Abstract
We examined the association between endogenous opioid β-endorphin, cancer progression and pain in a transgenic mouse model of breast cancer, with a rat C3(1) simian virus 40 large tumor antigen fusion gene (C3TAg). C3TAg mice develop ductal epithelial atypia at 8 weeks, progression to intra-epithelial neoplasia at 12 weeks, and invasive carcinoma with palpable tumors at 16 weeks. Consistent with invasive carcinoma at 4 months of age, C3TAg mice demonstrate a significant increase in hyperalgesia compared to younger C3TAg or control FVBN mice without tumors. Our data show that the growing tumor contributes to circulating β-endorphin. As an endogenous ligand of mu opioid receptor, β-endorphin has analgesic activity. Paradoxically, we observed an increase in pain in transgenic breast cancer mice with significantly high circulating and tumor-associated β-endorphin. Increased circulating β-endorphin correlates with increasing tumor burden. β-endorphin induced the activation of mitogenic and survival-promoting signaling pathways, MAPK/ERK 1/2, STAT3 and Akt, observed by us in human MDA-MB-231 cells suggesting a role for β-endorphin in breast cancer progression and associated pain.
Keywords: β-endorphin, breast cancer, pain, morphine, opioid
Introduction
Pain is the most devastating symptom of cancer, which impairs the quality of life, shortens survival and persists even with cancer treatment.[1, 2] With individuals living longer, pain control is critical to improve health related quality of life. Opioids are the mainstay of treatment for severe cancer pain.[3, 4] Use of opioids for pain dates back to 850 BCE in The lliad and The Odyssey by Homer. Since then, significant advancement has been made in understanding the pharmacology of analgesic opioids, and their potential for addiction. However, the role of endogenous opioids in normal physiology and pathobiology remains an enigma.
Endogenous opioid peptides Leu- and Met-enkephalin were recognized in the brain much before the discovery of opioid receptors.[5] Enkephalins, dynorphins, and β-endorphin cleaved from large molecule pro-peptides proenkephalin, prodynorphin and proopiomelanocortin (POMC), respectively, were subsequently recognized in the central nervous system (CNS).[6-8] β-endorphin acts through binding with mostly mu opioid receptor (MOR) in the CNS.[4, 9] However, peripheral (and local) analgesia can be modulated by increased levels of β-endorphin in the peripheral tissue,[10-12] supportive of its role in non-CNS tissues. In general, endogenous opioids interact with G-protein-coupled receptors: mu- (MOR), delta- (DOR), kappa- (KOR) and nociceptin opioid receptors (NOR).[13]
Of concern, MORs are also present on endothelial cells and in human tumors (peripheral MORs) including lung,[14-16] prostate[17] and breast[18] cancers. Compelling pre-clinical studies show that MOR activation stimulates mitogen-activated protein kinase/extracellular signal regulated kinase 1/2 (MAPK/ERK1/2) and Akt, the mitogenic and survival signaling pathways, respectively, leading to cell proliferation and cancer progression.[3, 16, 19-21] Additionally, MOR co-activates the receptor tyrosine kinases (RTKs) for vascular endothelial growth factor receptor 2 (VEGFR2), epidermal growth factor receptor (EGFR) and platelet derived growth factor receptor beta (PDGFR-β), which play a critical role in cancer progression and metastasis.[14, 16, 19, 22, 23] Complementary to the basic observations, morphine, a MOR agonist, promotes cancer progression, whereas MOR antagonists inhibit cancer progression in mouse models.[16, 19, 23-25] Consistent with pre-clinical findings, we found that higher opioid requirement is associated with poorer cancer-related outcomes and shorter survival in retrospective studies of prostate and lung cancer patients receiving opioids to treat pain. [17, 26]
Intriguingly, independent of opioid use, increased MOR-immunoreactivity (MOR-ir) in the tumors was associated with earlier time to progression and shorter progression-free survival and overall survival in prostate cancer patients [17] and increased metastasis in lung cancer patients.[20] However, a major gap in knowledge exists regarding the cause of constitutive activation of MOR in tumors, independent of pharmacological opioids. It is likely that increased endogenous ligand β-endorphin may be activating tumor MOR. Therefore, we hypothesized that β-endorphin may be associated with increased tumor burden and pain. We tested our hypothesis in transgenic mice with a rat C3(1) simian virus 40 large tumor antigen fusion gene (called C3TAg mice), which demonstrate the evolutionary spectrum of human infiltrating ductal breast carcinoma.[27] In C3TAg mice, morphine promoted tumor growth but not tumor initiation.[25] Of note, MOR expression in C3TAg mice was observed on larger growing tumors but not on the very small, early tumors, suggesting that the tumor microenvironment contributes to MOR expression much like other mitogenic growth factor receptors such as VEGFR2. We also examined the evolution of pain in this tumor model, which has translational potential to simulate the development of pain in human breast cancer. We demonstrate the development of pain in C3TAg mice with age, consistent with the advancement of tumor progression, and correlation of increasing β-endorphin with increasing tumor burden.
Materials and Methods
Mice
Female transgenic C3TAg mice which show the evolutionary spectrum of human infiltrating ductal breast carcinoma were used. [27-29] These mice express rat C3(1) simian virus 40 T-antigen fusion gene, which leads to the development of invasive mammary carcinoma in females and prostate cancer in males.[27] In this model, female mice develop multifocal mammary hyperplasia by about 3 months of age, which advances to adenocarcinoma by ~6 months of age and shows critical features of human basal-like triple negative breast cancer.[30, 31] Tumors metastasize hematogenously to multiple organs including lung, liver and heart with the end of survival at ~6 months of age. Female FVBN mice – the genetic background strain for C3TAg – were used as controls. We have used this model in previous studies for morphine-induced tumor progression via pericyte and mast cell activation.[25, 32] Animal use protocols were reviewed and approved by the Institutional Animal Care and Use Committee.
Human breast cancer cells
We used human MDA-MB-231 breast cancer cells, a highly-invasive model of triple-negative human breast cancer lacking estrogen receptor (ER), progesterone receptor (PR), and HER2 (human epidermal growth factor receptor 2).[33, 34] Cells were cultured in Dulbecco’s Modified Eagle Medium containing 10% fetal bovine serum and maintained at 37°C in 5% CO2.
Analysis of hyperalgesia in mice
Mice were acclimatized to each test protocol in a quiet room at constant temperature and tested for evoked and spontaneous hyperalgesia measures, including, thermal- (heat and cold), mechanical-, and deep tissue-hyperalgesia (grip force). All mice were familiarized with each testing procedure by performing a set of repeat measurements before collecting baseline measurements, as previously described.[35, 36]
Mechanical hyperalgesia:
Paw withdrawal frequency (PWF) evoked by calibrated von Frey (Semmes-Weinstein) monofilaments (Stoetling Co., Wood Dale, IL) applied to the plantar surface of each hind paw, was measured.
Grip force:
Peak forepaw grip force was measured using a computerized grip force meter (SA Maier Co., Milwaukee, WI). Mice held by the tail were made to pull on a wire-mesh connected to a force gauge with their forepaws, and the peak force (in g) exerted was recorded. Deep tissue hyperalgesia was defined as a decrease in the grip force.
Thermal hyperalgesia
Heat sensitivity:
Using the Hargreaves’ apparatus (PAW Thermal Stimulator System, Dept. of Anesthesiology, University of California, San Diego CA) a radiant heat stimulus was applied to the plantar surface of the hind paw and paw withdrawal latency (PWL) to the nearest 0.1 second was automatically recorded. A 20 s inter-stimulus cutoff was used to prevent tissue damage.
Cold sensitivity:
For cold sensitivity, mice were placed on a cold plate at 4°C (UGO Basile Model 35100, Collegeville, PA) and the paw withdrawal frequency (PWF) over a period of 2 minutes was determined.
Measurement of β-endorphin
Mouse blood was collected by cardiac puncture and transferred to tubes containing EDTA (Cat. No. EDTA-K2, Terumo Medical Corporation, Elkton, MD) and aprotinin (0.6 TIU/ml of blood, Cat. No. A6279, Sigma-Aldrich, St Louis, MO). The blood samples were centrifuged at 2000 rpm for 15 minutes at 4°C to separate plasma/supernatant. Tumors were collected from six month old C3TAg mice with tumors and control mammary tissue were collected from three month old C3TAg mice, which did not develop any tumors. Tissue lysates were prepared from the tumor and control tissue as described by us earlier using a cocktail of protease inhibitors.[23] Peptides were extracted from plasma and tissue lysates with SEP-COLUMN containing 200 mg of C18 (Phoenix Pharmaceuticals, Burlingame, CA) per the manufacturer’s protocol. Peptide samples were freeze-dried after extraction. Concentration of β-endorphin in peptide preparation was measured by enzyme immunoassay (EIA)(Phoenix Pharmaceuticals) per the manufacturer’s protocol. Fluorescence was measured using a microplate reader (Synergy HT, Biotek Instruments, Winooski, VT) at 360 nm for excitation and 460 nm for emission. Quantity of β-endorphin in samples was determined with Gen5 software (BioTek Instruments). All analyses and calibrations were performed in duplicate using suitable negative and positive controls.
Western blot analysis
MDA-MB-231 breast cancer cells were serum starved for 6 hours and stimulated with 1 nM human β-endorphin (Fisher Scientific, Waltham, MA) or sterile PBS. Cell lysates were prepared as previously described using a cocktail of protease inhibitors. [23] Protein lysates containing 30 μg of protein were separated on a 3–15% gradient SDS-PAGE gel and then transferred to a polyvinylidene fluoride membrane (Immobilon; Millipore, Bedford, MA). Protein bands were detected using 1:500 phospho-Akt, 1:500 Akt, 1:1000 phospho-STAT3, 1:1000 STAT3, 1:1000 phospho-MAPK/ERK1/2, and 1:1000 MAPK/ERK1/2; all from Cell Signaling Technology, Danvers, MA). Alkaline phosphatase-conjugated secondary antibodies (1:5000, Jackson ImmunoResearch, West Grove, PA) and ECF system (Amersham Bioscience, Buckinghamshire, UK) were used to detect chemiluminescent signals on a Storm 860 Phosphorimager (Molecular Dynamics, Sunnyvale, CA). Protein bands were quantitated by densitometric analysis using ImageJ Software (National Institutes of Health, Bethesda, MD).
Statistical analysis
We analyzed the data using Student’s two-tailed t-test, Pearson correlation, or One-way analysis of variance (ANOVA) with Bonferroni’s post-hoc multiple comparisons test, as necessary, using Prism 8 software (Graphpad, San Diego, CA). Data are expressed as mean ± SEM. P<0.05 is considered significant.
Results
Evolution of hyperalgesia in transgenic C3TAg mice
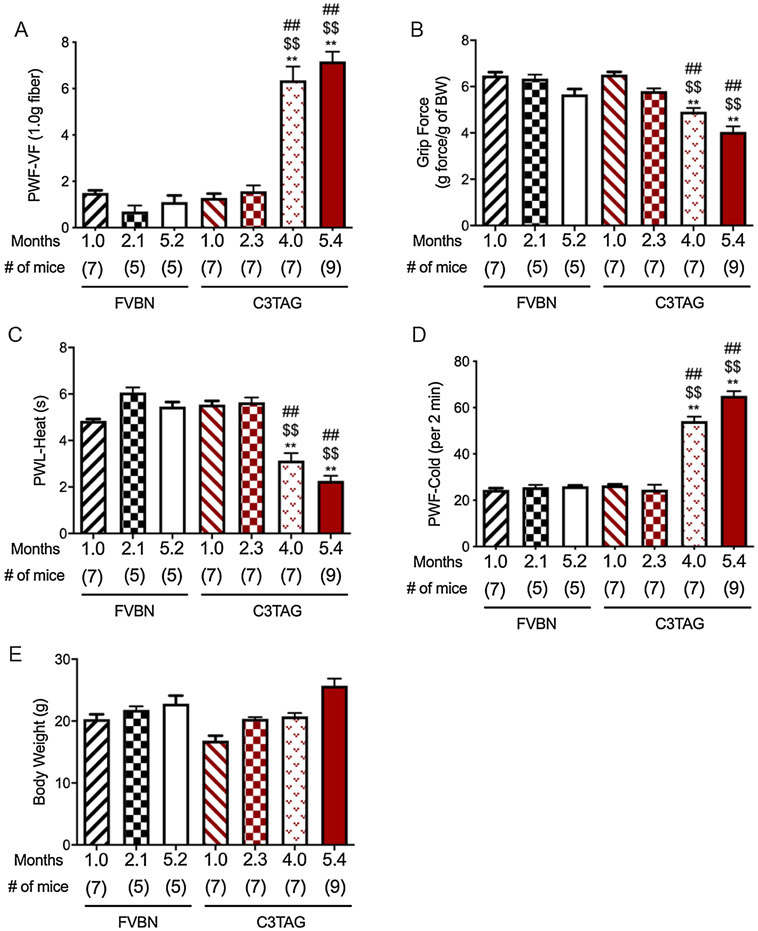
C3TAg mice develop ductal epithelial atypia at 8 weeks, progression to intra-epithelial neoplasia at 12 weeks (resembling human ductal carcinoma in situ), and invasive carcinoma with grossly palpable tumors at 16 weeks.[27] At approximately 6 months of age mice die because of universal development of multifocal mammary adenocarcinomas.[27] The evolution of pain in this mouse model may therefore represent the evolution of pain in human breast cancer. We evaluated temporal evolution of hyperalgesia in C3TAg and control FVBN mice with increasing age. Control FVBN mice from approximately 1 to 5 months of age did not exhibit hyperalgesia on any of the behavioral measures (Fig. 1A-D). Similarly, younger 1 to 2.3-month old C3TAg mice did not display hyperalgesia. However, 4 and 5.4-month old C3TAg mice with palpable tumors demonstrate significantly elevated mechanical, deep tissue and thermal (heat and cold) hyperalgesia compared to 5.4 month old FVBN control mice (P<0.0001) as well as compared to younger C3TAg mice at 1 or 2.3 months of age (P<0.0001, Fig. 1A-D). Between the 4 and 5.4-month old C3TAg mice, thermal hyperalgesia (both cold and heat) is significantly higher in 5.4-month old mice, however, neither mechanical nor deep tissue hyperalgesia show significant differences. Therefore, hyperalgesia develops progressively with tumor progression and metastasis. We did not observe a significant difference in the body weight between control and C3TAg mice.
Figure 1: Hyperalgesia increases with age in transgenic mice with breast cancer.
(A) Paw withdrawal frequency (PWF) in response to von Frey filament to test mechanical hyperalgesia. Higher PWF demonstrates more hyperalgesia. (B) Grip force to test musculoskeletal/deep tissue hyperalgesia. Lower grip force demonstrates more hyperalgesia. (C) Paw withdrawal latency (PWL) in response to a radiant heat stimulus applied to the plantar surface of the hind paw to test heat hyperalgesia. Lower PWL demonstrates more hyperalgesia. (D) Paw withdrawal frequency (PWF) over a period of 2 minutes when placed on a cold plate at 4°C. Higher PWF demonstrates more hyperalgesia. (E) Body weight of mice used for hyperalgesia measurements. Data are shown as mean ± SEM. Statistical analysis: One-way ANOVA/Bonferroni’s post-hoc analysis. A-D, ** P<0.0001 vs. 1.0-month old C3TAg; $$ P<0 .0001 vs. 2.1 months old C3TAg, ## P<0.0001 vs. 5.2 months old FVBN. FVBN, wild type genetic background mice and C3TAg, transgenic mice with breast cancer.
Circulating β-endorphin increases with tumor burden in C3TAg mice
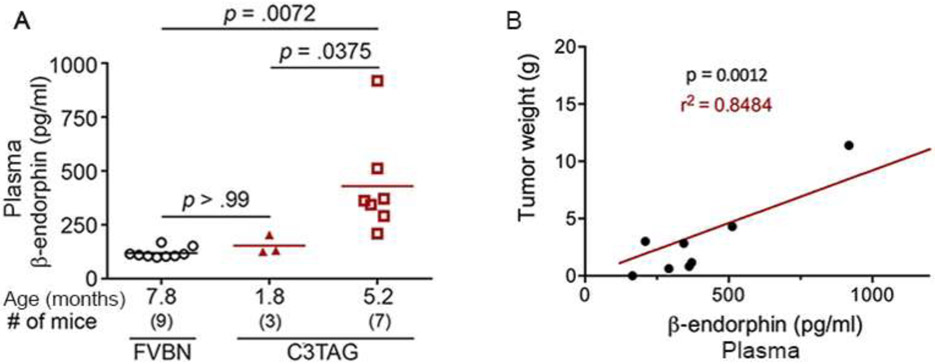
We observe that β-endorphin increases with age in the plasma, with a 3-fold increase at 5 months compared to 2-month old C3TAg mice (P<0.05, Fig. 2A). Plasma β-endorphin in 5-month old C3TAg mice is 4-fold higher compared to aged FVBN mice (P<0.01, Fig. 2A). Correspondingly, tumor burden in C3TAg mice increases with age.[25] Of note, we find that circulating β-endorphin has a significant, positive correlation with tumor burden in C3TAg mice (P<0.01, r2=0.85, Fig. 2B). Thus, these data indicate that plasma β-endorphin may be released from the tumors. Therefore, we next examined directly whether tumors of C3TAg mice express β-endorphin.
Figure 2: Constitutive increase in circulating β-endorphin with increasing tumor burden in transgenic mice with breast cancer.
(A) β-endorphin was analyzed in the plasma from C3TAg mice without tumors at 1.8 months and 5.2 month old with tumors and from control FVBN mice at 7.8 months of age. Plasma β-endorphin is significantly higher in 5.2 month old C3TAg mice with tumors compared to 1.8 month old C3TAg mice or FVBN mice without tumors. Notably, no significant difference in plasma β-endorphin concentration was observed between FVBN and 1.8 month old C3TAg mice. (B) Plasma and tumors were obtained from C3TAg mice at 5.2 months of age. Each value represents the plasma β-endorphin and correlative tumor weight from each mouse. Plasma β-endorphin increases with increasing tumor weight in C3TAg mice. Statistical analysis: (A) One-way ANOVA/Bonferroni’s post-hoc analysis; (B) Pearson’s correlation.
Tumors of mice express β-endorphin
We observe significantly higher β-endorphin (more than 5-fold) levels present in the tumor lysate of 6-month old C3TAg mice compared to mammary tissue from 3-month old mice without tumors (P<0.01, Fig. 3). Thus, tumor β-endorphin may be contributing to circulating levels of β-endorphin in the plasma of older mice, with increased tumor burden compared to younger C3TAg mice.
Figure 3: Increased β-endorphin expression in tumors of older transgenic breast cancer mice compared to control breast tissue from younger mice.
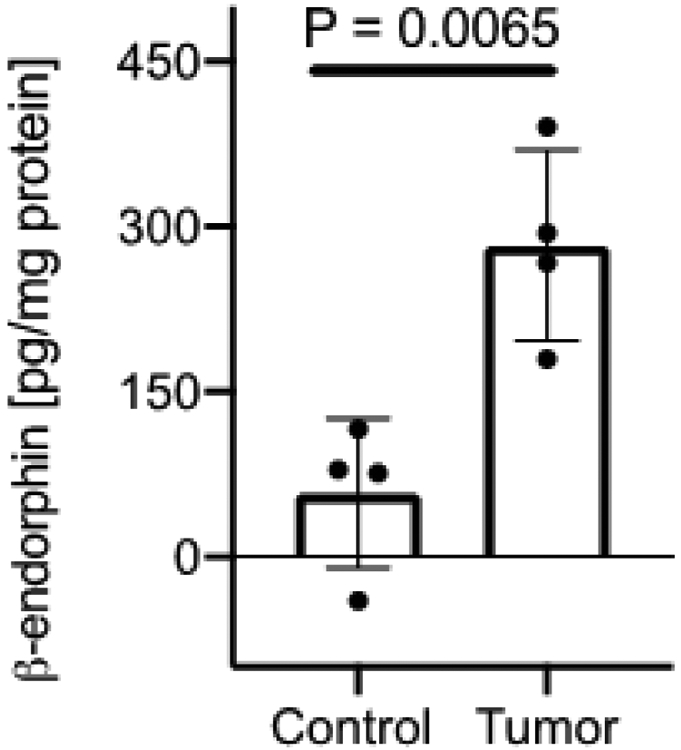
Breast tumors from ~6 month old C3TAg mice and non-tumor breast tissue from ~3 month old C3TAg mice were analyzed for β-endorphin expression. Significantly higher β-endorphin concentration was observed in the tumors compared to non-cancerous mammary tissue. Data are shown as mean ± SEM. Statistical analysis: Student’s two-tailed t-test.
β-endorphin stimulates mitogenic and survival-promoting signaling in human breast cancer cells
Cell survival and mitogenic signaling promoting PKB/Akt, JAK/STAT and MAPK/ERK pathways are known to contribute to cancer progression, metastasis, and drug resistance.[37-41] We incubated human MDA-MB-231 breast cancer cells with vehicle or 1 nM β-endorphin for 5-, 30- or 60-mins and analyzed the phosphorylation of Akt, STAT3 and MAPK/ERK signaling pathways. Relative to their respective total protein, we observed a time-dependent increase in the phosphorylation of each of the signaling pathways (Fig. 4A-C). β-endorphin significantly stimulated the phosphorylation of mitogenic MAPK/ERK and STAT3 after 30- and 5-minutes of incubation, respectively (P<0.05 and P<0.001, respectively vs. vehicle); and that of survival-promoting PKB/Akt after 5 min of incubation, which is sustained until 30 min (P<0.05). Thus, β-endorphin stimulates mitogenic and survival promoting signaling in human breast cancer cells.
Figure 4: β-endorphin stimulates mitogenic and survival promoting signaling in human MDA-MB-231 breast cancer cells.
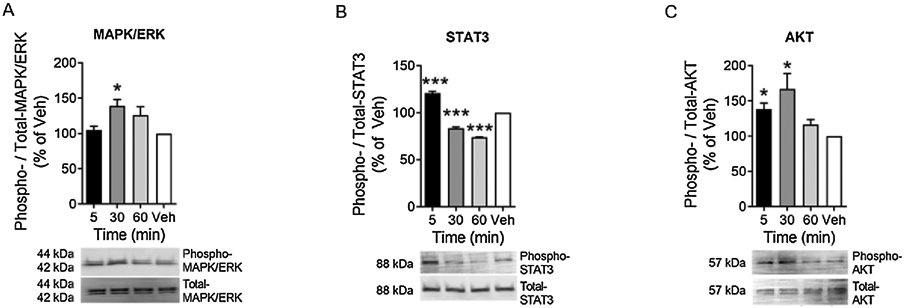
In vitro human MDA-MB-231 breast cancer cells were incubated with 1 nM β-endorphin for indicated time or with vehicle. Compared to total protein, phosphorylated signaling molecules are shown at 5, 30 or 60 minutes after β-endorphin stimulation: (A) Phospho- to total-MAPK/ERK1/2, (B) Phospho- to total-STAT3 and, (C) Phospho- to total-Akt. Each image represents 3 separate and reproducible experiments. Data are shown as mean ± SEM. Statistical analysis: Student’s two-tailed t-test. * P<0.05 and *** P<0.001, compared to vehicle.
Discussion
Most of the work on pain, opioids and cancer either investigates the impact of analgesic opioids on cancer biology or focuses on opioids and pain. In this novel study we have initiated the investigation on all three together as would happen in a clinical setting. We have found that circulating β-endorphin as well as pain increase with age in C3TAg mice consistent with the development of invasive carcinoma. The tumors from C3TAg mice exhibit significantly increased levels of β-endorphin compared to β-endorphin levels in mammary gland tissue extracted from mice without tumors, supporting the increase in circulating β-endorphin correlative to tumor burden. Also, β-endorphin-treatment of human MDA-MB-231 breast cancer cells led to the activation of mitogenic and survival-promoting signaling pathways known to promote cancer progression and metastases. To our knowledge, this is the first report on endogenous β-endorphin as a potential contributor of breast cancer progression and a biomarker of cancer progression and pain.
Most of the emphasis on the association of opioids with cancer pain or biology has targeted opioid receptors, mostly MOR.[3, 21, 42-44] Even though β-endorphin has the potential for activating MOR, hence the endogenous functional modulation of pain and cancer, but it has remained under-appreciated. β-endorphin mediates analgesic activity via MOR in the CNS and also in the periphery.[45-47] Mitogen-activated protein kinase (MAPKs: ERK1/2, p38 and JNK) and STAT3 have been suggested to contribute to β-endorphin expression in the inflammatory cells including leukocytes and glial cells in the periphery and the CNS, respectively.[48-52] Therefore, in the well-known inflammatory microenvironment of the tumor, inflammatory leukocytes may release β-endorphin, thus raising its levels as we observed in the tumors of C3TAg mice. β-endorphin has analgesic ability by binding to the MORs in the CNS. [53] However, in a human study including multiple solid tumor cohorts, high amounts of circulating β-endorphin correlated with more severe pain, and oxycontin treatment reduced pain as well as β-endorphin levels.[54] Increased MOR and DOR were expressed in human lung cancer tissue compared to adjacent normal tissue.[15] Therefore, peripheral and central β-endorphin expression may have opposite analgesic effects in cancer pain, perhaps due to the activation of tumor MOR leading to cancer progression. In low-back pain patients, low endogenous opioid function quantified by pharmacological OR blockade of pain response, was found to be associated with greater analgesic effect of morphine. [55] Thus, endogenous β-endorphin appears to be a critical determinant of pain, analgesic response to opioid therapy and cancer progression.
C3TAg mice do not develop bone metastases and perineural invasion is not known in these mice. However, previously our laboratory found that the tumors in C3TAg mice express inflammatory cytokines and neuropeptides, including substance P (SP) and interleukin-6 which are further exacerbated with morphine treatment.[25] SP is a soluble factor which is involved in the transmission and maintenance of chronic pain via its neurokinin receptor 1 (NK1R),[56] in addition to promoting cancer progression.[57] NK1R antagonists have been suggested for therapeutic potential to treat breast cancer including triple negative breast cancer which presents overexpressed NK1R.[58] Therefore, it is likely that SP and other cytokines released by the tumors contribute to pain, but the possibility of perineural invasion may co-exist, which requires investigation.
Our observation of increased β-endorphin release from tumors in older C3TAg mice is correlative of our earlier observation of higher MOR-immunoreactivity (ir) in advanced tumors compared to smaller tumors.[25] Such high MOR-ir may lead to consequent activation of the mitogenic signaling by intratumoral β-endorphin. On the other hand, in chronic pain, sustained increase of β-endorphin may desensitize MOR in the CNS, and therefore pain may continue to increase irrespective of increase in circulating β-endorphin. This appears to be the case because higher resting stage circulating β-endorphin levels are associated with increased mechanical sensitivity in older adults with chronic osteoarthritis pain.[59] In contrast, MOR expressed on newer cells formed continuously in advancing tumors will be sensitive to β-endorphin and continue to promote growth.
Sex differences in pain and analgesia are well-known,[60] with women experiencing more pain compared to men, due to hormonal variability and heightened inflammatory response in women.[59, 61-63] Male-specificity of endogenous analgesic systems have been observed in preclinical studies.[47, 50, 64] Moreover, response of opioid receptor subtypes to different chemical stimuli in the brain regions have been demonstrated to be sex-specific.[65, 66] Therefore, as breast cancer predominantly affect females, it is possible that ongoing gender-specific stimulation of the endogenous opioid system and subsequent expression of β-endorphin may be reflective of effects of gonadal hormones. POMC – the precursor of β-endorphin – has exhibited characteristics of sexual dimorphism. Interestingly, a recent study demonstrated that POMC helps to maintain tumor-initiating properties of cancer stem cells isolated from human breast cancers.[67] Hence, involvement of POMC and consequent β-endorphin expression in breast cancer is conceivable. Our data in a transgenic mouse model showing the positive correlation between circulating β-endorphin and tumor burden supports the role of POMC in human breast cancer patients.
We observed stimulation of Akt, STAT3 and MAPK/ERK in human MDA-MB-231 breast cancer cells by β-endorphin. Activation of PI3K/Akt/mTOR, JAK/STAT and MAPK/ERK pathways contribute to cell survival, growth, tumorigenesis, metastasis, cell cycle progression, cellular motility and drug resistance.[37-41] Together, the MAPK/ERK, PI3K/mTOR/Akt and JAK/STAT3 pathways promote cancer progression and metastasis and drug resistance while simultaneously stimulating peripheral (dorsal root ganglia, DRG) and central (spinal) nociceptive signaling, neuronal hypersensitivity, central sensitization, chronic pain and morphine tolerance,[68-76]. These pathways are activated in DRG neurons and the spinal dorsal horn in neuropathic pain models.[72-77] Pain is the most common problem in cancer, which can persist even with increasing cancer treatment options and following tumor excision in a phenomenon described as Postmastectomy Pain Syndrome.[1, 78] Moreover, persistence of pain is an outcome of neuronal hypersensitivity due to peripheral and/or central sensitization following nerve injury and/or inflammation.[79, 80] This may continue even after withdrawal of the source of injury due to neuronal sensitization continuing to discharge signals and release neurotransmitters such as SP from the central nervous system (CNS) to the periphery in an antidromic manner, thus promoting a vicious cycle of nerve activation and pain.[81-85] Therefore, the increased pain sensitivity concomitant to increased circulating β-endorphin with increasing tumor burden that we observed suggests β-endorphin induces activation of signaling pathways that promote cancer progression and pain.
The increased β-endorphin levels observed in tumors may contribute to sustained activation of tumor MORs. Blockade of tumor MOR with peripherally-acting mu-opioid receptor antagonists (PAMORAs) may potentially disrupt the vicious cycle of MOR activation and tumor progression and attenuate pain. Methylnaltrexone (MNTX) and naloxegol are two PAMORAs with antagonist activity approved by the FDA for opioid-induced constipation (OIC), since they do not impact opioid mediated analgesia.[86] The effect of PAMORAs on cancer progression has not been examined in a clinical setting, but in a post-hoc analysis of patients with advanced malignancies, MNTX use and improvement in OIC were associated with longer overall survival.[21] It is possible that longer survival upon MNTX treatment could be due to the blockade of β-endorphin-induced MOR signaling which may prevent cancer progression.
Earlier studies by us in this mouse model showed that larger tumors express higher MOR compared to smaller tumors.[25] In the present study, we have observed that plasma β-endorphin increases correlative to tumor burden, and tumors from older C3TAg mice express significantly higher β-endorphin compared to breast tissue from younger C3TAg mice. Based on this study and our previous study, there appears to be an association between MOR and β-endorphin in cancer, which is being examined in human cancers by our and other groups. Intriguingly, independent of opioid use, increased MOR-immunoreactivity (ir) in the tumors was associated with earlier time to progression and shorter progression-free survival and overall survival in prostate cancer patients,[17] and increased metastasis in lung cancer patients.[20] Retrospective clinical studies suggest tumor MOR as a molecular biomarker of poor prognosis including cancer progression and reduced survival in multiple types of human cancer including lung, prostate, laryngeal squamous cell carcinoma, and hepatocellular carcinoma.[17, 20, 26, 87-90] In 2 randomized placebo-controlled trials of several hundred cancer patients, response to MNTX was associated with increased overall survival, which could be due to limiting cancer progression via tumor MOR antagonism.[21] This could be the case because co-treatment with MNTX in docetaxel-treated mice xenografted with a human gastric cancer cell line, significantly prolonged survival and decreased visceral pain-related hunching behavior, in addition to reducing metastasis, compared to docetaxel alone treatment.[91] In this study, MNTX was effective in preventing resistance to chemotherapy in several human cancer cell lines expressing opioid growth factor (OGF)/ Met-enkephalin. Both, β-endorphin and Met-enkephalin are encoded by the same POMC gene and it is likely that both are simultaneously expressed in a highly active tumor microenvironment with a concomitant upregulation in their opioid receptors. Indeed, overexpression of MOR in human bronchoalveolar carcinoma cells led to increased primary tumor growth and lung metastases compared to vector control cells in nude mice. [19] Elegant in vitro studies showed that treatment with MNTX or silencing of MOR on Lewis lung carcinoma (LLC) cells, inhibited their invasion and anchorage-independent growth.[16] LLC tumor growth was suppressed in MOR knockout mice compared to wild type, and continuous infusion of MNTX reduced LLC tumor growth and lung metastasis.[16] Pre-clinical findings and retrospective analysis of human cancers thus suggest the contribution of MOR with cancer progression, metastasis, survival and pain, but the factor(s) activating MOR endogenously remained unknown. Our observations indicate that overexpression of endogenous β-endorphin in the tumors may be activating MOR, thus supporting the potential of MNTX to antagonize endogenous MOR activity leading to improved outcomes in cancer, which needs to be tested in clinical trials.
Together, our data show that pain persists despite high endogenous β-endorphin levels in C3TAg mice, correlative with tumor burden and previously observed high expression of MOR on advancing tumors. Thus, circulating β-endorphin and tumor MOR would be indicative of cancer severity and associated pain and serve as prognostic biomarkers of pain in breast cancer.
Highlights.
Transgenic mice with breast cancer show hyperalgesia with cancer progression
Increase in circulating β-endorphin correlates with tumor burden in transgenic mice
Tumors in transgenic mice with breast cancer express β-endorphin
β-endorphin stimulates mitogenic signaling in human breast cancer cells
β-endorphin may serve as a biomarker of pain and breast cancer progression
Acknowledgements
This work has been supported by NIH grants, UO1 HL117664, RO1 HL147562, U18 EB029354 to KG; a Diversity Supplement 3R01HL147562-03S to SK, University of California President’s Fellowship to DAA and University of California Provost’s Research Growth Fund Award to WZ and KG. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
Kalpna Gupta: Honoraria: Tautona Group, Novartis and CSL Behring. Research Grants: Cyclerion, 1910 Genetics and Grifols. Weian Zhao is a (co)founder of Velox Biosystems Inc., Amberstone Biosciences Inc., and Arvetas Biosciences Inc. Joshua Gu is a shareholder of Arvetas Biosciences Inc. All others: Nothing to disclose.
References
- 1.De Groef A, et al. , Best-Evidence Rehabilitation for Chronic Pain Part 2: Pain during and after Cancer Treatment. J Clin Med, 2019. 8(7): 979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zylla D, Steele G, and Gupta P, A systematic review of the impact of pain on overall survival in patients with cancer. Supportive Care in Cancer, 2017. 25(5): p. 1687–1698. [DOI] [PubMed] [Google Scholar]
- 3.Novy DM, et al. , Pain, opioid therapy, and survival: a needed discussion. Pain, 2020. 161(3): 496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hutchinson MR, et al. , Exploring the Neuroimmunopharmacology of Opioids: An Integrative Review of Mechanisms of Central Immune Signaling and Their Implications for Opioid Analgesia. Pharmacological Reviews, 2011. 63(3): p. 772–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hughes J, et al. , Identification of two related pentapeptides from the brain with potent opiate agonist activity. Nature, 1975. 258(5536): p. 577–579. [DOI] [PubMed] [Google Scholar]
- 6.Kakidani H, et al. , Cloning and sequence analysis of cDNA for porcine β-neo-endorphin/dynorphin precursor. Nature, 1982. 298(5871): p. 245–249. [DOI] [PubMed] [Google Scholar]
- 7.Horikawa S, et al. , Isolation and structural organization of the human preproenkephalin B gene. Nature, 1983. 306(5943): p. 611–614. [DOI] [PubMed] [Google Scholar]
- 8.Nakanishi S, et al. , Nucleotide sequence of cloned cDNA for bovine corticotropin-β-lipotropin precursor. Nature, 1979. 278(5703): p. 423–427. [DOI] [PubMed] [Google Scholar]
- 9.Sprouse-Blum AS, et al. , Understanding endorphins and their importance in pain management. Hawaii medical journal, 2010. 69(3): p. 70–71. [PMC free article] [PubMed] [Google Scholar]
- 10.Alves DP, et al. , Inflammation Mobilizes Local Resources to Control Hyperalgesia: The Role of Endogenous Opioid Peptides. Pharmacology, 2012. 89(1-2): p. 22–28. [DOI] [PubMed] [Google Scholar]
- 11.Ishikawa T, et al. , In-vivo transfection of the proopiomelanocortin gene, precursor of endogenous endorphin, by use of radial shock waves alleviates neuropathic pain. Journal of Orthopaedic Science, 2013. 18(4): p. 636–645. [DOI] [PubMed] [Google Scholar]
- 12.Slominski AT, et al. , Introduction, in Sensing the Environment: Regulation of Local and Global Homeostasis by the Skin's Neuroendocrine System, Slominski AT, et al. , Editors. Adv Anat Embryol Cell Biol, 2012. 212(v–115): p. 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peciña M, et al. , Endogenous opioid system dysregulation in depression: implications for new therapeutic approaches. Molecular Psychiatry, 2019. 24(4): p. 576–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujioka N, et al. , Morphine-induced epidermal growth factor pathway activation in non-small cell lung cancer. Anesth Analg, 2011. 113(6): p. 1353–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madar I, et al. , Imaging delta- and mu-opioid receptors by PET in lung carcinoma patients. J Nucl Med, 2007. 48(2): p. 207–213. [PubMed] [Google Scholar]
- 16.Mathew B, et al. , The novel role of the mu opioid receptor in lung cancer progression: a laboratory investigation. Anesth Analg, 2011. 112(3): p. 558–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zylla D, et al. , Opioid requirement, opioid receptor expression, and clinical outcomes in patients with advanced prostate cancer. Cancer, 2013. 119(23): p. 4103–4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farooqui M, et al. , Naloxone acts as an antagonist of estrogen receptor activity in MCF-7 cells. Molecular Cancer Therapeutics, 2006. 5(3): p. 611–620. [DOI] [PubMed] [Google Scholar]
- 19.Lennon FE, et al. , Overexpression of the mu-opioid receptor in human non-small cell lung cancer promotes Akt and mTOR activation, tumor growth, and metastasis. Anesthesiology, 2012. 116(4): p. 857–67. [DOI] [PubMed] [Google Scholar]
- 20.Singleton PA, et al. , Increased mu-opioid receptor expression in metastatic lung cancer. Br J Anaesth, 2014. 113 Suppl 1: p. i103–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janku F, et al. , Treatment with methylnaltrexone is associated with increased survival in patients with advanced cancer. Ann Oncol, 2016. 27(11): p. 2032–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen C, Farooqui M, and Gupta K, Morphine stimulates vascular endothelial growth factor-like signaling in mouse retinal endothelial cells. Curr Neurovasc Res, 2006. 3(3): p. 171–80. [DOI] [PubMed] [Google Scholar]
- 23.Gupta K, et al. , Morphine stimulates angiogenesis by activating proangiogenic and survival-promoting signaling and promotes breast tumor growth. Cancer Res, 2002. 62(15): p. 4491–4498. [PubMed] [Google Scholar]
- 24.Farooqui M, et al. , COX-2 inhibitor celecoxib prevents chronic morphine-induced promotion of angiogenesis, tumour growth, metastasis and mortality, without compromising analgesia. Br J Cancer, 2007. 97(11): p. 1523–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen J, et al. , Morphine stimulates cancer progression and mast cell activation and impairs survival in transgenic mice with breast cancer. British Journal of Anaesthesia, 2014. 113(Suppl 1): p. i4–i13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zylla D, et al. , Association of opioid requirement and cancer pain with survival in advanced non-small cell lung cancer. Br J Anaesth, 2014. 113(Suppl 1): p. i109–i116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maroulakou IG, et al. , Prostate and mammary adenocarcinoma in transgenic mice carrying a rat C3(1) simian virus 40 large tumor antigen fusion gene. Proc Natl Acad Sci U S A, 1994. 91(23): p. 11236–11240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aprelikova O, et al. , Development and Preclinical Application of an Immunocompetent Transplant Model of Basal Breast Cancer with Lung, Liver and Brain Metastases. PLoS One, 2016. 11(5): e0155262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoenerhoff MJ, et al. , Pathologic progression of mammary carcinomas in a C3(1)/SV40 T/t-antigen transgenic rat model of human triple-negative and Her2-positive breast cancer. Transgenic Res, 2011. 20(2): p. 247–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deeb KK, et al. , Identification of an integrated SV40 T/t-antigen cancer signature in aggressive human breast, prostate, and lung carcinomas with poor prognosis. Cancer Res, 2007. 67(17): p. 8065–8080. [DOI] [PubMed] [Google Scholar]
- 31.Green JE, et al. , The C3(1)/SV40 T-antigen transgenic mouse model of mammary cancer: ductal epithelial cell targeting with multistage progression to carcinoma. Oncogene, 2000. 19(8): p. 1020–7. [DOI] [PubMed] [Google Scholar]
- 32.Luk K, et al. , Influence of Morphine on Pericyte-Endothelial Interaction: Implications for Antiangiogenic Therapy. Journal of Oncology, 2012. 2012: 458385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chavez KJ, Garimella SV, and Lipkowitz S, Triple negative breast cancer cell lines: One tool in the search for better treatment of triple negative breast cancer. Breast Disease, 2011. 32: p. 35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu H, et al. , PPARγ Ligands and ATRA Inhibit the Invasion of Human Breast Cancer Cells in vitro. Breast Cancer Research and Treatment, 2003. 79(1): p. 63–74. [DOI] [PubMed] [Google Scholar]
- 35.Kohli DR, et al. , Pain-related behaviors and neurochemical alterations in mice expressing sickle hemoglobin: modulation by cannabinoids. Blood, 2010. 116(3): p. 456–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lei J, et al. , Comparative Analysis of Pain Behaviours in Humanized Mouse Models of Sickle Cell Anemia. PLoS One, 2016. 11(8): e0160608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McAuliffe PF, et al. , Deciphering the role of PI3K/Akt/mTOR pathway in breast cancer biology and pathogenesis. Clin Breast Cancer, 2010. 10 Suppl 3: p. S59–65. [DOI] [PubMed] [Google Scholar]
- 38.Laplante M and Sabatini DM, mTOR signaling in growth control and disease. Cell, 2012. 149(2): p. 274–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fruman DA, et al. , The PI3K Pathway in Human Disease. Cell, 2017. 170(4): p. 605–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ortega MA, et al. , Signal Transduction Pathways in Breast Cancer: The Important Role of PI3K/Akt/mTOR. J Oncol, 2020. 2020: 9258396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rahmani F, et al. , Role of regulatory miRNAs of the PI3K/AKT signaling pathway in the pathogenesis of breast cancer. Gene, 2020. 737: 144459. [DOI] [PubMed] [Google Scholar]
- 42.Xie N, et al. , Effect of Perioperative Opioids on Cancer-Relevant Circulating Parameters: Mu Opioid Receptor and Toll-Like Receptor 4 Activation Potential, and Proteolytic Profile. Clin Cancer Res, 2018. 24(10): p. 2319–2327. [DOI] [PubMed] [Google Scholar]
- 43.Moss J, Identifying and Treating Opioid Side Effects: The Development of Methylnaltrexone. Anesthesiology, 2019. 130(1): p. 142–148. [DOI] [PubMed] [Google Scholar]
- 44.Singleton PA, et al. , The mu opioid receptor: A new target for cancer therapy? Cancer, 2015. 121(16): p. 2681–2688. [DOI] [PubMed] [Google Scholar]
- 45.Bagley EE and Ingram SL, Endogenous opioid peptides in the descending pain modulatory circuit. Neuropharmacology, 2020. 173: 108131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Celik M, et al. , IL-4 induces M2 macrophages to produce sustained analgesia via opioids. JCI Insight, 2020. 5(4): e133093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scheff NN, et al. , Neutrophil-Mediated Endogenous Analgesia Contributes to Sex Differences in Oral Cancer Pain. Frontiers in integrative neuroscience, 2018. 12: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stein C, et al. , Opioids from immunocytes interact with receptors on sensory nerves to inhibit nociception in inflammation. Proceedings of the National Academy of Sciences, 1990. 87(15): p. 5935–5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith EM, Opioid peptides in immune cells. Advances in experimental medicine and biology, 2003. 521: p. 51. [PubMed] [Google Scholar]
- 50.Scheff NN, et al. , Granulocyte-Colony Stimulating Factor-Induced Neutrophil Recruitment Provides Opioid-Mediated Endogenous Anti-nociception in Female Mice With Oral Squamous Cell Carcinoma. Frontiers in Molecular Neuroscience, 2019. 12: 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gao F, et al. , Signaling Mechanism of Cannabinoid Receptor-2 Activation-Induced β-Endorphin Release. Mol Neurobiol, 2016. 53(6): p. 3616–3625. [DOI] [PubMed] [Google Scholar]
- 52.Apryani E, et al. , The spinal microglial IL-10/β-endorphin pathway accounts for cinobufagin-induced mechanical antiallodynia in bone cancer pain following activation of α7-nicotinic acetylcholine receptors. Journal of neuroinflammation, 2020. 17(1): 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Veening JG and Barendregt HP, The effects of beta-endorphin: state change modification. Fluids and barriers of the CNS, 2015. 12: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chong D, et al. , Correlations of cancer pain degree with levels of β-EP, CGRP and PGE2 and the effects of oxycontin on them. Age (years). 60(44): p. 46–32. [PubMed] [Google Scholar]
- 55.Bruehl S, et al. , Endogenous opioid inhibition of chronic low-back pain influences degree of back pain relief after morphine administration. Regional anesthesia and pain medicine, 2014. 39(2): p. 120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Millan MJ, Descending control of pain. Prog Neurobiol, 2002. 66(6): p. 355–474. [DOI] [PubMed] [Google Scholar]
- 57.Muñoz M and Coveñas R, Involvement of substance P and the NK-1 receptor in cancer progression. Peptides, 2013. 48: p. 1–9. [DOI] [PubMed] [Google Scholar]
- 58.Muñoz M, Rosso M, and Coveñas R, Triple Negative Breast Cancer: How Neurokinin-1 Receptor Antagonists Could Be Used as a New Therapeutic Approach. Mini Rev Med Chem, 2019. 20(5): p. 408–417. [DOI] [PubMed] [Google Scholar]
- 59.Ahn H, et al. , The Relationship Between β-Endorphin and Experimental Pain Sensitivity in Older Adults With Knee Osteoarthritis. Biol Res Nurs, 2019. 21(4): p. 400–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mogil JS, Qualitative sex differences in pain processing: emerging evidence of a biased literature. Nature Reviews Neuroscience, 2020. 21(7): p. 353–365. [DOI] [PubMed] [Google Scholar]
- 61.Dao TTT and LeResche L, Gender differences in pain. Journal of orofacial pain, 2000. 14(3): p. 169–195. [PubMed] [Google Scholar]
- 62.Grace PM, et al. , Pathological pain and the neuroimmune interface. Nature Reviews Immunology, 2014. 14(4): p. 217–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mogil JS, et al. , Sex differences in the antagonism of swim stress-induced analgesia: effects of gonadectomy and estrogen replacement. Pain, 1993. 53(1): p. 17–25. [DOI] [PubMed] [Google Scholar]
- 64.Long CC, Sadler KE, and Kolber BJ, Hormonal and molecular effects of restraint stress on formalin-induced pain-like behavior in male and female mice. Physiology & Behavior, 2016. 165: p. 278–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tershner SA, Mitchell JM, and Fields HL, Brainstem pain modulating circuitry is sexually dimorphic with respect to mu and kappa opioid receptor function. Pain, 2000. 85(1-2): p. 153–159. [DOI] [PubMed] [Google Scholar]
- 66.Loyd DR, Wang X, and Murphy AZ, Sex differences in micro-opioid receptor expression in the rat midbrain periaqueductal gray are essential for eliciting sex differences in morphine analgesia. The Journal of neuroscience : the official journal of the Society for Neuroscience, 2008. 28(52): p. 14007–14017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lin X, et al. , POMC maintains tumor-initiating properties of tumor tissue-derived long-term-cultured breast cancer stem cells. International Journal of Cancer, 2017. 140(11): p. 2517–2525. [DOI] [PubMed] [Google Scholar]
- 68.Kondo D, Saegusa H, and Tanabe T, Involvement of phosphatidylinositol-3 kinase/Akt/mammalian target of rapamycin/peroxisome proliferator-activated receptor γ pathway for induction and maintenance of neuropathic pain. Biochem Biophys Res Commun, 2018. 499(2): p. 253–259. [DOI] [PubMed] [Google Scholar]
- 69.Choi S, et al. , mTOR signaling intervention by Torin1 and XL388 in the insular cortex alleviates neuropathic pain. Neurosci Lett, 2020. 718: 134742. [DOI] [PubMed] [Google Scholar]
- 70.Xing X, et al. , Hyperactive Akt-mTOR pathway as a therapeutic target for pain hypersensitivity in Cntnap2-deficient mice. Neuropharmacology, 2020. 165: 107816. [DOI] [PubMed] [Google Scholar]
- 71.Kim K, et al. , Effects of mTOR inhibitors on neuropathic pain revealed by optical imaging of the insular cortex in rats. Brain Res, 2020. 1733: 146720. [DOI] [PubMed] [Google Scholar]
- 72.Liang L, et al. , mTOR and its downstream pathway are activated in the dorsal root ganglion and spinal cord after peripheral inflammation, but not after nerve injury. Brain Res, 2013. 1513: p. 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu JT, et al. , Expression and distribution of mTOR, p70S6K, 4E-BP1, and their phosphorylated counterparts in rat dorsal root ganglion and spinal cord dorsal horn. Brain Res, 2010. 1336: p. 46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu JT, et al. , Intrathecal rapamycin attenuates morphine-induced analgesic tolerance and hyperalgesia in rats with neuropathic pain. Transl Perioper Pain Med, 2015. 2(2): p. 27–34. [PMC free article] [PubMed] [Google Scholar]
- 75.Ji RR, et al. , ERK MAP kinase activation in superficial spinal cord neurons induces prodynorphin and NK-1 upregulation and contributes to persistent inflammatory pain hypersensitivity. J Neurosci, 2002. 22(2): p. 478–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shi X, et al. , Keratinocytes express cytokines and nerve growth factor in response to neuropeptide activation of the ERK1/2 and JNK MAPK transcription pathways. Regul Pept, 2013. 186: p. 92–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liang L, et al. , Protein kinase B/Akt is required for complete Freund's adjuvant-induced upregulation of Nav1.7 and Nav1.8 in primary sensory neurons. J Pain, 2013. 14(6): p. 638–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Meijuan Y, et al. , A retrospective study of postmastectomy pain syndrome: incidence, characteristics, risk factors, and influence on quality of life. ScientificWorldJournal, 2013. 2013: 159732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Latremoliere A and Woolf CJ, Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain, 2009. 10(9): p. 895–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Baron R, Binder A, and Wasner G, Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol, 2010. 9(8): p. 807–819. [DOI] [PubMed] [Google Scholar]
- 81.Pernow B, Substance P--a putative mediator of antidromic vasodilation. Gen Pharmacol, 1983. 14(1): p. 13–16. [DOI] [PubMed] [Google Scholar]
- 82.White DM, Release of substance P from peripheral sensory nerve terminals. J Peripher Nerv Syst, 1997. 2(3): p. 191–201. [PubMed] [Google Scholar]
- 83.Bruce AN, vaso-dilator axon-reflexes. Quarterly Journal of Experimental Physiology, 1913. 6(4): p. 339–354. [Google Scholar]
- 84.Jancsó G, et al. , Sensory Nerves as Modulators of Cutaneous Inflammatory Reactions in Health and Disease, in NeuroImmune Biology. NeurImmune Biology, 2009. 8(Suppl): p. 1–36. [Google Scholar]
- 85.Sousa-Valente J and Brain SD, A historical perspective on the role of sensory nerves in neurogenic inflammation. Semin Immunopathol, 2018. 40(3): p. 229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Naloxegol (Movantik) for Opioid-Induced Constipation. JAMA, 2016. 315(2): p. 194–195. [DOI] [PubMed] [Google Scholar]
- 87.Cata JP, et al. , Intraoperative opioids use for laryngeal squamous cell carcinoma surgery and recurrence: a retrospective study. J Clin Anesth, 2015. 27(8): p. 672–679. [DOI] [PubMed] [Google Scholar]
- 88.Shoffel-Havakuk H, et al. , Intravenous opioid drug abuse as an independent risk factor for supraglottic squamous cell carcinoma-A case-control study. Clin Otolaryngol, 2018. 43(2): p. 456–462. [DOI] [PubMed] [Google Scholar]
- 89.Chen DT, et al. , The mu-opioid receptor is a molecular marker for poor prognosis in hepatocellular carcinoma and represents a potential therapeutic target. Br J Anaesth, 2019. 122(6): p. e157–e167. [DOI] [PubMed] [Google Scholar]
- 90.Carli M, et al. , Opioid receptors beyond pain control: The role in cancer pathology and the debated importance of their pharmacological modulation. Pharmacol Res, 2020. 159: 104938. [DOI] [PubMed] [Google Scholar]
- 91.Suzuki M, et al. , Peripheral opioid antagonist enhances the effect of anti-tumor drug by blocking a cell growth-suppressive pathway in vivo. PLoS One, 2015. 10(4): e0123407. [DOI] [PMC free article] [PubMed] [Google Scholar]