Abstract
The severe acute respiratory syndrome novel coronavirus-2 pandemic has established a new set of challenges to health care delivery. Remotely monitored physiologic sensors on implantable cardiac devices can provide insight into the differential diagnosis of dyspnea in the heart failure population. We report on a unique pattern of sensor deviations that seem to occur specifically with severe acute respiratory syndrome novel coronavirus-2 infection.
The severe acute respiratory syndrome novel coronavirus-2 (SARS-CoV-2) pandemic has created evolving models of health care delivery with an increased reliance on telehealth.1 Individuals with underlying cardiovascular disease are at increased risk of complications from SARS-CoV-2 infection.2 As such, clinicians have been moving to virtual visits in these high-risk populations to avoid unnecessary exposure within the health care system. Infection with SARS-CoV-2 has a broad range of symptoms, with cough and shortness of breath among the top presenting symptoms.3 Given reports that only 48% of patients exhibit fever at the time of admission, the differentiation between congestive heart failure and SARS-CoV-2 infection becomes more difficult on a virtual platform.3
Many patients with heart failure due to reduced ejection fraction have implantable cardiac defibrillators and/or cardiac resynchronization therapy devices with physiologic sensors that are remotely monitored. HeartLogic is a multisensor heart failure index and alert algorithm that has been validated in predicting impending heart failure decompensations.4 The algorithm utilizes sensors that collect information on heart sounds (S1 and S3), respiration, thoracic impedance, heart rate, and activity level to create a daily index value. An alert to the clinician is automatically triggered when the index value crosses a configurable alert threshold. Sensor data are downloaded and transmitted remotely, with clinicians able to access individual sensor data via an online remote patient monitoring system.
The identification of a unique signature of senor patterns that differentiates heart failure from possible infection with SARS-CoV-2 may allow for a safer and more effective triage, testing, and treatment in these high-risk patients. We describe here the pattern of HeartLogic physiologic sensor changes in 3 patients with SARS-CoV-2 infection. Analysis of these sensors demonstrates a pattern with SARS-CoV-2 infection that is distinct from heart failure. Changes in sensor values were compared to a 60-day baseline average, 90 days before symptom onset.
Case Studies
Case 1
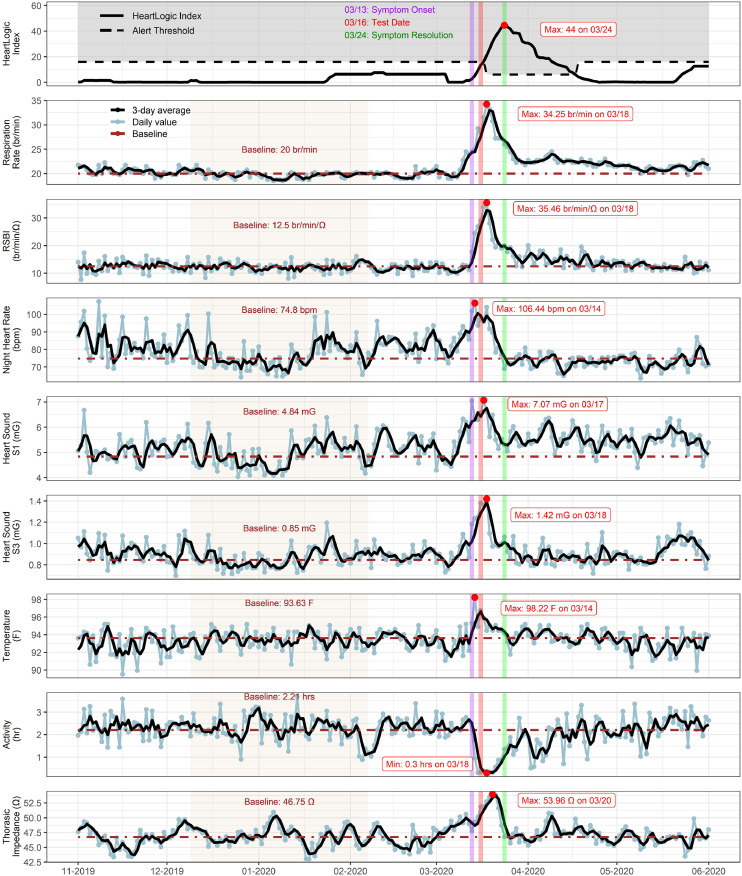
A 62-year-old Caucasian man with ischemic cardiomyopathy, a left ventricular ejection fraction of 30%, New York Heart Association functional class I, with comorbidities of hypertension and hyperlipidemia, who was implanted with a Boston Scientific RESONATE device for primary prevention. On March 13, he developed fever, chills, myalgia, anorexia, and severe fatigue. He was tested for SARS-CoV-2 on March 16, which ultimately returned as positive.
As seen in Fig. 1 , his symptom onset coincided with a substantial increase in the respiratory rate (from 20 to 34 bpm), rapid shallow breathing index (RBSI; from 12.5 to 35.5 br/min/Ω), nighttime heart rate (from 75 to 106 bpm), and temperature (from 93.6°F to 98.2°F), with a sudden decrease in his activity level (from 2.2 to 0.3 hours). Interestingly, a significant increase in thoracic impedance (from 46.7 to 54.0 Ω), S1 (from 4.8 to 7.0 mG) and S3 heart sounds (from 0.85 to 1.42 mG) was also noted with infection. This increase in thoracic impedance and S1 heart sound is atypical for decompensated heart failure, because thoracic impedance and S1 typically decrease as vascular congestion and fluid overload increase.5, 6, 7
Fig. 1.
Case 1. HeartLogic tracings depicting a sharp increase in respiratory rate, RSBI, nighttime heart rate, S1, S3, temperature and thoracic impedance with onset of severe acute respiratory syndrome novel coronavirus-2 symptoms (purple vertical line). Major symptom resolution (green vertical line) was associated with recovering sensor trends. Maximum and minimum values are indicated with red dots. A baseline was calculated for each sensor trend (tan shaded interval). RSBI, rapid shallow breathing index.
No change was made to his diuretic regimen. The majority of viral symptoms resolved by 10 days, but easy fatigability and decreased exercise tolerance continued for more than 8 weeks. As seen in Fig. 1, major symptom resolution coincided with recovering sensor trends. However, his respiratory rate, RSBI, and S1 remained increased for several weeks after this infection.
Case 2
A 67-year-old Caucasian man with ischemic cardiomyopathy, a left ventricular ejection fraction of 25%, New York Heart Association functional class II, with comorbidities of atrial fibrillation/flutter, hypertension, hyperlipidemia, chronic kidney disease, and type 2 diabetes was implanted with a Boston Scientific RESONATE implantable cardiac defibrillator device for primary prevention.
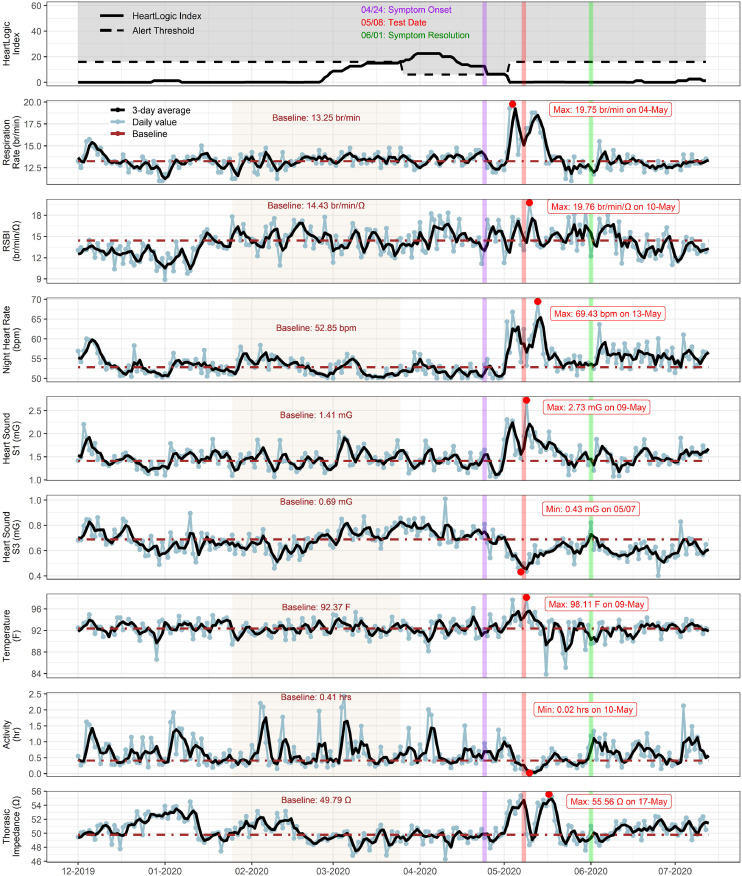
As seen in Fig. 2 , on April 24 he experienced symptoms of anosmia, rhinorrhea, and sore throat and self-isolated at home. He subsequently developed cough and shortness of breath. He was brought to the emergency department via emergency medical services on May 8 with hypoxia and respiratory distress, where he tested positive for SARS-CoV-2. He was diagnosed with pneumonia secondary to SARS-CoV-2 infection based on chest radiograph results and was hospitalized from May 8–12. He was treated with ceftriaxone and was able to be weaned off of supplemental oxygen within 48 hours of admission. No changes were made to his diuretic regimen.
Fig. 2.
Case 2. HeartLogic sensor tracings demonstrating trends with infection of severe acute respiratory syndrome novel coronavirus-2 (SARS-CoV-2). The onset of viral symptoms, including anosmia, is indicated by the purple vertical line. Two weeks after the initial symptoms, the patient presented with shortness of breath, testing positive for SARS-CoV-2 infection (red vertical line). A rapid increase in the respiratory rate, nighttime heart rate, S1, temperature, and thoracic impedance with a decrease in S3 and activity level were noted before the date of admission. Major symptom recovery is noted by the green vertical line. A baseline was calculated for each sensor trend (tan shaded interval). Maximum and minimum values are indicated with red dots. RSBI, rapid shallow breathing index.
An increase in respiratory rate (from 13.30 to 19.75 br/m), RSBI (from 14.4 to 35.5 br/min/Ω), nighttime heart rate (from 53 to 69 bpm), S1 heart sound (from 1.4 to 2.7 mG), and thoracic impedance (from 49.6 to 55.6 Ω) were seen in conjunction with a decrease in activity level (from 0.40 to 0.02 hours) and S3 heart sound (from 0.69 to 0.43 mG). The sensors showed a biphasic trend with initial peaks several days before hospitalization and a second peak 10 days later. The increased in thoracic impedance and S1, as well as the decrease in S3, are the opposite of what is seen typically in decompensated heart failure.5, 6, 7, 8
The patient had resolution of major symptoms by discharge on May 12. However, he continued to experience dyspnea on exertion and decreased exercise tolerance for 4 weeks. As seen in Fig. 2, his RSBI, S1 and thoracic impedance remain increased for 2–3 weeks after discharge from the hospital.
Case 3
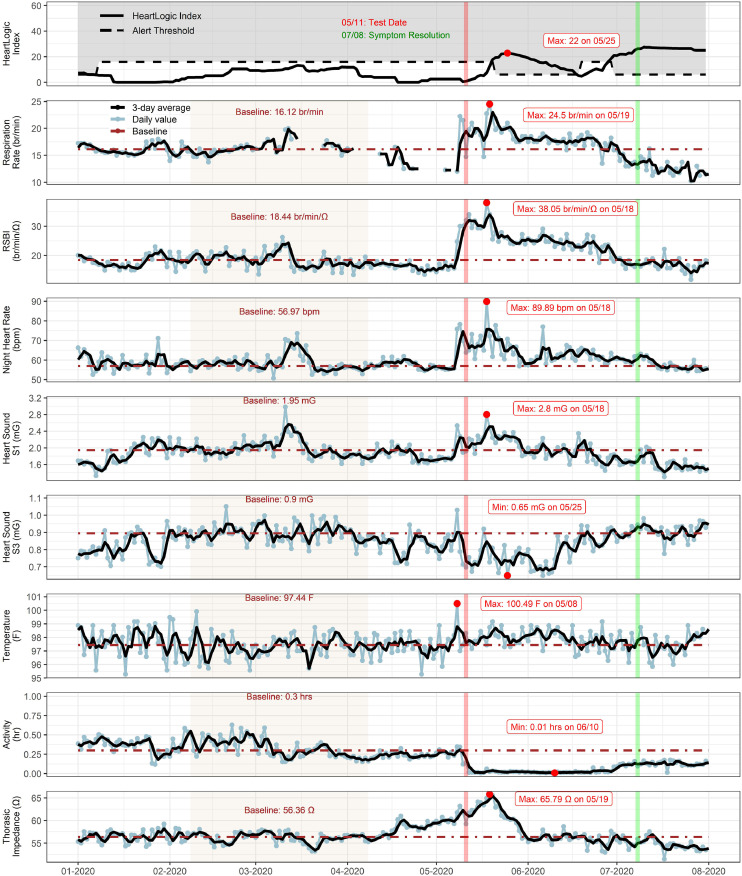
A 78-year-old Caucasian man had nonischemic cardiomyopathy, a left ventricular ejection fraction historically 10%–15%, recovered to 55%–60%, with a Boston Scientific RELIANCE implantable cardiac defibrillator, and had recently transitioned to a long-term care facility. As seen in Fig. 3 , the patient was screened for SARS-CoV-2 on May 11 per protocol, because another resident of the facility had tested positive. The patient is a limited historian and a full symptom assessment was not possible. However, nursing staff confirm that the patient was not complaining of symptoms at the time of testing. After a positive test result, the patient underwent a chest radiograph and was treated with ceftriaxone for pneumonia secondary to SARS-CoV-2 infection based on radiographic findings. He subsequently developed cough, shortness of breath, fatigue, decreased appetite, and nausea.
Fig. 3.
Case 3. HeartLogic sensor trends from a long-term care facility resident who was asymptomatic at the time of severe acute respiratory syndrome novel coronavirus-2 testing (red vertical line). A steep increase in respiratory rate, RSBI, nighttime heart rate, S1, temperature, and thoracic impedance and a decrease in S3 and activity level were noted with infection. Major symptom recovery (green vertical line) was associated with recovery of sensor trends. A baseline was calculated for each sensor trend (tan shaded interval). Maximum and minimum values are indicated with red dots. RSBI, rapid shallow breathing index.
The temperature recorded by the pulse generator spiked to 100.5°F on May 8. A simultaneous shift in all of the cardiovascular sensors occurred on the same day. Notably, there was an increase in respiratory rate (from 16.1 to 24.5 br/min), RSBI (from 18.4 to 38.1 br/min/Ω), nighttime heart rate (from 57 to 90 bpm), S1 heart sound (from 1.95 to 2.8 mG), and thoracic impedance (from 56.4 to 65.8 Ω) and a decrease in S3 heart sound (from 0.90 to 0.65 mG). As with the previous cases, thoracic impedance and S1 increased, with a decrease in S3 that is distinct from the pattern in heart failure.8
No change was made to his diuretic regimen. The patient had resolution of cough and shortness of breath but continued with symptoms of general malaise, anorexia, and prandial nausea at follow-up on July 7 .
Discussion
In the era of telemedicine, data derived from physiologic sensors can be extremely useful to clinicians in triaging and treating patients with heart failure complaining of dyspnea. Sensor data are monitored remotely and can provide insight into differential diagnoses without necessitating in-person contact. This technology holds the promise to decrease the spread of infection in the outpatient clinic setting by allowing clinicians to evaluate changes in physiologic sensors before patient presentation.
In our cases, infection with SARS-CoV-2 created a unique pattern of sensor changes that was distinct from decompensated heart failure. Thoracic impedance, known to decrease in volume overload,5 , 6 increased in all 3 cases. Interestingly, this increase in thoracic impedance also differs from the pattern seen with pneumonia, which has been noted to contribute to false-positive results with intrathoracic impedance monitoring devices for heart failure.8 , 9 The precise reason as to why the thoracic impedance increases with SARS-CoV-2 infection is not well-understood. However, this finding is consistent with findings from Heggermont et al,10 who described a similar phenomenon of increasing thoracic impedance in a hospitalized patient with SARS-CoV-2 infection. Furthermore, in all of our cases, S1 heart sound increased with infection, in contrast with the decrease expected in decompensated heart failure.7 An increasing heart rate and respiratory trends in conjunction with increasing thoracic impedance and S1 would argue against heart failure and points to an alternate diagnosis.
Our data suggest that a unique set of sensor trends is identifiable in the patient who is infected with SARS-CoV-2. Using this pattern of individual sensor changes, there is the potential that alerts configurable for specific respiratory conditions could be developed. However, this report is a retrospective observational review of 3 cases. Further studies are needed to determine if the trends seen with these 3 cases remain consistent in larger sample sizes.
With the recent literature focusing on the use of telemedicine in the patient with advanced heart failure, remotely monitored physiologic sensor data have become increasingly used in patient evaluation. Given the somewhat broad differential in the patient with heart failure presenting with dyspnea, these distinctive trends have the potential to be extremely useful to clinicians. The identification of patterns that are atypical for heart failure before patient presentation may expedite triage and decrease potential exposure to SARS-CoV-2.
References
- 1.Abraham WT, Fiuzat M, Psotka MA, O'Connor CM. Heart failure collaboratory statement on remote monitoring and social distancing in the landscape of COVID-19. JACC Heart Fail. 2020;8:692–694. doi: 10.1016/j.jchf.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aghagoli G, Gallo Marin B, Soliman LB, Sellke FW. Cardiac involvement in COVID-19 patients: risk factors, predictors, and complications: a review. J Card Surg. 2020;35:1302–1305. doi: 10.1111/jocs.14538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan W, Ni Z, Hu Y, Liang W-H, Ou C-Q, He J-X, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boehmer JP, Hariharan R, Devecchi FG, Smith AL, Molon G, Capucci A, et al. A multisensor algorithm predicts heart failure events in patients with implanted devices: results from the MultiSENSE Study. JACC Heart Fail. 2017;3:216–225. doi: 10.1016/j.jchf.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 5.Yu CM, Wang L, Chan RW, Kong SL, Tang MO, Christensen J, et al. Intrathoracic impedance monitoring in patients with heart failure: correlation with fluid status and feasibility of early warning preceding hospitalization. Circulation. 2005;9:841–848. doi: 10.1161/CIRCULATIONAHA.104.492207. [DOI] [PubMed] [Google Scholar]
- 6.Vollmann D, Nagele H, Schauerte P, Wiegand U, Butter C, Zanotto G, et al. Clinical utility of intrathoracic impedance monitoring to alert patients with an implanted device of deteriorating chronic heart failure. Eur Heart J. 2007;28:1835–1840. doi: 10.1093/eurheartj/ehl506. [DOI] [PubMed] [Google Scholar]
- 7.Luisada AA, MacCanon DM, Coleman B, Feigen LP. New studies on the first heart sound. Am J Cardiol. 1971;2:140–149. doi: 10.1016/0002-9149(71)90362-6. [DOI] [PubMed] [Google Scholar]
- 8.Abraham WT, Yancy CW. Intrathoracic impedance monitoring for early detection of impending heart failure decompensation. Congest Heart Fail. 2007;13:113–115. doi: 10.1111/j.1527-5299.2007.06255.x. [DOI] [PubMed] [Google Scholar]
- 9.Ha AC, Leather RA, Novak PG, Sterns LD, Tang AS. The role of device diagnostic algorithms in the assessment and management of patients with systolic heart failure: a review. Cardiol Res Pract. 2011;2011 doi: 10.4061/2011/908921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heggermont W, Nguyen P, Lau CW, Tournoy K. A steep increase in the HeartLogic index predicts Covid-19 disease in an advanced heart failure patient. Case Rep Cardiol. 2020;2020 doi: 10.1155/2020/8896152. [DOI] [PMC free article] [PubMed] [Google Scholar]