Abstract
Objective
To assess the utility of self-reported symptoms in identifying positive coronavirus disease 2019 (COVID-19) cases among predominantly healthy young adults in a military setting.
Methods
A questionnaire regarding COVID-19 symptoms and exposure history was administered to all individuals contacting the Israeli Defence Forces Corona call-centre, before PCR testing. Surveyed symptoms included cough, fever, sore throat, rhinorrhoea, loss of taste or smell, chest pain and gastrointestinal symptoms. Factors were compared between positive and negative cases based on confirmatory test results, and positive likelihood ratios (LR) were calculated. Results were stratified by sex, body mass index, previous medical history and dates of questioning, and a multivariable analysis for association with positive test was conducted.
Results
Of 24 362 respondents, 59.1% were men with a median age of 20.5 years (interquartile range 19.6–22.4 years). Significant positive LRs were associated with loss of taste or smell (LR 3.38, 95% CI 3.01–3.79), suspected exposure (LR 1.33, 95% CI 1.28–1.39) and fever (LR 1.26, 95% CI 1.17–1.36). Those factors were also associated with positive PCR result in a multivariable analysis (OR 3.51, 95% CI 3.04–4.06; OR 1.86, 95% CI 1.65–2.09; and OR 1.34, 95% CI 1.19–1.51, respectively). Reports of loss of taste or smell increased gradually over time and were significantly more frequent during the late period of the study (63/5231, 1.21%; 156/7941, 1.96%; and 1505/11 190, 13.45%: p < 0.001).
Conclusion
Loss of taste or smell, report of a suspicious exposure and fever (>37.5°C) were consistently associated with positive LRs for a positive SARS-CoV-2 PCR test result, in a population of predominantly young and healthy adults.
Keywords: Coronavirus disease 2019, Coronavirus disease 2019 confirmatory test shortage, Coronavirus disease 2019 in young adults, Coronavirus disease 2019 self-reported symptoms, Coronavirus disease 2019 symptoms, Coronavirus disease 2019 testing, Coronavirus disease 2019 testing criteria, Utility of self-reported symptoms
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first reported in China in December 2019 [1] and by 11 March 2020 was declared as a pandemic [2]. To date, the reported disease burden exceeds 96 million people, with a death toll of approximately 2 000 000 worldwide [3].
Insufficient viral RNA testing capacity [[4], [5], [6]], which results in propagation of the virus by undiagnosed infected individuals [7,8], cripples the ability of health authorities to control viral spread [9,10]. Hence, prioritizing testing and efficiently isolating positive cases is a vital aim. To this end, several studies examined the utility of self-reported symptoms in predicting coronavirus disease 2019 (COVID-19) and found a correlation between constitutional and respiratory symptoms with a positive test [[11], [12], [13]]. However, low response rates in young healthy adults [12,13] and their exclusion from studies [11], results in a dearth of data guiding testing in this population. As the proportion of young infected adults increases [[14], [15], [16]], and their potential in propagating disease spread grows [8,17,18], there is an impending need to better characterize the symptom distribution suggestive of infection in this group.
The Israel Defence Forces (IDF) is comprised mostly of young healthy individuals who have undergone extensive medical screening [19]. Therefore, in this study we used a retrospective cohort design to evaluate the association between symptoms in young adults and a positive test result.
Materials and methods
Study population
The special IDF COVID-19 centre (ICC) handled documentation of all individuals with suspicious symptoms, those quarantined, and all confirmed COVID-19 cases. This included collection of data obtained through contact tracing and an enquiries ‘hotline’. ICC referred soldiers for swab tests based on report of symptoms and exposure history. We included all individuals who were deemed eligible for COVID-19 testing by the ICC, including those voluntarily calling to report symptoms or a suspected exposure, or those actively addressed following an epidemiological investigation. Eligibility for confirmatory testing was directed by the surgeon general and approved by public health officers on a case-by-case basis. Testing was approved for those with: (a) presence of at least two acute respiratory symptoms (cough, shortness of breath, sore throat, fever >38°C); or (b) one symptom combined with a suspicious exposure; or (c) loss of taste or smell as a sole symptom.
Primary outcome
The primary outcome measure was the positive likelihood ratio (LR) of symptoms suggesting a positive real-time reverse transcript (rRT-) PCR result.
Data collection
Answers to questions asked by the ICC before swab testing were drawn from a digital database where each individual has a unique identification number. Symptoms surveyed included: cough, fever (>37.5°C), sore throat, rhinorrhoea, loss of taste or smell, chest pain and gastrointestinal symptoms (GI, i.e. abdominal pain, vomiting, or diarrhoea). Suspected exposure was defined as close contact with a confirmed COVID-19 patient or recent (<14 days) international travel (for questionnaire example see Supplementary material, Appendix S2). Data were collected between 26 March and 2 August 2020. A group of 6643 individuals had COVID-19 tests without answering the questionnaires so their symptoms and exposure history data were unavailable and they were excluded from the study. PCR test results were merged using the identification number from a digital laboratory database and classified as either positive or negative. For positive cases, only the first positive test was included, and data were collected only from the questionnaire obtained before this test. The following covariates were abstracted from the medical registry: body mass index (BMI), baseline health and comorbidities, as detailed in the Supplementary material (Appendix S1).
Viral RNA test
Viral RNA presence was examined using rRT-PCR based on the SARS-CoV-2 Centers for Disease Control and Prevention (CDC) protocol [20]; using viral markers for each of the three viral genes (RdRP, N gene and E gene). Cycle threshold values were reported, as well as an internal control marker. A threshold of 40 cycles and below was considered positive. Results in which only N gene was present within the range of 36–40 cycles, were defined as marginally positive. In those cases, a certified physician and a public-health officer interpreted the result according to the clinical context, and the individual was re-tested if deemed necessary. Additional details on testing methods are available elsewhere [21]. All swab materials were processed in the central IDF laboratory, throughout the entire study.
Statistical analysis
Prevalence of each reported symptom and exposure were compared between positive and negative rRT-PCR groups. Continuous variables were presented as medians and interquartile ranges, and categorical variables were presented as percentages and counts. Positive LR, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and 95% CI for test statistics were calculated using the methods of Simel et al. [22] and Wilson [23]. Results were stratified by sex, BMI category, previous medical history and dates of questioning. Univariate and multivariable logistic regression models were performed to assess the relationship between a positive PCR test result (dependent variable) and clinical (asthma, chronic sinusitis, hypertension and BMI), anamnestic (report of symptoms and exposure) and demographic factors (sex and age – analysed as continuous variable and as categorical ≤20; 21–22; ≥23) as independent variables. Six statistically significant independent variables were included in multivariable analysis and selected using forward stepwise method (p-in 0.05; p-out 0.10). No collinearity was found among independent variables included in multivariable analysis, maximum variance inflation factor = 1.039. Odds ratios (OR), 95% CI and p values are presented.
To assess trends in symptom distribution over time, we selected three consequent periods to represent different phases of the pandemic: the initial COVID-19 outbreak, mitigation of disease spread and the increased disease burden following the reopening of communities, as shown in Fig. 2 b. For each symptom, analysis of variance was used to compare the proportion of individuals reporting its presence between periods. As no homogeneity of variances was found, multiple comparisons were made with post hoc Dunnett T3 test.
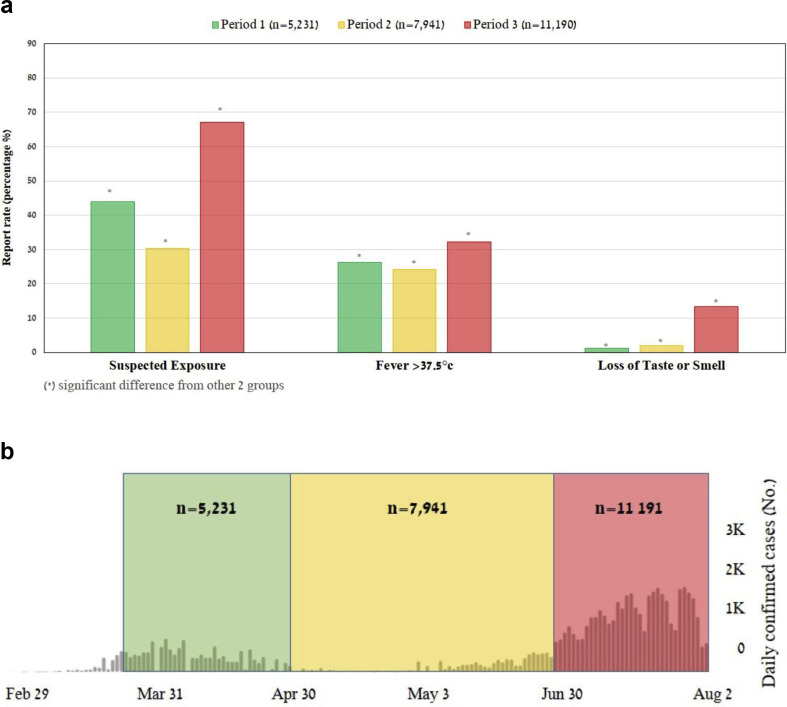
Fig. 2.
(a) Comparison of the report rate of surveyed factors against time. Presented here are factors that were associated with significant positive likelihood ratios for COVID-19 (see text). In general, overall report rates of suspected exposure and fever were parallel to the extent of disease spread in the country, i.e. decreasing between the first and second period, and raising drastically in the third period. All of these changes were statistically significant (all p ≤ 0.038). The exception is reports of loss of taste or smell, which increased gradually and significantly with time (all p < 0.001), (see Supplementary material, Table S3 for expanded results of statistical analysis). (b) Description of the time sections selected for comparison. This figure details the three consequent periods selected for comparison with the number of overall study participants in each. The background represents COVID-19 disease burden in Israel, as measured by daily confirmed cases between 29 February and 2 August 2020, according to the reports of WHO. Green – period 1: 26 March to 30 April, represents the initial COVID-19 outbreak. Yellow – period 2: 1 May to 30 June, represents mitigation of disease spread. Red – period 3: 1 July to 2 August, represents the increased disease burden following the reopening of communities. The second period is about twice as long because of the relatively small number of cases throughout May and we therefore summed those with the cases identified in the consequent month.
Values of p that were <0.05 and CI exclusive of the null were considered statistically significant. Statistical analyses were conducted using Microsoft Excel (version 2013; Microsoft, Redmond, WA, USA) and IBM SPSS Statistics for Windows (version 25.0; IBM, Armonk, NY, USA).
The institutional review board of the IDF approved this study (protocol number 2082-2020), and waived the requirement for written informed consent based on preserving participants' anonymity.
Results
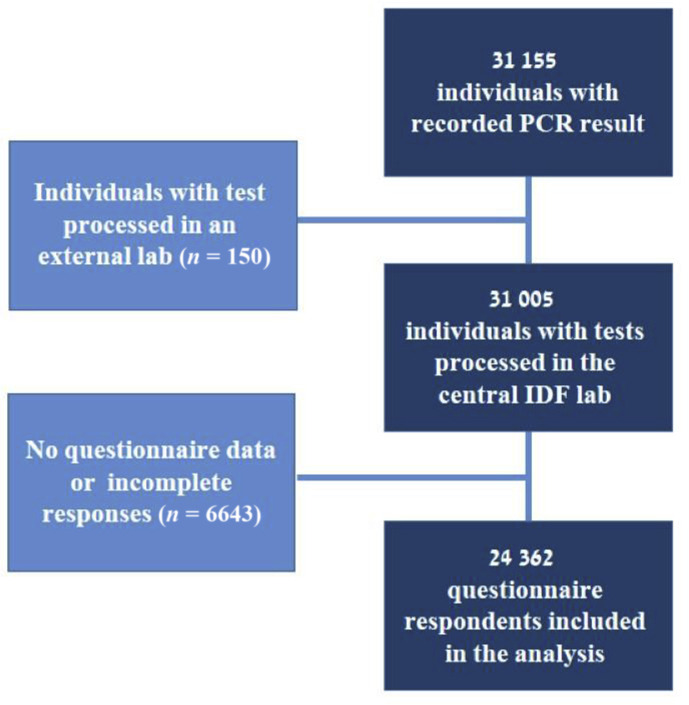
Overall, 31 155 individuals were identified between 26 March and 2 August 2020. We excluded those with no recorded result, or results processed in an external laboratory (n = 150) and individuals with missing questionnaire items (n = 6643, 21.4%) (Fig. 1, see Supplementary material, Table S1, for comparison with the included cohort). Ultimately, 24 362 respondents were included in the analysis. Over half (59.1%) were men, with a median age of 20.5 years (interquartile range 19.6–22.4 years) of whom 65% (n = 15 534) had unimpaired health at baseline. Of all PCR tests, 5.5% (n = 1338) were positive, 5.7% in men and 5.2% in women. During the first, second and third periods of the study, a total of 5231, 7941 and 11 190 tests were conducted with 2.25% (n = 118), 2.29% (n = 182) and 9.27% (n = 1038) positivity rates, respectively. Characteristics of the study population are shown in Table 1 .
Fig. 1.
Study population flow chart. Numbers represent respondents. A total of 31 155 individuals were identified between 26 March and 2 August 2020. Those with no recorded PCR test result, or whose tests were processed in an external laboratory (n = 150) were excluded. Questionnaires were not performed among 6643 (21.4%) individuals so their exposure and symptoms data were missing, resulting in their exclusion from the analysis, which ultimately included 24 362 respondents with a PCR test result and questionnaire data.
Table 1.
Baseline characteristics of study population
| Overall (n = 24 362) |
Positive cases (n = 1338; 5.5%)a |
Negative cases (n = 23 024) |
p value | |
|---|---|---|---|---|
| Age (years), median (IQR) | 20.5 (19.6–22.4) | 21 (20–23) | 21 (20–23) | 0.426 |
| Male sex, n (%) | 14 398 (59.1) | 817 (61.1) | 13 577 (59.0) | 0.075 |
| Unimpaired healthb, n (%) | 15 534 (63.7) | 869 (64.9) | 14 487 (62.9) | 0.135 |
| Asthmac, n (%) | 1786 (7.3) | 90 (6.7) | 1696 (7.4) | 0.383 |
| Allergic rhinitis, n (%) | 1756 (7.2) | 83 (6.2) | 1673 (7.3) | 0.144 |
| Chronic sinusitis, n (%) | 11 (0.05) | 1 (0.07) | 10 (0.04) | 0.463 |
| Hypertension, n (%) | 55 (0.22) | 1 (0.07) | 54 (0.23) | 0.371 |
| BMI (kg/m2), n (%) | ||||
| <18.5 | 2477 (10.2) | 140 (10.5) | 2337 (10.15) | 0.713 |
| 18.5–24.9 | 15 356 (63.0) | 851 (63.6) | 14 505 (62.9) | 0.657 |
| 25–29.9 | 3438 (14.1) | 172 (12.9) | 3266 (14.2) | 0.174 |
| ≥30 | 1309 (5.4) | 64 (4.8) | 1245 (5.4) | 0.325 |
Abbreviation: BMI, body mass index; IQR, interquartile range.
Percentage represents percentage of overall cases that were positive.
Definition and method for determination of unimpaired health is detailed in the Supplementary material, Appendix S1.
History of childhood asthma, with no use of inhaler for 3 or more years, and normal pulmonary function tests, do not affect medical fitness.
Reported symptoms
Cough was the most commonly reported symptom (13 675/24 362, 56.1%), followed by report of suspected exposure (12 211/24 362, 50.1%) and fever (6896/24 362, 28.3%). The item with highest sensitivity (65.5% (63-68.1%)) was suspected exposure, followed by cough (55.5% (52.9-58.2%)) and fever (35.1% (32.6-37.7%)). Highest specificity was seen with GI symptoms (98.6% (98.5-98.8%)), followed by chest pain (98.2% (98.1-98.4%)) and loss of taste or smell (93.7% (93.4-94.1%)). Loss of taste or smell was also found to have the highest PPV (16.4% (14.8-17.9%)). Suspected exposure was found to have the highest NPV (96.2% (95.9-96.5%)) followed by loss of taste or smell (95.3% (95.2-95.5%)) and fever (95% (94.8-95.2%)). The overall distribution of reports on surveyed factors, sensitivity, specificity, PPV and NPV, are summarized in Table 2 .
Table 2.
Reasons for contacting the IDF COVID-19 call centre and sensitivity, specificity, positive and negative predictive values for each (n = 24 362)
| Prevalence, n (%) | Sensitivity (95% CI) | Specificity (95% CI) | Positive predictive value (95% CI) | Negative predictive value (95% CI) | |
|---|---|---|---|---|---|
| Suspected exposurea | 12 211 (50.1) | 65.6% (63%–68.1%) | 50.8% (50.1%–51.4%) | 7.2% (6.9%–7.5%) | 96.2% (95.9%–96.5%) |
| Cough | 13 675 (56.1) | 55.5% (52.9%–58.2%) | 43.8% (43.2%–44.5%) | 5.4% (5.2%–5.7%) | 94.4% (94.1%–94.7%) |
| Fever >37.5°C | 6896 (28.3) | 35.1% (32.6%–37.7%) | 72.1% (71.5%–72.7%) | 6.8% (6.3%–7.3%) | 95.0% (94.8%–95.2%) |
| Sore throat | 5559 (22.8) | 21.2% (19.1%–23.4%) | 77.1% (76.5%–77.6%) | 5.1% (4.6%–5.6%) | 94.4% (94.2%–94.5%) |
| Rhinorrhoea | 2298 (9.4) | 7.9% (6.5%–9.4%) | 90.5% (90.1%–90.9%) | 4.6% (3.8%–5.5%) | 94.4% (94.3%–94.5%) |
| Loss of taste or smell | 1723 (7.1) | 21.2% (19.1%–23.4%) | 93.7% (93.4%–94.1%) | 16.4% (14.8%–17.9%) | 95.3% (95.2%–95.5%) |
| Chest pain | 430 (1.8) | 1.8% (1.2%–2.7%) | 98.2% (98.1%–98.4%) | 5.6% (3.8%–8.2%) | 94.5% (94.46%–94.54) |
| GI symptoms | 339 (1.4) | 1.6% (1%–23.9%) | 98.6% (98.5%–98.8%) | 6.2% (4.1%–9.3%) | 94.5% (94.47%–94.55%) |
Abbreviations: COVID-19, coronavirus disease 2019; GI, gastrointestinal symptoms, i.e. abdominal pain, vomiting, diarrhoea; IDF, Israel Defence Forces.
Suspected exposure in the survey was defined as a close contact with a confirmed COVID-19 patient or recent (<14 days) international travel.
Comparing the distribution of reports over time (Fig. 2a, see Supplementary material, Tables S2 and S3) demonstrated that suspected exposure decreased between first and second periods (2303/5231 (44.0%), 2411/7941 (30.4%), respectively, p < 0.001), and was significantly higher during the third period (7498/11 190 (67.0%), p < 0.001). The same trend was seen with reports of cough (2747/5321 (52.5%), 3591/7941 (45.2%), 7337/11 190 (65.6%), p < 0.001) and fever (1370/5321 (26.2%), 1927/7941 (24.3%), 3598/11 190 (32.2%), p 0.038, p < 0.001). For loss of taste or smell, a significant gradual increase in reporting during the three study periods occurred (63/5321 (1.2%), 156/7941 (1.96%), 1505/11 190 (13.5%), all p < 0.001). For context, disease burden in Israel during this timeline decreased between the first and second periods and increased drastically in the third period (Fig. 2b).
Likelihood ratio
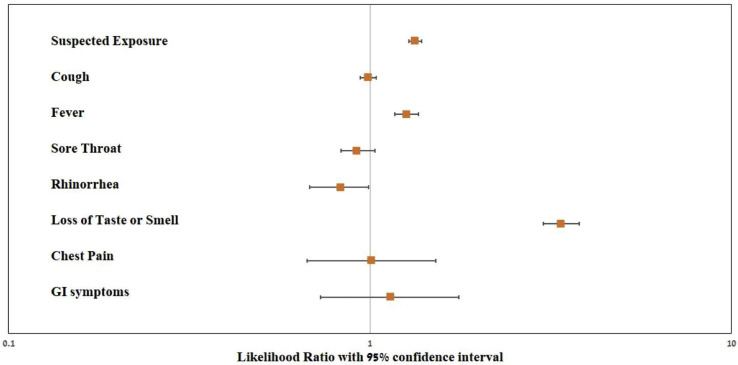
Of symptoms surveyed, loss of taste or smell had the highest positive LR (LR 3.38, 95% CI 3.01–3.79). Suspected exposure (LR 1.33, 95% CI 1.28–1.39) and fever (LR 1.26, 95% CI 1.17–1.36) were also found to have significant positive LR (Fig. 3 ). Stratification by sex, baseline health status, particular co-morbidities and BMI did not principally affect the results (see Supplementary material, Table S4–S9 and Fig. S1).
Fig. 3.
Positive likelihood ratios for surveyed factors (n = 24 362). Factors are shown on a logarithmic scale with 95% CI, those positively associated with COVID-19 are reports of suspected exposure (see text for definition), fever >37.5°C, and loss of smell or taste. LR, likelihood ratio. Error bars represent 95% CI.
Loss of taste or smell (LR 11.25, 95% CI 6.28–20.13) and suspected exposure (LR 1.44, 95% CI 1.25–1.70) were significantly associated with a positive test during the first period. During the second and third periods, the positive LR for loss of taste or smell decreased substantially but remained significant (LR 2.62, 95% CI 2.36–5.05, LR 2.05, 95% CI 1.83–2.31, respectively) (Table 3 ). Fever was also found to have significant positive LR (LR 1.4, 95% CI 1.2–1.3) during the third period whereas exposure shown significant positive LR in both earlier periods but not in the third, accompanied by lower specificity in the latest period (see Supplementary material, Table S2).
Table 3.
Positive likelihood ratios for symptoms and exposure in three consequent periods (see Fig. 2b legend for details)
| LR Period 1 (26 March to 30 April 2020) | LR Period 2 (1 May to 30 June 2020) | LR Period 3 (1 July to 2 August 2020) | |
|---|---|---|---|
| Suspected exposurea | 1.44 (1.25–1.66) | 1.85 (1.61–2.11) | 1.01 (0.97–1.06) |
| Cough | 0.97 (0.81–1.16) | 0.93 (0.79–1.11) | 0.88 (0.84–0.93) |
| Fever | 0.84 (0.6–1.18) | 1.18 (0.94–1.49) | 1.2 (1.1–1.3) |
| Sore throat | 0.85 (0.47–1.55) | 1.09 (0.86–1.38) | 0.79 (0.7–0.89) |
| Rhinorrhoea | 0.92 (0.52–1.63) | 0.83 (0.53–1.32) | 0.93 (0.75–1.17) |
| Loss of taste or smell | 11.25 (6.28–20.13) | 2.62 (1.36–5.05) | 2.05 (1.83–2.31) |
| Chest pain | 1.07 (0.27–4.31) | 0.71 (0.1–5.12) | 0.77 (0.5–1.2) |
| GI symptoms | 1.98 (0.27–14.5) | 0.44 (0.06–3.14) | 0.93 (0.59–1.49) |
Abbreviations: GI, gastrointestinal symptoms (i.e. abdominal pain, vomiting or diarrhoea); LR, positive likelihood ratio.
LR values are highlighted in bold when statistically significant.
Suspected exposure in the survey was defined as a close contact with a confirmed COVID-19 patient or recent (<14 days) international travel.
In a multivariable logistic regression loss of taste or smell (OR 3.51, 95% CI 3.04–4.06), fever (OR 1.34, 95% CI 1.19–1.51) and history of exposure (OR 1.86. 95%CI 1.65–2.09) were the only factors independently associated with a positive test (see Supplementary material, Table S10).
Discussion
In order to make better use of limited COVID-19 confirmatory tests and identify those at highest risk for infection, in Israel, like in many other countries, certain criteria were used to determine testing eligibility [[24], [25], [26]]. In this study, suspicious exposure, loss of taste or smell and fever were associated with a COVID-19 molecular diagnosis in over 24 000 young adults, suggesting that self-reported symptoms are an effective way to triage testing in this population.
Previous reports have been inconsistent with respect to the association between fever and a positive test [[11], [12], [13]]. These conflicting data may stem from the inclusion of populations with a variety of age groups, a factor shown to affect clinical manifestation and disease severity [27,28]. Varying definitions of fever between studies, and different survey components (where in some body temperature was not a mandatory question) also contribute to unequivocal findings. In the current study, fever >37.5°C was significantly associated with COVID-19 diagnosis. Yet, when three consequent periods were compared, fever had significant positive LR only in the late period. This could represent lack of power in earlier periods due to smaller participant numbers, or a true increase in fever as a manifestation of disease. Nonetheless, given the high likelihood observed we suggest prioritizing febrile or sub-febrile individuals for testing, especially if they are young and healthy at baseline. This recommendation should be taken with caution as the specificity of fever was 72% in our study and we hypothesize that with upsurge in seasonal cold and flu during wintertime this will decrease.
Our results suggest that exposure was associated with disease only in earlier periods and not in the latest one. When considering the countrywide disease burden, it is evident that by the third period infection was widespread and much more prevalent. Hence, naturally, exposures were more probable and overall reports were increased. However, compliance with measures taken to decrease transmission, such as mandated mask use in public places, and stricter enforcement perhaps resulted in many of these contacts being less endangering in the latest period. Based on our findings and the relatively low specificity of 51%, it is reasonable to refine the definition of an exposure to achieve better specificity in these circumstances.
In general, we found symptom reporting to be time dependent and mostly parallel to disease propagation in the country [3]. This trend was notable among positive cases but also within the entire cohort, implying a possible behavioural component. The phenomenon of healthy concerned individuals seeking medical attention was demonstrated previously in the setting of different catastrophic events and natural disasters [29], and our findings suggest similar behaviour during the COVID-19 pandemic. Notably, reporting of loss of taste or smell, increased over time, resulting in a decline in the magnitude of the positive LR. Among possible explanations is the increased media coverage and public recognition of this complaint as suggestive of COVID-19. Previously an opposite phenomenon was reported, where with mounting media reports of loss of smell the association between this complaint and a positive test increased [13]. The increase in loss of taste or smell in our cohort could have been attributed to an increase in prevalence of a different illness manifesting with these symptoms, but it is highly unlikely that this could explain a nine-fold increase within a month. Nonetheless, loss of taste or smell remains significantly and consistently associated with a positive likelihood for COVID-19, and fair PPV, despite the possible reporting bias. As this is a specific complaint [30] reaching as high as 94% in our cohort, unlike other flu-like symptoms, we suggest that young individuals presenting with loss of taste or smell should be prioritized for testing. In contrast to previous reports [11,13] we did not find respiratory symptoms to be associated with a positive test.
Our study includes a unique cohort comprised mainly of young healthy adults, a subgroup that has been overlooked in previous reports [[11], [12], [13]] but is the most common disease transmitting vector [8,17,18]. Given the mandatory duty to serve in the IDF, the included population is highly representative of young Israeli adults and can be generalized to young adults worldwide. Additional strengths are the systemic data collection and measurement throughout the study period and the consistency of laboratory examinations performed in the same protocol, facility and by the same staff. In addition, the free access to health services in the military is of importance in the light of evidence linking socioeconomic and racial disparities to COVID-19 morbidity and mortality [28]. A major shortcoming of this study is the use of self-reported symptoms, which is subject to response bias, especially when the presence of certain symptoms serves as the gatekeeper for PCR approval. In addition, risk for selection bias exists secondary to testing policy, which could underestimate the predictive value of symptoms that do not mandate a swab test. Furthermore, the group of excluded individuals without questionnaire data might bias the results despite being non-differential in origin and with similar characteristics overall. Also, because of the limitations of the SARS-CoV-2 rRT-PCR test, both false-negative and false-positive results could be included; however, PCR remains the reference standard for diagnosis. Finally, secondary to questionnaire phrasing, loss of taste and loss of smell are combined to a single symptom, so we could not appreciate the individual effect of report of either symptom.
In conclusion, reporting of loss of taste or smell, suspicious exposure and fever were strongly associated with a positive SARS-CoV-2 test in a population of predominantly young and healthy adults. We recommend using these self-reported symptoms to streamline testing in this population.
Transparency declaration
The authors declare that they have no conflicts of interest.
Funding: None received.
Author contributions
AF and MN were responsible for the conceptualization of the study. AF, MN and BT were responsible for the methodology and the investigation. ED performed the formal analysis, AF, RY, IT and MN curated the data. The original draft was written by MN, and it was reviewed and edited by MN, BT, IK, GG, GT, BG, EA and NF. Visualization was by MN, AF and GT. The study was supervised by AF, GT, NF, BG and EA, and project administration was by AF.
Editor: L. Scudeller
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2020.12.028.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Lu H., Stratton C.W., Tang Y.W. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J Med Virol. 2020;92:401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . WHO Dir Gen Speeches; Geneva: 2020. WHO Director-General’s opening remarks at the media briefing on COVID-19 – 11 March 2020. 4. [Google Scholar]
- 3.World Health Organization . WHO; Geneva: 2020. WHO coronavirus disease dashboard. [Google Scholar]
- 4.Esbin M.N., Whitney O.N., Chong S., Maurer A., Darzacq X., Tjian R. Overcoming the bottleneck to widespread testing: a rapid review of nucleic acid testing approaches for COVID-19 detection. RNA. 2020;26:771–783. doi: 10.1261/rna.076232.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Péré H., Péré H., Péré H., Podglajen I., Podglajen I., Wack M. Nasal swab sampling for SARS-CoV-2: a convenient alternative in times of nasopharyngeal swab shortage. J Clin Microbiol. 2020;58 doi: 10.1128/JCM.00721-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beetz C., Skrahina V., Förster T.M., Gaber H., Paul J.J., Curado F. vol. 10. 2020. Rapid large-scale COVID-19 testing during shortages. (Diagnostics (Basel, Switzerland)). Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han C., Duan C., Zhang S., Spiegel B., Shi H., Wang W. Digestive symptoms in COVID-19 patients with mild disease severity: clinical presentation, stool viral RNA testing, and outcomes 2020. 2020. Available from: [DOI] [PMC free article] [PubMed]
- 8.Gandhi M., Yokoe D.S., Havlir D.V. Asymptomatic transmission, the achilles’ heel of current strategies to control Covid-19. N Engl J Med. 2020;382:2158–2160. doi: 10.1056/NEJMe2009758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie J., Tong Z., Guan X., Du B., Qiu H., Slutsky A.S. Critical care crisis and some recommendations during the COVID-19 epidemic in China. Intensive Care Med. 2020;46:837–840. doi: 10.1007/s00134-020-05979-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grasselli G., Pesenti A., Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy. JAMA. 2020;323:1545. doi: 10.1001/jama.2020.4031. [DOI] [PubMed] [Google Scholar]
- 11.Shoer S., Karady T., Keshet A., Shilo S., Rossman H., Gavrieli A. Who should we test for COVID-19? A triage model built from national symptom surveys. MedRxiv. 2020 2020.05.18.20105569. [Google Scholar]
- 12.Tostmann A., Bradley J., Bousema T., Yiek W.-K., Holwerda M., Bleeker-Rovers C. Strong associations and moderate predictive value of early symptoms for SARS-CoV-2 test positivity among healthcare workers, the Netherlands, March 2020. Eurosurveillance. 2020;25:2000508. doi: 10.2807/1560-7917.ES.2020.25.16.2000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menni C., Valdes A.M., Freidin M.B., Sudre C.H., Nguyen L.H., Drew D.A. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat Med. 2020;26:1037–1040. doi: 10.1038/s41591-020-0916-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salvatore P.P., Sula E., Coyle J.P., PhD, Caruso E., Smith A.R. Recent increase in COVID-19 cases reported among adults aged 18–22 years – United States, May 31–September 5, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1419–1424. doi: 10.15585/mmwr.mm6939e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simchoni M., Klopstock I., Furer A. COVID-19 among young adults. J Isr Mil Med. 2020;17:11–15. [Google Scholar]
- 16.KS of Epidemiology Report on the epidemiological features of coronavirus disease 2019 (COVID-19) outbreak in the Republic of Korea from January 19 to March 2, 2020. J Korean Med Sci. 2020;35 doi: 10.3346/jkms.2020.35.e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giordano G., Blanchini F., Bruno R., Colaneri P., Di Filippo A., Di Matteo A. Modelling the COVID-19 epidemic and implementation of population-wide interventions in Italy. 2020. n.d. [DOI] [PMC free article] [PubMed]
- 18.Gao Z., Xu Y., Sun C., Wang X., Guo Y., Qiu S. A systematic review of asymptomatic infections with COVID-19. J Microbiol Immunol Infect. 2020 doi: 10.1016/j.jmii.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furer A., Afek A., Sommer A., Keinan-Boker L., Derazne E., Levi Z. Adolescent obesity and midlife cancer risk: a population-based cohort study of 2·3 million adolescents in Israel. Lancet Diabetes Endocrinol. 2020;8:216–225. doi: 10.1016/S2213-8587(20)30019-X. [DOI] [PubMed] [Google Scholar]
- 20.CDC . CDC; Atlanta, GA: 2020. CDC 2019-novel coronavirus (2019-nCoV) real-time RT-PCR diagnostic panel- for emergency use only. [Google Scholar]
- 21.Moore N.M., Li H., Schejbal D., Lindsley J., Hayden M.K. Comparison of two commercial molecular tests and a laboratory-developed modification of the CDC 2019-ncov reverse transcriptase PCR assay for the detection of sars-cov-2. J Clin Microbiol. 2020;58 doi: 10.1128/JCM.00938-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simel D.L., Samsa G.P., Matchar D.B. Likelihood ratios with confidence: sample size estimation for diagnostic test studies. J Clin Epidemiol. 1991;44:763–770. doi: 10.1016/0895-4356(91)90128-v. [DOI] [PubMed] [Google Scholar]
- 23.Wilson E.B. Probable inference, the law of succession, and statistical inference. J Am Stat Assoc. 1927;22:209–212. [Google Scholar]
- 24.Home Page Ministry of health. www.health.gov.il/English/Pages/HomePage.aspx n.d. Available from:
- 25.Testing in the U.S CDC. www.cdc.gov/coronavirus/2019-ncov/cases-updates/testing-in-us.html n.d. Available from:
- 26.Whittington A.M. Coronavirus: rolling out community testing for COVID-19 in the NHS. BMJ. 2020 blogs.bmj.com/bmj/2020/02/17/coronavirus-rolling-out-community-testing-for-covid-19-in-the-nhs/ n.d. Available from: [Google Scholar]
- 27.Riccardo F., Ajelli M., Andrianou X., Bella A., Del Manso M., Fabiani M. Epidemiological characteristics of COVID-19 cases in Italy and estimates of the reproductive numbers one month into the epidemic. MedRxiv. 2020 doi: 10.2807/1560-7917.ES.2020.25.49.2000790. 2020.04.08.20056861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 29.Lacy T.J., Benedek D.M. Terrorism and weapons of mass destruction: managing the behavioral reaction in primary care. South Med J. 2003;96:394–400. doi: 10.1097/01.SMJ.0000054783.69453.79. [DOI] [PubMed] [Google Scholar]
- 30.Salmon Ceron D., Bartier S., Hautefort C., Nguyen Y., Nevoux J., Hamel A.L. Self-reported loss of smell without nasal obstruction to identify COVID-19. The multicenter Coranosmia cohort study. J Infect. 2020 doi: 10.1016/j.jinf.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.