To the Editor:
Spain experienced a Coronavirus Disease 2019 (COVID-19)–related confinement for 14 weeks (March 14 to June 21, 2020) affecting all citizens irrespective of age, interrupting any kind of supervised physical activity programs for the older adults. This physical inactivity period could lead, among other complications, to disuse atrophy, functional decline, muscle wasting, and disability, which are all associated with longer hospitalization periods and a worse rehabilitation.1 More so than ever, implementing structured and supervised exercise programs for older adults is critical to improve/maintain the health status of patients at risk of COVID-19 and alleviate the consequences of this pandemic.1, 2, 3, 4, 5 In an attempt to improve physical and functional capacity, we recently developed the Vivifrail multicomponent tailored exercise program (www.vivifrail.com) to focus on providing training to older adults, and to design strategies to promote and prescribe such tailored physical exercise.6 , 7 We assessed the impact of a 4-week multicomponent, tailored exercise program on functional capacity and muscle strength in sarcopenic older adults residing in nursing homes after a 14-week COVID-19 confinement. We also compared the functional status of those who stopped the exercise program in the following 14 weeks with those who continued with exercise training for a similar period.
This is a randomized trial on sarcopenic older adults aged ≥75 years living in nursing homes (Supplementary Table 1). Participants (n = 24) completed 4 weeks of the tailored multicomponent exercise training program Vivifrail (www.vivifrail.com).6 , 7 One group (training, n = 12) continued the intervention for a further 14 weeks, whereas the other (confinement, n = 12) interrupted the intervention for 14 weeks because of the COVID-19 lockdown. Sarcopenia was determined according to the Foundation for the National Institutes of Health algorithm.8 Functional capacity and strength were evaluated at baseline, after 4 weeks of exercise, and after 14 weeks of training or detraining. This study is part of an ongoing multicenter trial (NCT03827499).9
Participants enrolled into one of the individualized Vivifrail training programs according to their frailty level: Disability (A), Frailty (B), Pre-frailty (C), and Robust (D). Exercise regimen and weight load were set according to the Vivifrail prescription guidelines (http://vivifrail.com/resources/). Each program combined individualized regimens of strength, power, balance, walking, stretching, and cardiovascular exercises. Functional capacity was measured using the Short Physical Performance Battery (SPPB) test scores (from 1 to 12 points), depending on performance in (1) gait speed 6 m, (2) 5-sit-to-stand test, (3) balance, and (4) timed up-and-go tests. Handgrip strength and sit-to-stand velocity (with a linear transducer) were also examined. A paired t-test was used to detect within-group differences along the time periods. Analysis of covariance was conducted to determine whether changes were different between the groups at each period after controlling for baseline scores. Effect sizes (ES) were computed by Cohen's d.
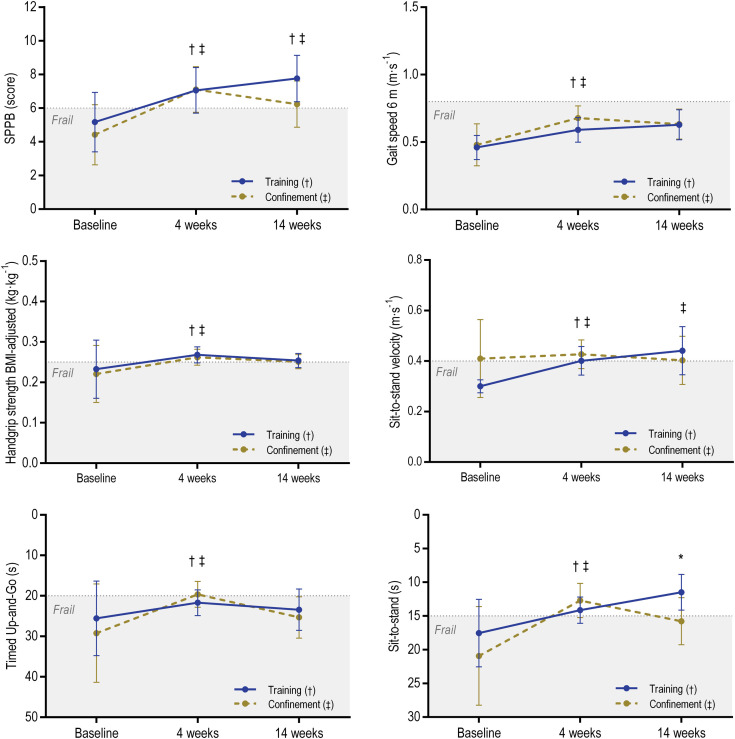
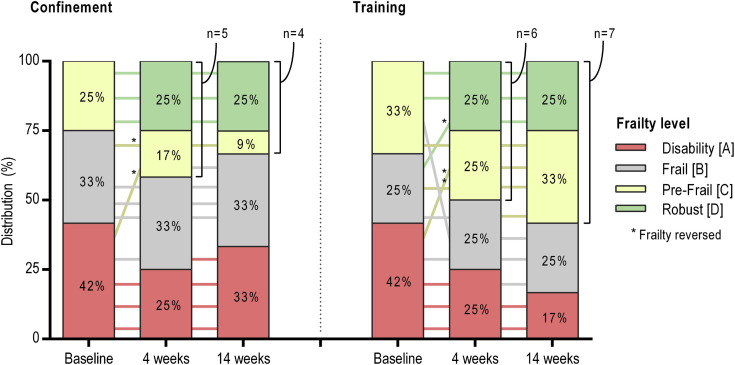
Postintervention changes are presented in Figure 1 and Supplementary Table 2. After 4 weeks of training, both groups significantly improved in all tests (P values from .001 to .042; ES from 0.33 to 1.08). After 14 weeks of further training, only the SPPB (P = .021, ES = 0.23) and the sit-to-stand velocity tests (P = .008, ES = 0.60) increased significantly. After 14 weeks of confinement, although the SPPB decreased (P = .034, ES = 0.24), participants maintained a better physical condition compared with baseline. Frailty status was reversed in 21% of participants after the 4-week exercise intervention, with 46% achieving high self- autonomy (C and D levels) (Supplementary Figure 1).
Fig. 1.
Changes in physical functional capacity and strength. Data are means and 95% confidence intervals adjusted for baseline values. Dotted line represents the cutoff points for frailty based on the literature.7,10 ∗Significant between-group differences (hierarchical analysis of covariance P < .05). †Significant time difference in Training group (paired t-test P < .05). ‡Significant time difference in Confinement group (paired t-test P < .05). BMI, body mass index.
Supplementary Fig. 1.
Changes in the frailty level according to the Vivifrail classification (http://vivifrail.com/resources/). Lines are the evolution of each participant across the timepoints. Frailty is considered reversed when upgrading from A or B to C or D levels.
The main findings are as follows: (1) The Vivifrail multicomponent tailored exercise program was very effective in the short-term (4 weeks) and produced a similar response to training in 2 groups of sarcopenic, frail, and institutionalized adults aged ≥75 years from 2 different nursing homes. This uniform improvement demonstrates the robustness of the Vivifrail tailored prescription guidelines (http://vivifrail.com/resources/); (2) short-term health improvements after 4 weeks of Vivifrail seemed to persist after 14 weeks of inactivity due to COVID-19 confinement, and may have prevented severe functional decline and strength loss in institutionalized older adults; (3) although overall functional capacity and strength declined along the 14-week confinement, the benefits of the previous exercise training persisted, with older adults in a better physical condition as compared with baseline; (4) frailty reversion (ie, recovery of autonomy) after 4 weeks of exercise was mostly maintained during the 14-week training cessation period. Overall, these results support the positive impact of acute exercise interventions in a sarcopenic and frail population. It would seem advisable to introduce face-to-face, multicomponent exercise programs into nursing homes and long-term care facilities11 as an essential activity to protect older adults from severe functional decline as a consequence of strict confinement conditions.
Acknowledgments
The authors are grateful to the health professionals and participants for their involvement in this study.
Footnotes
The authors declare no conflicts of interest.
This work was supported by the Autonomous Community of the Region of Murcia, Regional Program for the Promotion of Scientific and Technical Research (Action Plan 2018), Seneca Foundation-Agency of Science and Technology, Region of Murcia (ID: 20872/PI/18). Mikel Izquierdo was supported by a research grant PI17/01814 of the Ministerio de Economía, Industria y Competitividad (ISCIII, FEDER).
Supplementary Data
Supplementary Table 1.
Baseline Sample Characteristics of the Study Participations
| Variable | Confinement | Training | P value |
|---|---|---|---|
| Age (y) | 87.3 (7.7) | 87.2 (6.5) | .98 |
| Weight (kg) | 72.4 (11.5) | 65.3 (12.6) | .16 |
| BMI (kg·m−2) | 28.8 (3.5) | 28 (3.9) | .61 |
| BMD (g·cm−2) | 1.08 (0.16) | 1.06 (0.13) | .76 |
| Fat (%) | 37.6 (8.4) | 41.4 (8.3) | .28 |
| Lean mass (kg) | 39.8 (7.6) | 36 (6.3) | .19 |
| MNA (score) | 17.9 (3.8) | 18.8 (6.0) | .67 |
| SARC-F (score) | 4.5 (2.0) | 5.2 (3.0) | .69 |
| Barthel (score) | 66.7 (29.3) | 72.5 (24.6) | .61 |
| Lawton (score) | 2.1 (1.2) | 4.3 (5.6) | .23 |
| FES-I (score) | 12.8 (2.9) | 13.4 (7.2) | .82 |
| MMSE (score) | 25.9 (5.7) | 23.3 (7.2) | .34 |
| Yesavage (score) | 3.5 (3.6) | 5.1 (2.2) | .21 |
| SPPB (score) | 4.4 (2.8) | 5.2 (2.8) | .84 |
| Timed Up-and-Go (s) | 29.2 (18.1) | 25.6 (13.7) | .52 |
| Gait speed 6 m (m·s−1) | 0.48 (0.22) | 0.46 (0.14) | .79 |
| Sit-to-stand (s) | 20.9 (7.9) | 17.5 (7.0) | .76 |
| Sit-to-stand MPV (m·s−1) | 0.41 (0.15) | 0.30 (0.03) | .15 |
| Handgrip (kg) | 16.3 (8.4) | 15.2 (6.6) | .71 |
| Handgrip/BMI (kg·kg−1) | 0.22 (0.11) | 0.23 (0.11) | .80 |
BMD, bone mineral density; BMI, body mass index; FES-I, Falls Efficacy Scale International; MMSE, Mini Mental State Evaluation; MNA, Mini Nutritional Assessment; MPV, mean propulsive velocity; SARC-F, strength, assistance walking, rise from a chair, climb stairs, and falls.
Data are mean (SD).
Supplementary Table 2.
Changes in Functional Capacity and Strength in Response to the Training Interventions
| Variable | Group | n | Baseline [T0] vs. 4-week Training [T1] |
4-week Training [T1] vs.14-week Training/Detraining [T2] |
||||
|---|---|---|---|---|---|---|---|---|
| Change (95% CI) | P Value | ES | Change (95% CI) | P Value | ES | |||
| SPPB (score) | Confinement | 12 | 2.3 (0.8 to 3.8) | .006∗ | 0.71 | −.91 (−.08 to −1.7) | .034∗ | 0.24 |
| Training | 12 | 2.2 (3.3 to 1.1) | .001∗ | 0.78 | .74 (.13 to 1.4) | .021∗ | 0.23 | |
| Timed Up-and-Go (s) | Confinement | 11 | −8.6 (−1.6 to −15.5) | .021∗ | 0.59 | 6.2 (−.93 to 13.3) | .08 | 0.41 |
| Training | 11 | −4.8 (−.21 to −9.5) | .042∗ | 0.42 | 1.1 (−1.5 to 3.9) | .36 | 0.13 | |
| Gait speed 6 m (m·s−1) | Confinement | 11 | .21 (.11 to .32) | .001∗ | 0.76 | −.04 (−.14 to .05) | .34 | 0.14 |
| Training | 11 | .11 (.01 to .21) | .023∗ | 0.60 | .03 (−.05 to .12) | .36 | 0.13 | |
| Sit-to-stand (s) | Confinement | 7 | −7.0 (−3.6 to −10.4) | .002∗ | 1.08 | 2.8 (−1.1 to 6.7) | .13 | 0.45 |
| Training | 10 | −4.2 (−1.4 to 7.1) | .008∗ | 0.68 | −2.4 (−5.1 to .01) | .07 | 0.58 | |
| Sit-to-stand velocity† (m·s−1) | Confinement | 6 | .07 (.01 to .12) | .019∗ | 0.43 | −.02 (−.01 to .05) | .44 | 0.18 |
| Training | 6 | .05 (.01 to .11) | .037∗ | 0.91 | .04 (.01 to .06) | .008∗ | 0.60 | |
| Handgrip strength (kg) | Confinement | 12 | 2.7 (1.4 to 4.0) | .001∗ | 0.32 | −.91 (−2.1 to .22) | .10 | 0.11 |
| Training | 12 | 2.4 (.92 to 3.9) | .005∗ | 0.38 | −1.1 (−2.3 to .15) | .08 | 0.17 | |
| Handgrip/BMI (kg·kg−1) | Confinement | 12 | .03 (.01 to .05) | .001∗ | 0.33 | −.01 (−.01 to −.02) | .13 | 0.10 |
| Training | 12 | .04 (.01 to .06) | .002∗ | 0.36 | −.01 (−.01 to −.03) | .13 | 0.12 | |
BMI, body mass index; CI, confidence interval.
Significant differences (paired t-test P < .05).
Mean propulsive velocity measured with a linear transducer. From T0 to T1, both groups completed the same multicomponent exercise program; then, from T1 to T2 “Confinement” group interrupted the exercise program while “Training” maintained it.
References
- 1.Valenzuela P., Castillo-García A., Morales J.S. Physical exercise in the oldest old. Compr Physiol. 2019;9:1281–1304. doi: 10.1002/cphy.c190002. [DOI] [PubMed] [Google Scholar]
- 2.Cadore E.L., Casas-Herrero A., Zambom-Ferraresi F. Multicomponent exercises including muscle power training enhance muscle mass, power output, and functional outcomes in institutionalized frail nonagenarians. Age (Dordr) 2014;36:773–785. doi: 10.1007/s11357-013-9586-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.García-Hermoso A., Ramirez-Vélez R., Sáez de Asteasu M.L. Safety and effectiveness of long-term exercise interventions in older adults: A systematic review and meta-analysis of randomized controlled trials. Sport Med. 2020;50:1095–1106. doi: 10.1007/s40279-020-01259-y. [DOI] [PubMed] [Google Scholar]
- 4.Sáez de Asteasu M.L., Martínez-Velilla N., Zambom-Ferraresi F. Changes in muscle power after usual care or early structured exercise intervention in acutely hospitalized older adults. J Cachexia Sarcopenia Muscle. 2020;11:997–1106. doi: 10.1002/jcsm.12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martínez-Velilla N., Casas-Herrero A., Zambom-Ferraresi F. Effect of exercise intervention on functional decline in very elderly patients during acute hospitalization: A randomized clinical trial. JAMA Intern Med. 2019;179:28–36. doi: 10.1001/jamainternmed.2018.4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Izquierdo M., Rodriguez-Mañas L., Sinclair A.J., Vivifrail Investigators Group What is new in exercise regimes for frail older people—How does the Erasmus Vivifrail Project take us forward? J Nutr Heal Aging. 2016;20:736–737. doi: 10.1007/s12603-016-0702-5. [DOI] [PubMed] [Google Scholar]
- 7.Izquierdo M., Casas-Herrero A., Zambm-Ferraresi F. Multicomponent physical exercise program Vivifrail. A practical guide for prescribing a multicomponent physical training program to prevent weakness and falls in people over 70. 2017. http://vivifrail.com/wp-content/uploads/2019/11/VIVIFRAIL-ENG-Interactivo.pdf Available at:
- 8.Studenski S.A., Peters K.W., Alley D.E. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Bio Sci Med Sci. 2014;69:547–558. doi: 10.1093/gerona/glu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Courel-Ibáñez J., Pallarés J.G. Effects of β-hydroxy-β-methylbutyrate (HMB) supplementation in addition to multicomponent exercise in adults older than 70 years living in nursing homes, a cluster randomized placebo-controlled trial: the HEAL study protocol. BMC Geriatr. 2019;19:188. doi: 10.1186/s12877-019-1200-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramírez-Vélez R., Correa-Bautista J.E., García-Hermoso A. Reference values for handgrip strength and their association with intrinsic capacity domains among older adults. J Cachexia Sarcopenia Muscle. 2019;10:278–286. doi: 10.1002/jcsm.12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Izquierdo M., Morley J.E., Lucia A. Exercise in people over 85. BMJ. 2020;368:m402. doi: 10.1136/bmj.m402. [DOI] [PubMed] [Google Scholar]