Abstract
Direct-to-consumer (DTC) television ads must disclose a drug’s most important risks. Currently, the risks must be in audio at a minimum. Studies have shown that presenting information with both audio and superimposed risk text (dual-modality) improves recall beyond that of using audio alone. However, distracting elements in DTC ads may draw attention away from the superimposed risk text. This study combined eye-tracking data with questionnaire data to examine whether distracting elements decrease attention to the risk text in DTC ads, in turn affecting risk retention and risk perceptions. The authors randomly assigned 300 U.S. opt-in panel members to view either a low-distraction or a high-distraction DTC television ad. The authors found that distracting elements during risk presentation drew attention away from the risk text and, in turn, reduced retention of drug risk information. Risk perceptions were not affected. These results suggest that even if dual-modality is used to increase consumer’s comprehension of drug risk information, distracting visuals should still be avoided in order to help consumers focus on key information in the ad.
Keywords: eye tracking, prescription drug, direct-to-consumer advertising, distraction
Direct-to-consumer (DTC) television advertisements for prescription drugs are regulated by the U.S. Food and Drug Administration (FDA). Current regulations require that a major statement consisting of the risks of prescription drugs be included in at least the audio of DTC television ads (Prescription Drug Advertisements 2012). The Federal Food, Drug, and Cosmetic Act (the FD&C Act), as a result of the Food and Drug Administration Amendments Act of 2007 (FDAAA), requires that the major statement be presented in a clear, conspicuous and neutral manner (Congress 2007). In response to FDAAA, FDA introduced the idea of including the major statement in superimposed text as well as in the audio (dual-modality presentation) to aid in the clear, conspicuous, and neutral communication of risk information (FDA 2010). FDA (2010) has also stated that the major statement should not be presented at the same time as other features that would “arrest the attention and distract consumers.” In addition, results from a study required by FDAAA suggested that the following statement about FDA’s Medwatch reporting program for adverse events is appropriate for inclusion in DTC television ads: “You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch, or call 1–800-FDA-1088” (Aikin et al. 2016).
According to the Communication-Human Information Processing Model (C-HIP) model, individuals must notice risk information (attention switch) and then keep their attention on it (attention maintenance) for the information to be processed and understood (Conzola and Wogalter 2001; Wogalter 2006). The model states that the delivery of the risk information (such as whether it is delivered via dual-modality presentation) and external stimuli (such as distractors) can increase or decrease attention switch and maintenance. Thus, the combination of these recent FDA proposals could affect consumers’ ability to notice and attend to risk information.
Regulations state that prescription drug risks should be presented in similar scope, depth, or detail as the drug’s benefits (Prescription Drug Advertisements 2012). As noted earlier, the FD&C Act requires a “clear, conspicuous, and neutral” presentation of risk information in DTC television ads. Accordingly, FDA has issued warning and untitled letters to pharmaceutical companies for poor communication of risk information in television ads, including distracting from the risk information by presenting competing information during the major statement (Sheehan 2003). Distraction continues to be a relevant policy issue for FDA. Following Hoy and Park (2014), we reviewed promotion-related letters issued by FDA in 2015 and found one that cited distraction as a source of minimization of risk in a video segment. Specifically, the video potentially distracted the audience from the risk information (which was presented in small font without a contrasting background) by presenting it on the screen at the same time that non-risk information was discussed in an interview (FDA, 2015). This concern about distraction can also be seen in the Federal Trade Commission’s (FTC’s) “clear and conspicuous” standards (CCS; FTC 1970) and disclosure guidelines (FTC 2013), which state that distraction should be minimized by eliminating competing audio or video elements when a disclosure is presented. The CCS provides another framework that offers techniques for improving consumer understanding of disclosure statements (such as on-screen risk information).
Research has examined the role of distraction in advertising. In an analysis of prime-time advertising, Hoy and Andrews (2004) found that almost all disclosures were presented with distracting visuals, music, or scene changes. Royne and Myers (2008) note that advertising factors such as on-screen visuals and background music could distract consumers from the risk information. Indeed, research demonstrates how visuals (e.g., Hoyer, Srivastava, and Jacoby 1984), music (e.g., Cassidy and MacDonald 2007; Oakes and North 2006; Tavassoli and Lee 2003), and scene changes (e.g., Thomas, Fowler, and Kolbe 2011) in advertising can lead to lower retention of the information in the ad. In DTC television advertising, Wogalter, Shaver, and Kalsher (2014) found that when non-risk information (such as the branded drug website address) was presented at the same time as either audio or visual risk information it decreased risk recognition in comparison to risk information presented alone (either visually or both visually and in audio). Moreover, several content analyses of DTC television ads have raised concerns that drug risks are not well-communicated (Avery, Eisenberg, and Simon 2011; Frosch et al. 2007; Kaphingst et al. 2004; Macias, Pashupati, and Lewis 2007). For instance, almost every ad for antidepressants from 1995–2007 presented positive visuals during the risk statement (Avery, Eisenberg, and Simon 2012).
Distraction has long been considered as a factor that decreases individuals’ ability to process risk information (Stewart and Martin 1994). Therefore, our first goal was to replicate past research on the effects of distracting elements, extending it to the context of a DTC television ad.
H1: Distracting elements during the risk statement of a DTC television ad decrease retention of the risk information and risk perceptions.
As noted above, FDA (2010) contemplated the benefits of a dual-modality presentation of the major statement. FTC (1970) guidelines state that disclosures should be presented simultaneously in both the audio and video portions of the television ad for maximum effect. Conveying information in both audio and visual channels (dual-modality) has been found to increase memory, comprehension, and processing of that information across contexts (Brewer, Harvey, and Semmler 2004; Chung 2008; Frick 1984; Lang 1995; Murray, Manrai, and Manrai 1998; Walma van der Molen and Klijn 2004). Therefore, one potential way to increase consumers’ attention to the risk statement in DTC ads is to convey the risk information in both audio and superimposed text. Content analyses have found that few ads use dual-modality to convey risk information in DTC ads: 9% of DTC television ads broadcast during 2001 (Kaphingst et al. 2004) and 2% of DTC televisions ads in 2003 (Macias, Pashupati, and Lewis 2007). Researchers have found that individuals are better able to recall the drug risks in DTC ads when they are presented in superimposed text as well as in audio (Glinert and Schommer 2005; Morris, Mazis, and Brinberg 1989; Wogalter, Shaver, and Kalsher 2014). However, past studies have not included a behavioral measure of attention. In line with the C-HIP model (Conzola and Wogalter 2001; Wogalter 2006), we predict that one reason dual-modality is effective is that that the more people pay attention to the superimposed text the more information they retain. Wedel and Pieters (2008) argue that attention is a key construct for understanding marketing effects. Further, they argue that behavioral measures of attention with predictive validity in marketing research, such as eye tracking, are necessary because recall measures can be biased.
Eye tracking technology, which we incorporate in this study, can be used to determine the extent to which consumers pay attention to information by allowing researchers to unobtrusively detect and measure where and for how long a participant looks while viewing ads (Duchowski 2007). Previous research has demonstrated the value of eye tracking to understand consumers’ visual attention to advertising in print (Higgins, Leinenger, and Rayner 2014; Pieters, Wedel and Batra 2010; Thomsen and Fulton 2007), on the Internet (Simola, Kuisma, Öörni, Uusitalo, and Hyönä 2011; Wedel and Pieters 2008), and on television (Brasel and Gips 2008; Teixeria, Wedel, and Pieters 2010). Although it has been used to examine prescription drug packaging and labeling (Filik, Purdy, Gale and Gerrett 2004; Sundar, Becker, Bello and Bix 2012), we are unaware of any publications that used eye tracking to study DTC advertising.
H2: Attention to the superimposed risk information increases retention of the risk information and risk perceptions.
Even with dual-modality, consumers could still be distracted from the risk information by extraneous visuals and sounds presented during the risk statement, which could decrease their retention of this information. Murray and colleagues (1998) argued that dual-modality presentation should be resistant to interference from other ad content. Yet many psychological theories of information processing suggest that humans have a fixed pool of cognitive resources. For example, structural features of ads such as cuts, edits, narrator changes, and new music or scenes (all of which are classified as novel stimuli) can elicit automatic resource allocation to process that stimuli (Lang 2000; Lang et al. 2013). If scene, music, or character changes automatically prompt the allocation of cognitive resources to those elements, this would leave fewer cognitive resources available to process other elements in the ad, such as the superimposed text (thus resulting in poorer processing and retention of the risk information). Another exploration of distracting elements has focused on “seductive details” in education. These seductive details are text or images that are irrelevant to the learning material. Research suggests that these distractors can decrease attention and thus decrease individuals’ ability to remember or apply the information (Rey 2012; Rey 2014). Indeed, one study of credit card ads found that, in comparison to a visual disclosure, a dual-modality disclosure increased comprehension when no image or a still image was displayed; however, using a dual-modality disclosure had no effect when the credit card ad included music and scene changes (Thomas, Fowler, and Kolbe 2011).
Testing for mediation aids theory-building by expanding our knowledge of the mechanisms and processes through which advertising has its effects (Zhao, Lynch, and Chen 2010).We examined whether a behavioral measure of attention to the superimposed risk information mediates the relation between distracting visuals and retention of the risk information. It is also possible that distractors, by decreasing attention to the risk information, could alter perceptions of how likely or severe the risks are.
H3: In a dual-modality context, distracting elements during the risk statement decrease attention to the superimposed risk text, which accounts for the decrease in retention of the risk information and risk perceptions.
Also relevant to DTC advertising is the question of whether distracting elements during one segment of the ad can interfere with attention to and retention of information presented in a following segment. A study on the inclusion of the MedWatch statement in DTC television ads showed that approximately 15% of participants noticed the MedWatch statement and could report what it said when it was presented in text only (Aikin et al. 2016). However, that study did not test distraction or include a direct measure of attention. It is possible that distracting visuals during the risk statement could decrease consumers’ attention to superimposed text that follows the risk statement as well if they do not switch their attention back to the text.
H4: Distracting elements during the risk statement decrease attention to the MedWatch statement.
H5: Attention to the MedWatch statement increases retention of the MedWatch statement.
RQ1: Is attention to the MedWatch statement associated with retention of the risk information or risk perceptions?
The effects of dual-modality presentations, distraction, and the Medwatch statement have been studied separately; however, the effects of presenting these elements together in the same ad are unknown. Stakeholders repeatedly call for research on how separate policies may come together to affect DTC advertising (Federal Register Notice 2016). The current study is designed to examine how these multiple elements affect the communication of information in DTC television ads.
Methods
Participants
Participants were recruited from EyeTracking, Inc.’s opt-in panel across five cities representing various U.S. geographical areas (Chicago; Dallas; Phoenix; San Diego; and Washington, DC). To be eligible, participants had to be adults who self-reported needing to lose more than 30 pounds. They could not work in the health care, marketing, advertising, or pharmaceutical industries or wear bifocals or hard contact lenses to watch television (unless they could wear alternative glasses or soft contact lenses for the study). A total of 5,799 panelists were invited to participate; 1,807 responded to the invitation; 493 were eligible; and 301 consented to and participated in the study. One participant’s data were unusable, so the final sample was 300 participants.
Design
Two of the authors, along with an independent contractor, created a television ad for a fictional weight-loss drug (Escal). Consistent with real-world DTC television ads, the ad contained information about what condition the drug treats and a statement of important risks (major statement). The ad also contained superimposed text describing the risks that appeared on the screen at the same time as the audio risk information (e.g., “hip and spine fractures”). The MedWatch statement appeared on the bottom of the screen after the risk statement for 5 seconds. Participants were randomly assigned to view either a low-distractor ad or a high-distractor ad. To manipulate the level of distractors, we varied the visuals and sounds during the risk statement. In the low-distractor condition, a male actor (who was the main character throughout the ad) read the risk statement direct-to-camera while walking alone on a beach. With low distraction and a dual-modality presentation, this ad conformed to FDA’s dual modality consideration and FTC guidelines. In the high-distractor condition, the risk statement was read using a female voice-over while the visuals showed multiple quick-changing scenes on the beach, including the actor with his family, a volleyball game, someone throwing a football, someone else riding a horse, and the actor and his family petting the horse. A background music track was present in both ads, but the music was more up-tempo during the risk statement in the high-distractor ad. In addition, the high-distractor ad alone included other sounds during the risk statement, such as waves crashing and seagull calls. Both ads contained the same risk information presented in the audio and in the superimposed text.
The ads were developed using three sequential rounds of pretesting (with approximately 300 participants each)—with changes to the ads between each round—to ensure that participants rated the ads similarly high in quality and believability but differently in the amount of distraction during the risk statement. After these rounds of pretesting, a fourth pretest (N = 30) was conducted to provide a dry run of the main study and to identify any issues with the filler stimuli (two filler ads and a television clip) and the recruitment, screening, and data collection processes.
Procedure
We conducted in-person data collection in February and March 2015, with all study procedures approved by the appropriate ethics committees. Participants were seated in front of an eye tracker and computer monitor and tested individually. The monitor was a 24-inch HD LED television at a resolution of 1920 × 1080 chosen to most closely mimic a common interface that adults would typically use. Participants sat approximately 100 to 120 cm from the monitor and 60 to 70 cm from the eye tracker. FOVIO eye tracking equipment (manufactured by Seeing Machines) unobtrusively measured binocular eye movement at a sampling rate of 60 Hz, with no physical contact between the participant and the eye tracker.
To begin each testing session, we calibrated the eye tracker by asking participants to look at specific locations on the computer monitor—a process that usually took 1 to 2 minutes. Next, participants’ eye movements were tracked as they watched a 5.5-minute television clip, a 30-second filler ad, the 90-second study ad, and then a final 30-second filler ad (the presentation of the two filler ads was counterbalanced). The television clip was a non-health-related network news human interest story found in pretesting to be engaging. The filler ads were for non-health–related products. Finally, participants completed a questionnaire on a laptop, answering questions about their perceptions and retention of the information in the ad. They were not allowed to return to the ads or to previous questions while answering the questionnaire.
Measures
Attention to the Risk Text and the MedWatch Statement
Eye-tracking studies typically look at the amount of time each individual looks at defined features of an ad. These features are designated as regions or areas of interest (Holmqvist, Nyström, Andersson, Dewhurst, Jarodzka, and van de Weijer 2011). When an individual looks at an ad, one or more small unobtrusive cameras record all eye movements for one or both eyes. A simple calibration procedure ensures that the individual’s recorded gaze position is accurately mapped to the visual display at which he or she is looking. Recorded gaze position can be characterized by visual degrees or pixel units. When individuals attend to different elements or features in a visual display, they move their eyes to those features for at least a brief period of time (Duc, Bays, and Husain 2008). Attention is then measured by examining how often the eye data can be mapped into each region of interest. Either the data points themselves or an aggregation of data points that occur at the same location on the screen for a predetermined length of time (referred to as a fixation) may be measured.
We measured attention to the superimposed risk text during the risk statement and attention to the MedWatch statement using recorded gaze position data produced by the eye tracker. The present study reports gaze position in terms of horizontal and vertical pixel coordinates that show where the individual was looking at each recorded point in time with respect to the display being tracked (Holmqvist et al. 2011). A single region of interest was created for analysis. This region was a trapezoid that surrounded the text that appeared on the display. Both the risk text and the MedWatch statement appeared in the same area (roughly the lowest quarter of the television screen, extending from the left side to approximately the center of the screen), and their corresponding text-box regions were identical in location and size in both distractor conditions. The text box measured 1,245 pixels at the widest point (top of box); 1,085 pixels at the narrowest point (bottom of box); and 215 pixels in height. Only the text information ever appeared in this location. No other features of the ads could be seen in this location.
We created two variables to capture the percentage of attention given to the risk text and the attention given to the MedWatch statement. In each case, the variable was created by dividing the number of observations located in the region of interest by the total number of observations for the segment (defined as the portion of the ad during which the risk or MedWatch statement was displayed on the screen).
Risk Perceptions
Participants indicated how many people out of 100 would have a side effect from the drug (perceived risk likelihood—population). Participants then rated how likely it would be that they would experience side effects (1 = not at all likely, 7 = extremely likely; perceived risk likelihood—personal). Participants also indicated how serious the side effects would be (1 = most would not be serious, 7 = most would be very serious) and how bothersome the side effects would be (1 = not at all bothersome, 7 = extremely bothersome). We averaged these two items to create a measure of perceived risk magnitude (Cronbach’s α = 0.82). Finally, participants indicated their agreement with the statement, “I am certain that the good things about this medication outweigh the bad things” (1 = strongly disagree, 7 = strongly agree; risk-benefit assessment).
Risk Retention
We measured risk recall with an open-ended item asking participants to list the side effects and negative outcomes of the drug. We summed the number of correct responses (0–5 correct). We measured risk recognition by showing participants 10 statements about the risks of the drug (e.g., “Escal may increase heart rate”). Half the statements were true (i.e., mentioned in the ad) and half were not. Similarly, we measured contraindication recognition by showing participants 10 statements about the drug’s contraindications (e.g., “You should not take Escal if you have glaucoma”). Again, half were true and half were not. For both risk recognition and contraindication recognition, participants reported whether each statement was mentioned in the ad, not mentioned in the ad, or they did not know. Correct responses were summed to create a risk recognition score (0–10 correct) and a contraindication recognition score (0–10 correct).
MedWatch Retention
First, we asked participants if they noticed a statement about FDA in the ad. If they reported that they had, we next asked them to identify the correct reason for contacting FDA according to the statement (“to report side effects”) among six options. We created a MedWatch recall measure from these two questions, coded as correct (responded “yes” to the first question and chose the correct answer to the second question) or incorrect (responded “no” to the first question or chose the incorrect answer to the second question).
Demographics
Participants self-reported their age, gender, race/ethnicity, education, and height and weight (later used to calculate their body mass index [BMI]).
Analyses
All analyses were conducted using SPSS (Version 21; SPSS, Inc., an IBM Company, Chicago, IL) or SYSTAT (Version 13) statistical software. All tests of statistical significance were based on two-sided probability. Data analysis began with descriptive statistics and screening for outliers. Following previous research (e.g., Castellanos et al. 2009; Fleming, Bandy, and Kimble 2010), participants who were missing 25% or more of the eye-tracking data for the risk text and the MedWatch statement (n = 17, 5.7%) were excluded from analyses involving the attention variables. To test our research questions, we used analysis of variance (ANOVA), linear and logistic regression, and χ2 test. Finally, we conducted mediation analyses to test whether attention served as a mediator between level of ad distractors and recall. The simple mediation analyses were conducted using ordinary least squares path analysis using an SPSS macro developed by A. F. Hayes (PROCESS) (Hayes 2013). Output for these tests includes graphical models with unstandardized regression coefficients on the statistically significant links between variables along with the bias-corrected 95% bootstrap confidence intervals for the indirect effect that were generated using the PROCESS bootstrapping procedure with 1,000 replications (Hayes 2013).
Results
Table 1 presents the sample characteristics. The mean age of the sample was 44.4 years (SD = 11.9, range 19–72), and 65% were female. Participants were generally white, non-Hispanic (48%), with some college or an associate’s degree (42%). All participants self-reported needing to lose more than 30 pounds; based on the mean BMI, participants were on average considered obese (M = 34.32, SD = 7.21).
TABLE 1:
SAMPLE CHARACTERISTICS
| Demographics | N (%) |
|---|---|
| Age | |
| 18–34 | 71 (24%) |
| 35–44 | 75 (25%) |
| 45–54 | 94 (31%) |
| 55 or older | 60 (20%) |
| Gender | |
| Male | 105 (35%) |
| Female | 195 (65%) |
| Race/Ethnicity | |
| White (Non-Hispanic) | 140 (48%) |
| Black (Non-Hispanic) | 73 (25%) |
| Hispanic | 54 (19%) |
| Other (Non-Hispanic) | 25 (8%) |
| Education | |
| High school education or less | 38 (13%) |
| Some college or associate’s degree | 126 (42%) |
| Bachelor’s degree | 99 (33%) |
| Advanced degree | 37 (12%) |
| Body Mass Index | |
| 18.5 – 24.9 (Normal or healthy weight) | 11 (3.8%) |
| 25.0–29.9 (Overweight) | 74 (25.4%) |
| 30.0 and above (Obese) | 206 (70.8%) |
Notes: Eight participants did not report race/ethnicity information, and nine participants did not report body mass index information.
H1 was partially supported. Distracting elements during the risk text significantly affected retention of risk information. A one-way ANOVA found that risk recall (p = .001, η2 = .04) and risk recognition (p = .031, η2 = .02) differed significantly across the two distractor conditions (see Table 2). Participants in the high-distractor condition recalled and recognized significantly fewer risks than participants in the low-distractor condition. However, recognition of contraindications and risk perceptions (i.e., risk likelihood, risk magnitude, risk-benefit assessment) did not differ significantly by distractor condition.
TABLE 2:
MEANS (STANDARD ERRORS), PERCENTAGES, AND TEST STATISTICS FOR RISK-RELATED MEASURES BY AD CONDITION
| Measure | Low Level of Distractors M (SE) | High Level of Distractors M (SE) | Test Statistic | p-value |
|---|---|---|---|---|
| Attention | ||||
| Attention to risk text | 31.53 (1.74) | 24.66 (1.53) | F(1, 281) = 8.78 | .003 |
| Attention to MedWatch statement | 32.16 (2.53) | 27.13 (2.11) | F(1, 281) = 2.33 | .13 |
| Risk perceptions | ||||
| Perceived risk likelihood—population | 24.33 (1.42) | 26.66 (2.10) | F(1, 297) = .37 | .54 |
| Perceived risk likelihood—personal | 3.62 (.10) | 3.72 (.14) | F(1, 296) = .31 | .58 |
| Perceived risk magnitude | 4.09 (.14) | 3.99 (.13) | F(1, 288) = .28 | .60 |
| Risk-benefit assessment | 4.62 (.10) | 4.53 (.14) | F(1, 297) = .17 | .68 |
| Retention of risk information | ||||
| Risk recall | 1.43 (.10) | 0.99 (.09) | F(1, 298) =11.26 | .001 |
| Risk recognition | 3.92 (.18) | 3.35 (.20) | F(1, 298) = 4.68 | .03 |
| Contraindication recognition | 4.33 (.14) | 4.43 (.22) | F(1, 297) = .11 | .74 |
| MedWatch recall (% correct recall) | 19.1% | 12.2% | χ2(1, 300) = 2.72 | .10 |
Notes: Attention measures reflect the percentage of attention given to the risk text and the MedWatch statement, respectively. Perceived risk likelihood (population) was assessed on a scale of 0% (no people taking Escal will have side effects) to 100% (all people taking Escal will have side effects). Perceived risk likelihood (personal) was assessed on a scale of 1 (not at all likely [to experience any side effects or negative outcomes]) to 7 (extremely likely). Perceived risk magnitude was a composite measure ranging from 1 to 7, with higher scores equaling greater perceived severity of Escal’s side effects. The risk-benefit measure assessed how certain participants were that the good things about the medication outweighed the bad things, measured from 1 (strongly disagree) to 7 (strongly agree). Risk recall reported the number of correct risks recalled, from 0 (none correct) to 5 (maximum number correct). Risk and contraindication recognition are reported as the number of correct risks and contraindication recognized. Both items range from 0 (none correct) to 10 (all correct).
H2 posits that attention to the superimposed risk information increases retention of the risk information and risk perceptions. This hypothesis was also partially supported. Linear regression was used to test whether attention to the risk text was associated with increased retention of the risk information and increased perception of risk. Results from separate linear regression models, with attention to the risk text as the predictor and the respective risk retention and risk perception measures as the dependent variables, revealed that increased attention to the risk text was significantly associated with greater numbers of risks recalled (β = .31, p < .001) and more contraindications (β = .21, p < .001) and risks (β = .38, p < .001) recognized (Table 3). However, attention to the risk text was not a significant predictor of any of the four risk-perception measures.
TABLE 3:
LINEAR REGRESSION RESULTS: ATTENTION TO RISK TEXT PREDICTING RISK PERCEPTIONS AND RETENTION OF RISK INFORMATION
| Dependent Variablea | R2 | B | SE | Test Statistic | p-value |
|---|---|---|---|---|---|
| Risk perceptions | |||||
| Perceived likelihood—all people | 0 | −.00 | .07 | F(1, 281) = 0 | .98 |
| Perceived likelihood—personal | 0 | −.00 | .01 | F(1,280) = .70 | .40 |
| Perceived magnitude | 0 | .00 | .01 | F(1,273) = .47 | .49 |
| Risk-benefit assessment | .01 | .01 | .01 | F(1,281) = 1.72 | .19 |
| Retention of risk information | |||||
| Risk recall | .09 | .02 | .00 | F(1,258) = 26.74 | < .001 |
| Risk recognition | .14 | .05 | .01 | F(1,282) = 46.62 | < .001 |
| Contraindication recognition | .05 | .03 | .01 | F(1,281) = 13.23 | < .001 |
Each dependent variable was tested in a separate regression model with attention to risk text as the independent variable.
H3 posits that distracting elements during the risk statement decrease attention to the superimposed risk text, which accounts for the decrease in retention of the risk information. Supporting the first part of H3, one-way ANOVAs revealed that participants’ attention to the risk text differed significantly across the two distractor conditions (p = .003, η2 = .03). As predicted, participants spent a smaller percentage of time looking at the risk text in the high-distractor condition than in the low-distractor condition. Descriptively, two heat maps show the nature of participants’ viewing that occurred during the risk text presentation in the low-distractor condition (Figure 1) and in the high-distractor condition (Figure 2). As can be seen from the dispersion in the center of Figure 2, participants’ eyes were drawn to the many distracting elements in the high-distractor ad. In contrast, they stared directly at the face of the man in the low-distractor ad (who was shown in a close-up image walking down the beach) and viewed the risk text.
Figure 1.

Heat map for entire duration of the risk text for the low-distractor ad (n = 142). The heat maps utilize a blue-to-red scale to indicate the level of attention by the participants. Red indicates locations with the largest number of observations, followed by orange, yellow, and blue. The risk text appeared in the lower left corner of the screen.
Figure 2.
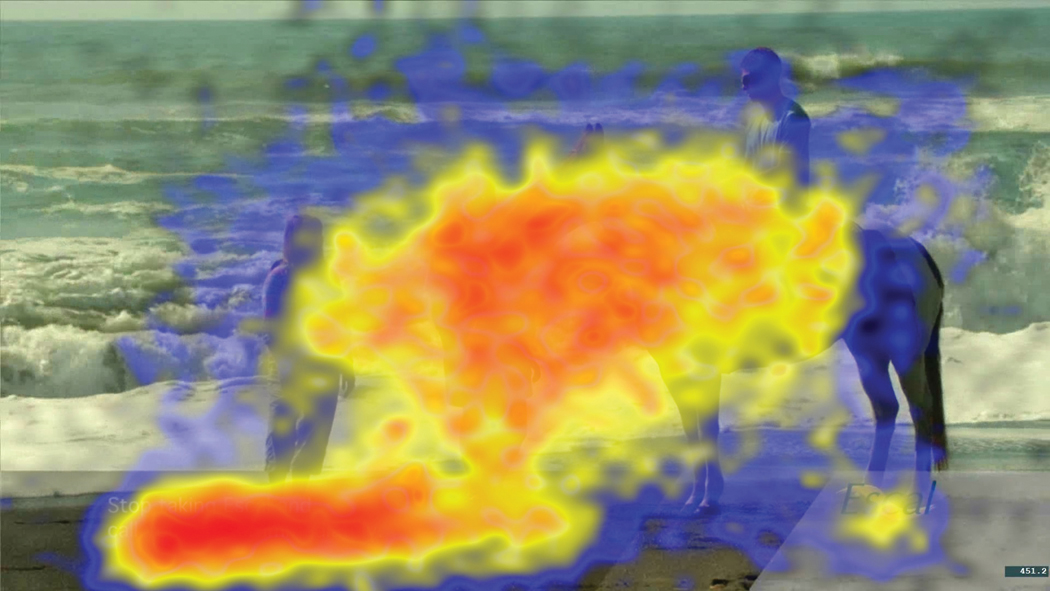
Heat map for entire duration of the risk text for the high-distractor ad (n = 141). The heat maps utilize a blue-to-red scale to indicate the level of attention by the participants. Red indicates locations with the largest number of observations, followed by orange, yellow, and blue. The risk text appeared in the lower left corner of the screen.
Mediation analyses with bootstrapping resampling procedures were used to examine whether attention to the risk text mediated the relationship between level of distractors and retention of the risk information. Separate simple mediation analyses conducted using ordinary least squares path analysis demonstrated that level of distractors indirectly influenced risk recall, risk recognition, and contraindication recognition through its effects on attention to risk text (Figure 3). Participants shown the high-distractor ad spent a smaller percentage of time paying attention to the risk text than those shown the low-distractor ad, and participants who spent a smaller percentage of time paying attention to the risk text had lower risk recall, risk recognition, and contraindication recognition. Bias-corrected bootstrap confidence intervals for the three indirect effects based on 10,000 bootstrap samples were below zero. For risk and contraindication recognition, there was no evidence that level of distractors influenced either outcome independently of its effect on attention (risk recognition: c’ = −.30, p = .26; contraindication recognition: c’ = −.34, p = .26). In contrast, attention to the risk text did not entirely account for the association observed between level of distractors and risk recall (c’ = −0.31, p = .02). Thus in support of H3 the decrease in attention to the superimposed risk text partially or completely accounted for the decrease in retention of the risk information. Mediation analyses with bootstrapping resampling procedures were nonsignficant when testing whether attention to the risk text mediated the relationship between level of distractors and risk perceptions.
Figure 3.
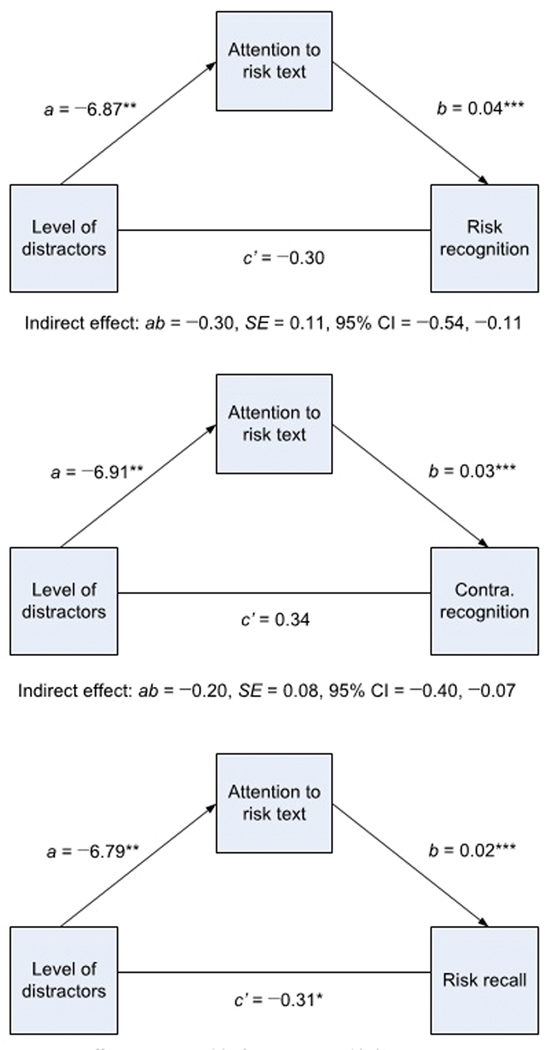
Mediation analyses for attention to the risk text mediating the relationship between level of distractors and risk recognition, contraindication recognition, and risk recall. An indirect effect was considered statistically significant at the 0.05 level if the 95% confidence interval for the estimate did not contain zero. Values are calculated through a bootstrapping routine with 10,000 replications. (**p < .01, ***p < .001; CI: confidence interval; Contra.: contraindication; MW: MedWatch statement; a: Unstandardized coefficient of level of distractors [0 = low; 1 = high] on attention to the risk text; b: Unstandardized coefficient of attention to risk text on retention of risk information variable; c’: Unstandardized coefficient of level of distractors [0 = low; 1 = high] on retention of risk information variable; ab: Indirect effect).
H4 was not supported: attention to the MedWatch statement and recall of the MedWatch statement did not differ significantly by distractor ad condition in one-way ANOVAs.
Logistic regression revealed that attention to the MedWatch statement was positively associated with improved MedWatch recall, supporting H5 (OR: 1.02; 95% CI: 1.01, 1.04, Wald χ2 (1) = 16.89, p < .001).
Finally, we explored whether attention to the MedWatch statement affected retention of risk information or risk perceptions (RQ1). Separate linear regression analyses were conducted for each dependent variable. To control for attention to the risk text, it was entered in the first step of the model. Attention to the MedWatch statement was entered in the second step. The results of these analyses suggest that attention to the MedWatch statement (controlling for attention to the risk text) was only significantly associated with one measure of risk perception (i.e., risk-benefit association). Namely, attention to the MedWatch statement was positively associated with perceptions that the positive attributes of the drug outweighed the negative ones (β = .21, p < .001).
Discussion
Previous studies have found that dual-modality increases the retention of risk information in DTC television ads (Glinert and Schommer 2005; Morris, Mazis, and Brinberg 1989; Wogalter, Shaver, and Kalsher 2014). In the current study, we presented risk information using dual-modality and found that visual attention to superimposed risk text led to higher retention of the risk information. We used eye-tracking as an unobtrusive behavioral measure of attention (Wedel and Pieters 2008). To our knowledge, this is the largest eye-tracking study to date to explore attention to, and retention of, risk information. Although we did not test attention to the voice-over, our finding demonstrates a method by which dual-modality improves retention of information (i.e., increasing attention maintenance to the text; Conzola and Wogalter 2001; Wogalter 2006), thus adding to the support for using dual-modality to present risk information in DTC television ads, as proposed by FDA (2010). Without the superimposed risk text, viewers do not have the opportunity to read the information. Because reading the information, as suggested by time spent viewing the risk text, is related to retention, it appears that the ability to see and read the risk information increases viewers’ ability to remember the information.
Although we found that attention to superimposed risk text increased retention of risk information, we also found that distracting elements during risk presentation drew attention away from the superimposed risk text (i.e., decreasing attention maintenance to the text; Conzola and Wogalter 2001; Wogalter 2006) and, in turn, reduced retention of drug risk information. This study extends our knowledge beyond previous research that showed that distractors in an ad lead to lower retention. These results help us gain insight into why the distractors lead to lower retention even in a dual-modality context: because the distractors draw attention away from the risk text. Testing two policy-related issues at once allows us to see that including risk text does not negate the effects of the distracting elements. This suggests that distracting visuals presented at the same time can interfere with the potential benefits of dual-modality presentations.
One interesting finding is that recognition of the drug’s contraindications was not significantly affected by the level of distractors; however, attention to the superimposed risk text did increase contraindication recognition. This finding may be a result of the order in which the risk information was conveyed in the ad. The contraindications were listed first in the risk statement, followed by other risk information. Participants, regardless of distractor ad condition, may have started off reading the superimposed text and then were distracted (or not) later in the risk statement.
Although the risk statements were identical in content across distractor ad conditions, it is possible that attention to the distracting visuals could downplay the severity or likelihood of risks. However, we found no support for the idea that distractors affected risk perceptions. We also found that attention to the superimposed risk text had no effect on risk perceptions. This is similar to previous studies on DTC ads that found differences in retention but not perceptions (e.g., O’Donoghue et al. 2014). It is possible that perceptions are more dependent on other factors, such as general attitudes toward prescription drugs or one’s own health status. It is also possible that perceptions are not easily changed by one ad viewing and may only be influenced by a series of exposures to an ad such as in a typical advertising campaign.
We also presented additional information (the MedWatch statement) in superimposed text after the risk statement. Distracting visuals were not present during the display of the MedWatch statement. As expected, attention to the MedWatch statement increased retention of the MedWatch information; however, there were no other significant effects related to the MedWatch statement. These results suggest that the impact of distracting images is time-sensitive. Interestingly, the distractors do not appear to have a trickle-over effect wherein the viewer is more likely to ignore subsequent superimposed text. Visual distracting elements may cause viewers to switch their attention from the text to the distractors, but they are able to switch their attention back to the text when the distraction subsides.
Limitations
This study had a few limitations. First, although we sought to create realistic ad-viewing by embedding the ad with a television clip and two other ads, it is possible that participants viewed the ad differently in a study environment than they would under their normal viewing circumstances. Second, our participants were recruited from an opt-in panel, and therefore our sample was not nationally representative. Third, we chose an ad for one medical condition (obesity) and one ad execution. We chose this medical condition because obesity is common and individuals are often motivated to lose weight, making the ad potentially personally relevant. However, it is possible that the results would vary depending on the medical condition, the population at risk or affected by the medical condition, or the severity of the drug’s risks. We encourage replication of this study with other medical conditions and populations to test the generalizability of the results. Fourth, although we included audio distractors in the high-distractor ad, our eye tracking measures focused on visuals. More research is needed on the role of audio distraction. Finally, in our low-distractor condition, the protagonist spoke directly to the camera, whereas in the high-distractor condition, a voice-over played while busy scenes emerged. Research has shown that viewing a talking face improves comprehension of the language (Massaro 1998). One explanation for this is that face appearance can have an orienting effect, meaning that it could enhance attention to ideas relayed by the person whose face is depicted or could distract from other material (Southwell 2005). Although our analyses suggest that attention to the risk text mediated the relation between the level of distractors and retention, we did not manipulate the presence or absence of a talking face in the low-distractor condition and thus cannot comment on this possibility. Future research should help to detangle these effects.
Implications for Policy
Consumers’ understanding of information in DTC advertising is critical because this advertising can affect the medical treatments they seek out and are prescribed (Daubresse et al. 2015; Gilbody, Wilson, and Watt 2005; Kim et al. 2016; Mintzes 2012; Niederdeppe et al. 2013). This research supports FDA’s efforts to ensure that risk information in DTC television advertising is communicated in a clear, conspicuous, and neutral manner.
This study provides additional support for the FDA’s (2010) consideration and FTC’s (1970) guidelines regarding dual-modality presentations. Although we did not directly assess dual-modality versus single-modality, eye tracking allowed us to measure exactly where and for how long viewers actually looked at the superimposed risk text, enabling us to assert confidently that those who looked at the risk text retained more risk information. Coupled with previous research, the advantage of dual-modality is clear. However, our study suggests that even when dual-modality is used, distracting visuals should still be avoided. Despite the availability of risk text, viewers in the high-distractor condition spent less time looking at the text and consequently retained less risk information. Thus, the advantage of utilizing dual-modality techniques in the presentation of risk information can be diminished by introducing flashy, compelling visuals, multiple scene changes, and faster music. All of these characteristics should be considered carefully to ensure that prescription drug information is presented clearly and without distracting elements in DTC television ads. The introduction of dual-modality in DTC television ads should not deter regulators from continuing to enforce fair balance regulations when competing visuals and audio are presented along with the risk information.
We also examined the inclusion of the MedWatch statement (Congress 2007). Overall, approximately 16% of participants correctly recalled the MedWatch statement after one viewing of an embedded ad. Exposing more consumers to the statement via multiple DTC television ads could increase consumers’ knowledge of the MedWatch information, even if distraction is present in other parts of the ad, thus having a positive effect on consumers’ ability to report drug safety concerns. Similar to Aikin et al. (2016), we found that attention to the MedWatch statement did not decrease risk retention, supporting the inclusion of the MedWatch statement in DTC television ads.
This study tested three policy-related issues together in one DTC television ad: dual-modality presentations, the effects of distraction, and the inclusion of the MedWatch statement. In doing so, the study replicated and extended past research on the effect of distractors to the context of DTC television ads. It provided a behavioral measure of attention, demonstrating that the way in which distractors lead to lower retention is by diverting attention away from written risk information. Further, these results suggest that implementing dual-modality in DTC television ads should not lessen regulators’ concerns about distractors presented along with a prescription drug’s risks in DTC television ads. Finally, the results support the inclusion of the MedWatch statement in DTC television ads, regardless of whether it is preceded by distractors. The lessons and findings from other studies on television advertising disclosures can inform this study just as findings from this study can inform research on broader product categories (such as truth-in-lending disclosures). This study highlights the fact that individual policies do not occur in a vacuum. The findings from this research should provide policy-makers and industry with a better idea of how separate policies can affect consumer understanding when presented in combination.
Acknowledgments
The authors would like to thank Better World Advertising and Ipsos for their help in constructing and pretesting the study stimuli and to thank Cassie Davis at EyeTracking, Inc. for her assistance with data collection and helpful feedback during the study.
Footnotes
Financial Disclosure
The study was funded by the Office of Prescription Drug Promotion, U.S. Food and Drug Administration.
References
- Aikin Kathryn J., O’Donoghue Amie C., Squire Claudia M., Sullivan Helen W., and Betts Kevin R. (2016), “An empirical examination of the FDAAA-mandated “toll-free statement” for consumer reporting of side effects in direct-to-consumer television advertisements,” Journal of Public Policy and Marketing, 35(1), 108–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery Rosemary J., Eisenberg Matthew, and Simon Kosali I. (2012), “Fair balance in direct-to-consumer antidepressant print and television advertising, 1995–2007,” Journal of Health Communication, 17, 250–277. [DOI] [PubMed] [Google Scholar]
- Brasel S. Adam, and Gips James (2008), “Points of view: where do we look when we watch TV?,” Perception, 37, 1890–1894. [DOI] [PubMed] [Google Scholar]
- Brewer Neil, Harvey Sophie, and Semmler Carolyn (2004), “Improving comprehension of jury instructions with audio-visual presentation,” Applied Cognitive Psychology, 18(6), 765–776. [Google Scholar]
- Cassidy Gianna and MacDonald Raymond A.R. (2007), “The effect of background music and background noise on the task performance of introverts and extraverts,” Psychology of Music, 35, 517–537. [Google Scholar]
- Castellanos EH, Charboneau E, Dietrich MS, Park S, Bradley BP, Mogg K, and Cowan RL (2009), “Obese adults have visual attention bias for food cue images: evidence for altered reward system function,” International Journal of Obesity, 33(9), 1063–1073. [DOI] [PubMed] [Google Scholar]
- Chung Kevin K.H. (2008), “What effect do mixed sensory mode instructional formats have on both novice and experienced learners of Chinese characters?” Learning and Instruction, 18(1), 96–108. [Google Scholar]
- Congress, U. S. (2007), “Food and Drug Administration Amendments Act of 2007,” Public Law, 115–85. [Google Scholar]
- Conzola Vincent C. and Wogalter Michael S. (2001), “A communication–human information processing (C–HIP) approach to warning effectiveness in the workplace,” Journal of Risk Research, 4, 309–322. [Google Scholar]
- Daubresse Matthew, Hutfless Susan, Kim Yoonsang, Kornfield Rachel, Qato Dima M., Huang Jidong, Miller Kay, Emery Sherry L., and Alexander G. Caleb (2015), “Effect of direct-to-consumer advertising on asthma medication sales and healthcare use,” American Journal of Respiratory and Critical Care Medicine, 192(1), 40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duc Albert, Bays Paul, and Husain Masud (2008), “Eye movements as a probe of attention,” In Kennard C and Leigh RJ (Eds.), Using Eye Movements as an Experimental Probe of Brain Function, Volume 171, (pp. 403–411). Amsterdam, The Netherlands: Elsevier Science; [DOI] [PubMed] [Google Scholar]
- Duchowski Andrew T. (2007), Eye Tracking Methodology: Theory and Practice. London: Springer. [Google Scholar]
- Filik Ruth, Purdy Kevin, Gale Alastair, and Gerrett David (2004), “Drug name confusion: evaluating the effectiveness of capital (“Tall Man”) letters using eye movement data,” Social Science & Medicine, 59, 2597–2601. [DOI] [PubMed] [Google Scholar]
- Fleming Kevin K., Bandy Carole L., and Kimble Matthew O. (2010), “Decisions to shoot in a weapon identification task: the influence of cultural stereotypes and perceived threat on false positive errors,” Social Neuroscience, 5(2), 201–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Drug Administration (2010), “Direct-to-consumer prescription drug advertisements; presentation of the major statement in television and radio advertisements in a clear, conspicuous, and neutral manner,” Federal Register, 75(59), 15376–15387. [Google Scholar]
- Food and Drug Administration (2015), “Untitled letter to Luitpold Pharmaceuticals, Inc,” (January 29), (accessed October 13, 2016), [available at http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/EnforcementActivitiesbyFDA/WarningLettersandNoticeofViolationLetterstoPharmaceuticalCompanies/ucm432949.htm#OPDP].
- Food and Drug Administration (2016), “Animation in direct-to-consumer advertising,” Federal Register, 81(41), 10867–10870. [Google Scholar]
- Frick Robert W. (1984), “Using both an auditory and a visual short term store to increase digit span,” Memory and Cognition, 12, 507–514. [DOI] [PubMed] [Google Scholar]
- Frosch Dominick L., Krueger Patrick M., Hornik Robert C., Cronholm Peter F., and Barg Frances K. (2007), “Creating demand for prescription drugs: a content analysis of television direct-to-consumer advertising,” Annals of Family Medicine, 5(1), 6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FTC (1970), “Commission enforcement policy statement in regard to clear and conspicuous disclosures in television advertising,” (accessed October 13, 2016), [available at https://www.ftc.gov/system/files/documents/public_statements/288851/701021tvad-pr.pdf].
- FTC (2013), “.com disclosures: How to make effective disclosures in digital advertising,” (accessed October 13, 2016), [available at https://www.ftc.gov/sites/default/files/attachments/press-releases/ftc-staff-revises-online-advertising-disclosure-guidelines/130312dotcomdisclosures.pdf].
- Gilbody S, Wilson P, and Watt I (2005), “Benefits and harms of direct to consumer advertising: a systematic review,” Quality and Safety in Health Care, 14(4), 246–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinert Lewis H., and Schommer Jon C. (2005), “Television advertisement format and the provision of risk information about prescription drug products,” Research in Social and Administrative Pharmacy, 1(2), 185–210. [DOI] [PubMed] [Google Scholar]
- Hayes Andrew F. (2013), Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. New York, NY: Guilford. [Google Scholar]
- Higgins Emily, Leinenger Mallorie, and Rayner Keith (2014), “Eye movements when viewing advertisements,” Frontiers in Psychology, 5, 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmqvist Kenneth, Nyström Marcus, Andersson Richard, Dewhurst Richard, Jarodzka Halszka, and van de Weijer Joost (2011), Eye Tracking, A Comprehensive Guide to Methods and Measures. New York, NY: Oxford University Press. [Google Scholar]
- Hoy Mariea Grubbs, and Andrews J. Craig (2004), “Adherence of prime-time televised advertising disclosures to the “Clear and Conspicuous” standard: 1990 versus 2002,” Journal of Public Policy and Marketing, 23(2), 170–182. [Google Scholar]
- Hoy Mariea Grubbs, and Park Jin Seong (2014), “Principles in action: an examination of Food and Drug Administration letters involving violative internet promotions from 1997 to 2012,” Journal of Public Policy and Marketing, 33, 127–142. [Google Scholar]
- Hoyer Wayne D., Srivastava Rajendra K., and Jacoby Jacob (1984), “Sources of miscomprehension in television advertising,” Journal of Advertising, 13(2), 17–26. [Google Scholar]
- Kaphingst Kimberly A., DeJong William, Rudd Rima E., and Daltroy Lawren H. (2004), “A content analysis of direct-to-consumer television prescription drug advertisements,” Journal of Health Communication, 9, 15–528. [DOI] [PubMed] [Google Scholar]
- Kim Yoonsang, Kornfield Rachel, Shi Yaru, Vera Lisa, Daubresse Matthew, Alexander G. Caleb, and Emery Sherry (2016), “Effects of televised direct-to-consumer advertising for varenicline on prescription dispensing in the United States, 2006–2009,” Nicotine & Tobacco Research, 18, 1180–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang Annie (1995), “Defining audio/video redundancy from a limited-capacity information processing perspective,” Communication Research, 22(1), 86–115. [Google Scholar]
- Lang Annie (2000), “The limited capacity model of mediated message processing,” Journal of Communication, 50(1), 46–70. [Google Scholar]
- Lang Annie, Kurita Satoko, Gao Ya, and Rubenking Bridget (2013), “Measuring television message complexity as available processing resources: dimensions of information and cognitive load,” Media Psychology, 16(2), 129–153. [Google Scholar]
- Macias Wendy, Pashupati Kartik, and Lewis Liza S. (2007), “A wonderful life or diarrhea and dry mouth? Policy issues of direct-to-consumer drug advertising on television,” Health Communication, 22, 241–252. [DOI] [PubMed] [Google Scholar]
- Massaro Dominic W. (1998), Perceiving Talking Faces: From Speech Perception to a Behavioral Principle, Cambridge, MA: MIT Press, Bradford Books. [Google Scholar]
- Mintzes Barbara. (2012), “Advertising of prescription-only medicines to the public: does evidence of benefit counterbalance harm?,” Annual Review of Public Health, 33, 259–277. [DOI] [PubMed] [Google Scholar]
- Morris Louis A., Mazis Michael B., and Brinberg David (1989), “Risk disclosures in televised prescription drug advertising to consumers,” Journal of Public Policy and Marketing, 8, 64–80. [Google Scholar]
- Murray Noel M., Manrai Lalita A., and Manrai Ajay K., (1998), “How super are video supers? A test of communication efficacy,” Journal of Public Policy and Marketing, 17(1), 24–34. [Google Scholar]
- Niederdeppe Jeff, Byrne Sahara, Avery Rosemary J., and Cantor Jonathan (2013), “Direct-to-consumer television advertising exposure, diagnosis with high cholesterol, and statin use,” Journal of General Internal Medicine, 28(7), 886–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes Steve and North Adrian C. (2006), “The impact of background musical tempo and timbre congruity upon ad content recall and affective response,” Applied Cognitive Psychology, 20(4), 505–520. [Google Scholar]
- O’Donoghue Amie C., Sullivan Helen W., Aikin Kathryn J., Chowdhury Dhuly, Moultrie Rebecca R., and Rupert Douglas J. (2014), “Presenting efficacy information in direct-to-consumer prescription drug advertisements,” Patient Education and Counseling, 95, 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieters Rik, Wedel Michel, and Batra Rajeev (2010), “The stopping power of advertising: Measures and effects of visual complexity,” Journal of Marketing, 74(5), 48–60. [Google Scholar]
- Prescription Drug Advertisements, 21 C.F.R. § 202.1 (2012).
- Rey Gunter D. (2012), “A review of research and a meta-analysis of the seductive detail effect,” Educational Research Review, 7, 216–237. [Google Scholar]
- Rey Gunter D. (2014), “Seductive details and attention distraction–an eye tracker experiment,” Computers in Human Behavior, 32, 133–144. [Google Scholar]
- Royne Marla B. and Myers Susan D. (2008), “Recognizing consumer issues in DTC pharmaceutical advertising,” Journal of Consumer Affairs, 42, 60–80. [Google Scholar]
- Simola Jaana, Kuisma Jarmo, Öörni Anssi, Uusitalo Liisa, and Hyönä Jukka (2011), “The impact of salient advertisements on reading and attention on web pages,” Journal of Experimental Psychology: Applied, 17(2), 174–190. [DOI] [PubMed] [Google Scholar]
- Sheehan Kim B. (2003), “Balancing acts: An analysis of Good and Drug Administration letters about direct-to-consumer advertising violations,” Journal of Public Policy and Marketing, 22(2), 159–169. [Google Scholar]
- Southwell Brian G. (2005), “Information Overload? Advertisement editing and memory hindrance,” Atlantic Journal of Communication, 13, 26–40. [Google Scholar]
- Stewart David W. and Martin Ingrid M. (1994), “Intended and unintended consequences of warning messages: A review and synthesis of empirical research,” Journal of Public Policy and Marketing, 13(1), 1–19. [Google Scholar]
- Sundar Raghav Prashant, Becker Mark W., Bello Nora M., and Bix Laura (2012), “Quantifying age-related differences in information processing behaviors when viewing prescription drug labels,” PLoS ONE, 7, e38819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavassoli Nader T. and Lee Yih H. (2003), “The differential interaction of auditory and visual advertising elements with Chinese and English,” Journal of Marketing Research, 40, 468–480. [Google Scholar]
- Teixeira Thales S., Wedel Michael, and Pieters Rik (2010), “Moment-to-moment optimal branding in TV commercials: preventing avoidance by pulsing,” Marketing Science, 29, 783–804. [Google Scholar]
- Thomas Veronica, Fowler Kendra, and Kolbe Richard H. (2011), “The implications of the FTC’s clear and conspicuous standards for the communication of credit card information to young consumers,” Journal of Financial Services Marketing, 16, 195–209. [Google Scholar]
- Thomsen Steven, and Fulton Kristi (2007), “Adolescents’ attention to responsibility messages in magazine alcohol advertisements: An eye-tracking approach,” Journal of Adolescent Health, 41, 27–34. [DOI] [PubMed] [Google Scholar]
- Walma van der Molen Julietter H., and Klijn Marlies E. (2004), “Recall of television versus print news: retesting the semantic overlap hypothesis,” Journal of Broadcasting and Electronic Media, 48(1), 89–107. [Google Scholar]
- Wedel Michel and Pieters Rik (2008), “A review of eye-tracking research in marketing,” in Review of Marketing Research, Vol. 4, Malhotra Naresh K., ed. Armonk, New York: M.E. Sharpe, 123–147. [Google Scholar]
- Wogalter Michael S. (2006), “Communication-human information processing (C-HIP) model,” Handbook of Warnings, 51–61. [Google Scholar]
- Wogalter Michael S., Shaver Eric F., and Kalsher Michael J. (2014), “Effect of presentation modality in direct-to-consumer (DTC) prescription drug television advertisements,” Applied Ergonomics, 45(5), 1330–1336. [DOI] [PubMed] [Google Scholar]
- Yarbus A (1967), Eye Movements and Vision. New York, NY: Plenum Press. [Google Scholar]
- Zhao Xinshu, Lynch John G. Jr., and Chen Quimei (2010), “Reconsidering Baron and Kenny: myths and truths about mediation analysis,” Journal of Consumer Research, 37, 197–206. [Google Scholar]


