Sir,
Hydroxychloroquine (HCQ) has been widely used to treat infection with SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) but most recently has been shown to be of no benefit in a large randomised clinical trial [1]. Detailed information on the pharmacodynamics of HCQ is lacking, but measures of the in vitro activity of HCQ against SARS-CoV-2 are available as well as pharmacokinetic data derived from analysis of patients with conditions other than SARS-CoV-2 infection who received HCQ. HCQ is much less active against SARS-CoV-2 in vitro than it is against Plasmodium spp. Pharmacokinetic data from patients with SARS-CoV-2 are sparse and are absent for those outside of critical care [2]. Here we report data on the serum concentrations of HCQ in patients who were recruited into the Randomised Evaluation of COVID-19 Therapy (RECOVERY) trial. Permission to take and use stored serum was ethically approved as part of the Diagnostic and Severity markers in COVID-19 to Enable Rapid triage (DISCOVER) study. Patients received HCQ at a dose of 800 mg 6 h apart, then 400 mg every 6 h later followed by 400 mg every 12 h subsequently.
HCQ was assayed using a recently developed liquid chromatography–tandem mass spectrometry (LCMS/MS) method. Serum was deproteinised with acetonitrile after addition of the internal standard (chloroquine). The method was validated over a working range of 0.005–1 mg/L. There was no evidence of drug loss in gel separator tubes compared with plain tubes or after 24 h at room temperature. The mean blood/plasma partitioning ratio was 1.64 ± 0.25.
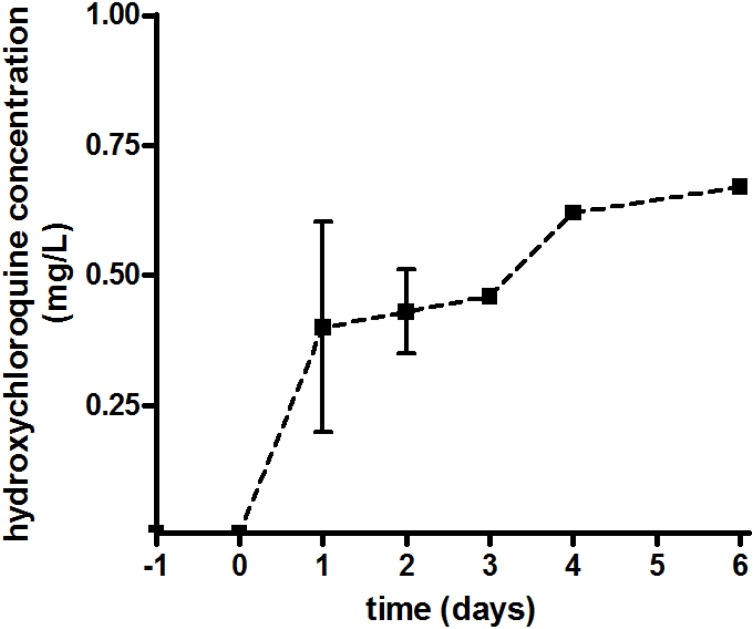
Seven patients were recruited into the HCQ treatment arm. Subsequently one patient (male, aged 58 years) had no blood samples drawn and a second patient (male, aged 84 years) only received a single dose of 800 mg. Of the five remaining patients, two were male and three were female with a mean ± standard deviation (range) age of 57.4 ± 20.7 years (26–83 years) and weight of 70.6 ± 4.2 kg (65.4–75.0 kg). All patients had an estimated glomerular filtration rate (eGFR) >80 mL/min on Day 0, and none had serum bilirubin, alkaline phosphatase or alanine aminotransferase enzyme levels more than three times the normal level. Three of the five patients had co-morbidities, namely cardiac, neurological or neoplastic disease. All HCQ serum concentrations were ≤0.005 mg/L either before or on the first day of dosing. Serum concentrations on subsequent days after starting treatment are shown in Fig. 1 .
Fig. 1.
Serum concentrations of hydroxychloroquine.
Concentrations on Days 1, 2, 3 and 4 were 0.4 ± 0.35 mg/L (n = 3), 0.43 ± 0.14 mg/L (n = 3), 0.46 mg/L (n = 2) and 0.62 mg/L (n = 2), respectively.
The timing of sampling after oral dosing was variable, being 2.9 ± 3.5 h (0.12–8.5 h), although three-quarters were taken within 4 h of the dose being administered.
Data on the pharmacokinetics of HCQ have been accumulated in patients with conditions other than SARS-CoV-2 infection. The European summary of product characteristics (SmPC) suggests that after a single 400 mg dose, the peak serum concentration was 0.105 mg/L at 1.8 h post-dose [3]. Pharmacokinetic and modelling data exist for SARS-CoV2-infected patients. Yoa et al. simulated several potential doses of HCQ, suggesting that in Chinese subjects (usually smaller and lighter than European or North American patients) that a dose of 400 mg twice daily and 200 mg twice daily may be optimal [4]. Interestingly, mean serum HCQ concentrations were not predicted to be >1 mg/L until 4–5 days of therapy with this regimen [4]. Downes et al. simulated doses more akin to those used in the RECOVERY trial, i.e. 1600 mg on Day 1 and 400 mg/day thereafter [5]. The predicted maximum concentration (C max) (interquartile range) was 2.7 mg/L (2.2–3.3 mg/L) and area under the concentration–time curve (AUC) was 109.9 mg/L h. Perinel et al. performed a study of HCQ serum concentrations in French patients in critical care receiving 200 mg three times daily; most were mechanically ventilated [2]. It took 2–7 days for serum concentrations to reach 1 mg/L. They also suggested a reference range of >1 mg/L and <2 mg/L but only 8 of 13 patients reached these concentrations [2]. HCQ dosing in the RECOVERY trial is higher than those in these studies and, despite this, all of our patients, none of whom were receiving critical care, had a HCQ concentration of <1 mg/L, which is a concentration well below the reported EC50 values for HCQ against SARS-CoV-2 in vitro [6]. It may be expected that patients not receiving critical care may have better absorption than those in the intensive care unit.
There are a number of limitations of our study, most obviously the small number of patients and samples available. However, our data indicate that HCQ concentrations are lower than might be expected, even with a high-dose regimen.
Given that HCQ is likely to have marginal pharmacodynamics in SARS-CoV-2 infection, these data may in part help explain the lack of benefit in randomised clinical trials and also further question its potential use in prophylaxis.
Funding
This study was supported by the Severn Infection Sciences Partnership, Southmead Research Foundation and North Bristol NHS Trust.
Competing interests
Bristol Centre for Antimicrobial Research & Evaluation (BCARE) receives grant funding for research on antimicrobials from Paratek, Wockhardt, InfectoPharm, Venatorx, Merck, AiCURIS, NosoPharm, Evotec, IMI JU, COMBACTE MAGNET and GNA NOW, MRC(UK) and NIHR(UK).
Ethical approval
Not required.
References
- 1.Statement from the Chief Investigators of the Randomised Evaluation of COVid-19 thERapY (RECOVERY) Trial on hydroxychloroquine, 5 June 2020. https://www.recoverytrial.net/files/hcq-recovery-statement-050620-final-002.pdf [Accessed 15 January 2021].
- 2.Perinel S., Launay M., BotelhoNevers E., Diconne E., Louf-Durier A., Lachand R. Towards optimization of hydroxychloroquine dosing in intensive care unit COVID19 patients. Clin Infect Dis. 2020;71:2227–2229. doi: 10.1093/cid/ciaa394/5816960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Electronic Medicines Compendium (EMC) Bristol Laboratories Ltd.; 2017. Summary of product characteristics (SmPC). Quinoric 200mg film-coated tablets.https://www.medicines.org.uk/emc/product/477/smpc [Google Scholar]
- 4.Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) Clin Infect Dis. 2020;71:732–739. doi: 10.1093/cid/ciaa237/5801998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Downes K.J., Chiotos K., Fitzgerald J.C., Scheetz M.H., Zuppa A.F. Rational dosing of hydroxychloroquine for treatment of COVID19. Open Science Framework PrePrints doi: 10.31219/osf.io/py3kv.
- 6.Clementi N., Criscuolo E., Diotti R.A., Ferrarese R., Castelli M., Dagna L. Combined prophylactic and therapeutic use maximises hydroxychloroquine anti-SARS-CoV-2 effects in vitro. Front Microbiol. 2020;11:1704. doi: 10.3389/fmicb.2020.01704. [DOI] [PMC free article] [PubMed] [Google Scholar]