Abstract
Background
Characteristics of critical Severe Acute Respiratory Syndrome-related Coronavirus 2 (SARS-CoV-2) infection in children is not well understood. This study described the clinical characteristics of children admitted to intensive care units (ICU) and explored factors associated with the need for invasive ventilation or mortality.
Methods
A multicenter, retrospective, cohort study was conducted over eight medical centers, including all patients younger than 18 years of age and admitted to the ICU due to a direct consequence of coronavirus disease 2019 (COVID-19). Patients who were admitted to the ICU for any alternate reason and tested positive for SARS-CoV-2 by screening test, and patients who were admitted due to multi-inflammatory syndrome in children, were excluded. Demographic, laboratory, imaging, and clinical data were collected. Descriptive statistics were used to compare survivors and non-survivors. Fine and Gray’s hazard model was used to estimate the association between clinical variables and ICU death.
Results
During the study period, 25 pediatric COVID-19 patients received care in the ICUs. The median age was 2.78 years (IQR 0.21–8.51), and 60% were male. Only three patients were reported to be previously healthy at admission. Nine (36%) patients required invasive mechanical ventilation, including two were on extracorporeal membrane oxygenation. Four (16%) patients died during ICU care. In univariate analysis, the presence of comorbidity (HR 0.0001; 95%CI 0.00001–0.00016), platelets count (HR 0.99; 95% CI 0.98–0.99), elevated procalcitonin (HR 1.05; 95%CI 1.016–1.09), and circulatory compromise (HR 16.34; 95%CI 1.99–134.35), all at the time of ICU admission, were associated with in-ICU mortality.
Conclusion
Our findings suggest that children admitted to the ICU with SARS-CoV-2 infection, generally, have a favorable outcome. Low platelets count, elevated procalcitonin, presence of comorbidity, and shock at the time of ICU admission were associated with death. This study may shed more light on the disease dynamics of critical pediatric COVID-19.
Keywords: Children, Intensive care, SARS-CoV-2, Mortality
Introduction
Severe Acute Respiratory Syndrome-related Coronavirus 2 (SARS-CoV-2) was first recognized in December 2019. Since then, it has caused a pandemic of respiratory illness, coronavirus disease 2019 (COVID-19), which causes a wide range of illness severity, including acute respiratory distress syndrome (ARDS) and respiratory failure. In older adults, SARS-CoV-2 infection is associated with high morbidity and mortality. About one-third of hospitalized adults require care in the intensive care unit (ICU) [1,2].
Children, in general, develop milder COVID-19 compared to adults [3]. National-level studies showed that pediatric COVID-19 cases represent less than 8% of all confirmed cases [[3], [4], [5]]. Among infected children, the proportion with severe or critical illness has ranged between 3.3%–8% [[6], [7], [8]]. Due to the relative rarity of pediatric critical SARS-CoV-2 infection, the exact proportion of pediatric COVID-19 cases needing care in an ICU and estimates of case fatality rate in children are difficult to ascertain. In a large European multicenter study that included 582 SARS-CoV-2-infected children, 8% required ICU admission, 4% were mechanically ventilated, and four patients died [8]. The Centers for Disease Control and Prevention (CDC) reported 2572 cases of COVID-19 in children, of which 15 were admitted to the ICU, and three died; however, hospitalization status was missing in 71% of subjects [9].
Risk factors for developing a critical disease in children are still not well understood. A mathematical model by Pathak et al. estimated that 0.6% of the detected pediatric COVID-19 cases would need ICU care [10]. The highest estimates were observed in children younger than one year of age [10,11]. Among 345 confirmed COVID-19 pediatric cases for whom data on underlying conditions were not missing, the most commonly reported underlying conditions were chronic lung disease (11.6%), cardiovascular disease (7.2%), and immunosuppression (2.9%) [9]. In a multicenter study that collected data from North American pediatric ICUs, 48 pediatric (<21 years) patients required ICU admission. Of those, 40 were admitted as a direct consequence of SARS-CoV-2, and two died [12].
A collaboration between eight pediatric intensive care units in Kuwait and the Kingdom of Saudi Arabia (KSA) was established to better understand the dynamics of critical pediatric COVID-19 and to improve the quality of care to those patients. Our objective was to describe the clinical characteristics, disease dynamics, and outcomes of critical pediatric COVID-19 cases admitted to one of the participating centers. Moreover, we evaluated predictors of mortality and intubation in this cohort of patients.
Materials and methods
A retrospective cohort, multicenter study was conducted in eight pediatric centers across Kuwait and the Kingdom of Saudi Arabia (KSA) between March 1st and August 1st, 2020. All centers combined provide medical service to around 12 million people. We included children aged less than 18 years with symptomatic newly diagnosed SARS-CoV-2 infection that leads to ICU admission. On the other hand, excluded subjects were patients with asymptomatic SARS-CoV-2 infection detected during a screening PCR test upon ICU admission, and patients who were admitted due to post-infectious complications of COVID-19, including multisystem inflammatory syndrome in children (MIS-C) [13].
Patients were identified through querying admission and discharge records from ICUs in six participating centers from Kuwait (Adan Hospital, Farwaniya Hospital, Jaber Al-Ahmad Hospital, Jahra hospital, Mubarak Al-Kabeer Hospital, and Sabah Medical Specialized Area), representing all pediatric ICUs in Kuwait, and two from KSA (Prince Sultan Military Medical City and King Saud University Medical City). As the pediatric age group differed between centers, records from both adult and pediatric ICUs were reviewed to ensure the inclusion of all patients less than 18 years of age. Ethics board approval from the Ministry of Health of Kuwait and each respective center in KSA were obtained.
Medical charts were reviewed to obtain demographic data, pre-existing comorbidities, clinical and laboratory information related to COVID-19 admission. Information also included the clinical course in relation to the date of admission and discharge, symptoms at admission, maximum respiratory support (Low-flow oxygen therapy, non-invasive ventilation including high flow nasal cannula, mechanical ventilation, and extracorporeal membrane oxygenation), the reason for respiratory support, nature of organ dysfunction, therapies received (antimicrobial, inotropic, bronchodilators, immunomodulatory therapies, and blood product transfusion). Patient outcome at discharge was recorded and categorized as full recovery, discharge with sequela, or death.
Disease severity was evaluated based on the classification suggested by the World Health Organization (WHO) [14]. Clinical and laboratory data required for the Pediatric Risk of Mortality Score (PRISM III) were also collected [15]. Organ dysfunction (cardiovascular, respiratory, neurological, hematological, renal, and hepatic dysfunction) was defined based on previously published consensus [16]. Physiological variables related to the pediatric risk of mortality score (PRISM) that occurred between two hours prior to ICU admission to four hours after were collected [15,17]. Reasons for ICU admission were categorized into respiratory failure, neurological disorder, or circulatory compromise. Patients were considered to have respiratory failure if they presented with acute hypoxemia, hypercarbia, or clinical respiratory distress requiring non-invasive or invasive respiratory support. Neurological disorder referred to any acute change of mental status or new neurological symptom not explained by low perfusion status or hypoxemia. Circulatory compromise is considered if the patient is presented with impaired perfusion, hypotension, or if started on vasoactive medication [18].
Statistical analysis was performed using R version 3.6.3. Counts and percentages were used to summarize categorical variables. Continuous variables were summarized using mean ± standard deviation (SD) or median and interquartile range (IQR) for normal and non-normal variables, respectively. Chi-square and Fisher-exact tests were used to assess the association between categorical clinical or laboratory parameters and study outcomes (survival or mechanical ventilation). Mann-Whitney test and unpaired t-test were used to determine the association of continuous non-normal and normal variables, respectively, with the study outcomes.
Fine and Gray's sub-distribution hazard model was used to assess the univariate association between clinical or laboratory data and mortality in the ICU. Survival, in days, was calculated as the time from ICU admission to in-ICU death, ICU discharge, or last follow-up date if the patient was not discharged from the ICU by the end of follow-up. Discharge from the ICU (alive) was considered a competing risk. Univariate analysis p-values were corrected for false discovery rate to avoid the inflation of the type I error. Multivariate analysis was not attempted due to the small sample size. Hypothesis testing was performed at 5% level of significance.
Results
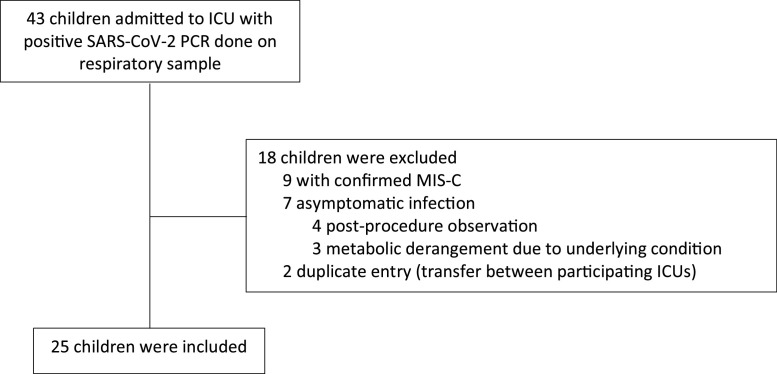
During the study period, 43 pediatric patients were admitted to the ICU with positive SARS-CoV-2 molecular test. Of those, 25 children with acute COVID-19, admitted to five of the participating ICUs, were included in the analysis (Fig. 1 ). Excluded subjects include 9 patients fulfilled MIS-C criteria, 2 duplicate entries, and 7 subjects had alternate reason for ICU admission and were identified to be SARS-CoV-2-infected by a routine screening. Thirteen patients were admitted to one of three participating centers in Kuwait, and 12 were admitted to two centers in KSA.
Fig. 1.
Flow diagram of patients included in the study.
The median age of subjects was 2.8 years (IQR 0.2–8.5 years), and 60% were male (Table 1 ). Most (88%) patients had underlying comorbidities. Six (24%) patients had an underlying neurological disease, five (20%) had hematological malignancies, and four (16%) had congenital heart disease. Noteworthy, three patients were not known to have any comorbidities at the time of ICU admission. By August 31st, 2020, 80% of patients were discharged home, of which two patients required home oxygen therapy. One patient is still admitted with a stable clinical condition. Four (16%) of the critical pediatric COVID-19 cases died. Median hospital and ICU length of stay were 16.5 (IQR 9.5–23.5) and 5 (IQR 3.75–21) days, respectively.
Table 1.
Demographic data of the study population.
| Variables | Total (n = 25) | Survivors (n = 21) | Non-survivors (n = 4) | p-Value |
|---|---|---|---|---|
| Age (median, IQR) | 2.78 [0.21;8.51] | 2.13 [0.21;7.3] | 10.3 [5.47;13.4] | 0.458 |
| Male (%) | 15 (60%) | 12 (57.1%) | 3 (75%) | 0.626 |
| Routine vaccination status | 1 | |||
| Fully vaccinated | 9 (36%) | 8 (38.1%) | 1 (25%) | |
| Missed vaccine | 7 (28%) | 6 (28.6%) | 1 (25%) | |
| Unknown | 9 (36%) | 7 (33.3%) | 2 (50%) | |
| Comorbid conditions | ||||
| Autoimmune disease | 2 (8.0%) | 2 (9.5%) | 0 (0.0%) | 1 |
| Chronic lung disease | 1 (4.0%) | 1 (4.8%) | 0 (0.0%) | 1 |
| Congenital heart disease | 4 (16%) | 4 (19.0%) | 0 (0.0%) | 1 |
| Hematological Malignancy | 5 (20%) | 4 (19.0%) | 1 (25%) | 1 |
| Medically complexa | 3 (12%) | 2 (9.5%) | 1 (25%) | 0.422 |
| Neurological disease | 6 (24%) | 4 (19.0%) | 2 (50%) | 0.234 |
| None | 3 (12%) | 3 (14.3%) | 0 (0.0%) | 1 |
| Obesity | 2 (8%) | 2 (9.5%) | 0 (0.0%) | 1 |
| Prematurity | 4 (16%) | 3 (14.3%) | 1 (25.0%) | 0.527 |
| Sickle cell disease | 3 (12%) | 3 (14.3%) | 0 (0.0%) | 1 |
| MRSA colonization | 1 (4%) | 0 (0.0%) | 1 (25%) | 0.16 |
| Medical Center: | 0.635 | |||
| 1 | 8 (32%) | 6 (28.6%) | 2 (50.0%) | |
| 2 | 11 (44%) | 10 (47.6%) | 1 (25.0%) | |
| 3 | 4 (16%) | 3 (14.3%) | 1 (25.0%) | |
| 4 | 1 (4%) | 1 (4.76%) | 0 (0.00%) | |
| 5 | 1 (4%) | 1 (4.76%) | 0 (0.00%) | |
| Hospital length of stay (median, IQR) (n = 18) | 16.5 [9.5;23.5] | 17.5 [11.5;23.5] | 5.50 [4.25;12.5] | 0.121 |
| ICU length of stay (median, IQR) (n = 24) | 5 [3.75;21] | 4.5 [3.75;21] | 5.5 [4.25;11.8] | 0.969 |
| Outcomes | < 0.001 | |||
| Full recovery | 18 (72%) | 18 (85.7%) | 0 (0.0%) | |
| Discharged with sequelae | 2 (8%)b | 2 (9.5%) | 0 (0.0%) | |
| Still Hospitalized | 1 (4%) | 1 (4.76%) | 0 (0.0%) | |
| Death | 4 (16%) | 0 (0.0%) | 4 (100%) | |
ICU: intensive care unit.
Defined as technically dependent children or those diagnosed with genetic or metabolic syndrome.
All required home oxygen therapy.
Fever and cough, were the most common presenting symptoms at the time of ICU admission (Table 2 ). Fever was present in 84% of subjects and lasted for a median of 4 days (IQR 1–7 days). The duration of fever was similar in survivors (median 4 days, IQR 1–7 days) and non-survivors (median 4 days, IQR 1–14.5 days, p = 0.9). Respiratory failure was the most common reason for ICU admission among survivors (95.2% in survivors vs. 50% in non-survivors, p = 0.057). Around 10% of the survivors presented with circulatory compromise compared to 75% of non-survivors (p = 0.016).
Table 2.
Clinical characteristics of the study population.
| Variables | Total (n = 25) | Survivors (n = 21) | Non-survivors (n = 4) | p-value |
|---|---|---|---|---|
| Symptoms at hospital presentation | ||||
| Abdominal pain | 2 (8%) | 2 (9.5%) | 0 (0.0%) | 1 |
| Chest pain | 3 (12%) | 3 (14.3%) | 0 (0.0%) | 1 |
| Cough | 15 (60%) | 13 (61.9%) | 2 (50%) | 1 |
| Cutaneous | 3 (12%) | 2 (9.52%) | 1 (25%) | 0.422 |
| Diarrhea | 6 (24%) | 5 (23.8%) | 1 (25%) | 1 |
| Fever | 21 (84%) | 17 (81%) | 4 (100%) | 1 |
| Duration of fever [median; IQR] | 4 [1;7] | 4 [2;6] | 4 [1;14.5] | 0.891 |
| Headache | 4 (16%) | 4 (19%) | 0 (0.0%) | 1 |
| Loss of appetite | 8 (32%) | 7 (33.3%) | 1 (25%) | 1 |
| Myalgia | 6 (24%) | 5 (23.8%) | 1 (25%) | 1 |
| Rhinorrhea | 6 (24%) | 5 (23.8%) | 1 (25%) | 1 |
| Seizure | 2 (8%) | 1 (4.76%) | 1 (25%) | 0.3 |
| Sore throat | 4 (16%) | 4 (19%) | 0 (0.0%) | 1 |
| Vomiting | 14 (56%) | 12 (57.1%) | 2 (50%) | 1 |
| The main reason for ICU admission | ||||
| Respiratory failure | 22 (88%) | 20 (95.2%) | 2 (50%) | 0.057 |
| Neurological compromise | 2 (8%) | 2 (9.5%) | 0 (0.0%) | 1 |
| Circulatory compromise | 5 (20%) | 2 (9.5%) | 3 (75%) | 0.016 |
| Diseases Severity | 0.081 | |||
| Severe pneumonia/ARDS | 17 (68%) | 16 (76.2%) | 1 (25%) | |
| Sepsis/septic shock | 8 (32%) | 5 (23.8%) | 3 (75%) | |
| Clinical characteristics at ICU admission | ||||
| PRISM III (median, IQR) | 12 [9;17] | 12 [9;16] | 15 [8.25;21.8] | 0.766 |
| GCS (median, IQR) | 13 [11;15] | 15 [11;15] | 12 [9.75;13.5] | 0.305 |
| Resuscitation within 24 hours | 2 (8%) | 1 (4.76%) | 1 (25%) | 0.3 |
| Hypotension | 4 (16%) | 3 (14.3%) | 1 (25%) | 0.527 |
| Highest respiratory support | 0.042 | |||
| None | 1 (4%) | 1 (4.8%) | 0 (0.0%) | |
| Low flow oxygen | 6 (24%) | 6 (28.6%) | 0 (0.0%) | |
| HFNC | 4 (16%) | 4 (19.0%) | 0 (0.0%) | |
| CPAP-BIPAP | 5 (20%) | 5 (23.8%) | 0 (0.0%) | |
| Intubation | 7 (28%) | 3 (14.3%) | 4 (100%) | |
| ECMO | 2 (8%) | 2 (9.5%) | 0 (0.0%) | |
| Reason for resp support | 0.52 | |||
| Hypoxic respiratory failure | 17 (70.8%) | 14 (70%) | 3 (75%) | |
| Hypercarbic respiratory failure | 4 (16.7%) | 4 (20%) | 0 (0.0%) | |
| Mixed respiratory failure | 1 (4.2%) | 1 (5%) | 0 (0.0%) | |
| Hemodynamic instability | 2 (8.3%) | 1 (5%) | 1 (25%) | |
| Type of organ failure | ||||
| Respiratory | 19 (76%) | 15 (71.4%) | 4 (100%) | 0.54 |
| Cardiac | 12 (48%) | 8 (38.1%) | 4 (100%) | 0.039 |
| Renal | 2 (8%) | 1 (4.8%) | 1 (25%) | 0.3 |
| Hepatic | 3 (12%) | 2 (9.5%) | 1 (25%) | 0.422 |
| Hematological | 4 (16%) | 3 (14.3%) | 1 (25%) | 0.527 |
| Neurological | 3 (12%) | 1 (4.8%) | 2 (50%) | 0.057 |
BiPAP: bilevel positive pressure ventilation, CPAP: continuous positive airway pressure, ECMO: extracorporeal membrane oxygenation, GCS: Glasgow coma scale, HFNC: high-flow nasal canula, PRISM III: The pediatric risk of mortality III score.
During ICU stay, nine patients were managed with non-invasive ventilation, nine required endotracheal intubation, of which two needed extracorporeal membrane oxygenation (ECMO). Hypoxic respiratory failure was the most common reason for requiring respiratory support, and it was observed more frequently in patients requiring invasive ventilation (88.9% vs. 60%, p = 0.19). One-third (32%) of the cohort, and all patients on invasive mechanical ventilation, received vasoactive agents (Table 3 ). Most (60%) patients requiring invasive ventilation had bilateral infiltrate or diffuse ground-glass opacities on chest radiographs.
Table 3.
Laboratory investigation and therapies provided to the study population.
| Variables | Total (n = 25) | Survivors (n = 21) | Non-survivors (n = 4) | p-value |
|---|---|---|---|---|
| Laboratory investigations within 24 h of ICU admission (mean, SD) | ||||
| Creatinine | 33.7 (17.3) | 30.8 (12.9) | 50.3 (32) | 0.400 |
| White blood cells | 10.0 (8.8) | 11.0 (9.1) | 4.75 (4) | 0.050 |
| Absolute lymphocyte count | 2.68 (2.5) | 3.04 (2.6) | 0.77 (0.8) | 0.005 |
| Absolute neutrophil count | 6.16 (6.2) | 6.62 (6.5) | 3.70 (3.9) | 0.263 |
| Hemoglobin | 111 (34.5) | 112 (35.8) | 108 (30.8) | 0.821 |
| Platelets | 243 (167) | 268 (169) | 107 (56.4) | 0.003 |
| INR | 1.30 (0.5) | 1.25 (0.2) | 1.53 (1.1) | 0.627 |
| D-dimer | 3106 (3491) | 3612 (3750) | 1086 (306) | 0.017 |
| Ferritin | 1229 (1689) | 1358 (1807) | 587 (796) | 0.277 |
| Lactate dehydrogenase | 518 (224) | 515 (226) | 546 (288) | 0.903 |
| Troponin | 24.7 (37.3) | 24.7 (37.3) | 0 (0) | NA |
| C-reactive protein | 84.7 (107) | 86.3 (116) | 74.7 (22.3) | 0.699 |
| Procalcitonin | 10.2 (21.2) | 5.36 (14.0) | 33.0 (35.7) | 0.220 |
| Erythrocyte sedimentation rate | 35.4 (25.9) | 29.8 (22.4) | 58.0 (35.4) | 0.451 |
| Alkaline phosphatase | 152 (78.2) | 151 (81.7) | 156 (68.7) | 0.900 |
| Alanine transferase | 40.3 (67.6) | 44.2 (74.0) | 21.8 (11.3) | 0.223 |
| Albumin | 33.3 (7.6) | 34.3 (7.1) | 28.8 (9.5) | 0.336 |
| pCO2 | 6.52 (2.2) | 6.78 (2.3) | 5.20 (1.3) | 0.1 |
| pH | 7.29 (0.1) | 7.30 (0.1) | 7.27 (0.2) | 0.843 |
| Chest X-ray | 0.84 | |||
| Normal | 1 (4.2%) | 1 (4.8%) | 0 (0.0%) | |
| Increased BV marking | 2 (8.3%) | 2 (9.5%) | 0 (0.0%) | |
| Unilateral infiltrate | 6 (25.0%) | 6 (28.6%) | 0 (0.0%) | |
| Bilateral infiltrate | 9 (37.5%) | 7 (33.3%) | 2 (66.7%) | |
| Diffuse ground-glass opacities | 6 (25.0%) | 5 (23.8%) | 1 (33.3%) | |
| Positive microbiological investigation | ||||
| Blood culture | 3 (12%) | 3 (14.3%) | 0 (0.0%) | 1 |
| Urine culture | 1 (4%) | 1 (4.8%) | 0 (0.0%) | 1 |
| Antibiotics | ||||
| Penicillins | 4 (16%) | 3 (14.3%) | 1 (25%) | 0.527 |
| Third-generation cephalosporins | 14 (56%) | 12 (57.1%) | 2 (50%) | 1 |
| Macrolides | 4 (16%) | 3 (14.3%) | 1 (25%) | 0.527 |
| Lincosamides | 3 (12%) | 1 (4.8%) | 2 (50%) | 0.057 |
| Beta lactam-beta lactamase inhibitor (anti-pseudomonal) | 11 (44%) | 8 (38.1%) | 3 (75%) | 0.288 |
| Glycopeptides | 11 (44%) | 10 (47.6%) | 1 (25%) | 0.604 |
| Carbapenems | 5 (20%) | 3 (14.3%) | 2 (50%) | 0.166 |
| Aminoglycosides | 2 (8%) | 1 (4.8%) | 1 (25%) | 0.3 |
| Trimethoprim-sulfonamide combinations | 2 (8%) | 1 (4.8%) | 1 (25%) | 0.3 |
| Fluroquinolones | 1 (4%) | 1 (4.8%) | 0 (0.0%) | 1 |
| Imidazoles | 1 (4.2%) | 1 (5%) | 0 (0.0%) | 1 |
| Antifungals | 4 (16%) | 3 (14.3%) | 1 (25%) | 0.527 |
| Antivirals | ||||
| Lopinavir/ritonavir | 1 (4%) | 1 (4.8%) | 0 (0.0%) | 1 |
| Favipiravir | 3 (12%) | 3 (14.3%) | 0 (0.0%) | 1 |
| Immunomodulators | ||||
| Tocilizumab | 6 (24%) | 5 (23.8%) | 1 (25%) | 1 |
| High dose steroids | 3 (12%) | 3 (14.3%) | 0 (0.0%) | 1 |
| Low dose steroids | 9 (36%) | 6 (28.6%) | 3 (75%) | 1 |
| Intravenous immunoglobulin | 5 (21.7%) | 4 (21.1%) | 1 (25%) | 1 |
| Convalescent plasma | 2 (8%) | 1 (4.8%) | 1 (25%) | 1 |
| Anticoagulation | 10 (43.5%) | 8 (42.1%) | 2 (50%) | 1 |
| Vasoactive agents | ||||
| Epinephrine | 4 (16%) | 2 (9.5%) | 2 (50%) | 0.106 |
| Norepinephrine | 5 (20%) | 3 (14.3%) | 2 (50%) | 0.166 |
| Dopamine | 3 (12%) | 2 (9.5%) | 1 (25%) | 0.422 |
| Dobutamine | 3 (12%) | 2 (9.5%) | 1 (25%) | 0.422 |
| Milrinone | 2 (8%) | 1 (4.8%) | 1 (2%) | 0.3 |
ICU: intensive care unit, INR: international normalized ratio, pCO2: carbon dioxide partial pressure.
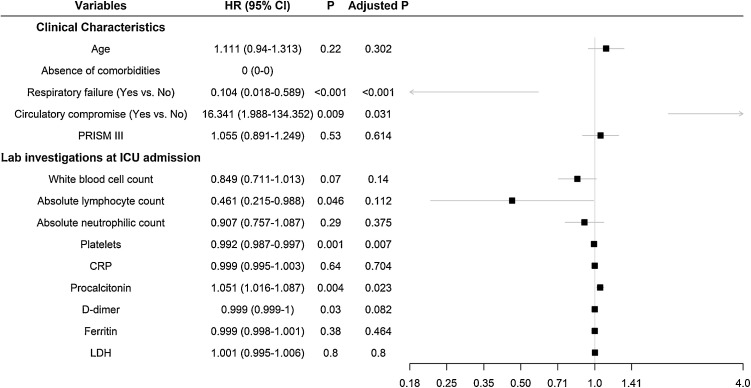
In univariate analysis, the presence of comorbidity, low platelets count, high procalcitonin, and circulatory compromise, all at the time of ICU admission, were associated with death during the ICU stay (Fig. 2 ). Elevated levels of D-dimer (≥500 ng/dl) were observed in 24 (96%) of patients, and it was higher in survivors (mean: 3612 vs. 1086 ng/mL, p = 0.017 using unpaired t-test). However, in univariate Fine-Gray analysis, D-dimer levels were not significantly associated with in-ICU mortality (HR 0.99, adjusted p = 0.08). Low levels of absolute lymphocytic count were associated with mortality (HR 0.46; 95% CI 0.21–0.98); however, this association was not statistically significant after p-value adjustment.
Fig. 2.
Forest plot diagram demonstrating unadjusted hazard ratio for factors associated with ICU mortality due to SARS-CoV-2 infection in children.
Co-infection was uncommon in the studied cohort. Three (12%) patients had bloodstream infections: one with Klebsiella pneumoniae, one with Staphylococcus lugdunensis, and one patient had polymicrobial bloodstream infection (K. pneumoniae and Escherichia coli). In the latter, similar organisms were isolated from a concomitant urine culture. Of all included patients, seven (28%) had an upper respiratory sample tested negative for a respiratory virus, other than SARS-CoV-2, by a molecular assay. However, the targeted viruses varied by centers and included, as minimum, influenza, and respiratory syncytial virus (RSV).
All patients in the cohort received antibiotics (Table 3). Third-generation cephalosporins (56%) and beta lactam-beta lactamase inhibitors (44%) were the most commonly prescribed antibiotic classes. Four patients received antifungals, of which three had proven or suspected pulmonary invasive fungal infection. The use of targeted antiviral therapy against SARS-CoV-2 was uncommon. However, almost two-thirds (60%) of subjects received immunomodulator therapy, of which low- and high-dose steroids were prescribed in 80% of those patients. There was no difference in the use of immunomodulator therapy between survivors and non-survivors, and between those with invasive ventilation and non-invasive ventilation.
Discussion
Critical SARS-CoV-2 infection in children is uncommon. In this multicenter, retrospective cohort study, we report the clinical characteristics and disease dynamics in 25 pediatric COVID-19 who required ICU care. We observed that the presence of comorbidities, low platelets count, high procalcitonin, and circulatory compromise on admission are associated with death in ICU. The D-dimer level was elevated in most patients admitted to ICUs; however, non-survivors had a lower rise in D-dimer levels when compared to survivors. In this study, 4 (16%) ICU-admitted children died. Our mortality, based on death-to-ICU admissions ratio, is higher than what was reported by Shekerdemian et al. [12]. This might be explained by our strict study inclusion criteria. We only included patients who were admitted to the ICU due to direct consequence of SARS-CoV-2 infection. This may be responsible for relatively inflating our reported COVID-19 ICU deaths. National-level data suggest a death-to-ICU admission ratio of 8–20% [8,9]. Furthermore, there were a total of 7934 SARS-CoV-2-infected children in Kuwait at the end of the our study, including 13 (0.16%; 95% CI 0.09–0.28%) ICU admissions part of this cohort, and three deaths (case fatality rate 0.04%, 95% CI 0.01–0.12%), which is lower than other reports [8,9].
Thrombocytopenia is commonly seen in critically ill SARS-CoV-2-infected patients. Our finding was consistent with what was described in adults with severe COVID-19 [19,20]. Many theories attempted to explain the association between severe COVID-19 and thrombocytopenia. Direct platelet destruction, either due to increased thrombosis or disseminated intravascular coagulation (DIC), and reduced platelet production were postulated as causes for thrombocytopenia in severe cases [19,20]. In our cohort, five patients (20%, one died) were diagnosed with hematological malignancy were infected with SARS-CoV-2 at different points of their treatment phase. It is difficult to assess if the underlying diseases contributed to the association between thrombocytopenia and mortality observed in this study. However, low platelet count was also observed in other patients that were supposed to have normal platelet counts. More studies are needed in children with SARS-CoV-2 infection to confirm this association. Once proven, we think that thrombocytopenia could be used as a marker to predict the unfavorable course of COVID-19 in children.
Reduction of lymphocyte and neutrophil counts in peripheral blood was observed in other respiratory virus infections in children, including influenza [21]. In SARS-CoV-2-infected adults, studies found that leukopenia is associated with a severe illness [22]. We found that non-survivors had lower mean white blood cell (4.75 vs 11 × 109/L, p = 0.05), lymphocyte (0.77 vs 3.04 × 109/L, p = 0.005) and neutrophil counts (3.7 vs 6.62 × 109/L, p = 0.263) than survivors. However, none were statistically significant in the univariate hazard model. Both groups have elevated D-dimer levels. Interestingly, we found that the degree of increase in D-dimer was significantly lower in the non-survival group (mean: 3612 vs 1086 ng/dl, p = 0.003). The reason for this difference between the two groups is not clear. This finding is not in keeping with a larger cohort of severe adult COVID-19 patients [23]. A possible explanation for our finding could be that non-survivors had rapid progression of refractory circulatory instability that did not allow sufficient time for the rise of D-dimer, as an increasing trend of D-dimer may associate with disease worsening [24]. In addition, the observed difference can be related to the unmeasured confounding effect. For instance, age-dependent normal ranges in D-dimer serum concentration is not fully described [25].
Procalcitonin, the precursor of the hormone calcitonin, synthesis can be triggered by inflammatory cytokines in response to severe bacterial infection. However, this response is not specific and was observed in severe respiratory viral infections [26]. Similarly, procalcitonin was found to be a predictor for ICU admission and mortality in adult patients with COVID-19 [27,28]. Our findings were in-line with these reports. We found that procalcitonin, done at the time of ICU admission, was associated with mortality.
The reason for ICU admission and type of organ dysfunction could be associated with patients' outcome. Circulatory failure was significantly associated with pediatric death in our cohort despite the small number of patients. A similar finding was observed in a study evaluating severe SARS-CoV-2 infection in adults by Zhou et al. Acute cardiac injury and heart failure were significantly associated with in-hospital death [23]. Significant expression of angiotensin-converting enzyme 2 (ACE2) receptor, the protein required for viral attachment, on the myocardial cells is believed to play a role in the increased cardiac morbidity in COVID-19 [29,30]. Also, cytokine release syndrome triggered by SARS-CoV-2 infection may contribute the circulatory failure [31].
This multicenter study has several limitations. The study is limited to its retrospective nature. Clinical data were dependent on the healthcare workers’ accuracy in recording daily medical information. In addition, since the severe and critical disease is uncommon in children, number of the study subjects was limited. For that reason, we were not able to accurately define all predictors for mortality during the ICU stay. Finally, study centers had variable supportive services. Not all ICUs were part of a tertiary pediatric center or had an ECMO service. Whether this contributed to patient mortality is unclear although patient transferring to another, more equipped, ICU was possible. Finally, study centers may have different laboratory assays with distinct test performance. This may contribute to some differences in laboratory results. However, there was insignificant differences in overall laboratory results among the study centers between the two patient groups. Also, all centers used commercial and verified assays. Finally, long-term follow-up for the discharged patients to assess the potential long-term effect of ICU admission was not possible.
Conclusions
Our findings showed that children admitted to the ICU with SARS-CoV-2 infection, generally, have a favorable outcome. Also, low platelet counts and circulatory compromise at the time of ICU admission were associated with death. Due to the small study population in this paper, larger prospective studies are needed to confirm our findings and better understand SARS-CoV-2 infection in children.
Funding
No funding sources.
Competing interests
None declared.
Ethical approval
Ethics board approval from the Ministry of Health of Kuwait and each respective center in KSA were obtained.
CRediT authorship contribution statement
Abdulla Alfraij: Conceptualization, Methodology, Investigation, Data curation, Writing - original draft. Abdulrahman A. Bin Alamir: Conceptualization, Investigation, Writing - review & editing. Abdulnasir M. Al-Otaibi: Conceptualization, Investigation, Writing - review & editing. Danah Alsharrah: Conceptualization, Investigation, Methodology, Writing - review & editing. Abdulrahman Aldaithan: Conceptualization, Investigation, Methodology, Writing - review & editing. Ahmed M. Kamel: Methodology, Data curation, Formal analysis. Muna Almutairi: Conceptualization, Investigation, Methodology, Writing - review & editing. Salman Alshammari: Conceptualization, Investigation, Writing - review & editing. Mohammed Almazyad: Conceptualization, Investigation, Writing - review & editing. Jara Mia Macarambon: Conceptualization, Investigation, Writing - review & editing. Mohammad Alghounaim: Conceptualization, Methodology, Data curation, Writing - original draft, Supervision.
Acknowledgement
We thank Dr Sarah Al Youha, MD, PhD, for her help with initial statistical analysis.
References
- 1.Abate S.M., Ahmed Ali S., Mantfardo B., Basu B. Rate of Intensive Care Unit admission and outcomes among patients with coronavirus: a systematic review and Meta-analysis. PLoS One. 2020;15(7) doi: 10.1371/journal.pone.0235653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim L., Garg S., O’Halloran A., Whitaker M., Pham H., Anderson E.J. Risk factors for intensive care unit admission and in-hospital mortality among hospitalized adults identified through the U.S. Coronavirus disease 2019 (COVID-19)-associated hospitalization surveillance network (COVID-NET) Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu X., Zhang L., Du H., Zhang J., Li Y.Y., Qu J. SARS-CoV-2 infection in children. N Engl J Med. 2020;382(17):1663–1665. doi: 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu Y., Li Y., Deng W., Liu M., He Y., Huang L. Symptomatic infection is associated with prolonged duration of viral shedding in mild coronavirus disease 2019: a retrospective study of 110 children in Wuhan. Pediatr Infect Dis J. 2020;39(7):e95–e99. doi: 10.1097/INF.0000000000002729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Children and COVID-19: State Data Report: American Academy of Pediatrics and the Children’s Hospital Association; July 16, 2020. Available from: https://downloads.aap.org/AAP/PDF/AAP%20and%20CHA%20-%20Children%20and%20COVID-19%20State%20Data%20Report%207.16.20%20FINAL.pdf.
- 6.de Souza T.H., Nadal J.A., Nogueira R.J.N., Pereira R.M., Brandao M.B. Clinical manifestations of children with COVID-19: a systematic review. Pediatr Pulmonol. 2020;55:1892–1899. doi: 10.1002/ppul.24885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun D., Li H., Lu X.-X., Xiao H., Ren J., Zhang F.-R. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center’s observational study. World J Pediatr. 2020:1–9. doi: 10.1007/s12519-020-00354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Götzinger F., Santiago-García B., Noguera-Julián A., Lanaspa M., Lancella L., Calò Carducci F.I. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. 2020;4(9):653–661. doi: 10.1016/S2352-4642(20)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Team CC-R Coronavirus Disease 2019 in children - United States, February 12-April 2, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(14):422–426. doi: 10.15585/mmwr.mm6914e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pathak E.B., Salemi J.L., Sobers N., Menard J., Hambleton I.R. COVID-19 in children in the United States: intensive care admissions, estimated total infected, and projected numbers of severe pediatric cases in 2020. J Public Health Manag Pract. 2020;26(4):325–333. doi: 10.1097/PHH.0000000000001190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong Y., Mo X., Hu Y., Qi X., Jiang F., Jiang Z. Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China. Pediatrics. 2020 doi: 10.1542/peds.2020-0702. [DOI] [Google Scholar]
- 12.Shekerdemian L.S., Mahmood N.R., Wolfe K.K., Riggs B.J., Ross C.E., McKiernan C.A. Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian Pediatric Intensive Care Units. JAMA Pediatr. 2020 doi: 10.1001/jamapediatrics.2020.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Multisystem inflammatory syndrome in children and adolescents with COVID-19: World Health Organization; May 15, 2020 [Available from: https://www.who.int/publications/i/item/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19.
- 14.Clinical management of COVID-19: interim guidance: World Health Organization; May 27, 2020 [Available from: https://www.who.int/publications/i/item/clinical-management-of-covid-19.
- 15.Pollack M.M., Holubkov R., Funai T., Dean J.M., Berger J.T., Wessel D.L. The pediatric risk of mortality score: update 2015. Pediatr Crit Care Med. 2016;17(1):2–9. doi: 10.1097/PCC.0000000000000558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldstein B., Giroir B., Randolph A. International Consensus Conference on Pediatric S. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6(1):2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 17.Pollack M.M., Patel K.M., Ruttimann U.E. PRISM III: an updated pediatric risk of mortality score. Crit Care Med. 1996;24(5):743–752. doi: 10.1097/00003246-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Weiss S.L., Peters M.J., Alhazzani W., Agus M.S.D., Flori H.R., Inwald D.P. Surviving Sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Pediatr Crit Care Med. 2020;21(2):e52–e106. doi: 10.1097/PCC.0000000000002198. [DOI] [PubMed] [Google Scholar]
- 19.Jiang S.Q., Huang Q.F., Xie W.M., Lv C., Quan X.Q. The association between severe COVID-19 and low platelet count: evidence from 31 observational studies involving 7613 participants. Br J Haematol. 2020;190(1):e29–e33. doi: 10.1111/bjh.16817. [DOI] [PubMed] [Google Scholar]
- 20.Lippi G., Plebani M., Henry B.M. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 2020;506:145–148. doi: 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao B., Li X.W., Mao Y., Wang J., Lu H.Z., Chen Y.S. Clinical features of the initial cases of 2009 pandemic influenza A (H1N1) virus infection in China. N Engl J Med. 2009;361(26):2507–2517. doi: 10.1056/NEJMoa0906612. [DOI] [PubMed] [Google Scholar]
- 22.Jesenak M., Brndiarova M., Urbancikova I., Rennerova Z., Vojtkova J., Bobcakova A. Immune parameters and COVID-19 infection - associations with clinical severity and disease prognosis. Front Cell Infect Microbiol. 2020;10:364. doi: 10.3389/fcimb.2020.00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Terpos E., Ntanasis-Stathopoulos I., Elalamy I., Kastritis E., Sergentanis T.N., Politou M. Hematological findings and complications of COVID-19. Am J Hematol. 2020;95(7):834–847. doi: 10.1002/ajh.25829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Camet C.N., Yee D.L. Focus on diagnosis: a primer on D-dimer. Pediatr Rev. 2011;32(1):31–33. doi: 10.1542/pir.32-1-31. [DOI] [PubMed] [Google Scholar]
- 26.Guervilly C., Coisel Y., Botelho-Nevers E., Dizier S., Castanier M., Lepaul-Ercole R. Significance of high levels of procalcitonin in patients with influenza A (H1N1) pneumonia. J Infect. 2010;61(4):355–358. doi: 10.1016/j.jinf.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 27.Almazeedi S., Al-Youha S., Jamal M.H., Al-Haddad M., Al-Muhaini A., Al-Ghimlas F. Characteristics, risk factors and outcomes among the first consecutive 1096 patients diagnosed with COVID-19 in Kuwait. EClinicalMedicine. 2020;24:100448. doi: 10.1016/j.eclinm.2020.100448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang I., Pranata R., Lim M.A., Oehadian A., Alisjahbana B. C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis. Ther Adv Respir Dis. 2020;14:1–14. doi: 10.1177/1753466620937175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu H., Zhong L., Deng J., Peng J., Dan H., Zeng X. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12(1):8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guzik T.J., Mohiddin S.A., Dimarco A., Patel V., Savvatis K., Marelli-Berg F.M. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020;116(10):1666–1687. doi: 10.1093/cvr/cvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanner T., Wahezi D.M. Hyperinflammation and the utility of immunomodulatory medications in children with COVID-19. Paediatr Respir Rev. 2020;35:81–87. doi: 10.1016/j.prrv.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]