Abstract
Objective
To study the risk of acquiring Corona Virus Disease 2019 (COVID-19) and its outcomes in patients on immunosuppressive therapy (IST) for chronic autoimmune neuromuscular disorders (aNMD) and multiple sclerosis (MS).
Methods
We used TriNetX, a global health collaborative clinical research platform collecting real-time electronic medical records data, which has one of the largest known global COVID-19 database. We included patients with chronic autoimmune neuromuscular disorders (aNMD) [myasthenia gravis (MG), inflammatory myositis, and chronic inflammatory neuropathies (CIN)] and MS, based on the International Classification of Disease-10 (ICD-10) coding for one year before January 20th, 2020. We examined the use of IST, rate of COVID- 19, hospitalization, intubation, and mortality among the patients with aNMD and MS.
Results
A total of 33,451 patients with aNMD and 42,899 patients with MS were included. Among them, 111 (0.33%) patients with aNMD and 115 patients (0.27%) with MS had COVID-19. About one third of them required hospitalization. IST did not appear to have a significant impact on overall infection risk in either group; however, risk of hospitalization for immunosuppressed patients with aNMD was higher (Odds ratio 2.86, p-value 0.011).
Conclusions
IST use does not appear to make patients with aNMD and MS more vulnerable to COVID-19. IST may be continued during the pandemic, as previously suggested by expert opinion guidelines. However, it is important to consider individualizing immunotherapy regimens in some cases. Additional physician reported registry-based data is needed to further confirm these findings.
Keywords: COVID-19, Multiple sclerosis, Neuromuscular disorders, Autoimmune disease, Immunosuppressive therapies, Neurological disorders
Highlights
-
•
Majority of patients with chronic autoimmune neurological disorders(cAND’s) likely have mild-to-moderate COVID-19.
-
•
Patients with cAND’s who had COVID-19, 33% were hospitalized 12.1% required intensive care unit stay and 8.5% died.
-
•
Immunosuppressive therapies did not appear to have a significant impact on overall infection risk.
1. Introduction
A novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causing COVID-19 (Corona Virus Disease 2019) emerged in December 2019 and soon led to a worldwide pandemic [1]. COVID-19 can be asymptomatic, may cause mild upper respiratory symptoms, or may lead to multi-system involvement including neurological manifestations, severe respiratory compromise requiring intubation and even death [[2], [3], [4]]. There is no established treatment for COVID-19 and vaccine development is in early stages [5].
Many patients with chronic autoimmune neurological disorders, such as chronic autoimmune neuromuscular disorders (aNMD) and multiple sclerosis (MS), require immunosuppression. Whether immunosuppression increases the risk of COVID-19 or has any impact on the disease outcome remains unclear. As there is a paucity of real-world data, evidence-based management of patients with chronic aNMD and MS has been particularly challenging during this pandemic [6,7]. Consensus guidelines, based largely on expert opinion and experience, were prepared to help clinicians [[8], [9], [10]]. However, there are no large-scale data to date reporting the impact of COVID-19 on patients with chronic autoimmune neurological disorders, in particular those on immunosuppressive therapy (IST). In this study, we have examined the risk of COVID-19 and clinical outcomes in patients with aNMD and MS.
2. Methods
2.1. Data source
De-identified patient information was extracted using TriNetX COVID-19 Research Network platform (www.trinetx.com), which is a global health collaborative clinical research platform collecting real-time electronic medical records data from a network of health care organizations (HCOs) across the USA and some outside the US territories. HCOs are typically academic medical centers and their affiliates. On March 24, 2020, TriNetX fast-tracked updates to its real-world data platform to incorporate specific COVID-19 terminology including the diagnosis and the World Health Organization (WHO) and Centers for Disease Control (CDC) specific coding guidelines to support COVID-19 related research. As a result, “COVID-19 Research Network” in TriNetX global network of HCOs represents the one of the largest global COVID-19 dataset. TriNetX is also being used by the Food & Drug Administration (FDA) Sentinel Operations Center at the Harvard Pilgrim Health Care Institute [11,12].
Queries were made through TriNetX using browser and accessing real-time features. TriNetX does not allow data downloads, or individual patient data for review. However, the TriNetX platform allows analysis in the form of queries. The TriNetX platform is described in detail elsewhere, and several similar studies on COVID-19 using TriNetX have been published to date [[13], [14], [15], [16], [17]]. At University of Arkansas for Medical Sciences the data from TriNetX is managed by the Arkansas Clinical Data Repository (AR-CDR) and maintained by the Department of Biomedical Informatics.
2.2. Study protocol
The University of Arkansas Institutional Review Board (IRB) reviewed this study and assigned it an ‘exempt’ status as it is limited to global de-identified COVID-19 Research Network data designated for research use.
The current study included data as of June 15th 2020 in the TriNetX COVID-19 Research Network. Individuals ≥18 years of age with a preexisting chronic autoimmune neurological disorders which included multiple sclerosis (MS), and other chronic aNMD (including myasthenia gravis (MG), chronic inflammatory neuropathies (CIN), or inflammatory myositis) were identified by using ICD-10 codes G35, G70, G61.8 and G61.9, G72.49, M60.8 and M60.9 between January 20th 2019 to January 19th 2020. We then examined the number of patients with a confirmed diagnosis of COVID-19 in this cohort using ICD-10 codes and laboratory codes B34.2 Coronavirus infection, unspecified, B97.29 Other coronavirus as the cause of diseases classified elsewhere, which also includes, U07.1 COVID-19, U07.2 COVID-19, virus not identified (WHO), J12.81Pneumonia due to SARS-associated coronavirus and Only positive lab value for 9088 SARS Coronavirus 2 and related RNA (presence), which includes Logical Observation Identifiers Names and Codes (LOINC).
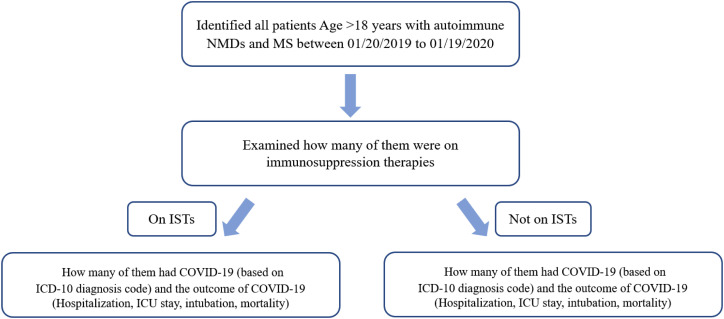
We also captured and examined hospitalization, critical care stay, intubation, and mortality data from the day of diagnosis to one month after the diagnosis of COVID-19. Details of search criteria using admission and procedure codes are provided in Fig. 1 as a flow chart.
Fig. 1.
Study flow chart.
Flow chart showing the search methodology. IST: Immunosuppressive Therapy, COVID-19: Corona Virus Disease 2019, ICD: International classification of disease, ICU: Intensive care unit.
We examined baseline demographics data and clinical outcomes in this cohort. Additional details regarding specific use of IST in the past year were identified using specific medication codes. If any group on the query had less than ten observations, a specific number could not be obtained, it was reported as ≤10 by the software.
These queries were performed independently by two researchers (SRO and KN) using same search criteria which yielded same results, with active support from a data scientist at the Arkansas Clinical Data Repository (AR-CDR). Moreover, direct guidance was obtained from TriNetX to ensure accuracy of the data.
2.3. Statistical analysis
Descriptive statistics were reported as number of observations and percentage or mean ± standard deviation as applicable. To compare the means between groups unpaired t-test was used. For nominal data chi squares test was performed. Odds ratio was calculated as applicable. To reduce false discovery rate, we have used Benjamini-Hochberg correction [18]. Statistical analyses were performed in SPSS (IBM Corporation) and R software (version 3.5.2, R Foundation for Statistical Computing, Vienna, Austria).
3. Results
A total 33,451 patients with aNMD and 42,899 patients with MS were identified. Of these 75,898 patients, 224 patients had COVID-19 (0.29%). Approximately one third (74/224, 33%) required hospitalization, and 19 (8.5%) died due to COVID-19 or COVID-19 related complications (Table 1 ). Of note, there were 452 patients who had both MS and an aNMD. Two patients with overlap disease had COVID-19 and were included in both groups for comparative analysis. Excluding patients with overlap disease did not make any significant difference to the overall outcome.
Table 1.
Baseline demographics and clinical outcome of patients with chronic autoimmune neurological disorders during the COVID-19 pandemic.
| Total patients with NMD/MS | NMD/MS + COVID-19 | |
|---|---|---|
| Number of patients | 75,898 | 224 |
| Age (years) | 53.5 ± 15.7 | 55 ± 16.8 |
| Woman | 52,588(69.3%) | 144(64.3%) |
| Race | ||
| White | 57,059(75.2%) | 132(58.9%) |
| African American | 9206(12.1%) | 58(25.9%) |
| Asian | 858(1.1%) | 0 |
| Hispanic (ethnicity) | 5037 (6.6%) | 18(8.0%) |
| Clinical outcomes | ||
| Hospitalization | NA | 74(33.0%) |
| ICU | NA | 27(12.1%) |
| Intubation | NA | 11(4.9%) |
| Death | NA | 19 (8.5%) |
NA-Not applicable.
The COVID-19 cohort comprised of 111 (0.33%) patients with aNMD and 115 patients (0.27%) with MS. Patients with aNMD were older and had a lower percentage of women when compared to MS (Table 2 ). To ensure the validity of our finding, we also examined the number of patients with aNMD and MS for the last 6 months and the last 24 months, and observed similar odd-ratios (6-month: 1.1, p-value 0.603, 24-month: 1.23, p-value 0.083). Next, we examined the impact of IST on the outcome of COVID-19 patients. In the aNMD group, patients on immunosuppression were more likely to be hospitalized (OR 2.86, CI 1.27–6.51). Similarly, patients with aNMD who were on additional IST (any IST but not on steroid) were also more likely to be hospitalized (OR 4.05, CI 1.42–11.58. No other significant differences in clinical outcomes were identified between those on IST and those not on IST in either the aNMD or the MS groups. Similarly, no significant impact of IST was noted between the subgroups of aNMD (CIN, MG, and myositis) in terms of risk of COVID-19 (Supplementary Table 1).
Table 3.
Comparison of COVID-19 patients with autoimmune neuromuscular disorders and MS based on immunosuppressive therapy use status.
| Autoimmune neuromuscular disorders (n = 111) with COVID-19 | |||
|---|---|---|---|
| On immunosuppression | Not on immunosuppression | p-value | |
| Number of patients (%) | 53/12839 (0.41%) | 58/20612 (0.28%) | 0.042* |
| Age (years) | 59.1 ± 16.3 | 57.7 ± 18.7 | 0.829 |
| Woman | 29 (54.7%) | 33 (56.9%) | 0.823 |
| Clinical outcome | |||
| Hospitalization | 24 (45.3%) | 13 (22.4%) | 0.011 |
| ICU | 12 (22.6%) | 6 (10.3%) | 0.079 |
| Intubation | ≤ 10 (≤18.9%) | ≤ 10 (≤17.2%) | NA |
| Death | ≤ 10 (≤18.9%) | ≤ 10 (≤17.2%) | NA |
| Multiple Sclerosis (n = 115) with COVID-19 | |||
|---|---|---|---|
| On immunosuppression | Not on immunosuppression | p-value | |
| Number of patients (%) | 42/15859 (0.26%) | 73/27040 (0.27%) | 0.920 |
| Age (years) | 45.5 ± 13.7 | 55.8 ± 15.3 | 0.005 |
| Woman | 32 (76.2%) | 51 (69.9%) | 0.467 |
| Clinical outcome | |||
| Hospitalization | 10 (23.8%) | 28 (38.4%) | 0.110 |
| ICU | ≤ 10 (≤23.8%) | ≤ 10 (≤ 13.7%) | NA |
| Intubation | 0 | ≤ 10 (≤ 13.7%) | NA |
| Death | 0 | ≤ 10 (≤ 13.7%) | NA |
Table 3: Baseline demographics and clinical outcome of COVID-19 in patients with autoimmune neurological disorders based on immunosuppression therapy.
NA: Not applicable, ICU: Intensive Care Unit, * did not remain statistically significant after correcting for false discovery, no definitive number could be obtained for an observation if the total number was below 10.
Table 2.
Comparison demographics and clinical outcomes of COVID-19 patients with chronic autoimmune neuromuscular disorders and MS.
| COVID-19 positive patients | Autoimmune neuromuscular disorders | Multiple sclerosis | p-value |
|---|---|---|---|
| Number of patients | 111/33451 (0.33%) | 115/42899 (0.27%) | 0.107 |
| Age (years) | 58.4 ± 17.5 | 52 ± 15.5 | 0.003 |
| Woman | 62 (56.9%) | 83 (72.2%) | 0.011 |
| Race | |||
| White | 61(55.9%) | 72 (62.6%) | 0.241 |
| African American | 29(26.1%) | 29 (25.2%) | 0.887 |
| Asian | 0 | 0 | 0.999 |
| Hispanic (ethnicity) | 12(10.8%) | 4 (3.5%) | 0.031 |
| Clinical outcomes | |||
| Hospitalization | 37 (33.3%) | 38 (33.0%) | 0.999 |
| ICU | 18 (16.2%) | ≤10 (≤8.7%) | NA |
| Intubation | ≤10 (≤9.0%) | ≤10 (≤8.7%) | NA |
| Death | 14 (12.6%) | ≤10 (≤8.7%) | NA |
NA-Not applicable, ICU-Intensive Care Unit, no definitive number could be obtained for an observation if the total number was below 10.
For patients with autoimmune NMD on different IST, percentage of COVID-19 ranged between 0% (plasma exchange, deflazacort, eculizumab) to ≤6.1% (cyclophosphamide) (Supplementary Table 2). For patients with MS, percentage of COVID-19 varied between 0% (cladribine, peginterferon beta-1a, siponimod) to ≤9.1% (mitoxantrone) (Supplementary Table 3).
4. Discussion
Current evidence examining the impact of COVID-19 on patients with aNMD and MS who are on IST is crucial to implement a safe and appropriate management strategy. Our findings, despite the inherent limitations of a large database-based study, can help to address some of the key knowledge gaps in managing these patients and provide important insights into possible risk factors and outcomes.
Overall the rate of COVID-19 infection in patients with aNMD and MS (0.29%) was similar to the rate of reported positive COVID-19 cases among the total US population (0.48% || 1,584,239/329,986,480) as of June 15, 2020 [19]. It appeared that IST did not have any differential impact on COVID-19 risk between aNMD and MS. While relative age and gender differences between the groups might have played a role, we could not adjust for such differences, and were only able to report risk for the group as a whole.
Patients with aNMD who were on immunosuppression were at greater risk of hospitalization. Factors that we were unable to account for might have played a role. For example, as about 10–15% of patients with MG may have uncontrolled disease with bulbar symptoms and respiratory muscle weakness, [20] it can be hypothesized that these patients would be more likely to have severe symptomatic COVID-19 secondary to respiratory compromise leading to hospitalization.
There are controversies regarding the role of IST in MS patients on the clinical outcome of COVID-19 [7,[21], [22], [23]]. Some have hypothesized a potential beneficial role of IST. The acute respiratory distress syndrome (ARDS) associated with COVID-19 can be related to the immune response triggered by the virus [24], and mild to moderate immunosuppression provided by disease modifying therapy (DMT) may be beneficial to prevent these severe COVID-19 related complications in MS patients [21,22,25] We did not observe a ‘protective’ effect of immunosuppression among the patients with MS [8,9].
While some medications were associated with a higher rate of COVID-19 in this cohort, further subgroup analysis was not pursued as we were unable to correct for other potential confounders (i.e., co-morbidities, combination immunotherapy) and as such these analyses would not be valid. Importantly, high intensity immunotherapy regimens are typically reserved for sicker patients and as such may not be a true indication of their inherent risk but rather a reflection of disease state. Similarly, combination IST are also used only to address uncontrolled disease.
Impact of IST on the risk of COVID-19 and its outcome is not well studied. Some small scale single center studies on patients with other immunological disorders and/or immunosuppressed reported possible higher mortality rate [26] and more frequent hospitalization for patients on glucocorticoids [27,28]. However, several other single center [29] and multi-center cohort studies did not find any definitive detrimental effects of IST in terms of risk of COVID-19 and related complications [30,31].
Overall, our findings largely reinforce the proposed guidelines by the expert communities where current IST (with a few exceptions) were recommended to be continued even during the pandemic [8,23]. Our study was limited to patients with chronic autoimmune neurological disorders and intentionally excluded acute autoimmune neurological disorders, such as Guillain-Barre Syndrome and immune-mediated encephalitis. First, these diseases are either monophasic, or the duration of treatment can considerably vary. The latter can be associated with an underlying malignancy, and require additional treatment for cancer [[32], [33], [34]]. Moreover, the use of immunosuppression in such diseases will essentially depend on severity of the presentation and having concomitant COVID-19 may or may not influence the management plan. We excluded Lambert-Eaton myasthenic syndrome (LEMS), where the mainstay of therapy is usually 3,4-Diaminopyridine. Moreover, it also can be related to underlying malignancy requiring chemotherapy [35].
There are several limitations of this study worth highlighting, and some of them are inherent to large databases. First, findings are based on reported data, with accuracy verification not being possible. The entered diagnosis may not have been accurate; however, such inaccuracies are likely to be of small scale and unlikely to significantly confound our findings. Second, there are variations on how COVID-19 was tested and some of the COVID-19 patients may just be false positive. Such limitations exist in the majority of reports on COVID-19 [22,23,36], and this is probably unavoidable during an ongoing pandemic. Third, we did not have any data on COVID-19 severity or underlying co-morbidities. We could not directly assess for respiratory symptoms. Fourth, we could not make statistical inferences in some cases where the number of observations were less than 10. The software did not provide a specific number in those cases. Fifth, this is a database-based study. Detailed important patient level information, such as severity of NMD, MS, timing of medication, treatment response, lymphocyte depletion etc. could not be assessed. Lastly, TriNetX covers a large number health care organization, but it may or may not include a particular tertiary care center who are providing care for more serious cases COVID-19. They may have higher intubation, ICU admission, and death, and there are reported variations between outcomes of COVID-19 between hospitals and also countries. While this limitation apparently can falsely reduce the rate of serious outcomes from COVID-19, overall, this possibility gets diluted by the inclusion of a big number HCOs providing care to different communities [[37], [38], [39], [40]].
These findings, albeit important, cannot replace the need for more controlled longitudinal studies to understand the true impact of COVID-19 in this complex patient cohort. Such efforts are already underway. European Academy of Neurology (EAN) Neuro COVID-19 registry [41], Coronavirus and MS Reporting Database (COViMS), COVID-19 Associated Risks and Effects in Myasthenia Gravis (CARE-MG) [42,43], COVID-19 Global Rheumatology Alliance [44] are among the major databases which are actively gathering data on COVID-19 in this population. The findings from these databases will help to further validate our observations.
5. Conclusions
Our findings indicate that the majority of patients with chronic autoimmune neurological disorders likely have mild-to-moderate COVID-19 and do not require hospitalization. Overall, the impact of immunosuppression in this cohort appears to be low and suggests that IST may be continued. The influence of specific immunosuppressive therapies, baseline autoimmune neurologic disease status/severity, and other co-morbidities on relative risk and outcome remains unanswered in this study. Re-thinking what defines a high-risk population and how this designation might affect their overall medical care are important considerations as definitive data emerges.
Author contributions
Sukanthi Kovvuru: Study concept and design, acquisition of data, interpretation of data, analysis of data, critical revision of manuscript. Krishna Nalleballe: Study concept and design, acquisition of data, interpretation of data, analysis of data, critical revision of manuscript. Sanjeeva Reddy Onteddu: Study concept and design, acquisition of data, interpretation of data, analysis of data, drafting of manuscript, critical revision of manuscript. Madhu Jasti: Interpretation of data, critical revision of manuscript. Nidhi Kapoor: Interpretation of data, critical revision of manuscript. Karthika Veerapaneni: Interpretation of data, critical revision of manuscript. Sisira Yadala: Interpretation of data, critical revision of manuscript. Vasuki Dandu: Study concept and design, critical revision of manuscript. Robert Archer: Interpretation of data, critical revision of manuscript. Richard J. Nowak: Interpretation of data, analysis of data, drafting of manuscript, critical revision of manuscript. Bhaskar Roy: Study concept and design, acquisition of data, interpretation of data, analysis of data, statistical analysis, drafting of manuscript, critical revision of manuscript.
Disclosures
Dr. Kovvuru, Dr. Nalleballe, Dr. Onteddu, Dr. Sharma, Dr. Jasti, Dr. Kapoor, Dr. Veerapaneni, Dr. Yadala, Dr. Dandu, and Dr. Archer have no conflicts of interest to report.
Dr. Nowak reports no conflicts directly related to this work. Dr. Nowak has received research support from the National Institutes of Health (NIH), Genentech, Alexion Pharmaceuticals, argenx, Annexon Biosciences, Ra Pharmaceuticals, Myasthenia Gravis Foundation of America, Momenta, Immunovant, and Grifols. He has served as consultant/advisor for Alexion Pharmaceuticals, argenx, CSL Behring, Grifols, Ra Pharmaceuticals, Immunovant, Momenta and Viela Bio.
Dr. Roy reports no conflicts directly related to this work. He has served as a consultant for Alexion Pharmaceuticals.
Ethical statement
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Grant support
None.
Acknowledgments
Data for the study were provided by the Arkansas Clinical Data Repository (AR-CDR) maintained by the Department of Biomedical Informatics in the College of Medicine at the University of Arkansas for Medical Sciences (UAMS). The AR-CDR is approved to operate as an enterprise data resource to support research across UAMS.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jns.2020.117230.
Appendix A. Supplementary data
Supplementary material
References
- 1.COVID-19. World Health Organization; 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports [Google Scholar]
- 2.Zaim S., Chong J.H., Sankaranarayanan V., Harky A. COVID-19 and multiorgan response. Curr. Probl. Cardiol. 2020 Apr 28;45(8) doi: 10.1016/j.cpcardiol.2020.100618. 100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jasti M., Nalleballe K., Dandu V., Onteddu S. A review of pathophysiology and neuropsychiatric manifestations of COVID-19. J. Neurol. 2020:1–6. doi: 10.1007/s00415-020-09950-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avula A., Nalleballe K., Narula N., Sapozhnikov S., Dandu V., Toom S. COVID-19 presenting as stroke. Brain Behav. Immun. 2020;87:115–119. doi: 10.1016/j.bbi.2020.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee Nelson, McGeer Allison. The starting line for COVID-19 vaccine development. Lancet. 2020;395(10240):1815–1816. doi: 10.1016/S0140-6736(20)31239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berger J.R., Brandstadter R. Bar-or a. COVID-19 and MS disease-modifying therapies. Neurol. Neuroimmunol. Neuroinflamm. 2020 Jul;7(4):e761. doi: 10.1212/NXI.0000000000000761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giovannoni G. Anti-CD20 immunosuppressive disease-modifying therapies and COVID-19. Mult. Scler. Rel. Disord. 2020 Jun;41 doi: 10.1016/j.msard.2020.102135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacob S., Muppidi S., Guidon A., Guptill J., Hehir M., Howard J.F. Guidance for the management of myasthenia gravis (MG) and Lambert-Eaton myasthenic syndrome (LEMS) during the COVID-19 pandemic. J. Neurol. Sci. 2020 May 15;412 doi: 10.1016/j.jns.2020.116803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guidon A., Amato A. COVID-19 and neuromuscular disorders. Neurology. 2020 May 5;10 doi: 10.1212/WNL.0000000000009566. [DOI] [PubMed] [Google Scholar]
- 10.http://www.msif.org/wp-content/uploads/2020/05/MSIF-Global-advice-on-COVID-19-for-people-with-MS-_-v2.pdf. Global COVID-19 Advice for People With MS, 2020.
- 11.https://www.trinetx.com/coronavirus/
- 12.https://trinetx.com/fda-sentinel/https://trinetx.com/fda-sentinel/https://trinetx.com/fda-sentinel/ Available at:
- 13.Nalleballe K., Reddy Onteddu S., Sharma R., Dandu V., Brown A., Jasti M. Spectrum of neuropsychiatric manifestations in COVID-19. Brain Behav. Immun. 2020;88:71–74. doi: 10.1016/j.bbi.2020.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stapff M., Hilderbrand S. First†line treatment of essential hypertension: a real†world analysis across four antihypertensive treatment classes. J. Clin. Hypertens. 2019;21(5):627–634. doi: 10.1111/jch.13531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Onteddu S.R., Nalleballe K., Sharma R., Brown A.T. Underutilization of healthcare for strokes during the COVID-19 outbreak. Int. J. Stroke. 2020;15(5):NP9–NP10. doi: 10.1177/1747493020934362. [DOI] [PubMed] [Google Scholar]
- 16.Ranabothu S., Onteddu S., Nalleballe K., Dandu V., Veerapaneni K., Veerapandiyan A. Spectrum of COVID-19 in children. Acta Paediatr. 2020;109(9):1899–1900. doi: 10.1111/apa.15412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turk M.A., Landes S.D., Formica M.K., Goss K.D. Intellectual and developmental disability and COVID-19 case-fatality trends: TriNetX analysis. Disab. Health J. 2020;13(3) doi: 10.1016/j.dhjo.2020.100942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995 Jan 1;57(1):289. [Google Scholar]
- 19.Cases in the US. 2020. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html Available at:
- 20.Anil R., Kumar A., Alaparthi S., Sharma A., Nye J.L., Roy B. Exploring outcomes and characteristics of myasthenia gravis: rationale, aims and design of registry – the EXPLORE-MG registry. J. Neurol. Sci. 2020 Jul 15;414 doi: 10.1016/j.jns.2020.116830. [DOI] [PubMed] [Google Scholar]
- 21.The COVID-19 pandemic and the use of MS disease-modifying therapiesMult. Scler. Relat. Disord. 2020;39:102073. doi: 10.1016/j.msard.2020.102073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montero-Escribano P., Matías-Guiu J., Gómez-Iglesias P., Porta-Etessam J., Pytel V., Matias-Guiu J.A. Anti-CD20 and COVID-19 in multiple sclerosis and related disorders: a case series of 60 patients from Madrid, Spain. Mult. Scler. Rel. Disord. 2020 Jul;42 doi: 10.1016/j.msard.2020.102185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sormani M.P. An Italian programme for COVID-19 infection in multiple sclerosis. Lancet Neurol. 2020 Jun;19(6):481–482. doi: 10.1016/S1474-4422(20)30147-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramanathan K., Antognini D., Combes A., Paden M., Zakhary B., Ogino M. Planning and provision of ECMO services for severe ARDS during the COVID-19 pandemic and other outbreaks of emerging infectious diseases. Lancet Respir. Med. 2020 May;8(5):518–526. doi: 10.1016/S2213-2600(20)30121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Novi G., Mikulska M., Briano F., Toscanini F., Tazza F., Uccelli A. COVID-19 in a MS patient treated with ocrelizumab: does immunosuppression have a protective role? Mult. Scler. Rel. Disord. 2020 Jul;42 doi: 10.1016/j.msard.2020.102120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vaid Nidhi, Ardissino Maddalena, Reed Thomas A.N., Goodall Jack, Utting Poppy, Miscampbell Megan, Condurache Dorina, Cohen David Leslie. Clinical characteristics and outcomes of immunosuppressed patients hospitalized with COVID-19: experience from London. J. Intern. Med. 2020 doi: 10.1111/joim.13172. [DOI] [PubMed] [Google Scholar]
- 27.Montero F., Martínez-Barrio J., Serrano-Benavente B., González T., Rivera J., Molina Collada J. Coronavirus disease 2019 (COVID-19) in autoimmune and inflammatory conditions: clinical characteristics of poor outcomes. Rheumatol. Int. 2020 Oct 1;40(10):1593–1598. doi: 10.1007/s00296-020-04676-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fraser J., Mousley J., Testro A., Smibert O.C., Koshy A.N. Clinical presentation, treatment, and mortality rate in liver transplant recipients with coronavirus disease 2019: a systematic review and quantitative analysis. Transplant. Proc. 2020;52(9):2676–2683. doi: 10.1016/j.transproceed.2020.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Veenstra J., Buechler C.R., Robinson G., Chapman S., Adelman M., Tisack A. Antecedent immunosuppressive therapy for immune-mediated inflammatory diseases in the setting of a COVID-19 outbreak. J. Am. Acad. Dermatol. 2020;83(6):1696–1703. doi: 10.1016/j.jaad.2020.07.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kates O.S., Haydel B.M., Florman S.S., Rana M.M., Chaudhry Z.S., Ramesh M.S. COVID-19 in solid organ transplant: a multi-center cohort study. Clin. Infect. Dis. 2020 Aug 7 doi: 10.1093/cid/ciaa1097. ciaa1097. [DOI] [Google Scholar]
- 31.Koker O., Demirkan F.G., Kayaalp G., Cakmak F., Tanatar A., Karadag S.G. Does immunosuppressive treatment entail an additional risk for children with rheumatic diseases? A survey-based study in the era of COVID-19. Rheumatol. Int. 2020 Oct 1;40(10):1613–1623. doi: 10.1007/s00296-020-04663-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Titulaer M.J., McCracken L., Gabilondo I., Armangué T., Glaser C., Iizuka T. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013 Feb;12(2):157–165. doi: 10.1016/S1474-4422(12)70310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burns T.M. Guillain-Barré syndrome. Semin. Neurol. 2008 Apr;28(2):152–167. doi: 10.1055/s-2008-1062261. [DOI] [PubMed] [Google Scholar]
- 34.Dalmau J., Rosenfeld M.R. Autoimmune encephalitis update. Neuro-Oncology. 2014 Jun;16(6):771–778. doi: 10.1093/neuonc/nou030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schoser B., Eymard B., Datt J., Mantegazza R. Lambert-Eaton myasthenic syndrome (LEMS): a rare autoimmune presynaptic disorder often associated with cancer. J. Neurol. 2017 Sep;264(9):1854–1863. doi: 10.1007/s00415-017-8541-9. [DOI] [PubMed] [Google Scholar]
- 36.Haberman R., Axelrad J., Chen A., Castillo R., Yan D., Izmirly P. Covid-19 in immune-mediated inflammatory diseases - case series from New York. N. Engl. J. Med. 2020 Jul 2;383(1):85–88. doi: 10.1056/NEJMc2009567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larsson E., Brattström O., Agvald-Öhman C., Grip J., Campoccia Jalde F., Strålin K. Characteristics and outcomes of patients with COVID-19 admitted to ICU in a tertiary hospital in Stockholm, Sweden. Acta Anaesthesiol. Scand. 2020 Sep 6 doi: 10.1111/aas.13694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mukherjee V., Toth A.T., Fenianos M., Martell S., Karpel H.C., Postelnicu R. Clinical outcomes in critically ill coronavirus disease 2019 patients: a unique New York city public hospital experience. Crit. Care Explor. 2020;2(8):e0188. doi: 10.1097/CCE.0000000000000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kayina C., Haritha D., Soni L., Behera S., Nair P., Gouri M. Epidemiological & clinical characteristics & early outcome of COVID-19 patients in a tertiary care teaching hospital in India: A preliminary analysis. Indian J. Med. Res. 1994:2020. doi: 10.4103/ijmr.IJMR_2890_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cummings M.J., Baldwin M.R., Abrams D., Jacobson S.D., Meyer B.J., Balough E.M. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020 Jun 6;395(10239):1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moro E., Deuschl G., de Visser M., Muresanu D., Soffietti R., Marson A. A call from the European academy of neurology on COVID-19. Lancet Neurol. 2020 Jun;19(6):482. doi: 10.1016/S1474-4422(20)30151-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.https://www.covims.org/. Coronavirus and MS Reporting Database, 2020.
- 43.https://myasthenia.org/For-Professionals/Resources-for-Professionals/CARE-MG. COVID-19 Associated Risks and Effects in Myasthenia Gravis (CARE-MG), 2020. [DOI] [PMC free article] [PubMed]
- 44.https://rheum-covid.org/. The COVID-19 Global Rheumatology Alliance, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material