Abstract
Primary aldosteronism (PA) is the most common form of endocrine hypertension. Agonistic autoantibodies against the angiotensin II type 1 receptor (AT 1 R-Abs) have been described in transplantation medicine and women with pre-eclampsia and more recently in patients with PA. Any functional role of AT 1 R-Abs in either of the two main subtypes of PA (aldosterone-producing adenoma or bilateral adrenal hyperplasia) requires clarification. In this review, we discuss the studies performed to date on AT 1 R-Abs in PA.
Key words: primary aldosteronism, angiotensin II receptor, autoantibodies, endocrine hypertension, adrenal cortex
Introduction
Primary aldosteronism (PA) displays a prevalence of around 5–10% in patients with hypertension increasing up to 20% in patients with treatment-resistant hypertension 1 2 3 . Patients with PA have an elevated risk of cerebrovascular and cardiovascular events relative to patients with hypertension with matched cardiovascular risk profiles 4 5 6 . Different subtypes of PA have been defined which affect one or both adrenal glands. The main subtypes are a unilateral aldosterone-producing adenoma (APA) or bilateral adrenal hyperplasia (BAH, also called idiopathic hyperaldosteronism). Other sporadic forms include unilateral adrenal hyperplasia and the very rare occurrences of aldosterone-producing carcinoma. Hereditary forms of familial hyperaldosteronism are rare and the genetic basis of the 4 described forms of familial hyperaldosteronism (FH types I–IV) has been identified 7 8 . A number of somatic mutations, mainly in ion channels and ATPases, have been reported which are likely to drive the aldosterone excess in the majority of APAs. Increasing interest in autoantibodies led to studies reporting a potential role for autoantibodies against the G-protein-coupled receptor (GPCR) angiotensin II type 1 receptor in PA.
G-Protein-Coupled Receptors
The largest superfamily of receptors in the human genome are GPCRs that are located in the plasma membrane of nearly all cell types 9 . The research of Kobilka and Lefkowitz about the molecular structure and function of GPCRs, especially β2-adrenergic G-protein-coupled receptors, was rewarded in 2012 by the Nobel prize in chemistry 10 . GPCRs are responsible for signal transduction to regulate numerous essential functions by mediating extracellular signals from hormones, neurotransmitters or environmental stimulants to the intracellular metabolic pathways 9 . Their structure consists of seven transmembrane-spanning helices bound by intra- and extracellular loops 11 . On the extracellular side, GPCRs are targeted by their ligands but also by autoantibodies, which may induce agonistic receptor stimulation or inhibition dependent on the autoantibody binding site to the first and second or third extracellular loops, respectively 9 .
Binding of extracellular agonists activate the receptor by initiating a conformational change that induce further signal transduction pathways 12 . Conformational changes result in the hetero-trimeric G-protein to exchange GDP for GTP at its Gα subunit 13 . The Gα subunit with GTP and the Gβγ subunit both dissociate from the receptor, resulting in the activation of specific signaling pathways such as, adenylyl cyclase (via cAMP generation) and phospholipase C [via diacylglycerol (DAG) and inositol trisphosphate (IP 3 ) production] 13 . The Gα subunit bound to GTP hydrolyses GTP back to GDP to reassociate with the Gβγ subunit 13 . Receptor signaling determination is mediated by G-protein-coupled receptor kinases (GRK) which phosphorylate the activated receptor to bind to a distinct scaffold protein for subsequent internalization into the cells 13 . These scaffold proteins are arrestins and are known to desensitize GPCRs and induce G-protein independent signaling 14 . Following internalization the receptor is either degraded or recycled back to the plasma membrane 13 . Besides its involvement in GPCR internalization, arrestins are able to interact directly with activated GPCRs resulting in a clear conformational change that could initiate further downstream signaling pathways 13 . There is some evidence for biased agonism of GPCRs towards β-arrestin-mediated signaling 15 16 .
The angiotensin II type 1 and 2 receptors (AT 1 R and AT 2 R) are GPCRs with opposing functions in blood pressure regulation and sodium excretion 17 18 19 . The two subtypes share 34% sequence homology and stimulate different signaling pathways to elicit distinct and counter-regulatory biological functions 17 . AT 2 R is highly expressed in the fetal state although lower levels are present in the adult brain, heart, kidney and the adrenal 17 . In some diseases, AT 2 R is upregulated acting as an anti-inflammatory and repairing factor for wound healing or after cardiac or vascular events 20 21 . In contrast, the AT 1 R is widely distributed, for example, in the adrenal gland, liver, kidney, fat, brain, placenta, spleen, or thyroid, and its physiological role as a component of the renin-angiotensin-aldosterone system (RAAS) is well characterized via binding of its cognate ligand angiotensin II for blood pressure regulation, vasoconstriction, inflammatory response and vascular and cardiac hypertrophy 21 .
The Discovery of Autoantibodies Against Angiotensin II Type 1 Receptor
The prevalence of autoimmune diseases in the population is around 2.5% although autoantibodies are also often present in healthy individuals 22 23 . Impaired B cell tolerance can allow autoantibody-producing B cells with medium or low binding affinity to self-antigens to escape from elimination or further anergy during B cell maturation thus becoming autoantibody-secreting plasma cells 22 . However, the pathogenic role of autoantibodies is mostly unknown.
The role of autoantibodies against the AT 1 R (AT 1 R-Abs) in hypertension has been investigated over the last decades. In 1999, Wallukat et al. described the presence of AT 1 R-Abs in patients with preeclampsia, which is discussed further below 24 . This discovery was extended one year later by Fu and coworkers who observed elevated AT 1 R-Ab levels in 33% patients with malignant secondary hypertension, but also found increased levels of AT 1 R-Abs in 14% of the control group 25 . Soon afterwards, an association of AT 1 R-Abs and renal graft failure was observed by many research groups, especially in patients without donor-specific human leukocyte antigen (HLA)-antibodies 26 27 28 29 . Dragun et al. reported a comparable prevalence of kidney rejections associated with either AT 1 R-Abs (3.6%) or donor-specific HLA-antibodies (3.2%) in a cohort of 278 kidney transplantations including 119 rejections 29 . High levels of AT 1 R-Abs (>17 units) without additional presence of donor-specific antibodies have been ascribed to an increased risk for antibody-mediated kidney rejection 27 . Other studies reported on AT 1 R-Ab levels of >9 U/ml or >10 U that have been shown to elevate the risk of graft loss 28 30 . Notably, in some patients (17–47%) AT 1 R-Abs were already present before transplantation 26 27 28 30 . A case report of Jobert et al. described a 28-year old male recipient of a well-matched renal allograft lacking anti-HLA-antibodies but with a high AT 1 R-Ab level of 14.1 U/ml prior transplantation who developed acute vascular rejection four days post-transplantation 31 . The authors hypothesized that the AT 1 R-Abs are the only factor that could have led to the allograft rejection due to the otherwise well-matched HLA-type and virus status 31 . Treatment with anti-thymocyte globulin, methylprednisolone, candesartan and plasma exchange was successful suggesting probably plasma exchange as a considerable option in patients with AT 1 R-Abs 31 . However, AT 1 R-Abs also appeared after kidney transplantation in prior AT 1 R-Ab-negative patients without an associated worse clinical outcome 32 . Of note is that Taniguchi et al. stressed that such studies report an association of AT 1 R-Ab levels and allograft failure and do not explain any causal relationship 26 . The occurrence of autoantibodies is widely distributed in transplantation medicine but also in autoimmune diseases such as Huntington, multiple sclerosis or systemic sclerosis 33 34 . A summary of diseased states widely reported as associated with increased AT 1 R-Ab levels is shown in the Fig. 1 .
Fig. 1.
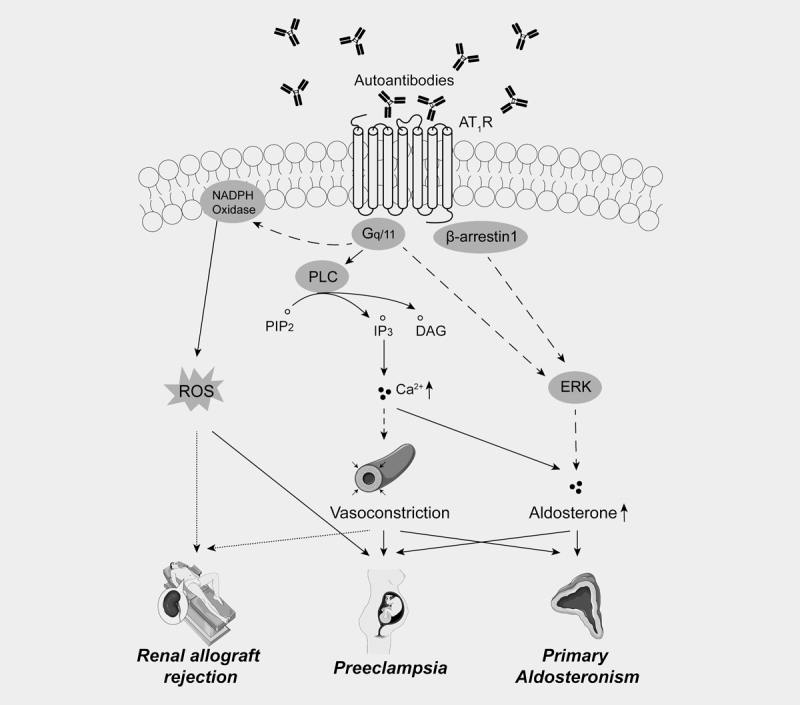
Potential pathological effects of agonistic AT 1 R autoantibodies: Autoantibodies to the AT 1 R have been described in a number of different pathological states as indicated. Continuous lines represent a direct effect, dashed lines indicate an indirect effect, dotted lines denote putative effects. Figure compiled by the authors using elements of https://smart.servier.com/ (licensed under Creative Commons Attribution 3.0 Unported License).
Comparison of Different Assays for AT1R-Ab Characterization
AT 1 R-Abs in patient serum have been widely measured by enzyme-linked immunosorbent assays (ELISA). For such ELISAs, the target antigens are immobilized on a solid phase, mainly on a microplate to detect binding autoantibodies from serum samples 22 . As antigens serve the human full-length AT 1 R or peptides comprising known epitopes (AFHYESQ or ENTNIT) for AT 1 R-Abs in the AT 1 R second extracellular loop 29 . The bound autoantibodies are then either directly labelled with reporter enzymes (direct ELISA) or by a secondary labelled antibody coupled to a reporter enzyme (indirect ELISA) for quantification. Internal laboratory assays have been developed as well as commercially available kits ( Table 1 ). Piazza et al. compared in a study with patients diagnosed with APA two of the commercially available Sandwich-ELISA kits used for AT 1 R-Abs determination 35 36 by CellTrend and Cusabio 37 . The results of both assays were consistent and both demonstrated higher AT 1 R-Ab levels in patients diagnosed with an APA compared with healthy controls 37 . In contrast, the same CellTrend kit and a second kit from ELISA-Creative Diagnostics revealed the detection of contrasting AT 1 R-Ab levels depending on the assay used for patients with pre-eclampsia relative to controls 38 . This highlights the inherent drawbacks of assays based on the detection of AT 1 R-Ab concentrations and not AT 1 R bioactivity. To investigate if AT 1 R-Abs activate the AT 1 R, assays have been developed, which measure cultured spontaneously beating neonatal rat cardiomyocytes in response to immunoglobulins G (IgGs) and the perfused rat cremaster arteriole assay to measure losartan-sensitive antibody-mediated vasoconstriction 24 39 . In vitro experiments using genetically engineered cells (for example, Chinese hamster ovary cells (CHO), or human bone osteosarcoma cells (U2OS)) stably transfected with human AT 1 R) to measure the functional activation of the AT 1 R in response to whole serum or purified IgGs have also been used 37 38 39 40 . Upon receptor activation, the transfected U2OS or CHO cells mediate chemiluminescent or fluorescent signals that can be quantitatively measured. Of note is that many cell-based assays used to quantify AT1R-Ab agonistic activity measure responses mediated by the β-arrestin signaling pathway. In addition, the production of aldosterone or increased expression of the aldosterone synthase gene ( CYP11B2 ) in response to treatment with IgG fractions or whole serum has been measured using human adrenocortical carcinoma cell line (HAC15) 37 .
Table 1 Angiotensin II type 1 receptor autoantibody measurements in primary aldosteronism.
| ELISA | |
|---|---|
| Indirect ELISA using immobilized AT 1 R peptides of extracellular loop 2 | |
| Rossitto et al. 2013 51 |
|
| Kem et al. 2014 39 |
|
| Li et al. 2015 40 |
|
| Sandwich-ELISA with full-length AT 1 R | |
| Sabbadin et al. 2018 35 | Human angiotensin II receptor 1 antibody, ATIIR1 Ab ELISA Kit
(Cusabio, Wuhan, China)
|
| Piazza et al. 2019 37 | Human angiotensin II receptor 1 antibody, ATIIR1 Ab ELISA Kit
(Cusabio, Wuhan, China)
|
| Piazza et al. 2019 37 | CellTrend, Luckenwalde, Germany
|
| Williams et al. 2019 38 | CellTrend, Luckenwalde, Germany
|
| Williams et al. 2019 38 | Creative Diagnostics
|
| Functional AT 1 R agonist measurements | |
| Cell-based functional assays | |
| Williams et al. 2019 38 | SERUM:
|
| Kem et al. 2014 39 | SERUM:
|
| Li et al. 2015 40 | SERUM:
|
| Piazza et al. 2019 37 | SERUM:
|
| Perfused rat cremaster arteriole assay | |
| Kem et al. 2014 39 | SERUM:
|
| CYP11B2-mRNA expression in HAC15 cells | |
| Piazza et al. 2019 37 | Purified IgG:
|
Ab: Antibody; APA: Aldosterone producing adenoma; AT 1 R: Angiotensin II type 1 receptor; BAH: Bilateral adrenal hyperplasia; ELISA: Enzyme-linked immunosorbent assay; HAC15: Human adrenocortical cell line; NT: Normotensive controls; PH: Primary hypertension.
AT 1 R-Abs and Pre-Eclampsia
Pre-eclampsia is the sudden appearance of hypertension in pregnant women after 20 weeks’ gestation week that is associated with increased risks of long-term hypertension, stroke, cardiovascular morbidity and proteinuria for the mother and uteroplacental dysfunction, preterm birth, fetal distress and fetal death for the unborn child 24 41 . In 1999, Wallukat et al. were the first who reported the presence of AT 1 R-Abs in pregnant women with pre-eclampsia compared to controls 24 . The purified IgG fractions from patients with preeclampsia demonstrated losartan-suppressible AT 1 R stimulation and identified the amino acid sequence AFHYESQ in the secondary extracellular loop of the AT 1 R as the possible binding site for these autoantibodies 24 . Peptides against the AFHYESQ epitope successfully abolished AT 1 R-Ab mediated activation of the AT 1 R suggesting a potential target for the treatment of patients with pre-eclampsia 42 43 . Despite postpartum persisting AT 1 R-Ab levels in 17% of women with previous pre-eclampsia 44 , the drop in AT 1 R-Ab levels after giving birth and the correlation of AT 1 R-Ab levels with the severity of the disease indicated a role for AT 1 R-Abs in pre-eclampsia 24 45 . Numerous studies subsequently supported the findings of Wallukat et al. with reports of AT 1 R-Abs in pregnant women with pre-eclampsia 43 46 47 . Diverse studies have reported a role for AT 1 R-Abs in pre-eclampsia in mediating intracellular Ca 2+ release 43 , induction of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and reactive oxygen species (ROS) production leading to the activation of the transcription factor nuclear factor Kappa B (NFκB) activation 46 and initiation of vasoconstriction 47 , all of which were inhibited by losartan. Further in vivo experiments ascribed a causative role for AT 1 R-Abs in the development of pre-eclampsia. Zhou et al. infused pregnant mice with human total IgG or affinity purified AT 1 R-Abs which resulted in the characteristic symptoms of pre-eclampsia including proteinuria, hypertension and abnormal appearance of the placenta 42 . In addition, Wenzel et al. injected angiotensin II, purified rabbit AT 1 R-Abs or both in pregnant rats which induced a phenotype resembling pre-eclampsia when a combination of both, angiotensin II and purified AT 1 R-Abs were applied 48 . In contrast to the aforementioned findings, in a population of Mexican-Mestizo women diagnosed with pre-eclampsia no AT 1 R-Abs were found 49 . Despite an apparently clear role for AT 1 R-Abs in pre-eclampsia, a recent retrospective analysis of 485 women with pre-eclampsia concluded that AT 1 R-Ab levels alone are not sufficient to predict hypertension in such patients, and additional risk factors for hypertension should be considered 50 .
AT 1 R-Abs in Primary Aldosteronism
Recently, AT 1 R-Abs were also detected in patients diagnosed with PA suggesting a possible role of AT 1 R activation in aldosterone production in some patients. Rossitto et al. described for the first time AT 1 R-Abs in patients with PA which was subsequently reported by other research groups 39 40 51 . In this study, the AT 1 R-Ab levels of serum from 46 patients with PA (26 APA, 20 BAH) as well as 62 patients with primary hypertension (PH) was measured by an indirect ELISA assay using an immobilized peptide of the second extracellular loop of AT 1 R. Thirteen pregnant women with preeclampsia and 45 normotensive control patients (NT) were used as positive and negative controls, respectively. Patients with PA and PH showed significantly higher AT 1 R-Ab levels than NT, with higher levels in patients with PA than in PH 51 . In 92.3% of patients with APA, AT 1 R-Abs were detected comprising a concentration 2-fold higher than of patients diagnosed with BAH or PH, despite comparable blood pressure levels ( Table 1 ) 51 . Interestingly, the AT 1 R-Ab levels of patients with APA and women with pre-eclampsia were similar (3.43±1.20 vs. 3.66±1.79) 51 . Another indirect ELISA using the peptide AFHYESQ located in the extracellular loop 2 was performed in the studies of Kem et al. and Li et al. 39 40 . Kem et al. described elevated AT 1 R-Ab levels in 4 of 13 patients with PA (31%) while the latter study focused on the subtypes of PA and measured elevated AT 1 R-Ab levels in 42% of BAH, 23% of APA and 7% of NT which contradicts the initial findings of the same group 40 .
Using a commercially available ELISA kit with full-length AT 1 R as antigen, Sabbadin et al. found higher AT 1 R-Ab levels in patients with PA compared with healthy controls which is in accordance with previous findings 35 . However, the authors could not distinguish patients with APA (n=15) and BAH (n=29) 35 . In contrast, a larger study comprising a cohort of 80 patients with PA (40 APA, 40 BAH), 40 with PH, 23 with pre-eclampsia and 25 NT observed equal levels of AT 1 R-Abs in all groups except for patients with pre-eclampsia using two different commercial available ELISA kits ( Table 1 ) 38 . Using the same CellTrend ELISA kit, Piazza et al. described higher AT 1 R-Ab levels in patients with APA (n=27) compared to healthy controls (n=7) 37 . Overall, it is clear that these studies using ELISA-based assays have yielded highly contrasting results.
AT 1 R-Ab levels pre- and post-adrenalectomy were investigated in 14 patients with APA 37 who were biochemically cured following surgery according to the PASO criteria 52 . The authors found no significant decrease in AT 1 R-Ab levels at one month after adrenalectomy indicating that the resected adrenal was not the source of antigens stimulating the immune response 37 .
When summarizing the results of AT 1 R-Ab quantification by ELISA it can be stated that there is a high variability in the AT 1 R-Ab levels of patients with PA with studies reporting contrasting AT 1 R-Ab levels for the different subtypes of PA 39 51 . Of note is that elevated AT 1 R-Ab levels were also described in healthy individuals without initiating subsequent AT 1 R activation 40 . Furthermore, Kem et al. found for instance more frequently elevated AT 1 R-bioactivity in patients with PA by a cell-based assay using AT 1 R-transfected CHO cells than autoantibodies were quantified by ELISA which is in agreement with a second study from the same group 39 40 . The authors hypothesized that the use of a linear peptide for the secondary extracellular loop instead of full-length AT 1 R could miss other potential binding sites for the autoantibodies in ELISA 39 . This is supported by the findings of multiple binding sites for IgG on the AT 1 R 53 and the recommendations to avoid using immobilized peptides in ELISA for GPCR-autoantibody detection 54 . However, using the full-length AT 1 R does not guarantee the functional activity of the captured autoantibodies which can be demonstrated in cell based-functional assays using whole serum and/or purified IgG 38 39 40 .
The rat cremaster arteriole assay or AT 1 R-transfected CHO cells both demonstrated elevated receptor activation when exposed to serum of patients with PA compared to controls, which was largely normalized by the AT 1 R-blockers losartan or candesartan 39 . Similarly, candesartan was able to reduce AT 1 R-Ab-stimulated aldosterone production in vitro in HAC15 cells 39 . AT 1 R-activity is more frequently observed in patients diagnosed with BAH (75%) compared to APA patients (46%), while serum of NT induced no AT 1 R-bioactivity 40 . However, one research group failed to measure AT 1 R-bioactivity in patients with APA and controls using this assay which might be due to the lack of activating function of the autoantibodies 37 . AT 1 R-transfected U2OS cells also showed no group differences when incubated with serum of patients with APA, BAH or pre-eclampsia or NT in the absence of losartan 38 . Notably, the administration of losartan only barely reduced AT 1 R-activation in the BAH group in comparison to APA, pre-eclampsia and NT indicating a losartan-independent activation pathway of the receptor 38 . Purified IgGs also caused an increase in AT 1 R-bioactivity in the BAH compared to APA group, independent of losartan 38 . This can be explained by different binding sites for IgG and losartan at the AT 1 R while angiotensin II and losartan share a common binding site on the AT 1 R 53 .
Autoantibody levels were not correlated with age, gender, BMI, blood pressure, baseline aldosterone and aldosterone-to-renin ratio (ARR) 35 51 . Mineralocorticoid receptor antagonist (MRA) treatment of patients with PA was also not associated with AT 1 R-Ab levels 35 . Agonistic AT 1 R-Abs displayed vasoconstrictive effects and correlate with the mean arterial pressure 39 40 . The previously mentioned elevated agonistic activity of AT 1 R-Abs in patients diagnosed with BAH and the higher responsiveness to angiotensin II in those patients 55 suggest an allosteric function of the autoantibodies which promotes the binding angiotensin II to its receptor. This is supported by the reduction of aldosterone levels after captopril-challenge in AT 1 R-Ab-positive compared with AT 1 R-Ab-negative patients with hypertension or PA 51 . In addition, treatment of HAC15 cells with angiotensin II and affinity-purified IgGs increased aldosterone production compared with angiotensin II treatment alone 39 .
As previously indicated, upon activation, the AT 1 R can initiate two distinct independent signaling pathways. More prominent is the G-protein signaling pathway in which PIP 2 is cleaved to DAG and IP 3 resulting in increased intracellular Ca 2+ and aldosterone production 15 . Secondly, biased signaling mediated by β-arrestin1 is independent of G-proteins and may occur in parallel to the G-protein signaling pathway 15 . Aldosterone production appears to be stimulated via extracellular signal-regulated kinase (ERK)-mediated activation of steroidogenic acute regulatory protein (StAR) to promote transfer of cholesterol to mitochondria 15 . When AT 1 Rs are activated by angiotensin II, both pathways appear to be initiated 15 . This has been demonstrated in vivo in rats with overexpression of β-arrestin1, which showed suppressed aldosterone production only through the administration of candesartan or valsartan and not by losartan or irbesartan despite belonging to the same pharmacological class 56 57 .
In conclusion, some evidence supports a pathological role for autoantibodies against the AT 1 R in different diseases. It has not been established if AT 1 R-Abs play a causative role or are a consequence of the pathology. Further studies are warranted to address the functional relevance of AT 1 R-Abs in PA and the significance of AT 1 R biased signaling.
Funding Statement
Funding: M. Reincke is supported by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement No. 694913). T. A. Williams and M. Reincke are supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) Projektnummer: 314061271-TRR 205.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
References
- 1.Calhoun D A, Nishizaka M K, Zaman M A et al. Hyperaldosteronism among black and white subjects with resistant hypertension. Hypertension. 2002;40:892–896. doi: 10.1161/01.hyp.0000040261.30455.b6. [DOI] [PubMed] [Google Scholar]
- 2.Rossi G P, Bernini G, Caliumi C et al. A prospective study of the prevalence of primary aldosteronism in 1125 hypertensive patients. J Am Coll Cardiol. 2006;48:2293–2300. doi: 10.1016/j.jacc.2006.07.059. [DOI] [PubMed] [Google Scholar]
- 3.Monticone S, Burrello J, Tizzani D et al. Prevalence and clinical manifestations of primary aldosteronism encountered in primary care practice. J Am Coll Cardiol. 2017;69:1811–1820. doi: 10.1016/j.jacc.2017.01.052. [DOI] [PubMed] [Google Scholar]
- 4.Mulatero P, Monticone S, Bertello C et al. Long-term cardio- and cerebrovascular events in patients with primary aldosteronism. J Clin Endocrinol Metab. 2013;98:4826–4833. doi: 10.1210/jc.2013-2805. [DOI] [PubMed] [Google Scholar]
- 5.Savard S, Amar L, Plouin P F et al. Cardiovascular complications associated with primary aldosteronism: a controlled cross-sectional study. Hypertension. 2013;62:331–336. doi: 10.1161/HYPERTENSIONAHA.113.01060. [DOI] [PubMed] [Google Scholar]
- 6.Catena C, Colussi G, Lapenna R et al. Long-term cardiac effects of adrenalectomy or mineralocorticoid antagonists in patients with primary aldosteronism. Hypertension. 2007;50:911–918. doi: 10.1161/HYPERTENSIONAHA.107.095448. [DOI] [PubMed] [Google Scholar]
- 7.Zennaro M C, Boulkroun S, Fernandes-Rosa F. Genetic causes of functional adrenocortical adenomas. Endocr Rev. 2017;38:516–537. doi: 10.1210/er.2017-00189. [DOI] [PubMed] [Google Scholar]
- 8.Prada ET A, Burrello J, Reincke M et al. Old and new concepts in the molecular pathogenesis of primary aldosteronism. Hypertension. 2017;70:875–881. doi: 10.1161/HYPERTENSIONAHA.117.10111. [DOI] [PubMed] [Google Scholar]
- 9.Wallukat G, Schimke I. Agonistic autoantibodies directed against G-protein-coupled receptors and their relationship to cardiovascular diseases. Semin Immunopathol. 2014;36:351–363. doi: 10.1007/s00281-014-0425-9. [DOI] [PubMed] [Google Scholar]
- 10.Clark R B. Profile of Brian K. Kobilka and Robert J. Lefkowitz, 2012 Nobel laureates in chemistry. Proc Natl Acad Sci USA. 2013;110:5274–5275. doi: 10.1073/pnas.1221820110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenbaum D M, Rasmussen S G, Kobilka B K. The structure and function of G-protein-coupled receptors. Nature. 2009;459:356–363. doi: 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whalen E J, Rajagopal S, Lefkowitz R J. Therapeutic potential of beta-arrestin- and G protein-biased agonists. Trends Mol Med. 2011;17:126–139. doi: 10.1016/j.molmed.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao P, Furness SG B. The nature of efficacy at G protein-coupled receptors. Biochem Pharmacol. 2019;170:113647. doi: 10.1016/j.bcp.2019.113647. [DOI] [PubMed] [Google Scholar]
- 14.Turu G, Balla A, Hunyady L. The Role of beta-Arrestin Proteins in Organization of Signaling and Regulation of the AT1 Angiotensin Receptor. Front Endocrinol (Lausanne) 2019;10:519. doi: 10.3389/fendo.2019.00519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maning J, Negussie S, Clark M A et al. Biased agonism/antagonism at the AngII-AT1 receptor: Implications for adrenal aldosterone production and cardiovascular therapy. Pharmacol Res. 2017;125:14–20. doi: 10.1016/j.phrs.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Lefkowitz R J, Rajagopal K, Whalen E J. New roles for beta-arrestins in cell signaling: not just for seven-transmembrane receptors. Mol Cell. 2006;24:643–652. doi: 10.1016/j.molcel.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Li X H, Yuan H. Angiotensin II type-2 receptor-specific effects on the cardiovascular system. Cardiovasc Diagn Ther. 2012;2:56–62. doi: 10.3978/j.issn.2223-3652.2012.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel S, Hussain T. Role of AT2R (Angiotensin Type 2 Receptor) in maintaining sodium-potassium balance. Hypertension. 2018;71:563–565. doi: 10.1161/HYPERTENSIONAHA.117.10552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liles C, Li H, Veitla V et al. AT2R Autoantibodies Block Angiotensin II and AT1R Autoantibody-Induced Vasoconstriction. Hypertension. 2015;66:830–835. doi: 10.1161/HYPERTENSIONAHA.115.05428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Terenzi R, Manetti M, Rosa I et al. Angiotensin II type 2 receptor (AT2R) as a novel modulator of inflammation in rheumatoid arthritis synovium. Sci Rep. 2017;7:13293. doi: 10.1038/s41598-017-13746-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levy B I. How to explain the differences between renin angiotensin system modulators. Am J Hypertens. 2005;18:134s–141s. doi: 10.1016/j.amjhyper.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Ludwig R J, Vanhoorelbeke K, Leypoldt F et al. Mechanisms of autoantibody-induced pathology. Front Immunol. 2017;8:603. doi: 10.3389/fimmu.2017.00603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagele E P, Han M, Acharya N K et al. Natural IgG autoantibodies are abundant and ubiquitous in human sera, and their number is influenced by age, gender, and disease. PLoS One. 2013;8:e60726. doi: 10.1371/journal.pone.0060726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wallukat G, Homuth V, Fischer T et al. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest. 1999;103:945–952. doi: 10.1172/JCI4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fu M L, Herlitz H, Schulze W et al. Autoantibodies against the Angiotensin Receptor (AT1) in patients with hypertension. J Hypertens. 2000;18:945–953. doi: 10.1097/00004872-200018070-00017. [DOI] [PubMed] [Google Scholar]
- 26.Taniguchi M, Rebellato L M, Cai J et al. Higher risk of kidney graft failure in the presence of anti-angiotensin II type-1 receptor antibodies. Am J Transplant. 2013;13:2577–2589. doi: 10.1111/ajt.12395. [DOI] [PubMed] [Google Scholar]
- 27.Reinsmoen N L, Lai C H, Heidecke H et al. Anti-angiotensin type 1 receptor antibodies associated with antibody mediated rejection in donor HLA antibody negative patients. Transplantation. 2010;90:1473–1477. doi: 10.1097/TP.0b013e3181fd97f1. [DOI] [PubMed] [Google Scholar]
- 28.Banasik M, Boratynska M, Koscielska-Kasprzak K et al. The influence of non-HLA antibodies directed against angiotensin II type 1 receptor (AT1R) on early renal transplant outcomes. Transpl Int. 2014;27:1029–1038. doi: 10.1111/tri.12371. [DOI] [PubMed] [Google Scholar]
- 29.Dragun D, Muller D N, Brasen J H et al. Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N Engl J Med. 2005;352:558–569. doi: 10.1056/NEJMoa035717. [DOI] [PubMed] [Google Scholar]
- 30.Giral M, Foucher Y, Dufay A et al. Pretransplant sensitization against angiotensin II type 1 receptor is a risk factor for acute rejection and graft loss. Am J Transplant. 2013;13:2567–2576. doi: 10.1111/ajt.12397. [DOI] [PubMed] [Google Scholar]
- 31.Jobert A, Rao N, Deayton S et al. Angiotensin II type 1 receptor antibody precipitating acute vascular rejection in kidney transplantation. Nephrology (Carlton) 2015;20 01:10–12. doi: 10.1111/nep.12421. [DOI] [PubMed] [Google Scholar]
- 32.Hesemann L E, Subramanian V, Mohanakumar T et al. De novo development of antibodies to kidney-associated self-antigens angiotensin II receptor type I, collagen IV, and fibronectin occurs at early time points after kidney transplantation in children. Pediatr Transplant. 2015;19:499–503. doi: 10.1111/petr.12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee D H, Heidecke H, Schroder A et al. Increase of angiotensin II type 1 receptor auto-antibodies in Huntington’s disease. Mol Neurodegener. 2014;9:49. doi: 10.1186/1750-1326-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riemekasten G, Philippe A, Nather M et al. Involvement of functional autoantibodies against vascular receptors in systemic sclerosis. Ann Rheum Dis. 2011;70:530–536. doi: 10.1136/ard.2010.135772. [DOI] [PubMed] [Google Scholar]
- 35.Sabbadin C, Ceccato F, Ragazzi E et al. Evaluation of angiotensin II type-1 receptor antibodies in primary aldosteronism and further considerations about their possible pathogenetic role. J Clin Hypertens (Greenwich) 2018;20:1313–1318. doi: 10.1111/jch.13351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lefaucheur C, Viglietti D, Bouatou Y et al. Non-HLA agonistic anti-angiotensin II type 1 receptor antibodies induce a distinctive phenotype of antibody-mediated rejection in kidney transplant recipients. Kidney Int. 2019;96:189–201. doi: 10.1016/j.kint.2019.01.030. [DOI] [PubMed] [Google Scholar]
- 37.Piazza M, Seccia T M, Caroccia B et al. AT1AA (Angiotensin II Type-1 Receptor Autoantibodies): Cause or Consequence of Human Primary Aldosteronism? Hypertension. 2019;74:793–799. doi: 10.1161/HYPERTENSIONAHA.119.13388. [DOI] [PubMed] [Google Scholar]
- 38.Williams T A, Jaquin D, Burrello J et al. Diverse responses of autoantibodies to the Angiotensin II type 1 receptor in primary aldosteronism. Hypertension. 2019;74:784–792. doi: 10.1161/HYPERTENSIONAHA.119.13156. [DOI] [PubMed] [Google Scholar]
- 39.Kem D C, Li H, Velarde-Miranda C, Liles C et al. Autoimmune mechanisms activating the angiotensin AT1 receptor in 'primary' aldosteronism. J Clin Endocrinol Metab. 2014;99:1790–1797. doi: 10.1210/jc.2013-3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li H, Yu X, Cicala M V et al. Prevalence of angiotensin II type 1 receptor (AT1R)-activating autoantibodies in primary aldosteronism. J Am Soc Hypertens. 2015;9:15–20. doi: 10.1016/j.jash.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fox R, Kitt J, Leeson P et al. Preeclampsia: Risk Factors, Diagnosis, Management, and the Cardiovascular Impact on the Offspring. J Clin Med. 2019;8:E1625. doi: 10.3390/jcm8101625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou C C, Zhang Y, Irani R A et al. Angiotensin receptor agonistic autoantibodies induce pre-eclampsia in pregnant mice. Nat Med. 2008;14:855–862. doi: 10.1038/nm.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thway T M, Shlykov S G, Day M C et al. Antibodies from preeclamptic patients stimulate increased intracellular Ca2+ mobilization through angiotensin receptor activation. Circulation. 2004;110:1612–1619. doi: 10.1161/01.CIR.0000142855.68398.3A. [DOI] [PubMed] [Google Scholar]
- 44.Hubel C A, Wallukat G, Wolf M et al. Agonistic angiotensin II type 1 receptor autoantibodies in postpartum women with a history of preeclampsia. Hypertension. 2007;49:612–617. doi: 10.1161/01.HYP.0000256565.20983.d4. [DOI] [PubMed] [Google Scholar]
- 45.Siddiqui A H, Irani R A, Blackwell S C et al. Angiotensin receptor agonistic autoantibody is highly prevalent in preeclampsia: correlation with disease severity. Hypertension. 2010;55:386–393. doi: 10.1161/HYPERTENSIONAHA.109.140061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dechend R, Viedt C, Muller D N et al. AT1 receptor agonistic antibodies from preeclamptic patients stimulate NADPH oxidase. Circulation. 2003;107:1632–1639. doi: 10.1161/01.CIR.0000058200.90059.B1. [DOI] [PubMed] [Google Scholar]
- 47.Yang X, Wang F, Chang H et al. Autoantibody against AT1 receptor from preeclamptic patients induces vasoconstriction through angiotensin receptor activation. J Hypertens. 2008;26:1629–1635. doi: 10.1097/HJH.0b013e328304dbff. [DOI] [PubMed] [Google Scholar]
- 48.Wenzel K, Rajakumar A, Haase H et al. Angiotensin II type 1 receptor antibodies and increased angiotensin II sensitivity in pregnant rats. Hypertension. 2011;58:77–84. doi: 10.1161/HYPERTENSIONAHA.111.171348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leanos-Miranda A, Campos-Galicia I, Alvarez-Jimenez G et al. Stimulating autoantibodies against the angiotensin II type 1 receptor are not associated with preeclampsia in Mexican-Mestizo women. J Hypertens. 2010;28:834–841. doi: 10.1097/HJH.0b013e3283376cc6. [DOI] [PubMed] [Google Scholar]
- 50.Birukov A, Muijsers HE C, Heidecke H et al. Regulatory antibodies against GPCR in women ten years after early-onset preeclampsia. Front Biosci (Landmark Ed) 2019;24:1462–1476. doi: 10.2741/4791. [DOI] [PubMed] [Google Scholar]
- 51.Rossitto G, Regolisti G, Rossi E et al. Elevation of angiotensin-II type-1-receptor autoantibodies titer in primary aldosteronism as a result of aldosterone-producing adenoma. Hypertension. 2013;61:526–533. doi: 10.1161/HYPERTENSIONAHA.112.202945. [DOI] [PubMed] [Google Scholar]
- 52.Williams T A, Lenders JW M, Mulatero P et al. Outcomes after adrenalectomy for unilateral primary aldosteronism: an international consensus on outcome measures and analysis of remission rates in an international cohort. Lancet Diabetes Endocrinol. 2017;5:689–699. doi: 10.1016/S2213-8587(17)30135-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dragun D, Catar R, Philippe A. Non-HLA antibodies against endothelial targets bridging allo- and autoimmunity. Kidney Int. 2016;90:280–288. doi: 10.1016/j.kint.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 54.Jahns R, Boege F. Questionable Validity of Peptide-Based ELISA Strategies in the Diagnostics of Cardiopathogenic Autoantibodies That Activate G-Protein-Coupled Receptors. Cardiology. 2015;131:149–150. doi: 10.1159/000376546. [DOI] [PubMed] [Google Scholar]
- 55.Wisgerhof M, Carpenter P C, Brown R D. Increased adrenal sensitivity to angiotensin II in idiopathic hyperaldosteronism. J Clin Endocrinol Metab. 1978;47:938–943. doi: 10.1210/jcem-47-5-938. [DOI] [PubMed] [Google Scholar]
- 56.Lymperopoulos A, Rengo G, Zincarelli C et al. Adrenal beta-arrestin 1 inhibition in vivo attenuates post-myocardial infarction progression to heart failure and adverse remodeling via reduction of circulating aldosterone levels. J Am Coll Cardiol. 2011;57:356–365. doi: 10.1016/j.jacc.2010.08.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lymperopoulos A, Sturchler E, Bathgate-Siryk A et al. Different potencies of angiotensin receptor blockers at suppressing adrenal beta-Arrestin1-dependent post-myocardial infarction hyperaldosteronism. J Am Coll Cardiol. 2014;64:2805–2806. doi: 10.1016/j.jacc.2014.09.070. [DOI] [PubMed] [Google Scholar]


