Abstract
Remarkable progress has been made in treating pancreatic cancer over the past century, including refinement of our surgical techniques and improvements in adjuvant and neoadjuvant therapies. Despite these advances, the incidence of pancreatic cancer is rising globally, and it remains a deadly disease. In this review, we highlight the historical perspectives of pancreatic cancer treatment and outline the areas of future advancement that will assist progression towards better outcomes. Areas of future advancement include improving prevention strategies and early detection, refining our molecular understanding of pancreatic cancer, identifying more effective systemic therapies, and improving quality of life and surgical outcomes. Furthermore, systems need to be put in place to ensure all patients with pancreatic cancer receive high quality care and are given the appropriate options and sequence of therapy. This is best achieved through multidisciplinary care.
Keywords: Pancreatic Cancer, Pancreaticoduodenectomy, Adjuvant Therapy, Neoadjuvant Therapy
INTRODUCTION
The Italian anatomist Giovanni Battista Morgagni was the first to describe a tumor of the pancreas, in 1761[1]. Nearly a century later, the microscopic features of pancreatic adenocarcinoma were defined [2]. Pancreatic tumors encompass cancers that arise from the endocrine or exocrine components of the pancreas with pancreatic adenocarcinoma, arising from the exocrine pancreas, being the most common and the most aggressive. Worldwide, the incidence of pancreatic cancer is increasing and has more than doubled over the past 30 years [3]. In 2018, pancreatic cancer was the 13th most common cancer globally, with 458,918 new cases, and the 7th most common cause of cancer-related mortality, with 432,242 deaths [4]. In the United States, pancreatic cancer was estimated in 2020 to be the third-leading cause of cancer-related death [5].
Since the early descriptions of pancreatic cancer, our surgical and medical treatment of this disease has evolved dramatically, making what was once uniformly a rapidly fatal malignancy, to now a more treatable disease. In this review, we highlight the historical perspectives in the treatment of pancreatic cancer, including the founders of pancreatic surgery, and the progress in adjuvant approaches towards treating pancreatic cancer. We also discuss the current progress and future goals of improving prevention and early detection, refining our molecular understanding of pancreatic cancer, identifying more effective systemic therapies, and improving quality of life and surgical outcomes.
HISTORICAL PERSPECTIVE
Founders of Pancreatic Surgery
Surgical resection of pancreatic cancer is the mainstay of treatment for patients with non-metastatic disease and includes pancreaticoduodenectomy for tumors in the head or body of the pancreas and distal pancreatectomy for tumors in the tail of the pancreas. It took a century of pioneering by the founders of pancreatic surgery to demonstrate that surgical resection of pancreatic cancer is not only technically feasible, but now a safe, effective, and key component of pancreatic cancer treatment.
The first successful anatomical resection of a pancreatic tumor was performed by Friedrich Trendelenberg in Germany in 1882 [6]. Trendelenberg performed a distal pancreatectomy to remove a tumor in the tail of the pancreas. Despite a poor postoperative outcome, which included wound infection, malnutrition, and ultimately, death, this procedure marked the birth of pancreatic surgery [7]. The first recorded attempt at partial pancreaticoduodenectomy was made by Alessandro Codivilla in Italy in 1898 [8]. Postoperatively, the patient had serious drainage from the surgical wound and subsequently died of “cachexia” after 21 days. Shortly thereafter, William Stewart Halsted, the first Chair of Surgery at Johns Hopkins University, performed successful resection of ampullary cancer in a jaundiced patient in 1898 [9]. Halsted performed a transduodenal local resection of the mass with reanastomosis of the pancreatic and bile ducts to the duodenum. Although the patient recovered from the operation, jaundice developed 3 months postoperatively, which required reoperation and cholecystoduodenostomy. The patient died 6 months later secondary to local recurrence.
In 1909, Walter Kausch, a professor of surgery at Viktoria Hospital in Berlin, performed the first successful en bloc resection of the head of the pancreas and partial resection of the duodenum to treat a patient with obstructive jaundice from ampullary cancer [10]. Kausch performed the procedure in 2-stages: the first stage consisting of cholecytojejunostomy with side-to-side enteroenterostomy; and the second stage consisting of resection of the head of the pancreas, pylorus, and first and second portions of the duodenum. Reconstruction was performed with gastroentrostomy, closure of the common bile duct, and anastomosis of the pancreatic remnant to the third portion of the duodenum. The patient survived for 9 months until dying of cholangitis, without evidence of visible tumor recurrence at autopsy.
Nearly three decades letter, Allen Whipple, the Surgeon-in-Chief at Columbia-Presbyterian Medical Center in New York, published his series of three patients with ampullary cancer and this marked the first report of a two-stage complete pancreaticoduodenectomy [11]. This two-stage approach included a gastroenterostomy, ligation of the common bile duct and cholecystogastrostomy in the first operation, followed by resection of the duodenum and partial excision of the pancreatic head in the second operation. Notably, the operation avoided a pancreatic anastomosis in favor of stump occlusion of the pancreas at its resection margin. The three patients in this series survived for 30 hours, 8 months, and 25 months, respectively. In 1942, Whipple reported the modification of this operation to a one-staged procedure, which he performed for the first time on a patient with non-functioning islet cell carcinoma who survived for 9 years [12]. This one-stage operation comprised distal gastrectomy, resection of the entire duodenum and head of the pancreas, followed by reconstruction with a gastrojejunostomy and choledochojejunostomy. Soon thereafter, Whipple further modified this procedure to include pancreaticojejunostomy [13] (Fig. 1).
Fig. 1. Historical timeline of the treatment of pancreatic cancer.
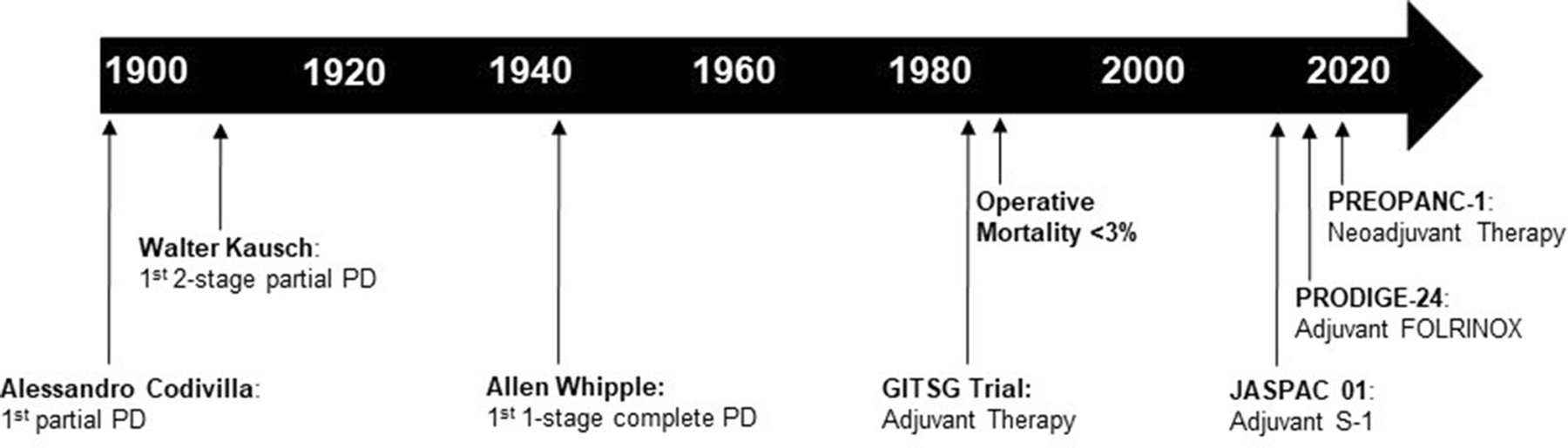
The first recorded attempt at a partial pancreaticoduodenectomy was by Alessandro Codivilla in Italy in 1898. Walter Kausch performed the first successful 2-stage partial pancreaticoduodenectomy in 1909. Allen Whipple was the first to perform a one-stage complete pancreaticoduodenectomy in 1942. The Gastrointestinal Tumor Study Group (GITSG) trial heralded a new era of adjuvant therapy and operative mortality fell to below 3% in the 1980s. The JASPAC 01 and PRODIGE-24 trials in the 2010s demonstrated significant improvements in median OS to over 3 years with adjuvant S-1 and adjuvant FOLFIRINOX. In 2019, PREOPANC-1 was the first phase III clinical trial to evaluate the efficacy of neoadjuvnt therapy.
Operative Morbidity and Mortality
The founders of pancreatic surgery were innovative, daring, and undoubtedly advanced the field, but these advancements in surgical technique did not come without consequences and criticism. In 1935, a review of all reported pancreaticoduodenectomies done until that time found that operative mortality was as high as 35% [11]. When Whipple later reported his experience of performing over 30 pancreaticoduodenectomies, his operative mortality remained high at 33% [14]. Through the 1960s, several groups consistently reported operative mortality of over 30%. This high mortality, coupled with few reports of long term survival prompted several to question the curative versus palliative nature of pancreaticoduodenectomy for pancreatic adenocarcinoma [15,16].
It was not until 50 years after Allen Whipple reported the first two-stage complete pancreaticoduodenectomy that dramatic reductions in operative mortality for the Whipple procedure were observed. Crist et al. reported their experience at Johns Hopkins Hospital over two time periods, 1969–1980 and 1981–1986. While operative mortality was 24% in the early time period, it dropped to about 2% in the more modern patient cohort [17]. This decrease in operative mortality coincided with an increase in case volume, shorter operative time, and reduced intraoperative estimated blood loss, all indicative of improved technical proficiency with this complex procedure. During this period there were also dramatic improvements in imaging, critical care, anesthetic techniques, and ability to rescue patients with interventional radiology procedures and better antibiotics. Currently, the operative mortality for pancreaticoduodenectomy is consistently reported as less than 3% at high volume facilities (Fig. 2). Numerous studies have demonstrated the association of higher surgical volume and improved postoperative outcomes [18–21]. Undoubtably, the centralization of care at centers of excellence has helped improve postoperative outcomes through better patient selection, overcoming the learning curve associated with technical proficiency, and early recognition and rescue from complications.
Fig. 2. Improvements in operative mortality following pancreaticoduodenectomy and median survival of patients with resectable pancreatic cancer.
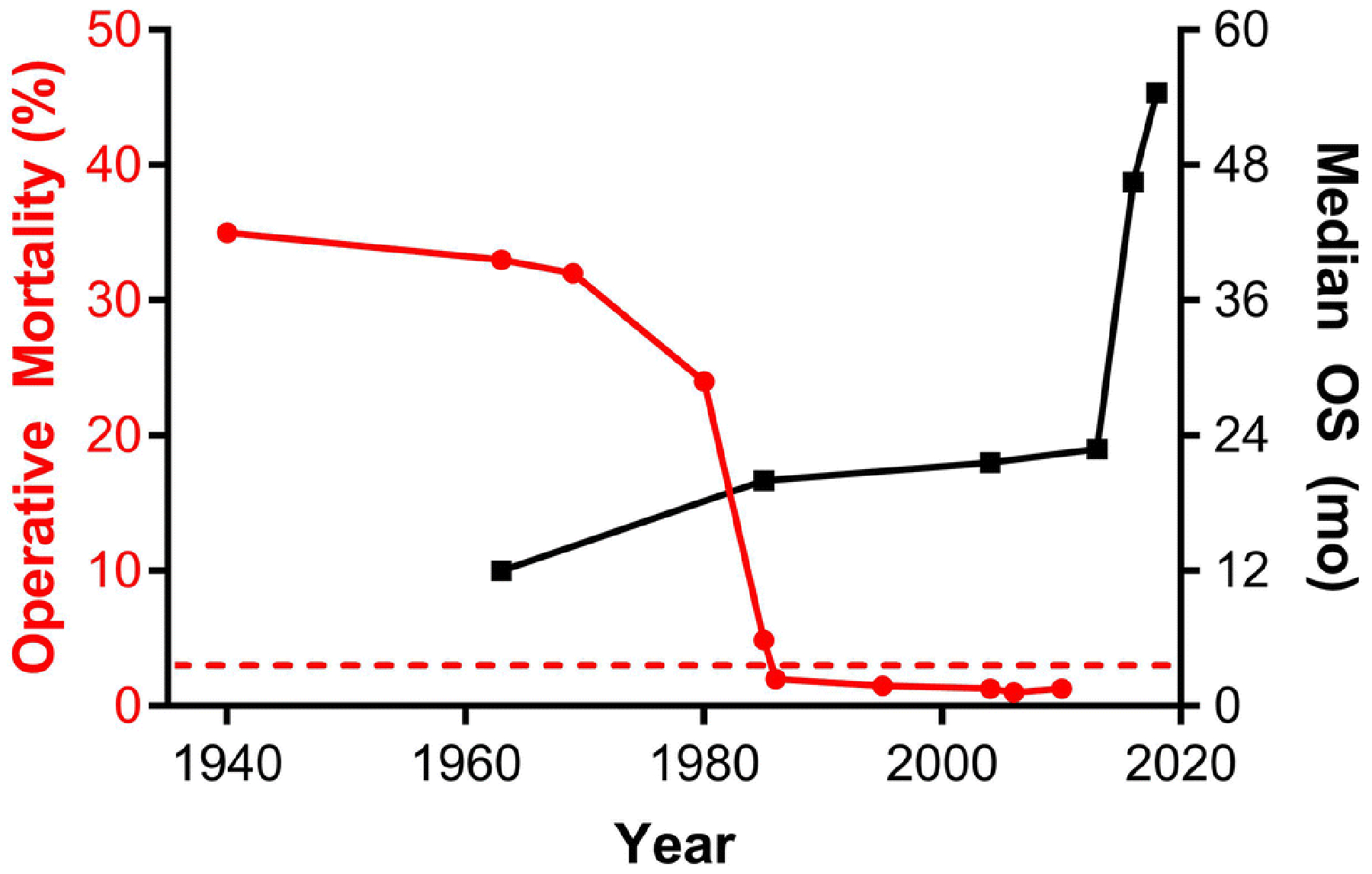
Operative mortality following pancreaticoduodenectomy was over 30% at its inception and did not improve significantly until the late 1980s when it fell below 3% (dashed line) at high volume centers. The median overall survival (OS) of patients with resectable pancreatic cancer was approximately 12 months in the 1960s, leading many to question the curative intent of pancreaticoduodenectomy. Clinical trials utilizing adjuvant chemotherapy and chemoradiotherapy resulted in steady improvements in median OS with dramatic improvements to 46.5 months and 54.5 months reported from the JASPAC 01 and PRODIGE-24 trials, respectively.
While operative mortality has improved remarkably, pancreaticoduodenectomy remains a highly morbid operation, with the most common complications including delayed gastric emptying, pancreatic fistula, and infection. In the current era, operative morbidity following pancreaticoduodenectomy ranges from 40–50%.
Progress in Long-Term Survival
In the 1960s, when the curative nature of pancreaticoduodenectomy for pancreatic cancer was being questioned, several surgeons astutely noted that “the tumor has extended beyond its primary site by the time diagnosis has been made [15].” This observation stemmed from the fact that there were very few long-term survivors of pancreatic cancer at this time and all developed recurrent disease. The 5-year survival rate for patients with resected pancreatic cancer in the 1960s ranged from 0–5% [15,16].
To improve long term outcomes for patients with surgically resectable pancreatic cancer, the Gastrointestinal Tumor Study Group (GITSG) performed a randomized trial comparing observation versus adjuvant chemoradiotherapy with 5-fluorouracil for patients with resected pancreatic cancer in the 1970s and early 1980s. Despite very poor patient accrual and early study termination, the GITSG trial demonstrated a significant survival benefit for patients who received adjuvant chemoradiotherapy, with median overall survival (OS) of 20 months versus 11 months in the observation cohort [22]. Subsequent clinical trials over the next several decades have pushed the boundaries of treatment of resectable pancreatic cancer, resulting in significant improvements in OS (Table 1; Figs. 1 & 2). Both the National Comprehensive Cancer Network (NCCN) and the American Society of Clinical Oncology (ASCO) recommend 6 months of adjuvant systemic therapy for patients with pancreatic cancer who have undergone surgical resection without preoperative therapy [23,24]. Currently, adjuvant treatment options include modified FOLFIRINOX, gemcitabine and capecitabine, and single-agent gemcitabine or fluorouracil. S-1, an oral 5-fluoruracil prodrug, is now the standard adjuvant treatment option in Asian countries following the JASPAC 01 trial [25].
Table 1:
Landmark Adjuvant and Neoadjuvant Clinical Trials in Pancreatic Cancer
| Trial | Accrual | Sequence | Comparison | Median OS# | Ref. |
|---|---|---|---|---|---|
| GITSG 9173 | 1974–1982 | Adjuvant | Chemoradiotherapy (Fluorouracil) vs Observation | Chemoradiotherapy: 20 mo | [22] |
| EORTC 40891 | 1987–1995 | Adjuvant | Chemoradiotherapy (Fluorouracil) vs Observation | Chemoradiotherapy: 24.5 mo | [64] |
| ESPAC-1 | 1994–2000 | Adjuvant | Chemotherapy alone (Fluorouracil) vs Chemoradiotherapy alone vs Combination vs Observation | Chemotherapy: 21.6 mo | [65] |
| CONKO-001 | 1998–2004 | Adjuvant | Gemcitabine vs Observation | Gemcitabine: 22.8 mo | [66] |
| ESPAC-3 | 2000–2007 | Adjuvant | Fluorouracil vs Gemcitabine | Fluorouracil: 23.0 mo | [67] |
| JASPAC 01 | 2007–2010 | Adjuvant | S-1 vs Gemcitabine | S-1: 46.5 mo | [68] |
| ESPAC-4 | 2008–2011 | Adjuvant | Gemcitabine vs gemcitabine & capecitabine | Gemcitabine & capecitabine: 28 mo | [69] |
| PRODIGE-24 | 2012–2016 | Adjuvant | Modified FOLFIRINOX vs Gemcitabine | Modified FOLFIRINOX: 54.4 mo | [25] |
| APACT* | 2014–2018 | Adjuvant | Gemcitabine & Nabpaclitaxel vs Gemcitabine | Gemcitabine & Nabpaclitaxel: 40.5 mo | [70] |
| PREOPANC-1 | 2013–2017 | Neoadjuvant | Neoadjuvant Chemoradiotherapy (Gemcitabine) vs Upfront Surgery | Chemoradiotherapy: 16 mo | [48] |
| Prep-02/JSAP-05 | 2013–2016 | Neoadjuvant | Neoadjuvant Gemcitabine & S1 vs Upfront Surgery | Gemcitabine & S1: 36.7 mo | [50] |
Arm with significant improvement in overall survival (OS) is shown in bold.
Preliminary results
The recent findings from the PRODIGE-24 trial highlight improvements in survival that can be achieved with novel multidrug regimens. The PRODIGE-24 trial randomized patients with resected pancreatic cancer to receive modified FOLFIRINOX (fluoruracil, leucovorin, irinotecan, and oxaliplatin) versus gemcitabine and demonstrated a significant improvement in median disease-free survival from 12.8 months in the gemcitabine group to 21.6 months in the modified FOLFIRINOX group. The median OS was also extended to 54.4 months in the modified FOLFIRINOX group [26].
FUTURE GOALS
Improving Prevention and Early Detection
Treating pancreatic cancer has proven challenging, in part because of its frequent presentation late in the disease course when surgical resection is no longer possible. While we have made progress in surgical techniques and systemic treatments, the incidence of pancreatic cancer continues to rise globally [3], highlighting the importance of a focus on prevention and early detection. Both modifiable risk factors and inherited genetic predisposition contribute to the pathogenesis of pancreatic cancer. Modifiable risk factors include smoking, obesity, a proinflammatory diet, chronic pancreatitis, and long-standing diabetes [27,28]. The burden of these modifiable risk factors is significant. Globally, it is estimated that 20% of pancreatic cancer deaths are attributable to smoking, 9% to diabetes, and 6% to obesity [3]. These risk factors in combination may even be multiplicative. Inherited genetic predisposition accounts for approximately 5–10% of pancreatic cancer cases [29]. Hereditary risk factors include multiple hereditary tumor predispositions syndromes, hereditary pancreatitis, and familial pancreatic cancer [28,30].
In patients with inherited germline mutations and in cases that arise from spontaneous somatic mutations, the pathogenesis of pancreatic cancer from precursor lesions is well defined. Precursor lesions include pancreatic intraepithelial neoplasia (PanIN), intraductal papillary mucinous neoplasm (IPMN), and mucinous cystic neoplasm (MCN) [31]. Screening and early detection in high risk individuals is an ideal mechanism to prevent the development of pancreatic cancer and improve the survival of those who develop cancer if it can be detected at an early stage. The International Cancer of the Pancreas Screening (CAPS) Consortium recommends surveillance for individuals with familial risk, from the age of 50–55 or from 10 years earlier than when the youngest relative with pancreatic cancer was diagnosed. Endoscopic ultrasound, magnetic resonance imaging (MRI), and magnetic resonance cholangiopancreatography (MRCP) are the recommended surveillance tests [32]. Surveillance programs in both Europe and the United States have demonstrated that surveillance of high-risk individuals, using endoscopic ultrasound and MRI/MRCP can lead to the early detection of pancreatic cancer with very high rates of resectability (75–90%) [33,34]. The recommended management of precursor IPMN and MCN lesions is outlined in the revised Fukuoka guidelines [35] and the guidelines on pancreatic cystic neoplasms from the European Study Group on Cystic Tumors of the Pancreas [36].
Surveillance programs for individuals with germline susceptibilities and the proper management of cystic neoplasms will offer better outcomes for high risk patients and those with precursor lesions. Equally important for the future of early diagnosis and prevention of pancreatic cancer is identifying novel biomarkers to aid in early detection. In the 1980s, the glycan carbohydrate antigen 19–9 (CA 19–9) was found to be present at increased levels in the serum of patients with pancreatic cancer and it is now the most clinically used biomarker for pancreatic cancer [37]. Serum CA 19–9 can provide important prognostic information and is useful for monitoring disease burden. Interestingly, CA 19–9 may also contribute to the pathogenesis of pancreatic cancer. This was recently revealed in a transgenic mouse model expressing the enzymes necessary to produce CA 19–9. In this model system, CA 19–9 expression led to the rapid development of pancreatitis and of pancreatic cancer when the mice also expressed the Kras oncogene [38]. Serum CA 19–9 alone is of limited value in the early diagnosis of pancreatic cancer because of its limited positive predictive value and its presence in other gastrointestinal diseases [39].
Additional biomarkers for pancreatic cancer are on the horizon and may add value to traditional CA 19–9 measurement, including plasma thrombospondin-2 (THBS2) and specific coagulation changes as assayed by thrombelastography (TEG). THBS2 is a promising biomarker that was found to accurately discriminate among all stages of human pancreatic ductal adenocarcinoma and distinguish resectable stage I disease from stage III/IV disease. When THBS2 was combined with CA 19–9, the sensitivity and specificity were 87% and 98%, respectively, in distinguishing pancreatic cancer patients from healthy controls [40]. Given the overall low incidence of pancreatic cancer in the general population, screening with serum biomarkers such as THBS2 will only provide clinically actionable information in high-risk populations. TEG is a viscoelastic assay used to measure coagulopathy in trauma, but it can also detect hypercoagulability in oncology patients [41]. TEG analysis of blood samples obtained from patients before pancreatic resection demonstrated increased coagulation indices for those with pancreatic adenocarcinoma compared with non-adenocarcinoma controls. Furthermore, TEG changes were associated with nodal disease burden and the probability of successful resection [42].
Refining our Molecular Understanding of Pancreatic Cancer
Historically, we have viewed pancreatic cancer as a single disease entity, but it is becoming increasing clear that pancreatic cancer has molecular diversity similar to other malignancies like breast cancer, for which treatment is tailored towards the biology of the tumor. The initial landmark study to evaluate the global genomic landscape of pancreatic cancer was published in 2008 and included genetic analysis of 24 advanced pancreatic cancers [43]. This study found that pancreatic cancers contained an average of over 60 genetic alterations, which equated to disruptions in 12 core cellular signaling pathways. Importantly, this study demonstrated the genetic diversity within pancreatic cancer and laid the foundation for future studies.
The advances in sequencing technology over the past decade have contributed greatly to our understanding of pancreatic cancer on a molecular level. Profiling the transcriptome (mRNA expression) of pancreatic tumors and performing unbiased classification of the mRNA expression data has allowed several groups to propose molecular subtypes of pancreatic cancer [44–47]. In 2011, Collisson et al. analyzed the transcriptional profile of microdissected primary pancreatic ductal adenocarcinoma tumors and defined three pancreatic cancer subtypes: “classical”, “quasi-mesenchymal”, and “exocrine like.” These three subtypes were enriched for gene expression associated with epithelial genes, mesenchymal genes, and digestive enzyme genes, respectively. The subtypes also had prognostic value for patients with classical subtype tumors, who have better survival following resection than patients with quasi-mesenchymal subtype tumors [45].
A modified approach using bulk tumor transcriptome profiling of primary and metastatic pancreatic adenocarcinomas by Moffitt et al., identified and validated two tumor-specific subtypes: “basal-like” and “classical”; and two stroma specific subtypes: “normal” and “activated”, and found that basal-like tumors were associated with worse survival than classical tumors. Interestingly, gene expression in basal-like tumors was also consistent with the gene expression of previously described basal subtypes of both breast and bladder cancer, suggesting similarities in tumor biology across organ sites. As pancreatic cancer is composed of abundant stroma, the identification of two stroma specific subtypes that are independent of the tumor-specific subtypes is significant as the microenvironment of pancreatic cancer is important in shaping tumor biology and treatment response [47]. Stromal subtypes may influence the effectiveness of therapies that target the tumor microenvironment in pancreatic cancer, including immunotherapies.
Ongoing and future efforts should be focused on revealing consensus pancreatic cancer subtypes that can be used in prospective clinical trials to understand if the tumor subtype contributes to the selection of optimal first-line systemic therapies. Early results suggest that this paradigm shift in understanding the molecular basis of pancreatic cancer may have important clinical benefits. The COMPASS trial enrolled patients with advanced pancreatic ductal adenocarcinoma and obtained core tumor biopsies prior to first-line chemotherapy. Real-time whole genome sequencing and RNA sequencing was performed on all samples. Results from this trial demonstrated the feasibility of real-time genomic profiling in pancreatic cancer and showed an association between tumor subtype and response to first line-therapy. Partial response was observed in only 8% of patients with basal-like tumors (1 of 12 patients) versus 34% of patients with classical tumors (13 of 38 patients). Patients treated with modified FOLFIRINOX who had a classical tumor had the best progression-free survival [48].
More Effective Systemic Therapies
The current multi-agent treatment options like modified FOLFIRINOX have improved the long-term survival of patients with pancreatic cancer, but more effective systemic therapies and treatment strategies are still needed. Current progress is being made in the area of neoadjuvant therapy for resectable and locally advanced pancreatic cancer and translational research efforts are focused on developing novel therapeutic approaches for treating pancreatic cancer. Preoperative neoadjuvant therapy is currently recommended for patients with radiographic findings suspicious, but not diagnostic of extrapancreatic disease, performance status not appropriate but potentially reversible for resection, vascular involvement, or a CA 19–9 level suggestive of disseminated disease. It is also now considered acceptable to offer neoadjuvant therapy as an alternative option to upfront surgery for patients who are surgical candidates [23]. In fact, for many centers, this is becoming the standard. The potential benefits of neoadjuvant therapy include increasing the number of patients with locally advanced disease that are able to proceed to surgical resection, early treatment of systemic micrometastatic disease, and ensuring postoperative complications do not prevent the delivery of systemic therapy.
Results from two randomized trials: PREOPANC-1 in the Netherlands and Prep-02/JSAP-05 in Japan, are now shedding light on the beneficial role of neoadjuvant therapy in treating pancreatic cancer (Table 1). The PREOPANC trial is a phase III randomized trial (NTR3709) comparing neoadjuvant gemcitabine therapy followed by chemoradiotherapy with gemcitabine versus upfront surgery for resectable and borderline resectable pancreatic cancer. In the immediate surgery arm, patients underwent pancreaticoduodenectomy, followed by adjuvant chemotherapy with six courses of gemcitabine. Patients randomized to the experimental arm received preoperative gemcitabine, chemoradiotherapy with gemcitabine, pancreaticoduodenectomy, and four remaining courses of gemcitabine. Intention-to-treat analysis for the primary outcome of OS showed no statistical difference between the neoadjuvant arm (16 months) and the upfront surgery arm (14.3 months). Neoadjuvant therapy demonstrated benefits in the secondary endpoints of disease-free survival, R0 resection rate, and lower rates of pathologic lymph nodes, perineural invasion, and venous invasion [49]. A significant limitation of the PREOPANC trial is the use of single-agent gemcitabine chemotherapy.[50] The PREOPANC-2 study will build on this work by evaluating neoajuvant FOLOFIRINOX versus neoadjuvant gemcitabine therapy followed by chemoradiotherapy.
The prep-02/JSAP-05 is a phase II/III randomized trial (UMIN000009634) comparing neoadjuvant chemotherapy with gemcitabine and S-1 followed by surgery and adjuvant S-1 versus upfront surgery followed by adjuvant S-1 for patients with resectable pancreatic cancer. Preliminary results demonstrate that neoadjuvant gemcitabine and S-1 improved OS significantly (median OS 36.7 months versus 26.6 months). There were no differences in resection rate, R0 resection rate or perioperative morbidity between the treatment groups [51].
The progress that has been made in prolonging the median OS for patients with resectable pancreatic cancer through optimizing the use of adjuvant and neoadjuvant approaches with conventional chemotherapy and chemoradiotherapy is significant. However, there remain very few long-term survivors of pancreatic cancer. Recently, several solid tumors have shown dramatic responses to immune checkpoint therapies, like anti-programmed death 1 (PD-1) and anti-cytotoxic T-lymphocyte associated antigen 4 (CTLA-4), but these immune checkpoint therapies have had disappointing results in pancreatic cancer [52–54]. Recent results from a phase II randomized trial showed poor response rates to combination therapy durvalumab (anti-PD-L1) and tremlimumab (anti-CTLA-4) in patients with metastatic pancreatic cancer [55].
Given the poor results of immune checkpoint therapies in pancreatic cancer, an area of potential future advancement is targeting the tumor microenvironment in combination with immunotherapy. Pancreatic cancer tumors have abundant desmoplastic stroma, which is composed of fibroblasts, pancreatic stellate cells, immune cells, endothelial cells, and extracellular matrix proteins. The failure of conventional chemotherapies and immunotherapies in treating pancreatic cancer is hypothesized to be partly due to this abundant desmoplastic stroma impairing drug delivery and creating an immunosuppressive microenvironment. Approaches toward targeting the desmoplastic stroma in pancreatic cancer include enzyme-based therapies such as PEGylated recombinant human hyaluronidase, which degrades the abundant glycosaminoglycan hyaluronic acid in the tumor microenvironment [56], and antifibrotic agents such as halofuginone, which inhibits the activation of pancreatic stellate cells and the deposition of extracellular matrix molecules [57]. A phase III trial of the PEGylated recombinant human hyaluronidase (PEGPH20) in combination with gemcitabine and nab-paclitaxel as first-line therapy for patients with metastatic pancreatic cancer failed to demonstrate an improvement in OS over gemcitabine and nab-paclitaxel, tempering optimism for this particular stromal directed therapy [58]. However, the future outlook is promising for new immune checkpoint and stroma directed therapies.
Improving Quality of Life and Surgical Outcomes
For over a century we have pushed the limits of treating pancreatic cancer and made substantial progress in improving operative techniques, postoperative morbidity and mortality, and OS. One area that has received relatively little attention is the affect that different treatment options have on quality of life and other patient-reported outcome measures such as symptoms and disability. Minimally invasive pancreatic resection, including robotic and laparoscopic approaches, is one modality that is gaining acceptance and may help improve surgical outcomes and reduce some of the negative impacts of major pancreatic resection on postoperative quality of life, particularly after distal pancreatic resection.
International evidence-based guidelines are now available for minimally invasive surgical techniques in pancreatic surgery [59]. Minimally invasive distal pancreatectomy is now recommended over open distal pancreatectomy for benign and low-grade malignant tumors in the tail of the pancreas; however, additional prospective studies are needed to evaluate its oncologic equivalency in treating pancreatic ductal adenocarcinoma. Several retrospective studies have demonstrated that minimally invasive distal pancreatectomy can be performed safely with a shorter hospital stay. Two studies also provide evidence that minimally invasive distal pancreatectomy results in better patient reported outcomes than an open approach. Braga et al. reported their single institution results of 100 consecutive laparoscopic distal pancreatectomies and found that patients who underwent laparoscopic resection had slightly higher quality of life scores as it related to feelings towards their general health and vitality [60]. The LEOPARD trial is a multicenter randomized trial that evaluated minimally invasive (laparoscopic) versus open distal pancreatectomy for benign and malignant tumors of the tail of the pancreas [61]. Results from this study demonstrated earlier functional recovery, reduced incidence in delayed gastric emptying and better postoperative quality of life in the minimally invasive cohort. Of note, there was a trend towards increased grade B/C pancreatic fistulas in the minimally invasive cohort, but no difference in the overall complication rate. Additional randomized trials are ongoing and will provide higher quality evidence on minimally invasive distal pancreatectomy [59].
Minimally invasive pancreaticoduodenectomy is also being performed increasingly to treat pancreatic cancer, particularly in the United States, including both robotic and laparoscopic approaches. Several large retrospective cohort studies have evaluated outcomes following laparoscopic and robotic pancreaticoduodenectomy versus an open approach for pancreatic cancer and have found that minimally invasive pancreaticoduodenectomy can be performed with equivalent morbidity, mortality, and oncologic outcomes [20,62]. The recent Dutch LEOPARD-2 study prospectively evaluated laparoscopic versus open pancreaticoduodenectomy for pancreatic and periampullary tumors but was stopped prematurely due to increased operative mortality in the laparoscopic cohort [63]. Despite the negative results from the LEOPARD-2 trial, minimally invasive pancreaticoduodenectomy has been shown to be safe when performed by experienced surgeons at high volume facilities, with operative mortality equivalent to modern standards for open pancreaticoduodenectomy [64]. Future trials evaluating minimally invasive pancreaticoduodenectomy should aim to include quality of life metrics as secondary outcome measures in addition to morbidity and mortality.
CONCLUSION
Collectively, we have made substantial progress in the treatment of pancreatic cancer. Surgically, we have progressed from the first reports of pancreatic resections in the late 1800s and early 1900s, to operative mortality rates of lower than 3% in the 1980s, and now to minimally invasive pancreatic resections. Medically, we have progressed from a nihilistic view of the treatment of pancreatic cancer in the 1960s, to the first randomized trials of adjuvant therapy in the 1980s, and now to new trials of neoadjuvant therapy. As we continue to make strides towards better treating pancreatic cancer, future areas of focus will include improving prevention and early detection, refining our molecular understanding of pancreatic cancer, developing more effective systemic therapies, improving quality of life and surgical outcomes, and multidisciplinary care of our patients.
ACKNOWLEDGEMENTS
Robert J. Torphy is supported by the National Institute of Health/National Center for Advancing Translational Science (NIH/NCATS) Colorado CTSA Grant Number TL1 TR002533.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
CONFLICT OF INTERESTS STATEMENT
We have no conflicts of interest to declare.
REFERENCES
- 1.Morgagni GB. De Sedibus, et Causis Morborum per Anatomen Indagatis Libri Quinque. Venetiis: Typog Remondiniana 1761. [Google Scholar]
- 2.Costa JMD. On the morbid anatomy and symptoms of cancer of the pancreas. Philadelphia: J.B. Lippincott & Co.; 1858. [Google Scholar]
- 3.Pourshams A, Sepanlou SG, Ikuta KS, Bisignano C, Safiri S, Roshandel G, et al. The global, regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet Gastroenterology & Hepatology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 5.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA: A Cancer Journal for Clinicians. 2020;70(1):7–30. [DOI] [PubMed] [Google Scholar]
- 6.Witzel O. Aus der Klinik des Herrn Prof. Trendelenburg. Beiträge zur Chirurgie der Bauchorgane. Deutsche Zeitschrift für Chirurgie. 1886;24(3):326–354. [Google Scholar]
- 7.Griffin JF, Poruk KE, Wolfgang CL. Pancreatic cancer surgery: past, present, and future. Chinese journal of cancer research = Chung-kuo yen cheng yen chiu. 2015;27(4):332–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schnelldorfer T, Sarr MG. Alessandro Codivilla and the First Pancreatoduodenectomy. JAMA Surgery. 2009;144(12):1179–1184. [DOI] [PubMed] [Google Scholar]
- 9.HALSTED WS. Contributions to the Surgery of the Bile Passages, Especially of the Common Bile-Duct. The Boston Medical and Surgical Journal. 1899;141(26):645–654. [Google Scholar]
- 10.Kausch W. Das Carcinom der Papilla duodeni und seine radikale Entfernung. Beitr Klin Chir. 1912;78:439–486. [Google Scholar]
- 11.Whipple AO, Parsons WB, Mullins CR. TREATMENT OF CARCINOMA OF THE AMPULLA OF VATER. Ann Surg. 1935;102(4):763–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whipple AO. Present-Day Surgery of the Pancreas. New England Journal of Medicine. 1942;226(13):515–526. [Google Scholar]
- 13.Whipple AO. Observations on radical surgery for lesions of the pancreas. Surg Gynecol Obstet. 1946;82:623–631. [PubMed] [Google Scholar]
- 14.Whipple AO. A reminiscence: pancreaticduodenectomy. Rev Surg. 1963;20:221–225. [PubMed] [Google Scholar]
- 15.Glenn F, Thorbjarnarson B. CARCINOMA OF THE PANCREAS. Annals of surgery. 1964;159(6):945–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallitano A, Fransen H, Martin RG. Carcinoma of the pancreas. Results of treatment. Cancer. 1968;22(5):939–944. [DOI] [PubMed] [Google Scholar]
- 17.Crist DW, Sitzmann JV, Cameron JL. Improved hospital morbidity, mortality, and survival after the Whipple procedure. Ann Surg. 1987;206(3):358–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Birkmeyer JD, Finlayson SR, Tosteson AN, Sharp SM, Warshaw AL, Fisher ES. Effect of hospital volume on in-hospital mortality with pancreaticoduodenectomy. Surgery. 1999;125(3):250–256. [PubMed] [Google Scholar]
- 19.Yoshioka R, Yasunaga H, Hasegawa K, Horiguchi H, Fushimi K, Aoki T, et al. Impact of hospital volume on hospital mortality, length of stay and total costs after pancreaticoduodenectomy. BJS. 2014;101(5):523–529. [DOI] [PubMed] [Google Scholar]
- 20.Torphy RJ, Friedman C, Halpern A, Chapman BC, Ahrendt SS, McCarter MM, et al. Comparing Short-term and Oncologic Outcomes of Minimally Invasive Versus Open Pancreaticoduodenectomy Across Low and High Volume Centers. Ann Surg. 2018. [DOI] [PubMed] [Google Scholar]
- 21.Hata T, Motoi F, Ishida M, Naitoh T, Katayose Y, Egawa S, et al. Effect of Hospital Volume on Surgical Outcomes After Pancreaticoduodenectomy: A Systematic Review and Meta-analysis. Ann Surg. 2016;263(4):664–672. [DOI] [PubMed] [Google Scholar]
- 22.Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985;120(8):899–903. [DOI] [PubMed] [Google Scholar]
- 23.Khorana AA, McKernin SE, Berlin J, Hong TS, Maitra A, Moravek C, et al. Potentially Curable Pancreatic Adenocarcinoma: ASCO Clinical Practice Guideline Update. J Clin Oncol. 2019;37(23):2082–2088. [DOI] [PubMed] [Google Scholar]
- 24.NCCN Clinical Practice Guidelines in Oncology: Pancreatic Adenocarcinoma. Improving Prevention and Early Detection.
- 25.Uesaka K, Boku N, Fukutomi A, Okamura Y, Konishi M, Matsumoto I, et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet. 2016;388(10041):248–257. [DOI] [PubMed] [Google Scholar]
- 26.Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul JL, et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N Engl J Med. 2018;379(25):2395–2406. [DOI] [PubMed] [Google Scholar]
- 27.Antwi SO, Oberg AL, Shivappa N, Bamlet WR, Chaffee KG, Steck SE, et al. Pancreatic cancer: associations of inflammatory potential of diet, cigarette smoking and long-standing diabetes. Carcinogenesis. 2016;37(5):481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raimondi S, Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: an overview. Nat Rev Gastroenterol Hepatol. 2009;6(12):699–708. [DOI] [PubMed] [Google Scholar]
- 29.Hruban RH, Canto MI, Goggins M, Schulick R, Klein AP. Update on familial pancreatic cancer. Adv Surg. 2010;44:293–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Torphy RJ, Schulick RD. Screening of Patients at Risk for Familial Pancreatic Cancer: What Is Beneficial? Surgical Clinics of North America. 2018;98(1):25–35. [DOI] [PubMed] [Google Scholar]
- 31.Basturk O, Hong SM, Wood LD, Adsay NV, Albores-Saavedra J, Biankin AV, et al. A Revised Classification System and Recommendations From the Baltimore Consensus Meeting for Neoplastic Precursor Lesions in the Pancreas. Am J Surg Pathol. 2015;39(12):1730–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goggins M, Overbeek KA, Brand R, Syngal S, Del Chiaro M, Bartsch DK, et al. Management of patients with increased risk for familial pancreatic cancer: updated recommendations from the International Cancer of the Pancreas Screening (CAPS) Consortium. Gut. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vasen H, Ibrahim I, Ponce CG, Slater EP, Matthai E, Carrato A, et al. Benefit of Surveillance for Pancreatic Cancer in High-Risk Individuals: Outcome of Long-Term Prospective Follow-Up Studies From Three European Expert Centers. J Clin Oncol. 2016;34(17):2010–2019. [DOI] [PubMed] [Google Scholar]
- 34.Canto MI, Almario JA, Schulick RD, Yeo CJ, Klein A, Blackford A, et al. Risk of Neoplastic Progression in Individuals at High Risk for Pancreatic Cancer Undergoing Long-term Surveillance. Gastroenterology. 2018;155(3):740–751.e742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanaka M, Fernandez-Del Castillo C, Kamisawa T, Jang JY, Levy P, Ohtsuka T, et al. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology. 2017;17(5):738–753. [DOI] [PubMed] [Google Scholar]
- 36.European evidence-based guidelines on pancreatic cystic neoplasms. Gut. 2018;67(5):789–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malesci A, Tommasini MA, Bonato C, Bocchia P, Bersani M, Zerbi A, et al. Determination of CA 19–9 antigen in serum and pancreatic juice for differential diagnosis of pancreatic adenocarcinoma from chronic pancreatitis. Gastroenterology. 1987;92(1):60–67. [DOI] [PubMed] [Google Scholar]
- 38.Engle DD, Tiriac H, Rivera KD, Pommier A, Whalen S, Oni TE, et al. The glycan CA19–9 promotes pancreatitis and pancreatic cancer in mice. Science. 2019;364(6446):1156–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ballehaninna UK, Chamberlain RS. The clinical utility of serum CA 19–9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: An evidence based appraisal. J Gastrointest Oncol. 2012;3(2):105–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim J, Bamlet WR, Oberg AL, Chaffee KG, Donahue G, Cao XJ, et al. Detection of early pancreatic ductal adenocarcinoma with thrombospondin-2 and CA19–9 blood markers. Sci Transl Med. 2017;9(398). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Francis JL, Francis DA, Gunathilagan GJ. Assessment of hypercoagulability in patients with cancer using the Sonoclot Analyzer and thromboelastography. Thromb Res. 1994;74(4):335–346. [DOI] [PubMed] [Google Scholar]
- 42.Moore HB, Paniccia A, Lawson PJ, Torphy RJ, Nydam TL, Moore EE, et al. Utility of Viscoelastic Assays Beyond Coagulation: Can Preoperative Thrombelastography Indices Predict Tumor Histology, Nodal Disease, and Resectability in Patients Undergoing Pancreatectomy? Journal of the American College of Surgeons. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321(5897):1801–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Collisson EA, Bailey P, Chang DK, Biankin AV. Molecular subtypes of pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2019;16(4):207–220. [DOI] [PubMed] [Google Scholar]
- 45.Collisson EA, Sadanandam A, Olson P, Gibb WJ, Truitt M, Gu S, et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat Med. 2011;17(4):500–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531(7592):47–52. [DOI] [PubMed] [Google Scholar]
- 47.Moffitt RA, Marayati R, Flate EL, Volmar KE, Loeza SG, Hoadley KA, et al. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat Genet. 2015;47(10):1168–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aung KL, Fischer SE, Denroche RE, Jang GH, Dodd A, Creighton S, et al. Genomics-Driven Precision Medicine for Advanced Pancreatic Cancer: Early Results from the COMPASS Trial. Clin Cancer Res. 2018;24(6):1344–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Versteijne E, Suker M, Groothuis K, Akkermans-Vogelaar JM, Besselink MG, Bonsing BA, et al. Preoperative Chemoradiotherapy Versus Immediate Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Results of the Dutch Randomized Phase III PREOPANC Trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2020:JCO1902274–JCO1902274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O’Reilly EM, Ferrone C. Neoadjuvant or Adjuvant Therapy for Resectable or Borderline Resectable Pancreatic Cancer: Which Is Preferred? Journal of Clinical Oncology. 2020:JCO.19.03318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Unno M, Motoi F, Matsuyama Y, Satoi S, Matsumoto I, Aosasa S, et al. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP-05). J Clin Oncol. 2019;37 (suppl 4; abstr 189). [DOI] [PubMed] [Google Scholar]
- 52.Royal RE, Levy C, Turner K, Mathur A, Hughes M, Kammula US, et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;33(8):828–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Torphy RJ, Zhu Y, Schulick RD. Immunotherapy for pancreatic cancer: Barriers and breakthroughs. Annals of Gastroenterological Surgery. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O’Reilly EM, Oh D-Y, Dhani N, Renouf DJ, Lee MA, Sun W, et al. Durvalumab With or Without Tremelimumab for Patients With Metastatic Pancreatic Ductal Adenocarcinoma: A Phase 2 Randomized Clinical Trial. JAMA Oncology. 2019;5(10):1431–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Infante JR, Korn RL, Rosen LS, LoRusso P, Dychter SS, Zhu J, et al. Phase 1 trials of PEGylated recombinant human hyaluronidase PH20 in patients with advanced solid tumours. Br J Cancer. 2018;118(2):e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Elahi-Gedwillo KY, Carlson M, Zettervall J, Provenzano PP. Antifibrotic Therapy Disrupts Stromal Barriers and Modulates the Immune Landscape in Pancreatic Ductal Adenocarcinoma. Cancer Res. 2019;79(2):372–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Doherty GJ, Tempero M, Corrie PG. HALO-109–301: a Phase III trial of PEGPH20 (with gemcitabine and nab-paclitaxel) in hyaluronic acid-high stage IV pancreatic cancer. Future Oncol. 2018;14(1):13–22. [DOI] [PubMed] [Google Scholar]
- 59.Asbun HJ, Moekotte AL, Vissers FL, Kunzler F, Cipriani F, Alseidi A, et al. The Miami International Evidence-Based Guidelines on Minimally Invasive Pancreas Resection. Ann Surg. 2019. [DOI] [PubMed] [Google Scholar]
- 60.Braga M, Pecorelli N, Ferrari D, Balzano G, Zuliani W, Castoldi R. Results of 100 consecutive laparoscopic distal pancreatectomies: postoperative outcome, cost-benefit analysis, and quality of life assessment. Surg Endosc. 2015;29(7):1871–1878. [DOI] [PubMed] [Google Scholar]
- 61.de Rooij T, van Hilst J, van Santvoort H, Boerma D, van den Boezem P, Daams F, et al. Minimally Invasive Versus Open Distal Pancreatectomy (LEOPARD): A Multicenter Patient-blinded Randomized Controlled Trial. Ann Surg. 2019;269(1):2–9. [DOI] [PubMed] [Google Scholar]
- 62.Nassour I, Wang SC, Christie A, Augustine MM, Porembka MR, Yopp AC, et al. Minimally Invasive Versus Open Pancreaticoduodenectomy: A Propensity-matched Study From a National Cohort of Patients. Ann Surg. 2018;268(1):151–157. [DOI] [PubMed] [Google Scholar]
- 63.van Hilst J, de Rooij T, Bosscha K, Brinkman DJ, van Dieren S, Dijkgraaf MG, et al. Laparoscopic versus open pancreatoduodenectomy for pancreatic or periampullary tumours (LEOPARD-2): a multicentre, patient-blinded, randomised controlled phase 2/3 trial. Lancet Gastroenterol Hepatol. 2019;4(3):199–207. [DOI] [PubMed] [Google Scholar]
- 64.Zureikat AH, Moser AJ, Boone BA, Bartlett DL, Zenati M, Zeh HJ 3rd. 250 robotic pancreatic resections: safety and feasibility. Annals of surgery. 2013;258(4):554–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Klinkenbijl JH, Jeekel J, Sahmoud T, van Pel R, Couvreur ML, Veenhof CH, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg. 1999;230(6):776–782; discussion 782–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350(12):1200–1210. [DOI] [PubMed] [Google Scholar]
- 67.Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. Jama. 2013;310(14):1473–1481. [DOI] [PubMed] [Google Scholar]
- 68.Neoptolemos JP, Stocken DD, Bassi C, Ghaneh P, Cunningham D, Goldstein D, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. Jama. 2010;304(10):1073–1081. [DOI] [PubMed] [Google Scholar]
- 69.Neoptolemos JP, Palmer DH, Ghaneh P, Psarelli EE, Valle JW, Halloran CM, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389(10073):1011–1024. [DOI] [PubMed] [Google Scholar]
- 70.Margaret A, Tempero MR, Hanno Riess, Uwe Pelzer, O’Reilly Eileen Mary, Winter Jordan Michael, Oh Do-Youn, Li Chung-Pin, Tortora Giampaolo, Chang Heung-Moon, Lopez Charles D., Tabernero Josep, Van Cutsem Eric, Philip Philip Agop, Goldstein David, Berlin Jordan, Ferrara Stefano, Li Mingyu, Lu Brian D., Biankin Andrew;. APACT: phase III, multicenter, international, open-label, randomized trial of adjuvant nab-paclitaxel plus gemcitabine (nab-P/G) vs gemcitabine (G) for surgically resected pancreatic adenocarcinoma. J Clin Oncol. 2019;37((suppl; abstr 4000)). [Google Scholar]


