVID-19. To date, there is minimal arsenal with no definite curative drugs, licensed-vaccines, or therapeutic conducts to combat the COVID-19 infections. Keeping in view the threats of this pandemic, various global organizations, physicians, researchers, and scientists, are trying to recognize the epidemiological characteristics and pathogenic mechanisms of COVID-19 to discover potential treatment regimens, vaccines, and therapeutic modes for future anticipation. Herein, we summarize a contemporary overview of curative invasions and vaccines for COVID-19 based on the earlier information and considerate of similar earlier RNA coronaviruses. The information reviewed here establishes a paramount intellectual basis to promote ongoing research to develop vaccines and curative agents. Thus, this review suggests the furthermost accessible frontiers in the vaccine development to tackle or combat the COVID-19/SARS-CoV-2.
Keywords: COVID-19, Transmission, Personal-level prevention, Therapeutics, Anti-viral drugs
Introduction
The COVID-19 caused by SARS-CoV-2 was first discovered in December 2019 and declared an epidemic by WHO on 11th March 2020 [1,2]. It is a quickly spreading disease with person-to-person contact [3]. Within months, it has extended to the whole world. The exact pathophysiology of infection with SARS-CoV-19 is unknown, thus, needing further investigation [4]. However, it is considered that angiotensin-converting enzyme 2 (ACE2) present in human lungs is the primary receptor for the virus. Therefore, it is suspected for a significant contribution of the virus to infect the upper and lower respiratory tracts. The symptoms associated with COVID 19 ranges from asymptomatic to severe disease leading to death [5]. They develop from 2 days up to 2 weeks after the exposure to the virus and commonly include high-grade fever, malaise, cough, and dyspnea [6]. Some studies have described an incubation period of 5.1 days, and almost all patients are developing symptoms up to 11.5 days after infection [7]. Nearly 81% of patients are either asymptomatic or mildly symptomatic. About 14% have severe disease, including hypoxia and dyspnea, almost 5% were having a critical illness of either cardiovascular or respiratory failure, and 2.3% of deaths were reported [8]. There are no drugs or vaccines available to treat this deadly contagious disease. However, multiple trials on different drugs are currently undergoing.
SARS-CoV-2 is an RNA virus that contains two protein groups, i.e., structural proteins and non-structural proteins. Structural proteins consist of a spike (S) protein, which binds host cells, nucleocapsid, matrix, and envelops. Non-structural proteins include proteins such as proteases (nsp3 and nsp5) and RdRp, which replicate the viral genome [9]. Recognition of receptors by the virus is crucial for viral infectivity. Previous studies show that SARS-CoV is dependent on the angiotensin-converting enzyme 2 (ACE2) receptor for its entry. This enzyme is present in the parenchyma of the lung, endothelium, epithelium, kidneys, and cardiovascular tissues [10]. ACE2 has 76% homology in amino acid sequence with the SARS-CoV-S. Upon entry in human cells, S-protein priming is done by the host cell transmembrane protease serine-type 2 (TMPRSS2), which causes initiation of infection and disease spread in human cells [11]. The epithelium of upper and lower airways shows an extensive expression of TMPRSS2 [12]. TMPRSS2 initiates a proteolytic activity at position S20 of SARS-CoV-2 S protein, causing membrane fusion and viral RNA release in host cells [13]. However, other studies show that clathrin-dependent and clathrin-independent endocytosis also helps other than membrane fusion [14]. After entry, the viral genome (RNA) is translated into structural proteins and polyproteins needed for viral replication. When host cells are infected with viral RNA, adaptive and innate immune responses are generated.
Upon entry of the virus in human cells, the antigen (peptides) is presented to antigen-presenting cells (APCs) via major histocompatibility complex (MHC) or human leukocyte antigen (HLA) [15]. The SARS-CoV antigen presentation mainly depends on MHC I molecules, but MHC II also contributes to its production [16]. Virus-specific cytotoxic T lymphocytes identify the antigen presented to them. In endosome, pathogen-associated molecular patterns (PAMPs) are recognized by membrane specific pattern recognition receptors (PRR). These include Toll-like receptor (TLR3,7,8,9) or the cytosolic RNA sensor (RIG-I/MDA5) or the secretory type PRR like Mannose-binding lectin (MBL) and C-reactive protein (CRP) [17]. The innate and adaptive immune responses are initiated by Interferon (IFN) type I. This recognition starts a signaling pathway using adaptor proteins such as mitochondrial antiviral signaling protein, IFN-β, and stimulator of interferon genes protein, thus initiating a downstream cascade of molecules. Transcription factors, such as nuclear factor-κB (NF-κB) and interferon regulatory factor 3 (IRF3), are activated to help nuclear translocation. These factors initiate the type I Interferons (IFN-α/β) and pro-inflammatory cytokines production, especially IL-6 [18]. The dysregulated immune response may lead to a cytokine storm. JAK-STAT pathway is initiated by type I IFN and also suppresses the viral replication, thus modulating the phagocytosis of antigens by macrophage and restriction of infected cells by NK cells [19]. Antigen-presenting cells mediate a cytokine release causing the response of T-cells. CD4 helper T cells assist cytotoxic T cells in initiating adaptive immunity. CD8 cytotoxic T cells secrete molecules including IFN-γ, granzymes, and perforins, necessary for viral eradication from host cells. B-cell mediated humoral immune response protects by the production of antibodies and thus reinfection [20].
Literature review methodology
A comprehensive bibliographic evaluation was performed to justify the work contents and theme. Several databases, including Google Scholar, Science Direct, PubMed, Taylor & Francis, Scopus, SciELO, Web of Science, SciFinder, Wiley, and Springer, were carefully searched to collect and discuss the information. From the citations of retrieved-literature additional sources were analyzed. Different search terms such as severe acute respiratory syndrome coronavirus 2, 2019-nCoV, SARS CoV 2, corona, viruses, COVID-2019, coronavirus were employed to search the literature.
Transmission of COVID-19
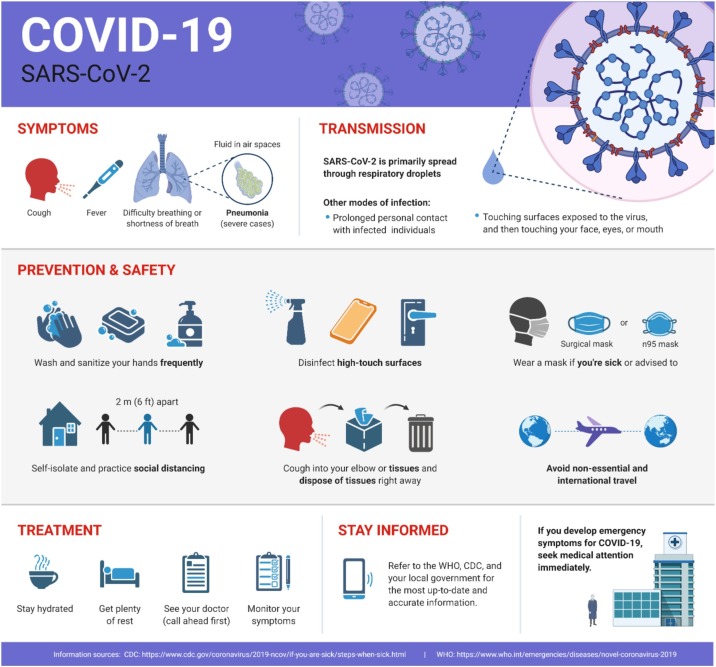
The COVID-19 can be transmitted to humans by two different routes, such as contact and respiratory. When infected people sneeze or cough, it generates tiny droplets, which can readily travel through the air and enter another person's respiratory system. Likewise, if a healthy person has close contact with a person who has respiratory symptoms (coughing and sneezing) has a greater chance of catching infection through inhaling the respiratory droplets of an infected person [21]. These droplets can quickly drop down on different surfaces, e.g., ground, walls, doors, the railing of stairs, etc. The COVID-19 can easily remain viable for several hours resulting in the immediate contamination of the environment. This, in turn, poses a severe threat of infection for the persons who may contact these surfaces. This transmission route is termed as contact transmission. On the other hand, the chances of infection from the waste (feces) of an infected person are relatively lower. There is very little evidence that a person got infected from COVID-19 from intestinal infection and is present in feces. Among the confirmed COVID-19 cases, nearly 2–10% of the patients suffer from diarrhea [[22], [23], [24]]. Whereas two investigations spotted COVID-19 viral RNA wreckages in the patients' feces [25,26]. Conversely, only a single study has cultured the COVID-19 from a single sampling [27]. No fecal-oral transmissions of the virus have been reported yet.
COVID-19 endurance on various surfaces
It is not evident that the human coronaviruses are present in surface or groundwater. Also, they are not transmitted through water bodies. The COVID-19 belongs to an enveloped class of viruses that have a fragile outer membrane. Typically, the enveloped viruses have lesser stability in an open atmosphere and are more vulnerable to the oxidizing agents, including chlorine. To date, there are no reports about the existence of COVID-19 in sewage or water. The waterborne transmission (hepatitis A, norovirus, adenoviruses, and rotavirus) of this class of viruses is significantly less than the viruses, which belong to the non-envelop class of viruses. For example, the existence of human coronavirus in dechlorinated water has been documented. It can only survive for two days at 20 °C in hospital wastewater [28]. Around 99.9% of infected individuals by a human coronavirus, mouse hepatitis virus, and gastroenteritis coronavirus died within two weeks at 23−25 °C [29,30]. The survival of COVID-19 is uncertain on various surfaces till now. Recently it has been documented about the survival of COVID-19 on multiple surfaces from 2 to 9 days. This document proves a large variability about the survival of COVID-19 [31]. This endurance of COVID-19 owes various factors such as surface type, relative humidity, temperature, and a particular strain of the virus. The COVID-19 can be inactivated within 1 min using common disinfectants, such as sodium hypochlorite or 70% ethanol. Person-to-person transmission behaviors and the impact of social distancing along with principles of herd immunity are shown in Fig. 1 .
Fig. 1.
Safety information and precautionary measures to put the current 2019-nCoV/COVID-19 outbreak in perspective. The Figure was created with the “BioRender.com” template and exported under the terms of premium subscription.
Protection of water supplies
Based on the present studies, COVID-19 in drinkable water bodies is out of the question; therefore, the risk of transmission from water supplies is low [32]. Correspondingly, the investigations are carried out in a well-controlled environment such as a laboratory reveals that the COVID-19 might persist contagious in water bodies polluted with feces for several days to weeks [30]. Water safety can easily be improved by observing various measures such as protection of water source, water treatment at distribution points, and storage of water with great care at homes in covered and frequently cleaned containers. Typically, integrated water treatment procedures, which use disinfection and filtration, should deactivate the COVID-19. The disinfection of other human coronaviruses can quickly be done with UV-light and chlorination [33].
The enveloped viruses have a fragile lipid-based cell membrane. Therefore, the coronavirus is more profound to oxidants and chlorine. The concentration and contact time for the disinfection of viruses should be in the range of ≥0.5 mg/L and 30 min, respectively, at pH < 8.0 [32]. The chlorine should be continuously maintained through the water distribution system. In the places or communities where central distribution systems are not available, different domestic treatment technologies such as ultra-filtration membranes, boiling, solar irradiation, UV irradiation, and nano-membrane filters, etc., prove best in destroying or removing viruses [21].
Safe management of wastewater and fecal waste
The transmission of COVID-19 has not been reported through sewerage systems with or without treatment. Moreover, there are no reports that wastewater or sewage treatment workers acquire acute respiratory syndrome (SARS). This type of illness has been caused by another class of coronavirus that triggered a massive outbreak of severe respiratory infection in 2003. The wastewater or sewage systems should be treated in well-managed, and well-designed consolidated wastewater treatment approaches for the better safety of public health. The potential contamination can quickly be decreased by carefully observing the treatment platforms. A large wastewater stabilization pool is usually considered to be a practical and straightforward wastewater treatment methodology. This type of pool is predominantly well appropriate to abolishing pathogens, as comparatively long retaining time combined with sunlight, high pH values, biological activity, and added factors assist the acceleration of pathogen demolition. Protective outwears such as masks, gloves, face shields, boots, goggles, etc., should be provided to the workers. Further, precautionary measures, such as hand hygiene, should also be observed to maintain sanitation workers' health. One should not touch his mouth, nose, eyes, etc., with unwashed hands.
WASH in health care settings
Current recommendations for hygiene and water sanitation dealings in health care sites are vital for providing proper care for patients and protecting staff, patients, and caretakers from infection risks [34]. The critical parameters such as, managing urine and feces carefully (should be disposed of with great care), arranging frequent hand hygiene, employing regular disinfection and cleaning practices; and carefully handling waste used in health care. Other recommended and necessary measures include providing plenty of drinking water to the caregivers, patients, and staff, certifying that individual hygiene can be sustained, containing hand hygiene for caregivers, staff, and patients. Other relevant personal hygiene includes regular laundering of patient’s bedsheets, clothing clean and easily accessible toilets, isolating, and careful disposal of waste used by health care officials. For detailed recommendations, please refer to the essential standards of environmental health [34].
Hand hygiene practices
Hand hygiene at regular intervals using various methods such as washing with soap, alcohol-based hand sanitizers, or pads is of extreme importance. This should be carried out according to documented instructions of health specialists known as “My 5 moments for hand hygiene” [35]. If there is no visible dirt present on hands, the alcohol-based hand sanitizers should be rubbed for 20–30 seconds [36]. On the other hand, if dirt is visible on hands, soap should be used to remove the dirt for 40–60 seconds [37]. On all five moments, hand hygiene should strictly be observed, such as changing gloves, before wearing and after removing personal protective equipment (PPE) after having any contact with a patient or suspected COVID-19 patient or after handling their waste or respiratory secretions. This practice must be carried out before eating anything and after using the toilet [38].
If sanitizers or soaps are not available, then chlorinated water (0.05%) is an alternative option for washing hands. But, the continuous use of chlorinated water is not a healthy activity as it can cause dermatitis. This can also increase the risk of asthma and infection. At all health-care units, functional facilities of hand hygiene should be provided to the medical workers, including the areas where medics gear up for treating patients. Furthermore, hand hygiene facilities should also be available to the patients, visitors, and family members of patients [39].
Sanitation and plumbing
Adequate sanitation facilities such as toilets, flush, or latrine should be provided to the confirmed or suspected patients of COVID-19. Toilets, flush should be operated well and have operative drain set-ups. After using the bathrooms, the lid should be properly drop down to reduce the spreading of droplet splash and aerosol clouds. If the availability of individual toilets is not feasible, then cleaning the toilets should be carried out by specialist cleaners wearing all safety gadgets. This task should be performed twice a day using a disinfectant.
Moreover, systematic with current regulation, health care workers, and staff should have separate toilet facilities from the patients. The WHO commends the use of well-maintained and standard plumbing, including closed bathroom pipes, and backflow stopcocks on atomizers to avoid aerosolized fecal material from arriving the ventilation or plumbing system, accompanied by the standard wastewater treatment [40,41]. In 2003, the spreading of aerosolized SARS coronaviruses was reported because of the lousy plumbing and air ventilation systems in high rise building situated in Hong Kong [42]. Therefore, similar apprehensions have been assumed about spreading the COVID-19 from poorly designed toilets in multistory apartment buildings [43].
As the health care facilities are associated with sanitation systems, one should have carried out the risk assessment to assure the confinement of wastewater within the sanitation system such as pipes (no leak) before its appearance at a disposal site or functioning treatment. Risks concerning the storage or disposal and treatment systems' suitability should be evaluated, ensuring a safety development methodology [44], accompanied by major controlling points highlighted for mitigation. The pit-toilets are a better option in the areas where health care facilities are not appropriate. One must take care of the standard safety measures in arranging the pit-flush to avoid environmental contamination due to the excreta. If both precautionary measures are available, then the storage tank for the wastes must be impenetrable. The feces of the patients should not get in contact with the groundwater table. Also, the storage tank must have the ability to reduce the contamination levels before the excreta's disposal. Another option is to adopt the two-tank system, which is parallels connected to facilitate inactivation by increasing the retention time. Furthermore, intensive care should be taken while emptying or cleaning the storage tanks so that droplets' splashing or release must be avoided.
Toilets and the handling of feces
Upon direct or suspected contact to hands with wastage of the COVID-19 patient, the hand hygiene must be carried out with due care by using soaps to wash hands rather than alcohol-based hand rubs (if dirt is visible). Suppose the latrine is not available to the patient. In that case, a diaper or clean bedpan must be used to collect excreta and be deposed in the separate toilet, which is only used by suspected or confirmed patients of COVID-19. In all the hospitals or health care units’ dealings with confirmed or suspected cases of COVID-19 patients, the toilet waste must be handled as biohazard waste, and contact should be avoided as little as possible. The personals dealing with COVID-19 patients wastage should follow the WHO precautions by using PPE to prevent exposure to droplets or direct contact with the feces [38]. There should be proper training of the workers about wearing and removing the PPE so that the purpose of the PPE use should not be breached [45]. Suppose all these protective gears (PPE) are not available or have limited supply. In that case, the other hygienic measures should be observed, which include hand wash, the medical workers should maintain 1 m distance from the patients regularly.
In case of using a bedpan, it must be washed and cleaned with some detergents, followed by using chlorinated water for the disinfection. It should be noticed that the water used for washing and final rinsing must be thrown into the drain or toilet. Some commercially available disinfectants, including cetylpyridinium chloride (quaternary ammonium salt) and peroxyacetic acid (500–2000 mg/L) can also be used for disinfection purposes according to the instructions provided by the manufacturers [46]. Chlorine is futile for disinfection of media that contain vast amounts of dissolved and solid organic matter. For that reason, the use of chlorine solution for disposing of fresh excreta is not suitable as it may create some health risks or possibly splashing.
Evacuating latrines/holding tanks and carrying excreta off-site
The holding tanks for waste used by the COVID-19 patients must not be emptied until their capacity. Generally, the best safety measures should be observed in safely managing the excreta. Both media should be prepared according to the patient requirement by considering a potential increase in the number of cases, and a regular timetable should be obeyed for evacuating them centered on the volumes of wastewater generated. The persons dealing with all this must wear PPE to avoid all the possible health risks. After carefully disposing of the waste and making sure that there is no risk of more exposure, persons should carefully take out their PPE and implement hand hygiene afore ingoing to the transport vehicle. A sealed bag should be used to put the dirty PPE for safe laundering afterward. In the areas where no off-site treatment facility is available, in-situ treatment can be carried out using lime. This treatment includes 10% lime slurry, in which 1-part lime slurry is added per 10 parts of waste.
Cleaning measures and practices
Current recommended disinfection and cleaning measures for health care services should be obeyed correctly and consistently [39]. Surfaces and laundry of the premises dealing with COVID-19 cases should be done at regular intervals of time (at least once a day) after discharging patients [47]. Many decontaminators are on the go in contrast to enveloped viruses, such as the COVID-19. At this time, WHO recommended the use of a 70% solution of ethanol for the disinfection of small areas such as reusable equipment. A 0.5% sodium hypochlorite solution can also be for disinfecting purposes. All persons dealing with stained bedding, clothes, and towels used by the patient of COVID-19 infection should wear proper PPE before moving it. This PPE may include a mask, heavy-duty gloves, face shield, goggles, a long-sleeved gown, and an apron if the gown is not fluid-resistant, and closed shoes or boots. On the other hand, they must wash their hands after having contact with the body fluid and further wastage after removing their PPE to reduce the risk of being infected. The clothing should be carefully packed in sealed containers that are clearly labeled.
Furthermore, the solid excreta should be put in a covered bucket to be disposed of in a toilet. The water used for washing purposes in machines should have a temperature of 60–90 °C accompanied by good-quality washing detergent. Correspondingly, laundry should be dried by adopting routine procedures. If the washing machines are not available, then the hand wash should be carried out in large drums having hot water. The laundry stuff should be put into the drums containing hot water and detergent with the help of a stick, and splashing should be avoided. After that, the wet laundry should be dipped in chlorinated water (30 min) followed by rinsing with fresh water and dried in sunlight. If the bed sheets or other clothes' surface is dirty with excreta, the stains should be cleaned with a towel and carefully disposed of in toilets. If the towels are disposable, they must be treated as contaminated waste, and if they are reusable, their washing must be carried out, as discussed earlier. Finally, the surfaces should be cleaned using a chlorine disinfection solution according to the documented guidelines [35].
Safe disposal of greywater
The reusable stuff such as plastic aprons, heavy-duty gloves, gowns, etc., should be cleaned with chlorinated water (0.5% sodium hypochlorite) and soap as per WHO recommendations. On the other hand, disposable stuff should be handled with care and dispose of as recommended. If the disinfectant is present in greywater due to prior washing, then the addition of chlorine is not necessary. Correspondingly, this greywater should be drained of in septic tanks or a soak away container or sewer system. In the case of soak away container, the container must follow the health policy to eradicate the chances of exposure and risk of infection.
Safe management of health care waste
The healthcare waste must be treated by observing the complete guidelines to assure environmental safety. No evidence of transmission of COVID-19 has been reported yet due to direct unprotected contact while handling the waste of health care personals. All the garbage of COVID-19 infected patients should be handled with care in specific containers and treated safely at the treatment places. While it is moved away from the operating site, it is difficult to understand the destroying of the waste. The detailed information about the handling and treatment measures can easily be found on the WHO guide of COVID-19 [48].
WASH practices in communities and homes
The maintenance of best washing practices should be observed in the community and home to prevent the transmission of COVID-19. Consistent and precise hand hygiene is of specific significance. In schools, homes, and crowded public places, including markets, worship places, bus or train stations, hand hygiene should be obeyed strictly. Similarly, before cooking food, before and after eating, changing the diapers of the child, touching animals after attending the toilets, etc. the regular handwashing should have followed. Operative handwashing services with soap and water should be accessible within 5 m vicinities of bathrooms.
Therapeutic potential, Trials, and current recommendations of different available drugs for COVID-19 treatment
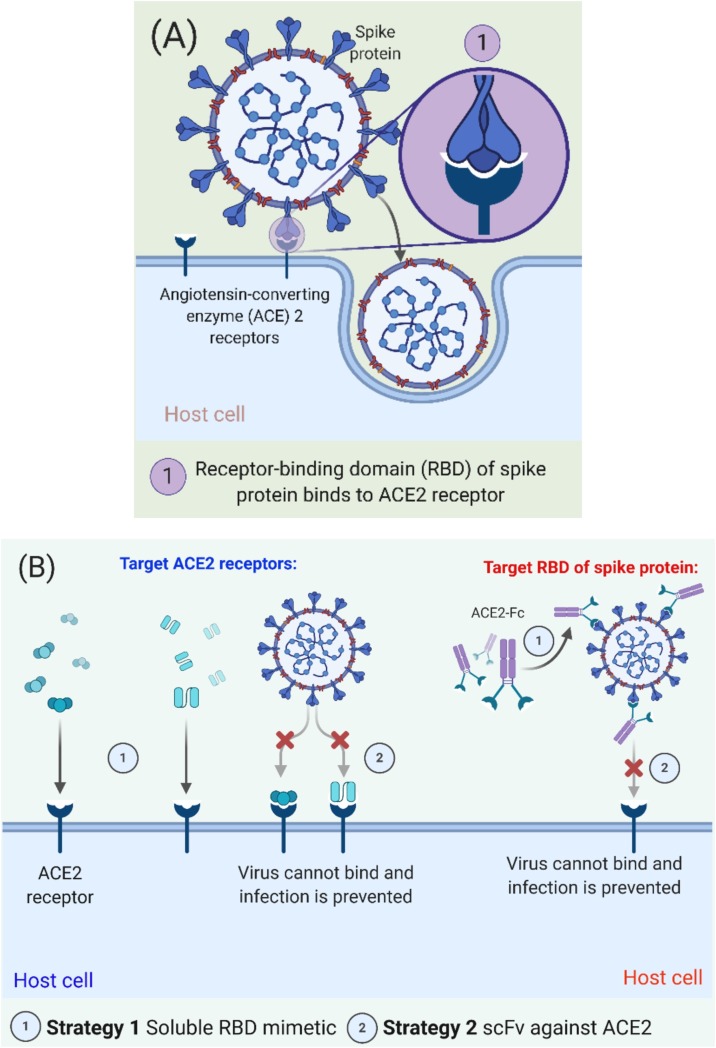
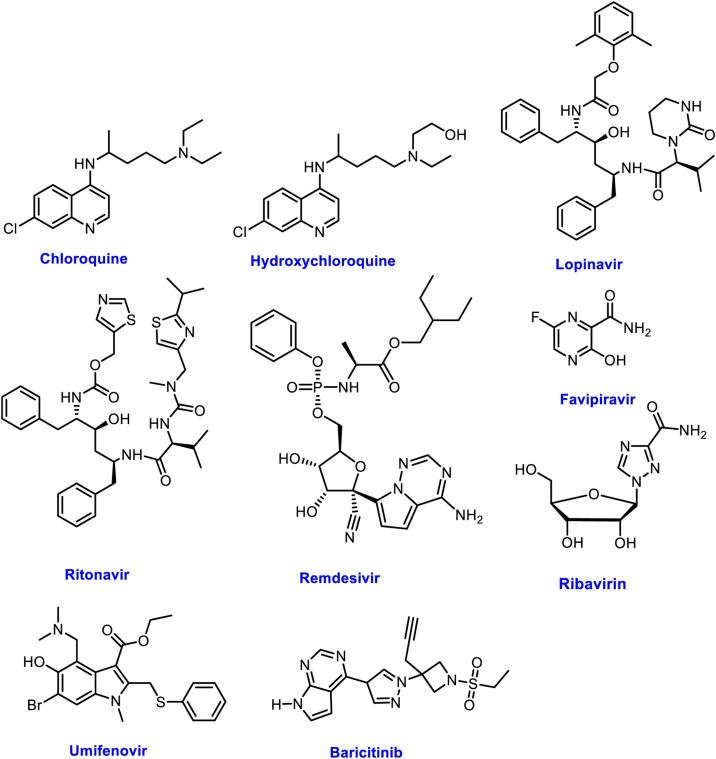
For treatment and control of COVID-19, there is an urgent need to develop therapeutic drugs until the development of a vaccine. Viral entry mechanism of SARS-CoV-2 and proposed treatments for COVID-19 targeting ACE2 receptors are illustrated in Fig. 2 . Considering available drugs, there is an opportunity to employ them against SARS-CoV-2 by considering their mechanism of action and SAR (structure-activity relationship). Following available medications have been used for the treatment of SARS-CoV-2 (Fig. 3 ). Data of their trials, recommendations, and outcomes have been presented in detail (Table 1 ).
Fig. 2.
(A) Viral entry mechanism of SARS-CoV-2, and (B) Proposed therapeutic treatments for COVID-19 targeting ACE2 receptors and RBD of spike protein. The Figure was created with “BioRender.com” template and exported under the terms of premium subscription.
Fig. 3.
Potential Drug candidates evaluated against COVID-19.
Table 1.
Clinical trials of available drugs against SARS-Cov-2 worldwide. Data was extracted from Refs. [52,53].
| Drugs | Trial No. | Topic | Date of Initiation | Current Status | Country or region |
|---|---|---|---|---|---|
| Chloroquine | NCT04323527 | Chloroquine Diphosphate for the Treatment of Severe Acute Respiratory Syndrome Secondary to SARS-CoV2 (CloroCOVID19) | 23/3/2020 | Recruiting | Brazil |
| ChiCTR2000031204 | A multicenter, single-blind, randomized controlled clinical trial for chloroquine phosphate in the treatment of novel coronavirus pneumonia (COVID-19) | 30/1/2020 | Recruiting | China | |
| ChiCTR2000030718 | Randomized controlled trial for Chloroquine Phosphate in the Treatment of novel coronavirus pneumonia (COVID-19) | 12/2/2020 | Recruiting | China | |
| ChiCTR2000030054 | A prospective, open label, randomized, control trial for chloroquine or hydroxychloroquine in patients with mild and common novel coronavirus pulmonary (COVIP-19) | 22/2/2020 | Recruiting | China | |
| ChiCTR2000029741 | Efficacy of Chloroquine and Lopinavir/Ritonavir in mild/general novel coronavirus (CoVID-19) infections: a prospective, open-label, multicenter randomized controlled clinical study | 12/2/2020 | Recruiting | China | |
| ChiCTR2000029939 | A Single-blind, Randomized, Controlled Clinical Trial for Chloroquine Phosphate in the treatment of Novel Coronavirus Pneumonia 2019 (COVID-19) | 06/2/2020 | Recruiting | China | |
| ChiCTR2000029988 | Clinical Study of Chloroquine Phosphate in the Treatment of Severe Novel Coronavirus Pneumonia (COVID-19) | 13/2/2020 | Recruiting | China | |
| ChiCTR2000029935 | A Single-arm Clinical Trial for Chloroquine Phosphate in the treatment of Novel Coronavirus Pneumonia 2019 (COVID-19) | 6/2/2020 | Recruiting | China | |
| ChiCTR2000029542 | Study for the efficacy of chloroquine in patients with novel coronavirus pneumonia (COVID-19) | 3/2/2020 | Recruiting | China | |
| HCQ | NCT04333225 | Hydroxychloroquine in the Prevention of COVID-19 Infection in Healthcare Workers | 3/4/2020 | Recruiting | USA |
| NCT04307693 | Comparison of Lopinavir/Ritonavir or Hydroxychloroquine in Patients With Mild Coronavirus Disease (COVID-19) | 11/3/2020 | Recruiting | Republic of Korea | |
| NCT04331834 | Pre-Exposure Prophylaxis With Hydroxychloroquine for High-Risk Healthcare Workers During the COVID-19 Pandemic | 3/4/2020 | Recruiting | Spain | |
| NCT04332094 | Clinical Trial of Combined Use of Hydroxychloroquine, Azithromycin, and Tocilizumab for the Treatment of COVID-19 | 2/4/2020 | Recruiting | Spain | |
| NCT04326725 | Proflaxis Using Hydroxychloroquine Plus Vitamins-Zinc During COVID-19 Pandemia | 30/3/2020 | Recruiting | Turkey | |
| NCT04332991 | Outcomes Related to COVID-19 Treated With Hydroxychloroquine Among In-patients With Symptomatic Disease | 3/4/2020 | Recruiting | United States | |
| NCT04321278 | Safety and Efficacy of Hydroxychloroquine Associated With Azithromycin in SARS-CoV2 Virus (Coalition Covid-19 Brasil II) | 25/3/2020 | Recruiting | Brazil | |
| NCT04333654 | Hydroxychloroquine in Outpatient Adults With COVID-19 | 3/4/2020 | Recruiting | USA | |
| NCT04322123 | Safety and Efficacy of Hydroxychloroquine Associated With Azythromycin in SARS-Cov-2 Virus | 26/3/2020 | Recruiting | Brazil | |
| NCT04261517 | Efficacy and Safety of Hydroxychloroquine for Treatment of Pneumonia Caused by 2019-nCoV (HC-nCoV) | 6/2/2020 | Completed | China | |
| ChiCTR2000030054 | A prospective, open label, randomized, control trial for chloroquine or hydroxychloroquine in patients with mild and common novel coronavirus pulmonary (COVIP-19) | 2/2/2020 | Recruiting | China | |
| ChiCTR2000029992 | A prospective, randomized, open label, controlled trial for chloroquine and hydroxychloroquine in patients with severe novel coronavirus pneumonia (COVID-19) | 18/2/2020 | Recruiting | China | |
| ChiCTR2000029899 | Evaluation the Efficacy and Safety of Hydroxychloroquine Sulfate in Comparison with Phosphate Chloroquine in Mild and Commen Patients with Novel Coronavirus Pneumonia (COVID-19): a Randomized, Open-label, Parallel, Controlled Trial | 16/2/2020 | Recruiting | China | |
| ChiCTR2000029898 | Evaluation the Efficacy and Safety of Hydroxychloroquine Sulfate in Comparison with Phosphate Chloroquine in Severe Patients with Novel Coronavirus Pneumonia (COVID-19): a Randomized, Open-Label, Parallel, Controlled Trial | 16/2/2020 | Recruiting | China | |
| ChiCTR2000029868 | Hydroxychloroquine treating novel coronavirus pneumonia (COVID-19): a randomized controlled, open label, multicenter trial | 15/2/2020 | Recruiting | China | |
| ChiCTR2000029803 | A prospective, randomized, open-label, controlled clinical study to evaluate the preventive effect of hydroxychloroquine on close contacts after exposure to the Novel Coronavirus Pneumonia (COVID-19) | 14/2/2020 | Recruiting | China | |
| ChiCTR2000029559 | Therapeutic effect of hydroxychloroquine on novel coronavirus pneumonia (COVID-19) | 4/2/2020 | Recruiting | China | |
| ChiCTR1900026938 | Efficacy of hydroxychloroquine pretreatment on improving pregnancy outcome in patients with polycystic ovary syndrome | 26/10/2019 | Recruiting | China | |
| ChiCTR1900026116 | The efficacy and safety of low-dose glucocorticoids combined withmethotrexate and hydroxychloroquine in the treatment of early rheumatoid arthritis: a randomized, double-blinded, controlled trial | 22/9/2019 | Recruiting | China | |
| ChiCTR1900021757 | A randomized controlled trial for hydroxychloroquine sulfate in the treatment of idiopathic membranous nephropathy | 8/3/2019 | Recruiting | China | |
| ChiCTR-IPR-17012224 | A multicenter, randomized, double-blind, double-mock test for the efficacy and safety of hydroxychloroquine in the treatment of rosacea | 2/8/2017 | Recruiting | China | |
| ChiCTR-IPR-17010622 | The effect of the treatment with hydroxychloroquine or compound glycyrrhisim on the blood glucose of patients with oral lichen planus companying diabetes mellitus | 13/2/2017 | Recruiting | China | |
| ChiCTR-IPR-15005757 | Prospective, Randomized, placebo-Controlled, double-blind, MultIcenter, parallel group Study to assess the Efficacy and safety of hydroxychloroquine in chinese patients with primary Sjogren's Syndrome | 20/11/2013 | Recruiting | China | |
| ChiCTR-TRC-13004257 | Hydroxychloroquine in the treatment of the lupus nephritis | 28/11/2013 | Recruiting | China | |
| ChiCTR-TRC-12002083 | An Open-Label, Randomized-Sequence, Single-Dose, Parallel Study of Generic and Branded Hydroxychloroquine Sulfate Tablet in Healthy Chinese Male Volunteers | 6/4/2012 | Recruiting | China | |
| Lopinavir/ Ritonavir | NCT04255017 | A Prospective/ Retrospective,Randomized Controlled Clinical Study of Antiviral Therapy in the 2019- nCoV Pneumonia | 1/2/2020 | Recruiting | China |
| NCT04307693 | Comparison of Lopinavir/Ritonavir or Hydroxychloroquine in Patients with Mild Coronavirus Disease (COVID-19) | 11/03/2020 | Recruiting | Republic of Korea | |
| NCT04328012 | COVID MED Trial -Comparison Of Therapeutics for Hospitalized Patients Infected With SARSCoV-2 | 6/4/2020 | Recruiting | USA | |
| NCT04315948 | Trial of Treatments for COVID-19 in Hospitalized Adults (DisCoVeRy) | 22/3/2020 | Recruiting | France | |
| NCT04276688 | Lopinavir/ Ritonavir, Ribavirin and IFN-beta Combination for nCoV Treatment | 10/2/2020 | Recruiting | Hong Kong | |
| EudraCT 2020-001113-21 | Randomised Evaluation Of Covid-19 Therapy (Recovery) | 23/3/2020 | Recruiting | UK | |
| ChiCTR2000030187 | Clinical study for Lopinavir and Ritonavir in the treatment of novel coronavirus pneumonia (COVID-19) | 24/2/2020 | Recruiting | China | |
| ChiCTR2000029741 | Efficacy of Chloroquine and Lopinavir/Ritonavir in mild/general novel coronavirus (CoVID-19) infections: a prospective, open-label, multicenter randomized controlled clinical study | 11/2/2020 | Recruiting | China | |
| ChiCTR2000029603 | A Randomized, Open-Label, Multi-Centre Clinical Trial Evaluating and Comparing the Safety and Efficiency of ASC09/Ritonavir and Lopinavir/Ritonavir for Confirmed Cases of Novel Coronavirus Pneumonia (COVID-19) | 6/2/2020 | Recruiting | China | |
| ChiCTR2000029548 | Randomized, open-label, controlled trial for evaluating of the efficacy and safety of Baloxavir Marboxil, Favipiravir, and Lopinavir-Ritonavir in the treatment of novel coronavirus pneumonia (COVID-19) patients | 4/2/2020 | Recruiting | China | |
| ChiCTR2000029541 | A randomised, open, controlled trial for darunavir/cobicistat or Lopinavir/ritonavir combined with thymosin a1 in the treatment of novel coronavirus pneumonia(COVID-19) | 3/2/2020 | Recruiting | China | |
| ChiCTR2000029539 | A randomized, open-label study to evaluate the efficacy and safety of Lopinavir-Ritonavir in patients with mild novel coronavirus pneumonia (COVID-19) | 3/2/2020 | Recruiting | China | |
| ChiCTR2000029468 | A real-world study for lopinavir/ritonavir (LPV/r) and emtritabine (FTC) / Tenofovir alafenamide Fumarate tablets (TAF) regimen in the treatment of novel coronavirus pneumonia (COVID-19) | 2/2/2020 | Recruiting | China | |
| ChiCTR2000029387 | Comparative effectiveness and safety of ribavirin plus interferon-alpha, lopinavir/ritonavir plus interferon-alpha and ribavirin plus lopinavir/ritonavir plus interferon-alphain in patients with mild to moderate novel coronavirus pneumonia | 1/29/2020 | Recruiting | China | |
| ChiCTR2000029308 | A randomized, controlled open-label trial to evaluate the efficacy and safety of lopinavir-ritonavir in hospitalized patients with novel coronavirus pneumonia (COVID-19) | 23/1/2020 | Recruiting | China | |
| Remdesivir | ISRCTN83971151 | Public health emergency SOLIDARITY trial of treatments for COVID-19 infection in hospitalized patients | 25/3/2020 | Recruiting | Argentina, Iran, Brazil, Canada, Peru, Qatar, Germany, Norway, Spain, Thailand, Indonesia,Switzerland South Africa, |
| NCT04315948 | Trial of Treatments for COVID-19 in Hospitalized Adults | 13/3/2020 | Recruiting | France | |
| EUCTR2020-000936-23-FR | Multi-centre, adaptive, randomized trial of the safety and efficacy of treatments of COVID-19 in hospitalized adults - DisCoVeRy | 9/3/2020 | Authorised | France | |
| NCT04292730 | Study to Evaluate the Safety and Antiviral Activity of Remdesivir (GS-5734™) in Participants With Moderate Coronavirus Disease (COVID-19) Compared to Standard of Care Treatment | 28/2/2020 | Recruiting | USA | |
| NCT04292899 | Study to Evaluate the Safety and Anti-viral Activity of Remdesivir (GS-5734™) in Participants With Severe Coronavirus Disease (COVID-19) | 28/2/2020 | Recruiting | USA | |
| NCT04280705 | Adaptive COVID-19 Treatment Trial (ACTT) | 20/2/2020 | Recruiting | USA | |
| NCT04252664 | Mild/Moderate 2019-nCoV Remdesivir RCT | 31/1/2020 | Recruiting | China | |
| Favipiravir | ChiCTR2000029600 | Clinical study for safety and efficacy of Favipiravir in the treatment of novel coronavirus pneumonia (COVID-19) | 30/1/2020 | Recruiting | China |
| ChiCTR2000030113 | Randomized controlled trial for safety and efficacy of Favipiravir in the treatment of novel coronavirus pneumonia (COVID-19) with poorly responsive ritonavir/ritonavir | 22/2/2020 | Recruiting | China | |
| ChiCTR2000030894 | Favipiravir Combined With Tocilizumab in the Treatment of novel coronavirus pneumonia (COVID-19)-A Multicenter, Randomized, Controlled Trial | 1/3/2020 | Recruiting | China | |
| ChiCTR2000030987 | A Randomized Controlled Trial for Favipiravir Tablets Combine With Chloroquine Phosphate in the Treatment of Novel Coronavirus Pneumonia (COVID-19) | 5/3/2020 | Recruiting | China | |
| JPRN-jRCTs031190226 | Favipiravir in patients infected with COVID-19 | 27/2/2020 | Recruiting | Japan | |
| JPRN-jRCTs041190120 | Favipiravir for SARS-CoV-infected patients | 2/3/2020 | Recruiting | Japan | |
| NCT04310228 | Favipiravir Combined With Tocilizumab in the Treatment of Corona Virus Disease 2019 | 8/3/2020 | Recruiting | China | |
| NCT04319900 | Clinical Trial of Favipiravir Tablets Combine With Chloroquine Phosphate in the Treatment of Novel Coronavirus Pneumonia | 5/3/2020 | Recruiting | China | |
| Ribavirin | ChiCTR2000030922 | Prospective, open-label, controlled, multicenter cohort study of long-acting interferon plus ribavirin in patients with novel coronavirus pneumonia (COVID-19) | 17/3/2020 | Recruiting | China |
| NCT04276688 | Lopinavir/ Ritonavir, Ribavirin and IFN-beta Combination for nCoV Treatment | 11/2/2020 | Recruiting | Hong Kong | |
| ChiCTR2000029387 | Comparative effectiveness and safety of ribavirin plus interferon-alpha, lopinavir/ritonavir plus interferon-alpha and ribavirin plus lopinavir/ritonavir plus interferon-alphain in patients with mild to moderate novel coronavirus pneumonia | 29/1/2020 | Recruiting | China | |
| Umefenovir | ChiCTR2000029621 | Clinical study of arbidol hydrochloride tablets in the treatment of novel coronavirus pneumonia (COVID-19) | 7/2/2020 | Recruiting | China |
| NCT04273763 | Evaluating the Efficacy and Safety of Bromhexine Hydrochloride Tablets Combined With Standard Treatment/ Standard Treatment in Patients With Suspected and Mild Novel Coronavirus Pneumonia (COVID-19) | 14/2/2020 | Recruiting | China | |
| Tocilizumab | NCT04317092 | Tocilizumab in COVID-19 Pneumonia (TOCIVID-19) | 19/3/2020 | Recruiting | Italy |
| NCT04331795 | Tocilizumab to Prevent Clinical Decompensation in Hospitalized, Non-critically Ill Patients With COVID-19 Pneumonitis | 4/4/2020 | Recruiting | USA | |
| NCT04332094 | Clinical Trial of Combined Use of Hydroxychloroquine, Azithromycin, and Tocilizumab for the Treatment of COVID-19 | 2/4/2020 | Recruiting | Spain | |
| NCT04320615 | A Study to Evaluate the Safety and Efficacy of Tocilizumab in Patients With Severe COVID-19 Pneumonia | 3/3/2020 | Recruiting | USA | |
| NCT04310228 | Favipiravir Combined With Tocilizumab in the Treatment of Corona Virus Disease 2019 | 8/3/2020 | Recruiting | China | |
| NCT04306705 | Tocilizumab vs CRRT in Management of Cytokine Release Syndrome (CRS) in COVID-19 | 20/2/2020 | Recruiting | China | |
| NCT04330638 | Treatment of COVID-19 Patients With Anti-interleukin drug | 04/2020 | Recruiting | Belgium | |
| NCT04322773 | Anti-il6 Treatment of Serious COVID-19 Disease With Threatening Respiratory Failure | 5/4/2020 | Recruiting | Denmark | |
| ChiCTR2000030894 | Favipiravir Combined With Tocilizumab in the Treatment of novel coronavirus pneumonia (COVID-19) - A Multicenter, Randomized, Controlled Trial | 16/3/2020 | China | ||
| EUCTR2020-001039-29-GR | Management Of Novel Sars Coronavirus | 31/3/2020 | Germany | ||
| Interferons | ChiCTR2000029387 | Comparative effectiveness and safety of ribavirin plus interferon-alpha, lopinavir/ritonavir plus interferon-alpha and ribavirin plus lopinavir/ritonavir plus interferon-alpha in in patients with mild to moderate novel coronavirus pneumonia | 1/29/2020 | Recruiting | China |
| ChiCTR2000029638 | A Multicenter, Randomized, Controlled trial for Recombinant Super-Compound Interferon (rSIFN-co) in the Treatment of 2019 Novel Coronavirus (2019-nCoV) Infected Pneumonia | 2/8/2020 | Recruiting | China | |
| ChiCTR2000030262 | Clinical study for combination of anti-viral drugs and type I interferon and inflammation inhibitor TFF2 in the treatment of novel coronavirus pneumonia (COVID-19) | 2/26/2020 | Recruiting | China | |
| ChiCTR2000030480 | Randomized, open, blank controlled trial for the efficacy and safety of recombinant human interferon alpha 1beta in the treatment of Wuhan patients with novel coronavirus pneumonia (COVID-19) | 3/3/2020 | Recruiting | China | |
| ChiCTR2000030922 | Prospective, open-label, controlled, multicenter cohort study of long-acting interferon plus ribavirin in patients with novel coronavirus pneumonia (COVID-19) | 3/17/2020 | Recruiting | China | |
| ChiCTR2000031196 | Efficacy and optimization of anti-viral therapy for novel coronavirus pneumonia (COVID-19) patients | 3/23/2020 | Recruiting | ||
| EUCTR2020-001023-14-GB | a trial of an inhaled anti-viral drug to treat or prevent severe respiratory difficulties in patients with COVID-19 | 3/17/2020 | Authorised | UK | |
| IRCT20100228003449N27 | Interferon ß in treatment of COVID-19 | 3/16/2020 | Recruiting | Iran | |
| IRCT20100228003449N28 | Interferon in treatment of COVID-19 | 3/19/2020 | Recruiting | Iran | |
| IRCT20151227025726N12 | Evaluating the therapeutic and adverse effects of Interferon beta 1-a in patients with novel Coronavirus(COVID-19) | 3/23/2020 | Recruiting | Iran | |
| Barcitinib | NCT04320277 | Baricitinib in Symptomatic Patients Infected by COVID-19: an Open-label, Pilot Study | 20/3/2020 | Recruiting | Italy |
Chloroquine (CQ)
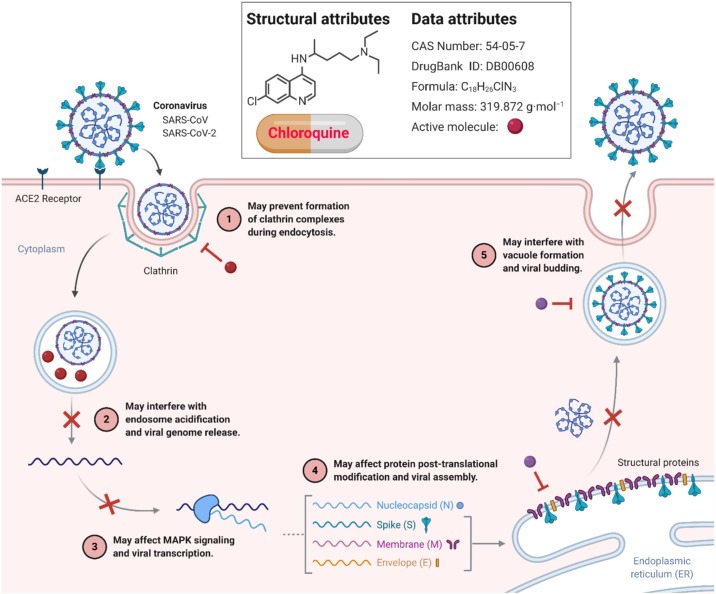
Chloroquine is an aminoquinoline, which has been widely used in malaria treatment, prophylaxis of malaria, and treatment of extraintestinal amoebiasis. It is an old drug, but its use has been reduced after introducing novel anti-malarial drugs. However, it is associated with a prolonged QT interval, which may be fatal when prescribed with other medications, causing prolonged QT interval. The anti-viral properties of chloroquine are well established, and it has been tested against many viruses in vitro [49]. There have been multiple mechanisms explaining its anti-viral properties that need further research. As SARS-CoV-2 is found to use ACE2 receptors for its binding [50,51], it has been hypothesized that it may interfere with glycosylation of ACE2 receptor stopping SARS-CoV-2. One study reported that other coronaviruses such as SARS-CoV and MERS-CoV increase the number of ACE2 receptors, thus speeding up replication and spreading the virus [50]. Inhibition of this step by chloroquine may lead to decreased replication and spread of SARS-CoV-2. Although chloroquine is being used for the treatment and prophylaxis of COVID-19, no randomized controlled trials (RCTs) prove its efficacy have been conducted. We searched the National Clinical Trial Database [52] and the Chinese Clinical Trial Database [53] for completed trials, but none were found. Although many trials for the efficacy of Chloroquine phosphate are underway where patients are currently recruited, none of the completed trials can be found. After the results of these trials, any recommendations can be made regarding the use of chloroquine in COVID-19 (Table 1). Currently, the WHO does not recommend Chloroquine Phosphate in prophylaxis or treatment of COVID-19 at any stage. Potential mechanisms of action of chloroquine against coronaviruses/SARC-CoV/SARC-CoV-2 are shown in Fig. 4 .
Fig. 4.
Potential Mechanisms of action of Chloroquine (CQ) against Coronaviruses. CQ can interfere with the glycosylation of ACE2 and reduce the binding efficiency between ACE2 on the host cells and the spike protein on the surface of the coronavirus. They can also increase the pH of endosomes and lysosomes, through which the fusion process of the virus with host cells and subsequent replication are prevented. As a result, administration of CQ not only blocks the invasion and replication of coronavirus, but also attenuates the possibility of cytokine storm. The Figure was created with “BioRender.com” template and exported under the terms of premium subscription.
Hydroxychloroquine (HCQ)
Hydroxychloroquine (HCQ) is an analog of chloroquine. It has been widely used to treat rheumatoid arthritis, systemic lupus erythematosus, porphyria cutanea tarda and malaria prophylaxis and treatment. It is given orally in different doses in different diseases. Its mechanism is unclear in the case of treating rheumatological conditions. However, in the case of COVID-19, HCQ can increase the intracellular pH and impede lysosomal activity in antigen-presenting cells preventing antigen processing and MHC class II-mediated autoantigen presentation to T cells. It reduces activation of T cells, decreases expression and differentiation of co-stimulatory proteins, and also reduces the production of cytokines by T and B cells [54,55]. It also disrupts the interaction between DNA in cytosols and the cyclic GMP-AMP synthase [56,57].
One recent ongoing French study demonstrated the HCQ use in patients with COVID-19. It revealed a significant decrease in the viral load of patients with COVID-19 who were given HCQ 600 mg orally daily. Although only 36 patients were included and divided into three groups of mild disease, respiratory infection of the upper tract (URTI), and respiratory infection of the lower tract (LRTI), 70% of the study population were given HCQ were virologically cured compared to control group. Furthermore, the response was better in patients with respiratory infection of the upper and lower tract [58]. However, one pilot study conducted in China did not determine any differences using HCQ [59,60]. There are many trials whose results are awaited regarding the use of HCQ in prophylaxis and COVID-19 treatment and efficacy of use along with other drugs. These trials are tabulated in Table 1. Despite its promise as a drug for COVID-19 and widespread use, there is no evidence currently for its use in COVID-19.
Lopinavir/Ritonavir
Lopinavir is used in combination with ritonavir for the treatment of HIV infection. One open-label trial confirmed the effective use of this combination and ribavirin in patients with SARS CoV infection and decreased the incidence of acute respiratory distress syndrome and poor outcomes in those patients [61]. This combination has also shown in-vitro efficacy against MERS-CoV infection [62]. Lopinavir is an aspartate protease inhibitor of HIV type 1 virus, which makes an inhibitor-enzyme complex, thus blocking the activity of the enzyme. Ritonavir inhibits the cytochrome P450 enzyme and increases the plasma half-life of lopinavir. There is no precise mechanism defined regarding its action against SARS-CoV-2. However, due to some promising in vitro results in the treatment of SARS and MERS [61,62], it has been considered a therapy for the treatment of SARS-CoV-2. Furthermore, on recent study established that efficacy of these drugs may be due to inhibition of coronavirus endopeptidase C30 by ritonavir [63]. Researchers performed an RCT in China regarding the efficacy of Lopinavir/Ritonavir in patients having a severe stage of COVID-19. This well-performed study showed no benefit of Lopinavir/Ritonavir use in patients with COVID-19 [64]. However, the case report of a patient infected with SARS CoV 2 showed that this combination had decreased viral loads in a patient with COVID-19 [65]. In one case series done in Taiwan, this combination was not found to reduce the viral loads of SARS-CoV-2 [66]. Still, this combination needs to evaluate via randomized controlled trials. Following trials (Table 1) are currently recruiting COVID 19 patients to be assessed or efficacy of lopinavir/ritonavir with other drugs. This regimen of Lopinavir/Ritonavir is not recommended by WHO and should not be prescribed except for clinical trials. No recommendation can be made until enough data is obtained for these drugs.
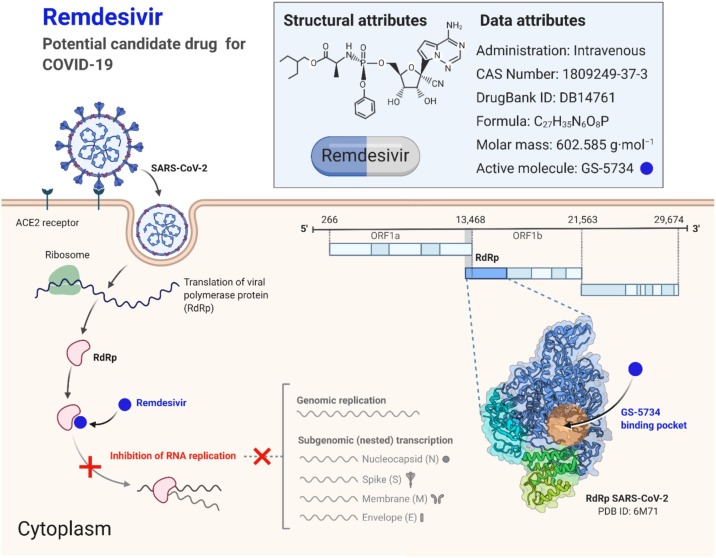
Remdesivir
Remdesivir is a new anti-viral drug that is an adenosine analog and has shown efficacy against many viruses. It was initially launched as treatment of the Ebola virus [67] but was later discovered to work against many other viruses, including Marburg virus, RSV, Lassa fever virus, Junin Virus, Hendra Virus, Nipah virus, MERS, and SARS viruses [[68], [69], [70]]. Due to its activity against other coronaviruses, it has been considered an option against SARS-CoV-2. It is an adenosine analog and a prodrug that metabolizes to its active component. It masks viral RNA polymerase and is not proofread by viral exonuclease, thus decreasing the production of viral RNA production. Its anti-viral activity is due to a delayed chain cessation of the virus's nascent viral RNA, which incorporates itself in viral RNA and leads to premature termination of viral RNA [71]. Randomized clinical trials have not been conducted in patients with SARS-CoV-2 using Remdesivir. One recent study published showed that Remdesivir, when used compassionately, showed improvement in almost 68% of patients with severe disease [72]. However, many clinical trials are being undertaken for its probable efficacy against this virus (Table 1). WHO does not currently recommend the use of Remdesivir in the treatment of COVID-19. The potential mechanism of action of Remdesivir against coronaviruses is shown in Fig. 5 .
Fig. 5.
Potential mechanisms of action of Remdesivir against Coronaviruses. The Figure was created with “BioRender.com” template and exported under the terms of premium subscription.
Favipiravir
Favipiravir is a purine nucleic acid analog and was developed for the treatment of severe influenza infection. It is effective against RNA viruses but not against DNA viruses. Due to its anti-viral activity against RNA viruses, it was used against SARS-CoV-2 [73]. Favipiravir is a potent inhibitor of RNA polymerase of virus and inhibits the viral genome replication of influenza virus [74]. It is activated by ribosylation and phosphorylation within the cell to its active form favipiravir-RTP. The RNA polymerase of virus recognizes this activated form and incorporates it into emerging viral RNA as a purine. It causes chain termination during the synthesis of viral RNA. It has a broad spectrum of activity against other RNA viruses. The drug is currently approved for influenza infection in Japan but is not approved in the US [75]. Due to its efficacy against RNA viruses, it has been considered for SARS CoV 2 infections. One recent RCT was conducted in China, which compared favipiravir versus arbidol in moderate COVID-19 infections. The trial showed that favipiravir had better efficacy in treating moderate COVID-19 infections as compared to arbidol. Multiple trials are being conducted and are tabulated in Table 1. It is currently not recommended in treatment for COVID-19 until the results of the above trials are available.
Ribavirin
This drug has been widely used in several indications. It is being used for chronic Hepatitis C treatment, HIV treatment, and in cases of several viral hemorrhagic fevers. However, the data for use in viral hemorrhagic fevers is lacking, and it may be only effective in early disease stages [76]. It is a prodrug, and when converted to activate form, it resembles purine nucleotide. It inhibits viral mRNA capping and RNA synthesis in the virus. More than five mechanisms have been postulated for its exact mechanism of action [77]. This drug has been recommended for repurposing in SARS-CoV-2 infection [78]. Although its efficacy has been shown along interferons in inhibiting replication of coronavirus in human and animal cell lines [79], there are no trials that have been conducted to prove its efficacy in humans against COVID 19. However, the following trails are being shown (Table 1).
Umefenovir
Umefenovir (arbidol) is an anti-viral drug with a broad spectrum and is used to treat influenza in China and Russia [80]. It is, however, not recommended for this use in other countries. Arbidol inhibits the fusion of membranes [68]. It inhibits the contact of viral and host cell membranes, thus inhibiting their fusion. It prevents the entry of the virus into target cells; therefore, protecting it from viral infection. Few studies suggest that it is also more effective against RNA viruses as compared to DNA viruses [81]. Multiple studies have been conducted regarding the use of arbidol in COVID-19. One retrospective cohort study determined that it speeds up the clearance of virus, improves radiologic images, and decreases the oxygen demand, particularly in those with a mild disease on admission [64]. One comparative study of arbidol against favipiravir showed that favipiravir is superior to arbidol in combating SARS-CoV-2 [82]. Another retrospective cohort study showed that the combination of lopinavir/ritonavir with arbidol is more effective in treating SARS CoV 2 than arbidol alone [83]. One randomized control trial showed no arbidol or lopinavir/ritonavir efficacy in patients with mild to moderate COVID 19 [84]. Tests that are being conducted to evaluate its efficacy are presented in Table 1. Arbidol is currently not recommended for the treatment of COVID 19.
Tolicizumab
It is an immunosuppressant that is used to treat rheumatoid arthritis and systemic sclerosis. It is a monoclonal antibody that is directed against interleukine-6 receptor (IL-6R), thus blocking the cytokine related inflammatory process. Cytokine Response is an inflammatory response that leads to a sudden increase in the levels of multiple pro-inflammatory cytokines [85,86]. It is common among diseases related to the immune system and therapy associated with the immune system, such as sepsis-associated transplantation of organs [87] and viral infections. The SARS-CoV-2 binds epithelial cells of alveoli in the lungs via ACE2, and activates the adaptive and innate immune system. It leads to the release of cytokines, including interleukin 6 (IL-6). Usually, the signal of IL-6 is only limited to the cells that express the IL-6 receptor (IL-6R). This combination leads to gp130 homologous dimerization and initiates the downstream pathway. However, when the levels of IL-6 increase, its signal is expressed because gp130 is present everywhere. Tocilizumab binds cell-related IL-6R and soluble IL-6R, thus inhibiting their signals and cytokine response [88]. The efficacy of tocilizumab in randomized clinical trials is being investigated [89]. No trial has been completed yet. However, one clinical study was conducted in patients with severe disease, and it is found to be effective in patients with severe COVID-19 [90]. Thus, several studies are under investigation for its possible role in severe SARS-CoV-2 infection (Table 1). It is being investigated for use in severe COVID 19 disease. There are no current recommendations for its use in patients.
Interferons
Interferons (IFN-α/β) have broad-spectrum anti-viral activity against RNA viruses, inducing an anti-viral response across several cell types and mediating adaptive immune response. Humans produce 13 kinds of IFN-α and a singular IFN-β [91]. Clinically, type 1 interferons have been approved to treat certain cancers, viral infections, and autoimmune disorders. One recent study also demonstrated the in vitro effects of interferons on SARS-CoV-2. It showed that it inhibits the viral replication at the same doses used for chronic hepatitis B and C treatment [92]. Another study also showed a significant effect of interferon on replication of SARS-CoV-2 [93]. No trials have been completed regarding the role of interferons in COVID-19 management. However, many attempts are under process to look for their potential as treatment or prophylaxis (Table 1). Interferons are currently not suggested for COVID-19 treatment.
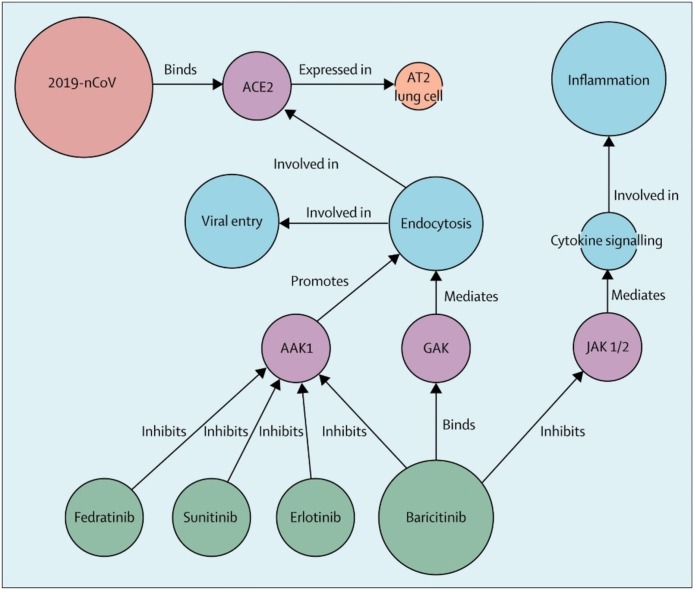
Baricitinib and other drugs
Baricitinib is a drug primarily approved for use in rheumatoid arthritis. It is an immunosuppressant drug. Cytokine storm has been postulated in patients with COVID 19 [94]. Thus, baricitanib has been considered a potential treatment for COVID 19 (Fig. 6 ) [95]. However, some researchers suggest that it may not be an ideal drug to treat COVID 19 [96]. As cytokine storm is considered in the pathology of COVID 19, baricitinib inhibits the activity of Janus Kinase 1 and 2 enzymes, which interfere with the JAK-STAT signaling pathway, thus reducing disease severity. There are no trials conducted in this regard; however, one study is being conducted in Italy, recruiting the patients (Table 1). It is currently not recommended for the treatment of SARS-CoV-2 infection. Other drugs like traditional Chinese medicines [97] and oseltamivir [98] have been used in China without any efficacy in the treatment of COVID-19. There are hundreds of ongoing studies regarding the efficacy of different drugs such as losartan, tocilizumab, hyperbaric oxygen, and others. However, no evidence regarding the use of specific therapy has emerged till now.
Fig. 6.
Baricitnib effect on COVID-19 and CSS by suppressing JAK 1/2 kinases pathway. Reprinted from Ref. [95] with permission from Elsevier. Copyright © 2020 Elsevier Ltd.
COVID-19 vaccines
There are many efforts ongoing in the development of a vaccine for COVID-19. Most of these studies are either in phase 1 or 2 trials. However, multiple types of vaccines are being developed and tested throughout the world. These developed or under-development vaccines include protein subunit vaccines, viral vectored vaccines, mRNA vaccines, DNA vaccines, live attenuated vaccines, and other types such as self-assembling vaccines [99]. Many phase 1 & 2 results of trials have shown promise. One single centered phase 2 study done in Wuhan, China, showed that Ad5-vectored COVID-19 vaccine given in two different doses elicited an immune response in the majority of its participants. No serious adverse events were noted [100]. Another phase 1/2 trial conducted in Russia also supported the use of vector-based rAd26 and rAd5 heterologous prime-boost COVID-19 vaccine [101]. Moreover, another phase 1/2 trial was conducted in the UK using a viral vectored vaccine expressing the spike protein of SARS-CoV-2 induced both cellular and humoral immunity among its participants [102]. Another phase 1 trial conducted in the USA using RNA-based vaccines (BNT162b1 and BNT162b2) showed effective immunogenicity [103]. Although these are preliminary results of different vaccine trials that have been conducted throughout the world, the vaccines have shown promise. There are hundreds of trials ongoing for other vaccines for COVID-19 all over the world. Maybe it is just a matter of time until phase 3/4 studies are conducted, and the world will be free from this deadly pandemic.
Conclusion
In conclusion, the details provided in this review are based on the literature and information available about the COVID-19. The topic's inputs are further required from virologists, microbiologists and infection control experts, and the persons having better practical knowledge about water, sanitation, and health-care waste management. Furthermore, in the present situation, the worldwide collaborations dealing with the solution of the COVID-19 pandemic should be carried out instead of solving the problem independently. However, the epidemic has re-emphasized the significance of evolving broad-spectrum anti-viral agents to fight present and future coronaviruses.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgment
The listed author(s) are thankful to their representative universities for providing the facilities for the literature survey.
References
- 1.Chinazzi M., Davis J.T., Ajelli M., Gioannini C., Litvinova M., Merler S. The effect of travel restrictions on the spread of the 2019 novel coronavirus (2019-nCoV) outbreak. medRxiv. 2020;368:395–400. doi: 10.1126/science.aba9757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bilal M., Nazir M.S., Parra-Saldivar R., Iqbal H.M. 2019-NCOV/COVID-19-Approaches to viral vaccine development and preventive measures. J Pure Appl Microbiol. 2020;14(1):25–29. [Google Scholar]
- 3.Shah S.T.A., Mansoor M., Mirza A.F., Dilshad M., Khan M.I., Farwa R. Predicting COVID-19 spread in Pakistan using the SIR model. J Pure Appl Microbiol. 2020;14(2):1423–1430. [Google Scholar]
- 4.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bilal M., Nazir M.S., Ahmed I., Iqbal H. Coronaviruses and COVID-19–Complications and lessons learned for the future. J Pure Appl Microbiol. 2020;14:725–731. [Google Scholar]
- 6.Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;5(7):831–840. doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 7.Lauer S.A., Grantz K.H., Bi Q., Jones F.K., Zheng Q., Meredith H.R. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172(9):577–582. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 9.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tikellis C., Thomas M.C. Angiotensin-Converting Enzyme 2 (ACE2) is a keymodulator of the renin angiotensin systemin health and disease. Int J Pept. 2012;2012 doi: 10.1155/2012/256294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gue Y.X., Kanji R., Markides V., Adrienne G.D. Angiotensin converting enzyme 2 may mediate disease severity in COVID-19. Am J Cardiol. 2020;130:161–162. doi: 10.1016/j.amjcard.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belouzard S., Chu V.C., Whittaker G.R. Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinct sites. Proc Natl Acad Sci USA. 2009;106:5871–5876. doi: 10.1073/pnas.0809524106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang H., Yang P., Liu K., Guo F., Zhang Y., Zhang G. SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Res. 2008;18:290–301. doi: 10.1038/cr.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar S., Nyodu R., Maurya V.K., Saxena S.K. In: Host immune response and immunobiology of human SARS-CoV-2 infection, in Coronavirus Disease 2019 (COVID-19) Saxena S.K., editor. Springer; Singapore: 2020. pp. 43–53. [Google Scholar]
- 16.Liu J., Wu P., Gao F., Qi J., Tachikawa A.I., Xie J. Novel immunodominant peptide presentation strategy: a featured HLA-A*2402-restricted cytotoxic T-lymphocyte epitope stabilized by intrachain hydrogen bonds from severe acute respiratory syndrome coronavirus nucleocapsid protein. J Virol. 2010;84:11849–11857. doi: 10.1128/JVI.01464-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perlman S., Netland J. Coronaviruses post-SARS: update on replication and Pathogenesis. Nat Rev Microbiol. 2009;7:439–450. doi: 10.1038/nrmicro2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li G., Fan Y., Lai Y., Han T., Li Z., Zhou P. Coronavirus infections and immune responses. J Med Virol. 2020;92:424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou Z., Ren L., Zhang L., Zhong J., Xiao Y., Jia Z. Heightened innate immune responses in the respiratory tract of COVID-19 patients. Cell Host Microbe. 2020;27:883–890. doi: 10.1016/j.chom.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chatterjee S.K., Saha S., Munoz M.N.M. Molecular pathogenesis, immunopathogenesis and novel therapeutic strategy against COVID-19. Front Mol Biosci. 2020;7:196. doi: 10.3389/fmolb.2020.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iqbal H.M., Romero-Castillo K.D., Bilal M., Parra-Saldivar R. The emergence of novel-coronavirus and its replication cycle: an overview. J Pure Appl Microbiol. 2020;14(1):13–16. [Google Scholar]
- 22.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tarar S.H., Atta H., Khalid M., Saeed S., Shah S.M.A., Rizwan K. A case report of pregnant lady having COVID-19 delivered via cesarean section in tertiary care hospital in Pakistan. J Pure Appl Microbiol. 2020;14(2):1121–1123. [Google Scholar]
- 25.Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158(6):1831–1833. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:e53. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y., Chen C., Zhu S., Shu C., Wang D., Song J. Isolation of 2019-nCoV from a stool specimen of a laboratory-confirmed case of the coronavirus disease 2019 (COVID-19) China CDC Weekly. 2020;2(8):123–124. [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X.-W., Li J.-S., Jin M., Zhen B., Kong Q.-X., Song N. Study on the resistance of severe acute respiratory syndrome-associated coronavirus. J Virol Methods. 2005;126(1–2):171–177. doi: 10.1016/j.jviromet.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gundy P.M., Gerba C.P., Pepper I.L. Survival of coronaviruses in water and wastewater. Food Environ Virol. 2009;1(1):10. [Google Scholar]
- 30.Casanova L., Rutala W.A., Weber D.J., Sobsey M.D. Survival of surrogate coronaviruses in water. Water Res. 2009;43(7):1893–1898. doi: 10.1016/j.watres.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and its inactivation with biocidal agents. J Hosp Infect. 2020;382:929–936. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Organization, W.H . 2017. Guidelines for drinking-water quality: first addendum to the fourth edition. ( http://apps.who.int/iris/bitstream/10665/254637/1/9789241549950-eng.pdf, accessed 3 March 2020) [PubMed] [Google Scholar]
- 33.SARS-CoV-2 − water and sanitation. Adelaide: Water Research Australia; 2020 (http://www.waterra.com.au/_r9544/media/system/attrib/file/2199/WaterRA_FS_Coronavirus_V10.pdf, accessed 3 March 2020).
- 34.Bilal M., Nazir M.S., Rasheed T., Parra-Saldivar R., Iqbal H.M. Case studies in chemical and environmental engineering. 2020. Water matrices as potential source of SARS-CoV-2 transmission–An overview from environmental perspective. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.In: WHO/Infection prevention and control [website] World Health Organization; Geneva: 2020. My 5 moments for hand hygiene. ( https://www.who.int/infectionprevention/campaigns/cleanhands/5moments/en/, accessed 3 March 2020) [Google Scholar]
- 36.Siddharta A., Pfaender S., Vielle N.J., Dijkman R., Friesland M., Becker B. Virucidal activity of world health organization–recommended formulations against enveloped viruses, including Zika, Ebola, and emerging coronaviruses. J Infect Dis. 2017;215(6):902–906. doi: 10.1093/infdis/jix046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Health Organization; Geneva: 2009. WHO guidelines on hand hygiene in health care settings. ( https://apps.who.int/iris/bitstream/handle/10665/44102/9789241597906_eng.pdf?sequence=1&isAllowed=y, accessed 3 March 2020) [Google Scholar]
- 38.Shahbaz M., Bilal M., Akhlaq M., Moiz A., Zubair S., Iqbal H.M. Strategic measures for food processing and manufacturing facilities to combat coronavirus pandemic (COVID-19) J Pure Appl Microbiol. 2020;14(2):1087–1094. [Google Scholar]
- 39.Shahbaz M., Bilal M., Moiz A., Zubair S., Iqbal H.M. Food safety and COVID-19: precautionary measures to limit the spread of coronavirus at food service and retail sector. J Pure Appl Microbiol. 2020;14:749–756. [Google Scholar]
- 40.Organization W.H., Council W.P. World Health Organization; 2006. Health aspects of plumbing. [Google Scholar]
- 41.Organization W.H. 2018. Guidelines on sanitation and health. [Google Scholar]
- 42.Yu I.T., Li Y., Wong T.W., Tam W., Chan A.T., Lee J.H. Evidence of airborne transmission of the severe acute respiratory syndrome virus. N Engl J Med. 2004;350(17):1731–1739. doi: 10.1056/NEJMoa032867. [DOI] [PubMed] [Google Scholar]
- 43.Regan H. How can the coronavirus spread through bathroom pipes? Experts are investigating in Hong Kong. CNN. 12 February 2020 (https://edition.cnn.com/2020/02/12/asia/hong-kong-coronaviruspipes-intlhnk/index.html).
- 44.Organization W.H. World Health Organization; 2015. Sanitation safety planning: manual for safe use and disposal of wastewater greywater and excreta. [Google Scholar]
- 45.Organization W.H. World Health Organization; 2008. How to put on and take off personal protective equipment (PPE) [Google Scholar]
- 46.Control, C. f. D.; Prevention, Guideline for disinfection and sterilization in healthcare facilities, 2008. URL: https://www.cdc.gov/infectioncontrol/pdf/guidelines/disinfection-guidelines.pdf (19.12. 2017) 2008.
- 47.US Centers for Disease Control and Prevention; Atlanta: 2019. Best practices for environmental cleaning in health-care facilities in resource-limited settings. ( https://www.cdc.gov/hai/pdfs/resourcelimited/environmental-cleaning-508.pdf, accessed 3 March 2020) [Google Scholar]
- 48.Organization W.H. World Health Organization; 2017. Safe management of wastes from health-care activities: a summary. [Google Scholar]
- 49.Devaux C.A., Rolain J.-M., Colson P., Raoult D. New insights on the anti-viral effects of chloroquine against coronavirus: what to expect for COVID-19? Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang P.-H. Increasing host cellular receptor—angiotensin-converting enzyme 2 (ACE2) expression by coronavirus may facilitate 2019-nCoV infection. bioRxiv. 2020:1–22. doi: 10.1002/jmv.26139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li R., Qiao S., Zhang G. Analysis of angiotensin-converting enzyme 2 (ACE2) from different species sheds some light on cross-species receptor usage of a novel coronavirus 2019-nCoV. J Infect. 2020;80(4):469–496. doi: 10.1016/j.jinf.2020.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Available at: https://clinicaltrials.gov/ Last accessed on 07 Apr 2020.
- 53.Available at: http://www.chictr.org.cn/enindex.aspx Last accessed on 07 Apr 2020.
- 54.Wu S.-F., Chang C.-B., Hsu J.-M., Lu M.-C., Lai N.-S., Li C. Hydroxychloroquine inhibits CD154 expression in CD4+ T lymphocytes of systemic lupus erythematosus through NFAT, but not STAT5, signaling. Arthritis Res Ther. 2017;19(1):183. doi: 10.1186/s13075-017-1393-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kužnik A., Benčina M., Švajger U., Jeras M., Rozman B., Jerala R. Mechanism of endosomal TLR inhibition by antimalarial drugs and imidazoquinolines. J Immunol. 2011;186(8):4794–4804. doi: 10.4049/jimmunol.1000702. [DOI] [PubMed] [Google Scholar]
- 56.An J., Woodward J.J., Sasaki T., Minie M., Elkon K.B. Cutting edge: antimalarial drugs inhibit IFN-β production through blockade of cyclic GMP-AMP synthase–DNA interaction. J Immunol. 2015;194(9):4089–4093. doi: 10.4049/jimmunol.1402793. [DOI] [PubMed] [Google Scholar]
- 57.Dijkmans B., Verweij C. Chloroquine and hydroxychloroquine equally affect tumor necrosis factor-alpha, interleukin 6, and interferon-gamma production by peripheral blood mononuclear cells. J Rheumatol. 1997;24(1):55–60. [PubMed] [Google Scholar]
- 58.Gautret P., Lagier J.-C., Parola P., Meddeb L., Mailhe M., Doudier B. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen J., Liu D., Liu L., Liu P., Xu Q., Xia L. A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease-19 (COVID-19) J Zhejiang Univ (Med Sci) 2020;49(2):215–219. doi: 10.3785/j.issn.1008-9292.2020.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou D., Dai S.-M., Tong Q. COVID-19: a recommendation to examine the effect of hydroxychloroquine in preventing infection and progression. J Antimicrob Chemother. 2020;75:1667–1670. doi: 10.1093/jac/dkaa114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chu C., Cheng V., Hung I., Wong M., Chan K., Chan K. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59(3):252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chan J.F.-W., Yao Y., Yeung M.-L., Deng W., Bao L., Jia L. Treatment with lopinavir/ritonavir or interferon-β1b improves outcome of MERS-CoV infection in a nonhuman primate model of common marmoset. J Infect Dis. 2015;212(12):1904–1913. doi: 10.1093/infdis/jiv392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lin S., Shen R., Guo X. Molecular modeling evaluation of the binding abilities of ritonavir and Lopinavir to Wuhan pneumonia coronavirus proteases. bioRxiv. 2020 doi: 10.1101/2020.01.31.929695. [DOI] [Google Scholar]
- 64.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lim J., Jeon S., Shin H.-Y., Kim M.J., Seong Y.M., Lee W.J. Case of the index patient who caused tertiary transmission of COVID-19 infection in Korea: the application of lopinavir/ritonavir for the treatment of COVID-19 infected pneumonia monitored by quantitative RT-PCR. J Korean Med Sci. 2020;35(6):e79. doi: 10.3346/jkms.2020.35.e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cheng C.-Y., Lee Y.-L., Chen C.-P., Lin Y.-C., Liu C.-E., Liao C.-h. Lopinavir/ritonavir did not shorten the duration of SARS CoV-2 shedding in patients with mild pneumonia in Taiwan. J Microbiol Immunol Infect. 2020;53:488–492. doi: 10.1016/j.jmii.2020.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Warren T.K., Jordan R., Lo M.K., Ray A.S., Mackman R.L., Soloveva V. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016;531(7594):381–385. doi: 10.1038/nature17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lo M.K., Jordan R., Arvey A., Sudhamsu J., Shrivastava-Ranjan P., Hotard A.L. GS-5734 and its parent nucleoside analog inhibit Filo-, Pneumo-, and Paramyxoviruses. Sci Rep. 2017;7:43395. doi: 10.1038/srep43395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sheahan T.P., Sims A.C., Graham R.L., Menachery V.D., Gralinski L.E., Case J.B. Broad-spectrum anti-viral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med. 2017;9(396):eaal3653. doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Agostini M.L., Andres E.L., Sims A.C., Graham R.L., Sheahan T.P., Lu X. Coronavirus susceptibility to the anti-viral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. MBio. 2018;9(2):e00221–18. doi: 10.1128/mBio.00221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Al-Tawfiq J.A., Al-Homoud A.H., Memish Z.A. Remdesivir as a possible therapeutic option for the COVID-19. Travel Med Infect Dis. 2020 doi: 10.1016/j.tmaid.2020.101615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med. 2020;382:e101. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Furuta Y., Takahashi K., Fukuda Y., Kuno M., Kamiyama T., Kozaki K. In vitro and in vivo activities of anti-influenza virus compound T-705. Antimicrob Agents Chemother. 2002;46(4):977–981. doi: 10.1128/AAC.46.4.977-981.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Baranovich T., Wong S.-S., Armstrong J., Marjuki H., Webby R.J., Webster R.G. T-705 (favipiravir) induces lethal mutagenesis in influenza A H1N1 viruses in vitro. J Virol. 2013;87(7):3741–3751. doi: 10.1128/JVI.02346-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ramirez J.A. Current and future anti-virals medications to treat influenza: mechanisms of action. Univ Louisville J Respir Infect. 2019;3(1):9. [Google Scholar]
- 76.Ascioglu S., Leblebicioglu H., Vahaboglu H., Chan K.A. Ribavirin for patients with Crimean–congo haemorrhagic fever: a systematic review and meta-analysis. J Antimicrob Chemother. 2011;66(6):1215–1222. doi: 10.1093/jac/dkr136. [DOI] [PubMed] [Google Scholar]
- 77.Graci J.D., Cameron C.E. Mechanisms of action of ribavirin against distinct viruses. Rev Med Virol. 2006;16(1):37–48. doi: 10.1002/rmv.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Elfiky A.A. Anti-HCV, nucleotide inhibitors, repurposing against COVID-19. Life Sci. 2020 doi: 10.1016/j.lfs.2020.117477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Morgenstern B., Michaelis M., Baer P.C., Doerr H.W., Cinatl J., Jr. Ribavirin and interferon-β synergistically inhibit SARS-associated coronavirus replication in animal and human cell lines. Biochem Biophys Res Commun. 2005;326(4):905–908. doi: 10.1016/j.bbrc.2004.11.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Leneva I.A., Russell R.J., Boriskin Y.S., Hay A.J. Characteristics of arbidol-resistant mutants of influenza virus: implications for the mechanism of anti-influenza action of arbidol. Antiviral Res. 2009;81(2):132–140. doi: 10.1016/j.antiviral.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 81.Shi L., Xiong H., He J., Deng H., Li Q., Zhong Q. Anti-viral activity of arbidol against influenza A virus, respiratory syncytial virus, rhinovirus, coxsackie virus and adenovirus in vitro and in vivo. Arch Virol. 2007;152(8):1447–1455. doi: 10.1007/s00705-007-0974-5. [DOI] [PubMed] [Google Scholar]
- 82.Chen C., Huang J., Cheng Z., Wu J., Chen S., Zhang Y. Favipiravir versus Arbidol for COVID-19: a randomized clinical trial. medRxiv. 2020 doi: 10.1101/2020.03.17.20037432. [DOI] [Google Scholar]
- 83.Deng L., Li C., Zeng Q., Liu X., Li X., Zhang H. Arbidol combined with LPV/r versus LPV/r alone against Corona Virus Disease 2019: a retrospective cohort study. J Infect. 2020;81:e1–e5. doi: 10.1016/j.jinf.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li Y., Xie Z., Lin W., Cai W., Wen C., Guan Y. An exploratory randomized, controlled study on the efficacy and safety of lopinavir/ritonavir or arbidol treating adult patients hospitalized with mild/moderate COVID-19 (ELACOI) medRxiv. 2020 doi.org/10.1101/2020.03.19.20038984. [Google Scholar]
- 85.Shimabukuro-Vornhagen A., Gödel P., Subklewe M., Stemmler H.J., Schlößer H.A., Schlaak M. Cytokine release syndrome. J Immunother Cancer. 2018;6(1):56. doi: 10.1186/s40425-018-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Norelli M., Camisa B., Barbiera G., Falcone L., Purevdorj A., Genua M. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med. 2018;24(6):739–748. doi: 10.1038/s41591-018-0036-4. [DOI] [PubMed] [Google Scholar]
- 87.Chousterman B.G., Swirski F.K., Weber G.F. Seminars in immunopathology, 2017; Springer; 2017. In Cytokine storm and sepsis disease pathogenesis; pp. 517–528. [DOI] [PubMed] [Google Scholar]
- 88.Zhang C., Wu Z., Li J.-W., Zhao H., Wang G.-Q. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. 2020;55(5) doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhou M., Zhang X., Qu J. Coronavirus disease 2019 (COVID-19): a clinical update. Front Med. 2020:1–10. doi: 10.1007/s11684-020-0767-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xu X., Han M., Li T., Sun W., Wang D., Fu B. Effective treatment of severe COVID-19 patients with tocilizumab. ChinaXiv. 2020;117(20):10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pestka S., Krause C.D., Walter M.R. Interferons, interferon‐like cytokines, and their receptors. Immunol Rev. 2004;202(1):8–32. doi: 10.1111/j.0105-2896.2004.00204.x. [DOI] [PubMed] [Google Scholar]
- 92.Mantlo E.K., Bukreyeva N., Maruyama J., Paessler S., Huang C. Potent anti-viral activities of type I interferons to SARS-CoV-2 infection. bioRxiv. 2020 doi: 10.1101/2020.04.02.022764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lokugamage K.G., Hage A., Schindewolf C., Rajsbaum R., Menachery V.D. SARS-CoV-2 sensitive to type I interferon pretreatment. BioRxiv. 2020 doi: 10.1101/2020.03.07.982264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lippi G., Sanchis-Gomar F., Henry B.M. Coronavirus disease 2019 (COVID-19): the portrait of a perfect storm. Ann Transl Med. 2020;8(7):497. doi: 10.21037/atm.2020.03.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Richardson P., Griffin I., Tucker C., Smith D., Oechsle O., Phelan A. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet (London, England) 2020;395(10223):e30. doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Praveen D., Chowdary P.R., Aanandhi M.V. Baricitinib-a januase kinase inhibitor-not an ideal option for management of COVID 19. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ren J.-l., Zhang A.-H., Wang X.-J. Traditional Chinese medicine for COVID-19 treatment. Pharmacol Res. 2020;155 doi: 10.1016/j.phrs.2020.104743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bai Y., Yao L., Wei T., Tian F., Jin D.-Y., Chen L. Presumed asymptomatic carrier transmission of COVID-19. Jama. 2020;323(14):1406–1407. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kaur S.P., Gupta V. COVID-19 vaccine: a comprehensive status report. Virus Res. 2020;288 doi: 10.1016/j.virusres.2020.198114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhu F.C., Guan X.H., Li Y.H., Huang J.Y., Jiang T., Hou L.H. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396(10249):479–488. doi: 10.1016/S0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Logunov D.Y., Dolzhikova I.V., Zubkova O.V., Tukhvatullin A.I., Shcheblyakov D.V., Dzharullaeva A.S. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet. 2020;396(10255):887–897. doi: 10.1016/S0140-6736(20)31866-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Folegatti P.M., Ewer K.J., Aley P.K., Angus B., Becker S., Belij-Rammerstorfer S. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396(10249):467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Walsh E.E., Frenck R.W., Falsey A.R. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020 doi: 10.1056/nejmoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]