As the COVID-19 pandemic has escalated, an unmatched surge of severe cases requiring intensive care unit (ICU) admission has been observed.1 Currently, more than 50% of patients in the ICU require invasive mechanical ventilation and up to 20% need dialysis. ICU capacity has been increased in many hospitals; however, due to the increased severity of illness,1, 2 even ICUs that are adequately staffed for their usual routine might not have enough trained professionals to deliver the complex care required by ventilated patients with COVID-19-related acute respiratory failure or acute respiratory distress syndrome (ARDS). The challenges can be even greater in developing countries with limited resources. In Brazil, the surge of patients has overwhelmed the health system and worsened the already inadequate access to an ICU bed in a public hospital.
Despite promising results for the antiviral remdesivir,3 no specific and effective treatment exists for the disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Patient care relies mainly on advanced life-sustaining therapies that support organ functions while the immune system controls and eliminates the viral infection.1, 4 The severity of clinical presentations and exceedingly high mortality rates have triggered discussions about the use of traditional adjunctive therapies (eg, corticosteroids) and novel interventions (eg, convalescent plasma infusion), but all lack strong evidence of efficacy and robust safety data in patients with COVID-19.4 Improvements in the outcomes of patients with sepsis and non-COVID-19-related ARDS have been achieved by the use of quality-of-care measures to decrease the duration of mechanical ventilation and ICU length of stay, as well as ICU-acquired complications.5
The current pandemic is a significant burden on health-care systems worldwide and a strain on their ICUs. Strain can be associated with decreased adherence to the implementation of protocols and evidence-based care measures, and potentially worse patient outcomes.6 Thus, it is crucial to focus on protocol implementation and adherence to basic care principles for mechanically ventilated patients.
The severity of COVID-19-associated lung injury can result in long periods of mechanical ventilation and prolonged stay in the ICU. Severe hypoxia can require more aggressive ventilation strategies to improve oxygenation in the short term. Higher tidal volumes (VT) and driving pressures are more likely to be associated with prolonged ventilation, more profibrotic stimuli due to high VT in the dysregulated inflammatory response of the lungs, and increased mortality rates.7, 8 In this context, intensivists should aim to reduce the evidence-to-practice gap by implementing lung-protective ventilator strategies and building bedside protocols based on the most recent recommendations, ensuring that VT lower than 6 mL/kg and plateau pressures of less than 30 cm H2O are applied.9 In severe ARDS, a short course of paralysis and deep sedation might be associated with improved outcomes.10
Aiming at light sedation strategies should be a goal for most ICU patients, as recommended by recent guidelines.11 The use of deep sedation, even for a short duration, is independently and strongly associated with increased mortality rates for mechanically ventilated patients.11 However, patients with severe COVID-19 might need sedation to avoid asynchrony and improve the application of invasive mechanical ventilation. In elderly patients with COVID-19-related ARDS and increased systemic inflammation, delirium and its complications are likely to develop. Moreover, studies show that in severe cases of COVID-19, SARS-CoV-2 can be identified in the central nervous system, potentially leading to direct brain injury. These factors might contribute to a high incidence of post-intensive care syndrome and decreased quality of life in survivors of COVID-19. Therefore, ICUs should, whenever possible, ensure the use of targeted sedation with strategies to reduce the use of sedatives and benzodiazepines, in order to limit the duration of mechanical ventilation and its associated complications, including the frequency of delirium.12 The above-mentioned strategies should be applied judiciously, as the increased burden of care in the ICU might unintentionally be associated with a proportional reduction in patient monitoring, which could pose a safety issue in patients under light sedation.
Wise implementation of general preventive measures is of the utmost importance. Patients with COVID-19 present with hypercoagulable states and are particularly prone to vascular thrombosis, including venous thromboembolism (VTE).12 Ensuring maximal implementation of VTE prophylaxis should thus be a priority goal of care. Hand hygiene and use of masks continue to be fundamental measures to protect patients and staff from nosocomial infections; the use of usual processes of care, such as checklists, before central venous line catheterisation must also be applied. Finally, there is evidence that a combination of evidence-based protocol implementation and multidisciplinary care is associated with improved outcomes and reduced length of stay for mechanically ventilated patients.13, 14 The application of such measures might allow early discharge of patients with COVID-19 and admission of new patients without the investment required to provide additional ICU beds. Optimising the use of scarce resources is even more challenging, but vital, in developing countries, where adherence to low VT and other process-of-care measures can be suboptimal.15
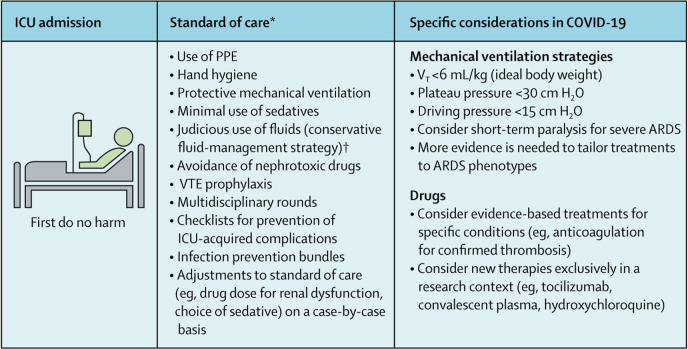
Considering the severity and unparalleled number of cases of COVID-19 pneumonia in ICUs, we must ensure the delivery of high-quality care for mechanically ventilated patients (figure ). More than adjunctive treatments or expensive immune therapies, for which evidence of efficacy is lacking, the focus should be on the careful application of evidence-based approaches associated with improved outcomes in ARDS over the past three decades.
Figure.
Effective care measures to improve outcomes in mechanically ventilated patients with COVID-19
ARDS=acute respiratory distress syndrome. ICU=intensive care unit. PPE=personal protective equipment. VT=tidal volume. VTE=venous thromboembolism. *Must be done if no contraindications are present. †Conservative fluid management is associated with reduced duration of mechanical ventilation.16
Acknowledgments
JIFS is supported in part by individual research grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ). We declare no competing interests.
References
- 1.Phua J, Weng L, Ling L. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med. 2020;8:506–517. doi: 10.1016/S2213-2600(20)30161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grasselli G, Zangrillo A, Zanella A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beigel JH, Tomashek KM, Dodd LE. Remdesivir for the treatment of Covid-19 — preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2007764. published online May 22. [DOI] [PubMed] [Google Scholar]
- 4.Alhazzani W, Møller MH, Arabi YM. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19) Intensive Care Med. 2020;46:854–887. doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Máca J, Jor O, Holub M. Past and present ARDS mortality rates: a systematic review. Respir Care. 2017;62:113–122. doi: 10.4187/respcare.04716. [DOI] [PubMed] [Google Scholar]
- 6.Weissman GE, Gabler NB, Brown SES, Halpern SD. ICU capacity strain and adherence to prophylaxis guidelines. J Crit Care. 2015;30:1303–1309. doi: 10.1016/j.jcrc.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellani G, Laffey JG, Pham T. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 8.Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 9.Papazian L, Aubron C, Brochard L. Formal guidelines: management of acute respiratory distress syndrome. Ann Intensive Care. 2019;9:69. doi: 10.1186/s13613-019-0540-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moss M, Huang DT, Brower RG. Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med. 2019;380:1997–2008. doi: 10.1056/NEJMoa1901686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devlin JW, Skrobik Y, Gélinas C. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46:825–873. doi: 10.1097/CCM.0000000000003299. [DOI] [PubMed] [Google Scholar]
- 12.Helms J, Tacquard C, Severac F. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020 doi: 10.1007/s00134-020-06062-x. published online May 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soares M, Bozza FA, Angus DC. Organizational characteristics, outcomes, and resource use in 78 Brazilian intensive care units: the ORCHESTRA study. Intensive Care Med. 2015;41:2149–2160. doi: 10.1007/s00134-015-4076-7. [DOI] [PubMed] [Google Scholar]
- 14.Zampieri FG, Salluh JIF, Azevedo LCP. ICU staffing feature phenotypes and their relationship with patients' outcomes: an unsupervised machine learning analysis. Intensive Care Med. 2019;45:1599–1607. doi: 10.1007/s00134-019-05790-z. [DOI] [PubMed] [Google Scholar]
- 15.Cavalcanti AB, Bozza FA, Machado FR. Effect of a quality improvement intervention with daily round checklists, goal setting, and clinician prompting on mortality of critically ill patients: a randomized clinical trial. JAMA. 2016;315:1480–1490. doi: 10.1001/jama.2016.3463. [DOI] [PubMed] [Google Scholar]
- 16.Wiedemann HP, Wheeler AP, Bernard GR. Comparison of two fluid-management strategies in acute lung injury. N Eng J Med. 2006;354:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]