Summary
ER tubules form and maintain membrane contact sites (MCSs) with endosomes. How and why these ER-endosome MCSs persist as endosomes traffic and mature is poorly understood. Here we find that a member of the reticulon protein family, Reticulon-3L (Rtn3L), concentrates ER-endosome MCSs as endosomes mature. We show that this localization is due to the long divergent N-terminal cytoplasmic domain of Rtn3L. We found that Rtn3L is recruited to ER-endosome MCSs by endosomal protein Rab9a, which marks a transition stage between early and late endosomes. Rab9a utilizes an FSV region to recruit Rtn3L via its six LC3-interacting region motifs. Consistent with our localization results, depletion or deletion of RTN3 from cells results in endosome maturation and cargo sorting defects, similar to RAB9A depletion. Together our data identify a tubular ER protein that promotes endosome maturation at ER MCSs.
Graphical Abstract
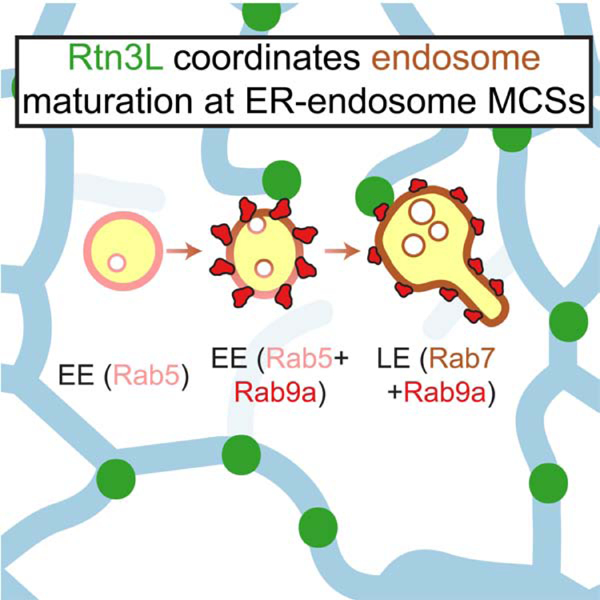
eTOC Blurb
Wu and Voeltz characterize a tubular endoplasmic reticulum protein Reticulon-3L that is recruited to endosomes by Rab9a to coordinate endosome maturation.
Introduction
The endoplasmic reticulum (ER) is organized into three subdomains with distinct morphologies and functions: the nuclear envelope, which surrounds chromatin; ER sheets, which are studded with ribosomes and are sites of protein translocation; and ER tubules, which regulate lipid and ion homeostasis as well as organelle trafficking and biogenesis (Westrate et al., 2015; Wu et al., 2018). ER tubules can perform their functions by forming membrane contact sites (MCSs) with other organelles (Phillips and Voeltz, 2015; Wu et al., 2018). ER tubules can be tightly tethered to other organelles that the two organelles will remain tethered as they traffic along microtubules (MTs) (Friedman et al., 2010; Wu et al., 2018).
Endosomes maintain MCSs with ER tubules as they traffic and mature on MTs. These ER-endosome MCSs have diverse functions, including regulating endosome trafficking, lipid composition, and fission during cargo sorting (Friedman et al., 2013; Hoyer et al., 2018; Jongsma et al., 2016; Raiborg et al., 2015; Rocha et al., 2009; Rowland et al., 2014). Endosome trafficking and maturation on MTs is tightly linked to essential cellular functions, including cell signaling, cargo sorting, and lipid metabolism, and dysfunctions in these processes are linked to many diseases (Huotari and Helenius, 2011).
The prevalence of ER-endosome MCSs increases as endosomes mature (Friedman et al., 2013). However, how and why endosomes recruit and maintain tubular ER MCSs as they move and mature on MTs remains elusive. Identification of tubular ER proteins that form MCSs could provide insight on how and why organelles are attached to ER tubules while trafficking on MTs. The Reticulon (Rtn) proteins reside exclusively on tubular ER due to a conserved C-terminal reticulon homology domain (RHD). The RHD forms a characteristic hairpin transmembrane domain that restricts Rtn protein localization to regions of high membrane curvature like tubular ER membranes (Voeltz et al., 2006). The prototype of Rtn protein function is Rtn4a, which generates and maintains the structure of ER tubules (Voeltz et al., 2006). Functional Rtn4a homologs are present in various eukaryotes, including yeast (Rtn1 and Rtn2 in S. cerevisiae), flies (Rtnl1 in D. melanogaster), worms (RET-1 in C. elegans), and plants (RTNLB13 in A. thaliana) (Audhya et al., 2007; O’Sullivan et al., 2012; Tolley et al., 2008; Voeltz et al., 2006). However, Rtn paralogs exist with highly diverse N-terminal cytoplasmic domains in mammalian cells, and it has been unclear whether Rtn paralogs function redundantly. Rtn proteins are ideal candidates to function at ER MCSs due to their tubular ER-specific localization. Here, we find that one mammalian Rtn paralog, Rtn3L, does not shape ER tubules. Instead, Rtn3L is recruited by Rab9a to directly tether MCSs between ER tubules and endosomes. Our data reveal that Rtn3L functions at ER MCSs to regulate endosome maturation and cargo sorting.
Results
RTN3 and RTN4 have non-redundant functions
Rtn proteins are defined by their conserved C-terminal RHDs but also contain variable N-terminal cytoplasmic domains. Hence, we compared the functional properties of the longest isoform of Rtn3 (Rtn3L) with the ER tubule-shaping protein Rtn4a. Rtn3 is highly conserved in vertebrates (Figure S1A). By sequence comparison, the RHD of Rtn3L shares 66% similarity to Rtn4a, while the cytoplasmic domains are only 28% similar (Figure S1B). To test if RTN3 and RTN4 function similarly to promote ER tubule formation, we generated RTN3 and RTN4 knockout HeLa and U-2 OS cell lines using CRISPR-Cas9 technology (Ran et al., 2013) (Figure S1C). We first compared ER morphology visualized with a fluorescently tagged ER membrane protein [mNeonGreen (mNG)-Sec61β]. In wild-type (WT) cells, a typical peripheral ER includes perinuclear ER sheets interconnected with an extensive network of branched tubules that is joined by three-way junctions (Westrate et al., 2015). Consistent with previous studies (Jozsef et al., 2014; Lee et al., 2020; Rämö et al., 2016), RTN4Δ cells had a reduced tubular network and an expansion of peripheral ER sheets (Figure 1A, HeLa; Figure S1D, U-2 OS). In contrast, RTN3Δ cells retained an extensive tubular ER network and did not display an expansion of ER sheets (Figure 1A, Figure S1D). We scored whether RTN3 deletion affected the organization of the tubular ER network by comparing the number of three-way junctions within 10 x 10 µm boxes placed in the peripheral ER of WT or RTN3Δ HeLa cells. The WT and RTN3Δ cells had similar numbers of three-way junctions per box (~140, Figure 1A–1B). These data show that RTN3 deletion does not affect the branching of the tubular ER network.
Figure 1. Rtn3L localizes to discrete domains on ER tubules.
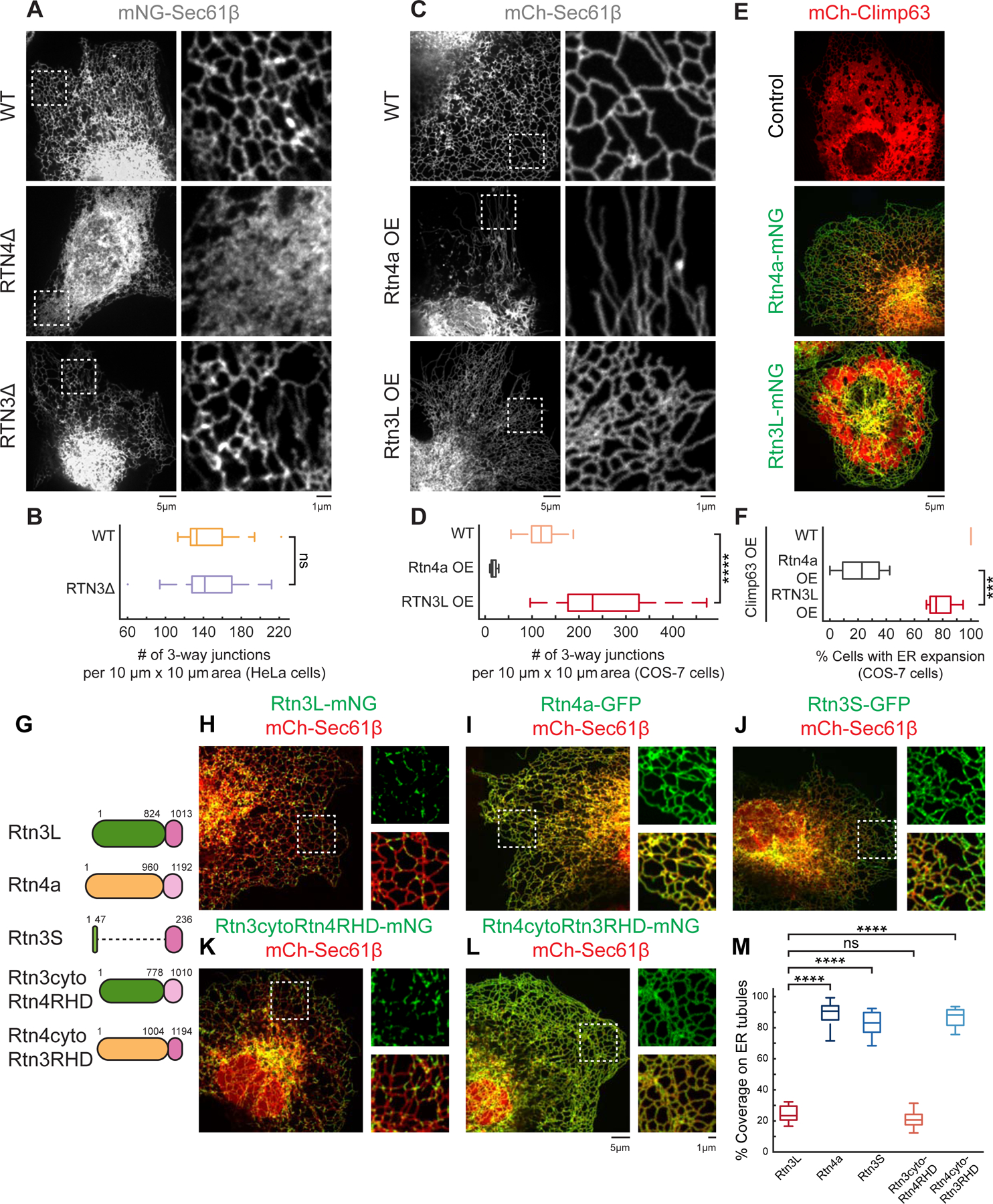
(A) Representative images and 10 x 10 µm insets of ER morphology in WT, RTN4Δ, and RTN3Δ HeLa cells transfected with a general ER marker mNeonGreen (mNG)-Sec61β. (B) Quantification of number of three-way junctions in 10 x 10 µm peripheral insets (A) in WT (n=17 cells) and RTN3Δ cells (n=18 cells). (C) Representative images and 10 x 10 µm insets of ER morphology in COS-7 cells co-transfected with an ER marker (mCh-Sec61β, WT) and either overexpressing (OE) Rtn4a-mNG (Rtn4a OE) or Rtn3L-mNG (Rtn3L OE). (D) Quantification as in (B) for (C) from WT (n=19 cells), Rtn4a OE (n=10 cells) and Rtn3L OE (n=21 cells). (E) Representative images of ER morphology in COS-7 cells OE mCh-Climp63 alone (control), and either with OE of Climp63/Rtn4a (Rtn4a-mNG) or Climp63/Rtn3L (Rtn3L-mNG). (F) Quantification of percent of cells with expanded peripheral ER sheets (E) for Climp63-OE (n=14 cells), Climp63/Rtn4a-OE (n=113 cells) and Climp63/Rtn3L-OE (n=113 cells). (G-L) Cartoon diagrams (with amino acid numbers) and representative images of Rtn variants’ localization for (H) Rtn3L-mNG, (I) Rtn4a-GFP, (J) Rtn3S-GFP, (K) Rtn3cytoRtn4RHD-mNG and (L) Rtn4cytoRtn3RHD-mNG relative to a mCh-Sec61β (ER) in COS-7 cells. 10 µm x 10 µm insets are shown for each Rtn variant alone (green, top right) and overlaid with Sec61β (red, bottom right). (M) Quantification of Rtn variants pixel coverage over the general ER marker (Sec61β) (H-L, n=15 cells for each condition). A lower percent coverage indicates that the Rtn variant is not evenly distributed on ER tubules. Statistical analyses were performed with one-way ANOVA, p-value from Tukey’s test: ns=not significant, ***p<0.001, ****p<0.0001. Scale bars = 5 µm, 1 µm for insets. See also Figure S1–S2. Video S1–S2.
Rtn4a overexpression is sufficient to convert peripheral ER tubules and sheets into long unbranched tubules (Voeltz et al., 2006); this phenotype can also be scored by measuring the number of 3-way junctions. As expected, COS-7 cells overexpressing Rtn4a had significantly fewer three-way junctions than in control cells (Figure 1C–1D). By comparison, Rtn3L overexpression did not result in long unbranched tubules, but these cells did have twice the density of 3-way junctions, indicating more branching upon Rtn3L overexpression compared to control cells (Figure 1C–1D).
In complementary experiments, we also tested whether Rtn3L overexpression can suppress ER sheet proliferation. We used Climp63 overexpression to drive ER sheet formation (Figure 1E) (Shibata et al., 2010). Climp63-propagated ER sheets are eliminated if Climp63 is overexpressed together with the Rtn4a [(Shibata et al., 2010) and Figure 1E–1F]. In contrast, overexpression of Rtn3L did not suppress Climp63-driven ER sheets (Figure 1E–1F). Taken together, these data demonstrate that, unlike Rtn4a, Rtn3L does not drive ER tubulation.
Rtn3L localizes to discrete domains on ER tubules
To gain insight into Rtn3L’s function, we investigated its localization in COS-7 cells transfected with mCherry (mCh)-Sec61β (ER) and low levels of Rtn3L-mNG. Surprisingly, unlike Rtn4a which distributes homogenously along the length of ER tubules at low expression levels, Rtn3L concentrates in discrete puncta along ER tubules (Figure 1G–1I). To score this distribution, we measured the percent ER area (Sec61β) that is covered by Rtn3L. Rtn3L puncta cover 23% of the tubular ER network, which is significantly lower than Rtn4a coverage (91%) (Figure 1H–1I and 1M, note that all numbers referred in main text are medians of data represented in boxplots). A similar distribution can be seen for Rtn3L in U-2 OS and HeLa cells (Figure S2A). We tested whether the divergent long cytoplasmic domain of Rtn3L is responsible for its punctate distribution by comparing the localization of Rtn3L to Rtn3S, the short splice isoform of Rtn3, which lacks the N-terminal cytoplasmic domain (Figure 1G). Similar to Rtn4a, Rtn3S has a high coverage along the ER tubules (83%, Figure 1J and 1M). Finally, we tested whether the cytoplasmic domain of Rtn3L is sufficient to confer punctate localization by fusing the cytoplasmic domain of Rtn3L to the Rtn4 RHD (Rtn3cytoRtn4RHD-mNG) (Figure 1G). Rtn3cytoRtn4RHD-mNG localized to punctate structures along ER tubules similar to Rtn3L (21% coverage, Figure 1K and 1M). In contrast, fusion of the cytoplasmic domain of Rtn4a to the Rtn3 RHD (Rtn4cytoRtn3RHD-mNG) displayed a homogeneous distribution along ER tubules similar to Rtn4a (88% coverage, Figure 1L–1M). These data demonstrate that the conserved RHDs of Rtn3L and Rtn4a function similarly to localize Rtn proteins to ER tubules, but the divergent long Rtn3L cytoplasmic domain uniquely confers a punctate distribution along ER tubules.
We next used time-lapse microscopy to determine whether Rtn3L puncta are dynamic. An overlay of Rtn3L signals at time = 0 sec versus time = 60 sec later showed little overlap, indicating that Rtn3L puncta are dynamic on ER tubules (Figure S2B and Video S1). We tested whether dynamic Rtn3L puncta co-localized with MTs by imaging live cells co-transfected with Rtn3L-mNG, BFP-Sec61β, and mCh-Ensconsin (MTs). Rtn3L puncta moved along MTs (Figure S2C and Video S2), and the majority (86%) overlapped with positions where ER tubules and MTs intersected (Figure S2C). By comparison the overlap is much lower for the general ER marker with MTs (24%, Figure S2C). We measured the speed of Rtn3L puncta that traveled both towards and away from the nucleus. Anterograde and retrograde Rtn3L puncta trafficked at similar speeds (0.18 µm/s and 0.24 µm/s, respectively, Figure S2D) and were comparable to ER sliding dynamics on MTs (Friedman et al., 2010; Waterman-Storer and Salmon, 1998). These data suggest that Rtn3L may function at intersections between the ER and MTs.
Interestingly, a subset of dynamic Rtn3L puncta localized to the leading edge of moving ER tubules (Figure S2D and Video S1). Hence, we tested whether RTN3 is required for ER tubule dynamics. We collected snapshots of the tubular ER network in control versus RTN3Δ HeLa cells at time = 0 sec versus time = 60 sec later and overlaid the snapshots to score how dynamic the tubular ER network is (Figure S2E). A higher level of ER overlap indicates fewer ER dynamic events (Rowland et al., 2014). The level of ER network overlap in WT is similar to RTN3Δ cells indicating that RTN3 is not required to maintain ER tubule dynamics (Figure S2E).
Rtn3L enriches at ER MCSs with endolysosomal pathway organelles
ER MCSs often form at the leading edge of dynamic ER tubules. These ER MCSs persist when other organelles traffic along MTs, causing ER tubules and organelles to move together (Friedman et al., 2013; Wu et al., 2018). Since dynamic Rtn3L puncta localize to the leading edge of dynamic ER tubules and move along MTs (Figure S2C–S2D), we visualized whether Rtn3L puncta label specific ER MCSs. COS-7 cells were co-transfected with fluorescently tagged markers for Rtn3L, the general ER (Sec61β), and lysosomes (LAMP1), autophagosomes (LC3), or peroxisomes (SKL) (Figure 2A). Live cell movies were taken to visualize the positions of dynamic Rtn3L puncta (green) relative to each of these organelles (red) that often track with ER tubules (blue) (Figure 2B–D). We scored the percent of time where Rtn3L puncta remained associated with a moving organelle during time-lapse movies. These data revealed that Rtn3L puncta are enriched at ER MCSs with moving lysosomes (95%, Figure 2B, 2F) or autophagosomes (88%, Figure 2C, 2F) and to a much lower extent with peroxisomes (36%, Figure 2D–2F), indicating a preference for late maturation stages of the endolysosomal pathway. Since all three organelles can maintain contact with the ER as they traffic (Figure 2E), we aimed to determine the co-localization between Rtn3L and organelles due to chance. Accordingly, for each experiment, we quantified the coverage of Rtn3L on the general ER, which gave a range of ~20–25% (Figure 2F). Thus, the frequency of Rtn3L puncta that track at MCSs with moving lysosomes and autophagosomes is significantly higher than what would be expected due to chance.
Figure 2. Rtn3L enriches at ER membrane contact sites.

(A) Cartoon diagram of peroxisome (SKL) and endolysosomal system including early endosome (Rab5), late endosome/lysosome (Rab7/LAMP1) and autophagosome (LC3). (B-D) Representative whole cell and time-lapse images of 10 µm x 10 µm insets show Rtn3L localization relative to other ER-associated trafficking organelles in COS-7 cells co-transfected with Rtn3L-mNG (green), a general ER marker (BFP-Sec61β, blue) and (B) lysosomes (LAMP1-mCh, red), (C) autophagosomes (GFP-LC3, red) or (D) peroxisomes (mRuby-SKL, red). (E) Graph of the percent of time each organelle maintains contact with the ER during time-lapse movies. (F) Graph of percent coverage of Rtn3L pixels over the general ER marker (blue bars) and the percent of time that each dynamic organelle tracks with an Rtn3L puncta over time (orange bars). Quantified from data represented in (B-D) for 60 lysosomes in n=9 cells; 18 autophagosomes in n=7 cells; 23 peroxisomes in n=13 cells. Scale bars = 5 µm, 1 µm for insets.
Rtn3L accumulates at MCSs as endosomes mature
Recently, Rtn3 was shown to regulate the nonclathrin endocytic (NCE) pathway (Caldieri et al., 2017). Since we found that Rtn3L is enriched at ER MCSs with late stage endocytic compartments (Figure 2) and that endosomes acquire ER contacts as they mature (Friedman et al., 2013), we sought to more precisely define the timing of Rtn3L recruitment during endosome maturation (Figure 3A). First we visualized whether Rtn3L localized to MCSs with Rab7-labeled late endosomes (LEs) during live cell movies. COS-7 cells were co-transfected with Rtn3L-mNG, BFP-Sec61β (ER), and mCh-Rab7 (LEs). We scored the percent of time where Rtn3L remained associated with moving LEs during time-lapse movies (Fig 3B, 3D). These data revealed that Rtn3L puncta were enriched at ER MCSs with dynamic LEs (94%, Figure 3B, Figure 3D, and Video S3). To further pinpoint the timing of Rtn3L recruitment to endosomes we labeled endosomes with Rab5 [early endosomes (EEs)] and EEA1, which is a “late” EE marker that is recruited to EEs after Rab5 but before Rab7 (Figure 3A) (Friedman et al., 2013; Zoncu et al., 2009). Movies were collected in live COS-7 cells co-transfected with Rtn3L-mNG, BFP-Sec61β (ER), mScarlet-EEA1, and SNAP-Rab5 to compare Rtn3L recruitment to Rab5-labeled EEs that are either negative (less mature, blue, no magenta) or positive for EEA1 (more mature, blue and magenta) (Figure 3C). We observed that Rtn3L puncta were more frequently associated with more mature EEA1+/Rab5+ EEs (94%) over time than with less mature EEA1-/Rab5+ EEs (27%) (Figure 3C–3D and Video S4). We conclude that Rtn3L puncta are recruited to endosomes as they mature.
Figure 3. Rtn3L puncta are recruited to ER-endosome MCSs during maturation.
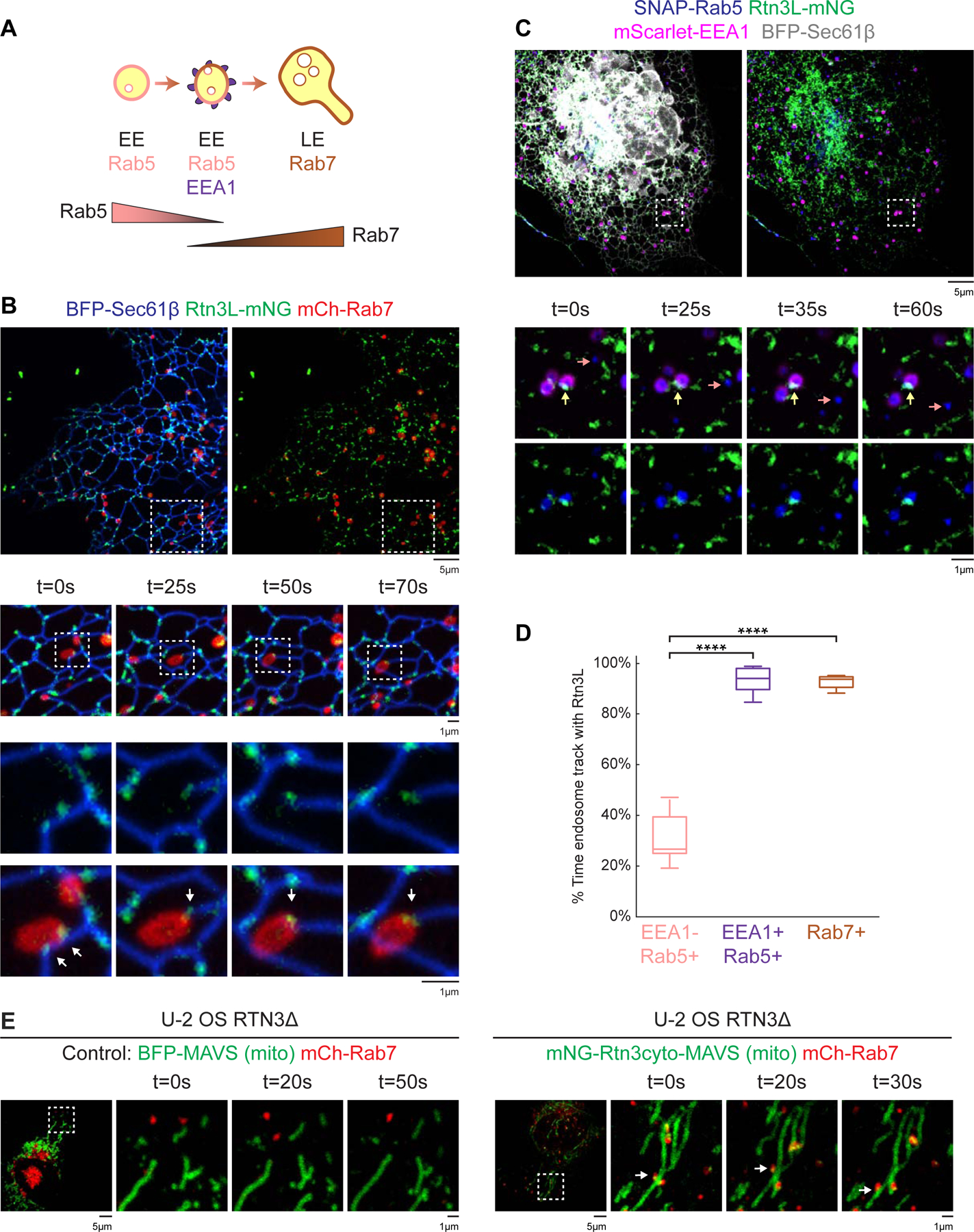
(A) Cartoon diagram of Rab5, EEA1, and Rab7 recruitment during endosome maturation. (B) Rtn3L puncta were tracked relative to LEs in live COS-7 cells co-transfected with Rtn3L-mNG (green), BFP-Sec61β (ER, blue), and mCh-Rab7 (LEs, red). Images below show time-lapse 10 µm x 10 µm and 3 µm x 3 µm insets, respectively. (C) Rtn3L puncta localization relative to moving early endosomes (EEs) in COS-7 cells co-transfected with Rtn3L-mNG (green), BFP-Sec61β (ER, grey), SNAP-Rab5 (blue), and mScarlet-EEA1 (magenta). Representative time lapse insets are 5 µm x 5 µm. Note that a Rab5 EE that is EEA1+ (magenta, marked by yellow arrows) tracks with an Rtn3L punctum over time. In contrast, a Rab5+ EE that is EEA1- (marked by pink arrows) did not associate with an Rtn3L punctum over time. (D) The percent of time that each endosome population associates with Rtn3L puncta during 2min movies (from data represented in B and C). Endosomes are binned according to their maturation stages: EEA1-/Rab5+ (early maturation stage EEs, 84 endosomes from n=10 cells), EEA1+/Rab5+ (late maturation stage EEs, 87 endosomes from n=10 cells) and Rab7+ endosomes (LEs, 82 endosomes from n=10 cells). (E) Representative time-lapse images of U-2 OS RTN3Δ cells expressing mCh-Rab7 (LEs) and BFP-MAVS (left panels) or mNG-Rtn3cyto-MAVS (right panels) reveals that ectopically localized Rtn3cyto-MAVS can tether LEs (red) to mitochondria (green). Statistical analyses were performed with one-way ANOVA, p-value from Tukey’s test: ****p<0.0001. Scale bars were 5 µm, 1 µm for insets. See also Figure S2, Movie S3-S6.
Rtn3L puncta localize to the intersection between ER tubules and trafficking LEs, and between ER tubules and MTs (Figure S2C and Fig 3B). Hence, we tracked the position of Rtn3L relative to trafficking LEs (mCh-Rab7) and MTs (SNAP-Ensconsin) in live cell movies. Consistently, we find Rtn3L localizes to a compelling location at the tripartite junctions between ER tubules, MTs, and moving LEs (Figure S2F, Video S5).
The cytoplasmic domain of Rtn3L is sufficient to tether LEs
Rtn3L localizes to the intersection between ER tubules and trafficking LEs, suggesting that Rtn3L could be part of a tethering complex. We therefore tested whether the cytoplasmic domain of Rtn3L is sufficient to tether LEs. We fused the cytoplasmic domain of Rtn3L to an outer mitochondrial membrane (OMM) targeting sequence (the MAVS transmembrane domain), which targets the cytoplasmic domain of Rtn3L to the OMM (mNG-Rtn3cyto-MAVS). We then asked if mNG-Rtn3cyto-MAVS would recruit LEs to the OMM in RTN3Δ U-2 OS cells (to avoid interference from endogenous Rtn3L). RTN3Δ U-2 OS cells were co-transfected with mNG-Rtn3cyto-MAVS and mCh-Rab7 (LEs). Live movies revealed LEs (red) that were tightly tethered to Rtn3cyto-MAVS-labeled mitochondria (green) over time (Figure 3E, right panels, Video S6); compared to control cells (Figure 3E, transfected with BFP-MAVS and mCh-Rab7, left panels). These data demonstrate that the Rtn3L’s cytoplasmic domain is sufficient to tether LEs.
Rab9a promotes Rtn3L recruitment to endosomes
Our data demonstrate that Rtn3L is recruited to endosomes as they mature and is sufficient to tether Rab7-positive LEs. To gain further insight into when and how Rtn3L puncta are recruited during endosome maturation, we labeled endosomes with Rab9a, which is recruited to maturing endosomes after Rab5 but before Rab7 (Kucera et al., 2016; Lombardi et al., 1993). Rab9a stays on Rab7 LEs throughout endosome maturation (Figure S3A) (Kucera et al., 2016). COS-7 cells were co-transfected with Rtn3L-mNG, mScarlet-Rab9a, and BFP-Sec61β (ER). Strikingly, not only did Rtn3L co-localize with Rab9a-labeled endosomes, but Rtn3L, together with co-labeled ER tubules, wrapped extensively around these Rab9a-marked endosomes (Figure 4A). This effect is also observed in HeLa and 293T cells (Figure S3B–S3C). This wrapping effect is not observed when Rtn3L is co-expressed with Rab7 (Figure 3B & S3B–S3E). We quantitated the relative localization of Rtn3L signal with expression of Rab9a versus Rab7 for representative examples by linescan (Figure S3D–S3E). Rab9a expression caused Rtn3L to circumscribe endosomes (Figure 4A, S3B–S3E) in a manner that is similar to other cases where ER-endosome MCS protein pairs are co-expressed, e.g. VAPB/SNX2, Protrudin/Rab7, and PDZD8/Rab7 (Dong et al., 2016; Elbaz-Alon et al., 2020; Guillén-Samander et al., 2019; Raiborg et al., 2015). Expression of Rab9a with Rtn3S-mNG, Rtn4a-mNG, or the ER-endosome MCS protein VAPB did not cause the ER to wrap around endosomes (Figure S3F–S3H). These data demonstrate that Rab9a, through Rtn3L’s cytoplasmic domain, promotes Rtn3L recruitment to maturing endosomes.
Figure 4. Rab9a promotes Rtn3L recruitment to endosomes.
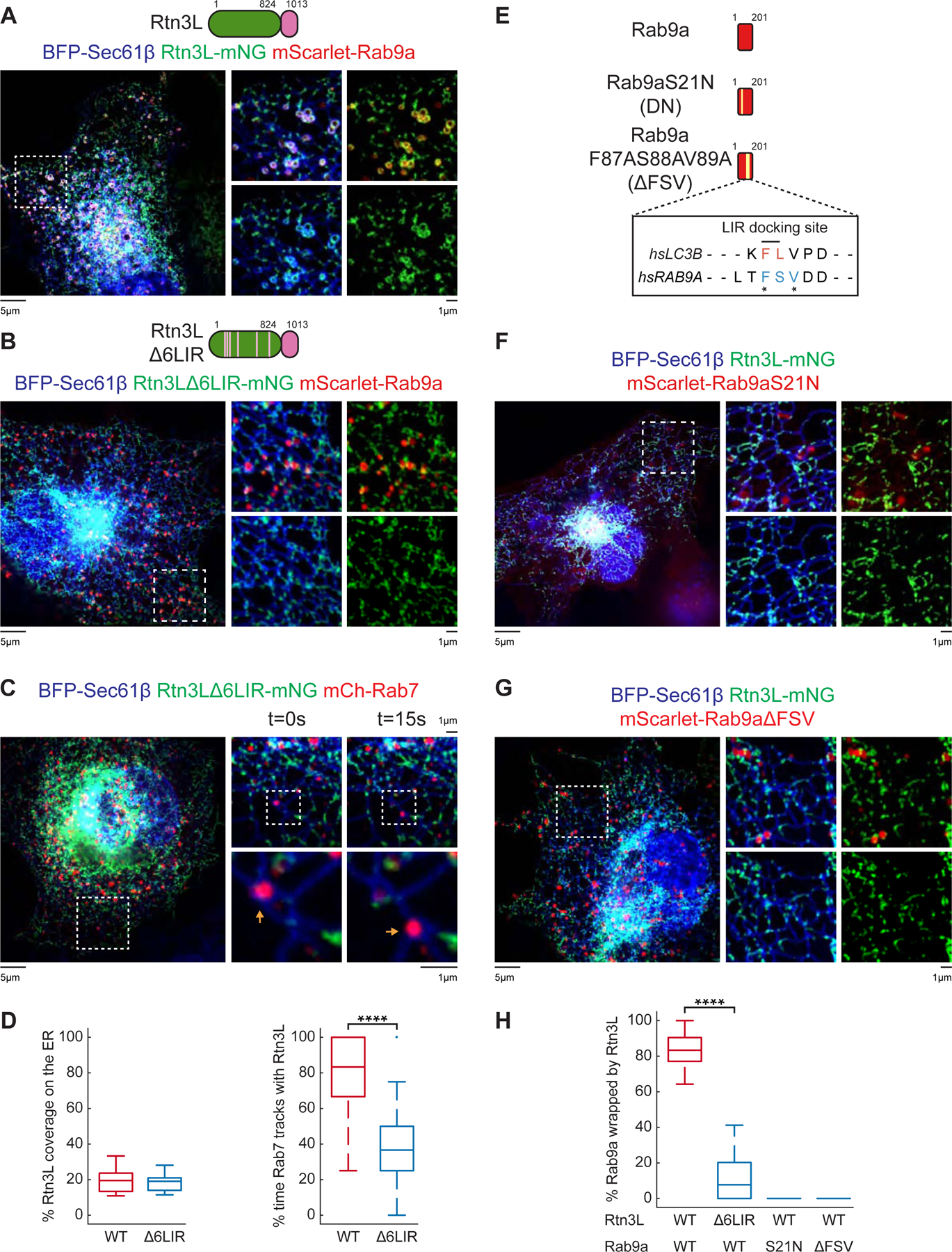
(A-B) Representative images of COS-7 cells co-transfected with mScarlet-Rab9a (red), BFP-Sec61β (ER, blue), and (A) Rtn3L-mNG (green) or (B) Rtn3LΔ6LIR-mNG (green). 10 x 10 µm insets were shown in right panels. (C) Rtn3LΔ6LIR puncta were tracked relative to LEs in live COS-7 cells co-transfected with Rtn3LΔ6LIR-mNG (green), BFP-Sec61β (ER, blue), and mCh-Rab7 (LEs, red). Time-lapse insets of 10 µm x 10 µm and 3 µm x 3 µm are shown on the right. (D) Quantification of Rtn3L (WT versus Δ6LIR) pixel coverage over an ER marker and the percent of time that Rtn3L (WT versus Δ6LIR) maintain contact with Rab7 LEs over time. Data taken from WT Rtn3L (50 endosomes in n=10 cells) and Rtn3LΔ6LIR (54 endosomes in n=14 cells). (E) Domain structure of human Rab9a, Rab9aS21N (dominant negative, DN) and Rab9aF87AS88AV89A (ΔFSV). Amino acid numbers are indicated. Pairwise sequence alignment between LC3 and Rab9a were performed using Clustal-Omega and its default parameters (Söding, 2005). *indicate identical amino acids. (F) The percent of Rab9a-positive endosomes that are wrapped by both Rtn3L (green) and ER (Sec61β, blue) was quantified from data represented in (A) (Rtn3L, 242 endosomes from n=15 cells), (B) (Rtn3LΔ6LIR, 214 endosomes from n=16 cells), (F) (Rab9aS21N, 202 endosomes from n=13 cells), and (G) (Rab9aΔFSV, 233 endosomes from n=14 cells). Statistical analyses were performed with two-tailed student t-test: ****p<0.0001. Scale bars = 5 µm, 1 µm for insets. See also Figure S3–S4.
We then asked what regions of the Rtn3L cytoplasmic domain are required for its recruitment to ER-endosome MCSs. Surprisingly, truncating the first or second halves of Rtn3L’s cytoplasmic domain still allowed Rtn3L truncations to wrap ER around Rab9a endosomes (Figure S4A–S4B). These data led us to test whether the six LIR (LC3-interacting region) motifs, which are distributed throughout the cytoplasmic domain of Rtn3L (Grumati et al., 2017), are required for driving Rtn3L to wrap around endosomes. These motifs were previously shown to regulate an interaction between Rtn3L and LC3 during starvation-induced ER-phagy (Grumati et al., 2017). We mutated the six LIR motifs (Rtn3LΔ6LIR) and co-transfected COS-7 cells with BFP-Sec61β (ER), mScarlet-Rab9a, and Rtn3LΔ6LIR-mNG. We measured a 10-fold reduction in levels of Rtn3L/ER wrapping around Rab9a endosomes in Rtn3LΔ6LIR-expressing cells compared to WT Rtn3L (Figure 4A–4B, 4H). Mutating only five out of the six LIR motifs retained Rtn3L’s ability to wrap around Rab9a endosomes (Figure S4C). We also tested whether the Rtn3LΔ6LIR could still be recruited to Rab7-positive LEs. COS-7 cells were co-transfected with BFP-Sec61β (ER), mCh-Rab7 (LEs) and either WT Rtn3L-mNG or Rtn3LΔ6LIR-mNG, and WT versus Δ6LIR Rtn3L recruitment to dynamic Rab7 LEs was scored during live cell movies. We observed a more 2-fold reduction of Rtn3LΔ6LIR (37%) recruitment to endosome contact sites compared to WT Rtn3L (83%) (Figure 4C–D). The ER coverage of WT Rtn3L (20%) and Rtn3LΔ6LIR (19%) was similar (Figure 4D). Together, these data support a model whereby the cytoplasmic six LIR motifs are important for Rtn3L recruitment to ER-LE MCSs.
Conversely, we asked what regions of Rab9a are important for Rtn3L recruitment. First we tested if Rab9a GTP binding is required. We made a dominant negative Rab9a (Rab9aS21N) that favors GDP over GTP (Riederer et al., 1994) and co-transfected COS-7 cells with BFP-Sec61β (ER), Rtn3L-mNG, and mScarlet-Rab9aS21N (Figure 4E). Rab9aS21N was unable to drive Rtn3L to wrap around endosomes (Figure 4E–4F, 4H). These data show that Rab9a’s GTP binding activity is important for the formation of MCSs with Rtn3L. The requirement of six LIR motifs for Rtn3L to wrap Rab9a at MCSs also provided an intriguing clue. Hence, we searched for Rab9a sequences that are similar to LC3’s LIR docking site and found an FSV region that could potentially function similarly to a LIR docking site (Figure 4E, S4D). We mutated this FSV region, co-transfected COS-7 cells with BFP-Sec61β (ER), Rtn3L-mNG, and mScarlet-Rab9aΔFSV (F87A, S88A, V89A) and compared the ability of this Rab9a mutant to recruit Rtn3L/ER to wrap around LEs. mScarlet-Rab9aΔFSV has completely abolished its ability to drive wrapping between Rtn3L and Rab9a (Figure 4G–H). Together, these data show that Rtn3L relies on six LIR motifs to form ER-endosome MCSs with an FSV region on Rab9a-positive endosomes.
Rab9a recruits Rtn3L to endosomes
Since Rab9a promotes Rtn3L recruitment to MCSs, we wondered whether Rab9a depletion would conversely decrease Rtn3L recruitment. We depleted RAB9A using siRNA and tested whether depletion would reduce Rtn3L recruitment to dynamic Rab7-positive LEs. COS-7 and HeLa cells were co-transfected with BFP-Sec61β (ER), Rtn3L-mNG, mScarlet-Rab7 (LEs) and control or RAB9A siRNA (Figure 5A–B, Figure S5A–B). We observed a more than 2-fold reduction in Rtn3L association with dynamic LEs in cells depleted of RAB9A (33% versus 88% in control cells, in COS-7 cells, Fig 5A–5B; 37% versus 100% in control cell, in HeLa cells, Figure S5A–B). RAB9A depletion did not alter Rtn3L protein levels (Figure S5E) or its punctate distribution (Figure 5A–B, Figure S5A–S5B). Conversely, we did not observe an effect of RTN3 depletion on Rab9a (mScarlet-Rab9a) recruitment to LEs (GFP-Rab7) (Figure S5C–D) or Rab9a protein levels (Figure S5E). We conclude that Rab9a acts upstream of Rtn3L and regulates its recruitment to ER-endosome MCSs.
Figure 5. Rab9a recruits Rtn3L to ER-endosome MCSs.
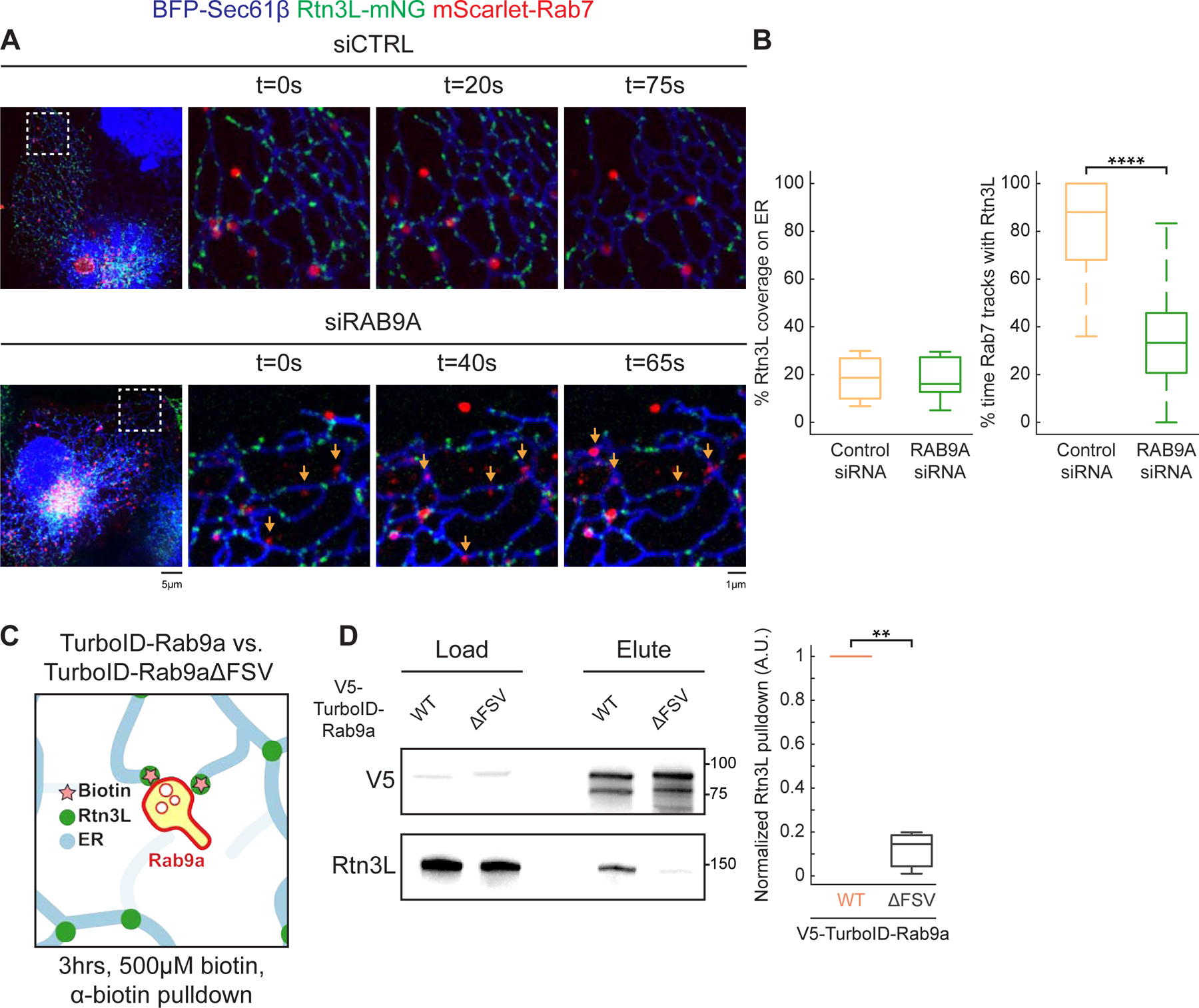
(A) Representative images of control siRNA (siCTRL) and RAB9A siRNA (siRAB9A) treated COS-7 cells co-transfected with BFP-Sec61β (ER, blue), mCh-Rab7 (LEs, red) and Rtn3L-mNG (green). Time-lapse 10 x 10 µm insets are shown on the right. Orange arrows point at LEs in RAB9A-depleted cells that do not track with Rtn3L. (B) Quantification of Rtn3L coverage on ER tubules (left) and the percent of time Rab7 LEs track with Rtn3L in 2min movies (right). Data quantitated from siCTRL (66 endosomes, n= 10 cells) versus siRAB9A (56 endosomes, n=11 cells). (C) Cartoon diagram of experiment testing whether Rtn3L and Rab9a are within tethering distance. Briefly: HeLa cells expressing TurboID-Rab9a or V5-TurboID-Rab9aΔFSV (negative control) were treated with 500µM biotin for 3hrs. Biotinylated proteins were pulled-down by anti-biotin agarose beads. Eluted biotinylated proteins are assayed by immunoblot. (D) Representative V5 and Rtn3L immunoblot of load and elute of biotin pulldown (left), and the quantification of normalized Rtn3L biotinylated by V5-TurboID-Rab9a WT versus V5-TurboID-Rab9aΔFSV. All numbers normalized to V5-TurboID-Rab9a WT. Statistical analyses were performed with two-tailed student t-test: **p<0.01, ****p<0.0001. Scale bars = 5 µm, 1 µm for insets. See also Figure S5.
Our complementary analyses of Rtn3L and Rab9a recruitment to MCSs suggest that Rtn3L and Rab9a could interact physically to promote ER-endosome MCS formation. We thus tested if Rtn3L and Rab9a are in close proximity in cells. We fused TurboID, a promiscuous biotin ligase, to WT Rab9a, or Rab9aΔFSV as a negative control (Branon et al., 2018) (Figure 5C). TurboID constructs were transfected into HeLa cells, and these cells were treated with biotin, and biotinylated proteins were bound and eluted from biotin antibody agarose beads and biotinylated endogenous Rtn3L levels were analyzed by immunoblot. The levels of Rtn3L biotinylation by WT Rab9a were 10-fold higher than by the Rab9aΔFSV mutant (Figure 5C–5D). These data consistently support a model that the Rab9a FSV region regulates recruitment of Rtn3L through its six LIR motifs.
Rtn3L and Rab9a regulate endosome maturation and cargo sorting
Rtn3L (Figure 3B–3D) and Rab9a (Kucera et al., 2016) are recruited to ER-endosome MCSs coincident with endosome maturation, which suggest that Rab9a could recruit Rtn3L to MCSs to regulate endosome maturation. We therefore tested whether RTN3 depletion alters the endosome maturation process. HeLa cells were co-transfected with BFP-Rab5 (EEs), GFP-Rab7 (LEs) and with control or RTN3 siRNA. Cells were then pulse-labeled with EGF-647 (epidermal growth factor conjugated with Alexa Fluor™ 647) to stimulate endocytosis. After pulse-labeling, EGF-647 will only label the newly formed and actively trafficking endosomes. Cells were fixed at 10min and 20min post EGF-647 addition and the percent of EGF-647-positive endosomes that are labeled with Rab5 and Rab7 were scored. Normally, two distinct endosomal populations should exist: Rab5+ EEs and Rab7+ LEs. These markers display very low levels of co-localization (Schmid et al., 1988). As expected, in control siRNA-treated cells, EGF-647-positive endosomes were mainly labeled by Rab5 at 10 minutes and Rab7 at 20 minutes (Figure 6A, Figure S6A–S6B); only a low percent of endosomes were simultaneously labeled with both Rab5 and Rab7 (~10–20%) (Figure 6A–6B, Figure S6A–S6B). In contrast, in RTN3-depleted cells, the majority of EGF-647-positive endosomes were simultaneously labeled with Rab5 and Rab7 (71%, Figure 6A–6B, Figure S6A–S6C). Furthermore, Rab7 was recruited to EGF-647-positive structures prematurely (at 10min), and these Rab7 endosomes are associated with Rab5 even at the 20 min time point (Figure 6A–6B, Figure S6A–S6C). This phenotype was rescued by re-introduction of Rtn3L back into the RTN3-depleted cells (Figure 6A–6B, Figure S6A–S6C).
Figure 6. RTN3 and RAB9A are required for endosome maturation.
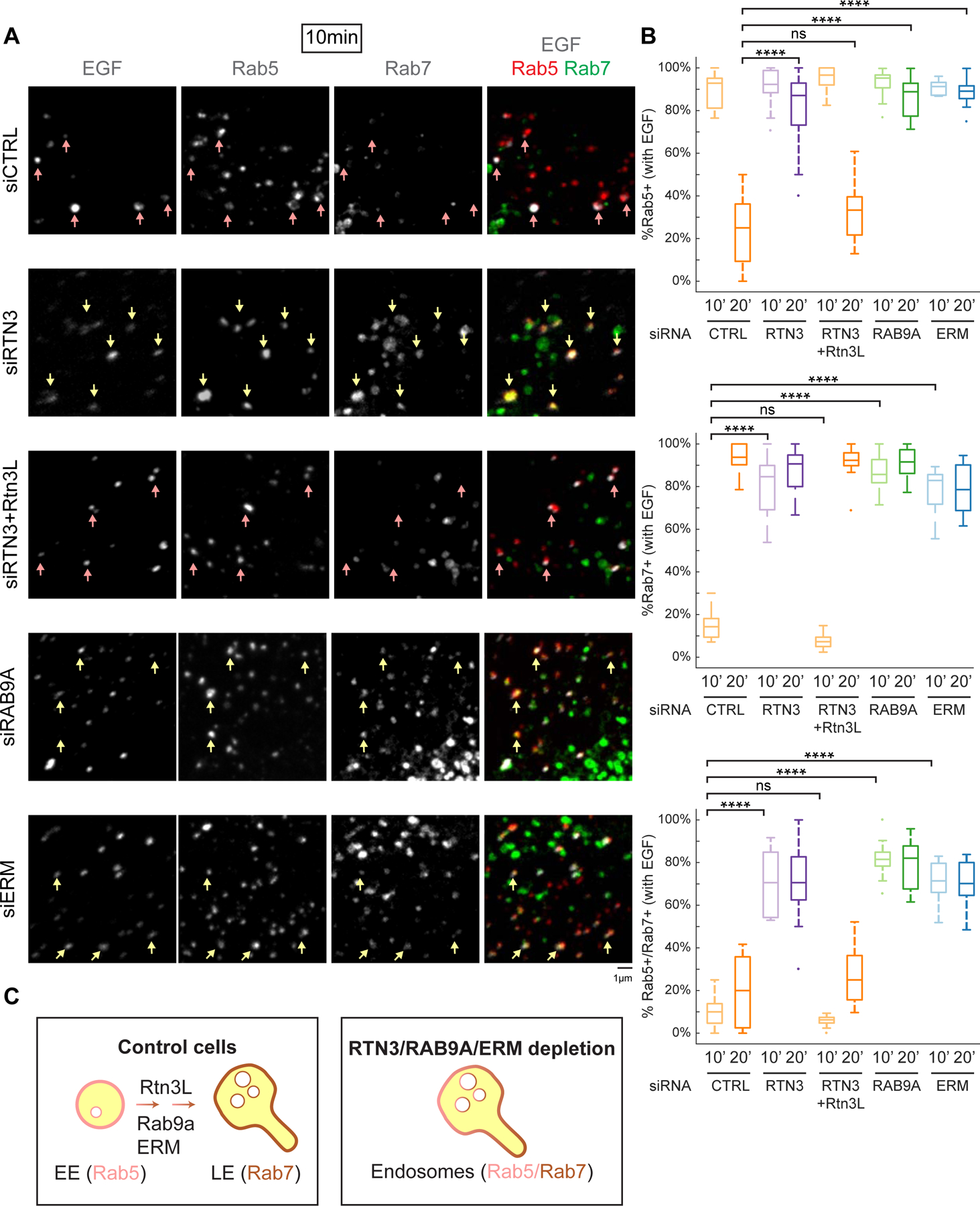
(A) The effect of RTN3 and RAB9A on endosome maturation. HeLa cells were co-transfected with BFP-Rab5 (EEs), GFP-Rab7 (LEs) and with control siRNA (siCTRL), RTN3 siRNA (siRTN3), RTN3 siRNA with re-expression of siRNA resistant Rtn3L-mCh (siRTN3+Rtn3L), RAB9A siRNA (siRAB9A), or ERM siRNA (siERM). To visualize newly formed and actively trafficking endosomes, cells were pulse-labeled with EGF-647 and then fixed 10 min or 20 min later (see 20 min images in Figure S6). Representative max-projected z-stacks are shown. Individual channels are shown in grey the merge of all three are shown on the right (EGF in grey, Rab5 in red and Rab7 in green). Only EGF-647-positive endosomes are scored. Examples of EGF+ endosomes are marked by pink arrows (EGF+ and Rab5+) or yellow arrows (EGF+, Rab5+ and Rab7+). For whole cell images see Figure S6A. (B) Quantification of data represented in (A) and from 20 min time point (data represented in Figure S6). Endosomes were binned into EGF+/Rab5+, EGF+/Rab7+, or EGF+/Rab5+/Rab7+ at the 10 min and 20 min time points. Data presented in 10min/20min graphs are from: control siRNA (239/201 endosomes, n=11/15 cells) RTN3 siRNA (166/276 endosomes, n=11/16 cells), RTN3 siRNA + Rtn3L-mCh (470/249 endosomes, n=14/13 cells), RAB9A siRNA (319/352 endosomes, n=11/16 cells) and ERM siRNA (352/414 endosomes, n=12/13 cells). (C) Model of endosome maturation defect in cells depleted of RTN3, RAB9A or ERM: Rab7 recruitment is premature, and Rab5 displacement (Rab5/7 conversion) is delayed. Statistical analyses were performed with one-way ANOVA, p-value from Tukey’s test: ns=not significant, ****p<0.0001. Scale bars = 5 µm, 1 µm for insets. See also Figure S6.
Our experiments have shown that Rab9a recruits Rtn3L to ER-endosome MCSs. We therefore tested whether RAB9A depletion would cause a similar phenotype during endosome maturation to RTN3 depletion. HeLa cells were transfected with either control or Rab9a siRNA, and endosome maturation was analyzed as before. Similar to RTN3 depletion, RAB9A siRNA depletion also caused Rab7 to be prematurely recruited to newly formed endosomes (10 min after EGF-647 addition), and Rab5 remained associated with most (82%) of the Rab7 endosomes even after 20 min (Figure 6A–6B, Figure S6A–S6C). Together, these results show that Rtn3L and Rab9a similarly compromise the processes of endosome maturation and Rab5/7 recruitment (Figure 6C).
ERM proteins (ezrin, radixin, moesin) depletion is known to compromise endosome maturation and result in higher levels of early and late endosome marker co-localization (Chirivino et al., 2011). Thus, as a control for an endosome maturation defect in our assays, we depleted ERM proteins by siRNA and similarly measured endosome maturation. Indeed, ERM depletion conferred a similar defect (compared to RTN3 and RAB9A depletion) and led to premature Rab7 recruitment at 10min and a retention of Rab5 on endosomes even at 20 min (~70%, Figure 6A–6B, Figure S6A–S6C).
Our data so far identify Rtn3L as an ER MCS protein that is involved in endosome maturation. A major cellular role for endosome maturation is endocytic cargo sorting (Huotari and Helenius, 2011). Thus, we asked whether RTN3 depletion would also disrupt cargo sorting. We monitored two cargoes: EGF receptor (EGFR) and cation-independent mannose 6-phosphate receptor (CI-MPR). A previous report has shown that RTN3 depletion delays EGFR degradation (Caldieri et al., 2017). We similarly assayed EGFR degradation by treating cells with EGF and harvesting cells at 0, 30, 60 and 120 minutes. Consistently, immunoblot analysis confirmed that EGFR degradation is compromised in RTN3-deleted or RTN3-depleted HeLa cells compared to control cells (Figure S7A–B). We tested whether RAB9A depletion also alters EGFR degradation. Indeed, RAB9A depletion in HeLa cells attenuates EGFR degradation (Figure S7C).
Next, we tested whether RTN3 depletion would compromise CI-MPR sorting. CI-MPR can be internalized from the plasma membrane (PM) and onto endosome membranes; as endosomes mature CI-MPR gets trafficked from LEs to the Golgi (Kornfeld and Mellman, 1989). We monitored CI-MPR trafficking by incubating cells with CI-MPR antibody. Normally, the antibody is internalized with CI-MPR and trafficked to the Golgi within 1hr (Dong et al., 2016; Hoyer et al., 2018). Compared to WT, CI-MPR failed to be sorted to the Golgi and accumulated in peripheral vesicles, indicative of a sorting defect in RTN3Δ or in RTN3 siRNA treated HeLa cells (Figure 7A, 7C, Figure S7D, S7F). Importantly, this CI-MPR sorting defect can be rescued by re-introduction of Rtn3L into RTN3Δ or RTN3-depleted cells (Figure 7A, 7C, Figure S7D, S7F). Rtn3cytoRtn4RHD also rescued the CI-MPR sorting defect in RTN3 depleted cells (Figure 7B–7C), corroborating a key role of Rtn3’s cytoplasmic domain in regulating endosome maturation and cargo sorting. Consistent with previous literature, we observed RAB9A is also important for CI-MPR sorting (Figure S7E–S7F) (Lombardi et al., 1993). Due to a similar defect in CI-MPR sorting caused by RTN3 or RAB9A depletion and Rab9a’s role in recruitment of Rtn3L to MCSs (Figure 5), we tested if a constitutively active form of Rab9aQ66L can rescue CI-MPR sorting in RTN3 depleted cells (Espinosa et al., 2009). Expression of Rab9aQ66L in RTN3 siRNA treated cells partially rescued CI-MPR sorting to the Golgi, while a constitutively active Rab7Q67L did not (Figure 7B–7C). These data support a model that a constitutively active form of Rab9a can partially bypass the requirement for RTN3 to regulate endosomal cargo sorting at ER-endosome MCSs.
Figure 7. Rab9a and Rtn3L regulate endosomal cargo sorting.
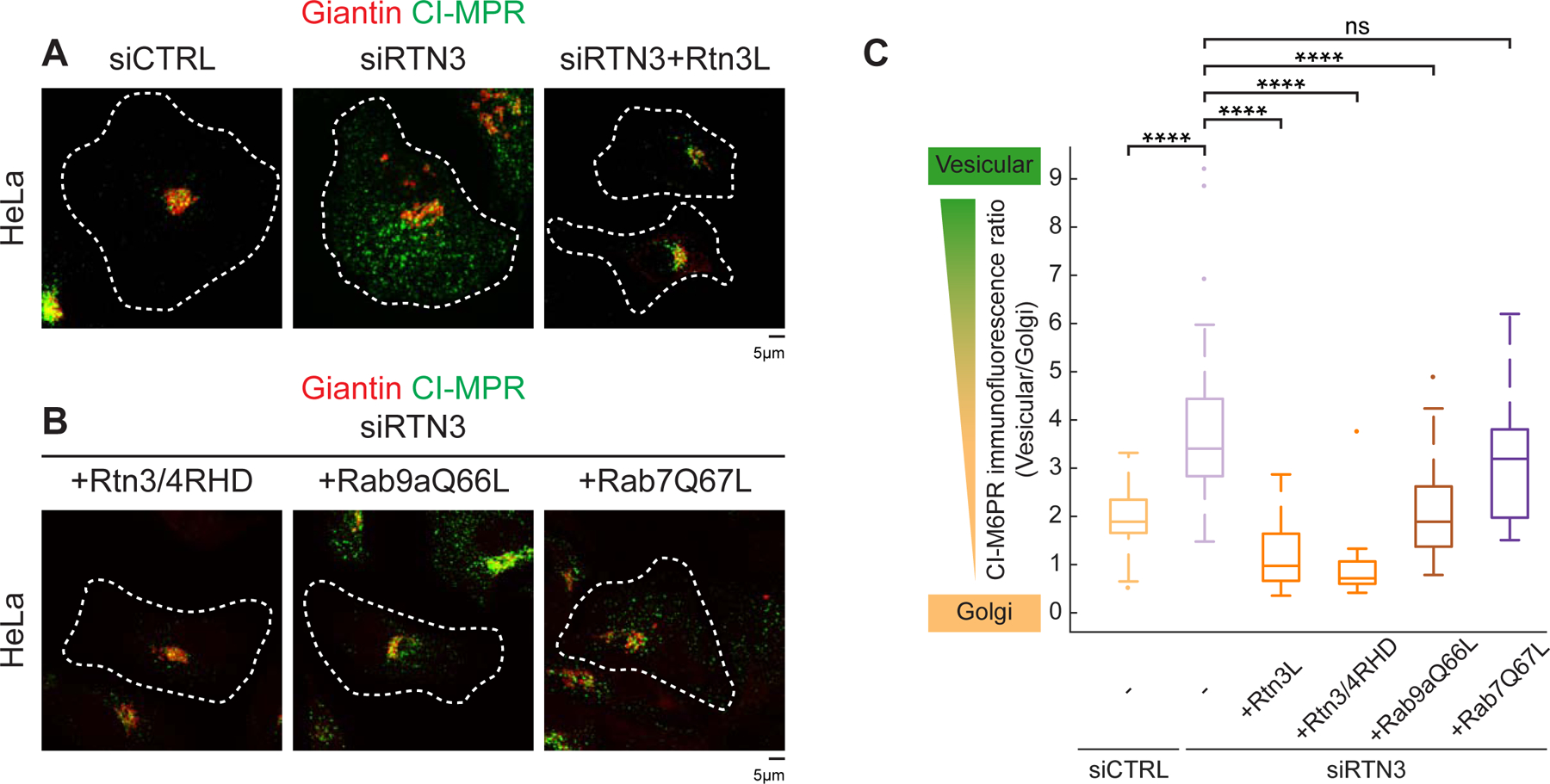
(A) CI-MPR trafficking assay. The relative fluorescence intensity of internalized anti-CI-MPR antibody (green) by immunostaining shows the localization of internalized anti-CI-MPR relative to the Golgi (Giantin, red) in HeLa cells treated with control (left, siCTRL), RTN3 siRNA (middle, siRTN3) or RTN3 siRNA and re-expressing exogenous Rtn3L (right, siRTN3+Rtn3L). Note that a block in cargo trafficking causes CI-MPR to stall in peripheral vesicles and not traffic to the perinuclear Golgi. (B) As in (A) for HeLa cells treated with RTN3 siRNA and rescued with Rtn3cytoRtn4RHD-mNG (+Rtn3/4RHD, left), constitutively active GFP-Rab9aQ66L (+Rab9aQ66L, middle), or constitutively active GFP-Rab7Q67L (+Rab7Q67L, right). (C) Quantification of the ratio between fluorescence intensity in vesicular structures relative to that of the Golgi for siCTRL (1.9, n=35 cells), siRTN3, (3.4, n=53 cells); siRTN3+Rtn3L (1.0, n=44 cells), siRTN3+Rtn3/4RHD (0.7, n=21 cells), siRTN3+ Rab9aQ66L (1.9, n=34 cells), and siRTN3+Rab7Q67L (3.2, n=23 cells). Statistical analyses were performed with one-way ANOVA, p-value from Tukey’s test: ns = not significant, ****p<0.0001. Scale bars = 5 µm, 1 µm for insets.
Discussion
Rtn3L localizes to the tubular ER through its RHD. Some RHD can directly shape the tubular ER and generate membrane curvature (Hu et al., 2008; Voeltz et al., 2006; Zurek et al., 2011). For example, Rtn1 in yeast possess strong membrane shaping activity, while containing a minimal cytoplasmic sequence. However, mammalian Rtns have evolved long, divergent cytoplasmic domains. These cytoplasmic domains can be localized to ER tubules through the RHDs to perform diverse functions that ER tubules are well suited for. Many other RHD-containing tubular ER proteins also fit this model: Atlastin, which mediates ER tubule fusion (Betancourt-Solis et al., 2018; Hu et al., 2009; Orso et al., 2009), FAM134B which regulates ER-phagy (Khaminets et al., 2015), E-Syts that regulate lipid transfer at ER-PM MCSs (Giordano et al., 2013) and protrudin, which regulates anterograde trafficking of LEs at ER-endosome MCSs (Raiborg et al., 2015). Through these segregated tubular ER specific RHD containing proteins, tubular ER is maintained and perform a variety of functions effectively.
Mechanistically, Rtn3L could regulate a few aspects of endosome maturation. Firstly, endosome maturation is coupled to endosome trafficking on MTs towards the perinuclear region (Bayer et al., 1998; Collinet et al., 2010; Driskell et al., 2007; Vonderheit and Helenius, 2005). Rtn3L localizes to tripartite junctions between ER tubules, MTs and LEs (Figure S2F). At these junction structures, Rtn3L could coordinate endosome maturation by regulating motor loading and dissociation, potentially together with other ER-endosome MCS proteins that regulate similar processes including protrudin, RILP and ORP1L (Johansson et al., 2007; Raiborg et al., 2015; Rocha et al., 2009). Secondly, an assortment of factors can regulate endosome maturation including Rab guanine nucleotide exchange factors, Rab GTPase-activating proteins, guanine nucleotide dissociation inhibitor (GDI), GDI-displacement factor and endosome homotypic fusion machineries (Gougeon et al., 2002; Horiuchi et al., 1997; Hutt et al., 2000; Kinchen and Ravichandran, 2010; Nickerson et al., 2009; Poteryaev et al., 2007, 2010; Sivars et al., 2003; Wang et al., 2003). Rtn3L-mediated MCSs could provide a platform for the recruitment of these factors. Importantly, these two possibilities of regulation of endosome maturation by Rtn3L are not mutually exclusive, which could emphasize the versatility of ER MCSs.
Notably, we do observe a subset of Rtn3L puncta that move around the ER but are not yet associated with endosomes. This could represent the dynamic pools of Rtn3L that are poised to “capture” newly formed endosomes. However, we have shown that Rtn3L also localizes to other MCSs, including LAMP1-marked lysosomes and LC3-marked autophagosomes (under non-starvation conditions). Consistently, Rtn3L has also been implicated in other biological processes other than endosome maturation upon serum starvation, including the NCE pathway and ER-phagy (Caldieri et al., 2017; Grumati et al., 2017). There are a few key differences between the experimental conditions of these data and ours. NCE happens at high concentration of EGF (20–100ng/mL), and we used a lower concentration of EGF-647 that corresponds to 10ng/mL of EGF to score endosome maturation (Figure 6); at our EGF-647 concentrations, the NCE pathway should not be active (Caldieri et al., 2017). Moreover, RTN3’s involvement in NCE was carefully studied with short EGF stimulations (5–8 minutes) in effort to focus on the population of cargoes that resides on the PM (Caldieri et al., 2017). We focused on cargo-containing endosomes that have already detached from the PM and have gained endosome markers including Rab5 and Rab7. As for the interaction that occurs between Rtn3L and LC3 during ER-phagy, these experiments were all performed during extended periods of starvation (6hrs) plus Bafilomycin A1 treatment (Grumati et al., 2017). Despite these differences, one should conclude that Rtn3L may have multiple binding partners at multiple locations on endocytic and autophagic organelles, and which partner it binds may be a reflection of the metabolic state of the cell.
Rtn3L’s six LIR motifs are important for its recruitment to endosome contact sites, through Rab9a’s FSV region. One could imagine a model wherein Rtn3L binds Rab9a-marked endosomes in non-starved conditions, whereas it is poised to bind LC3-marked autophagosomes under extended starvation. This dual binding model could be beneficial and efficient because autophagosomes need to fuse with LEs/lysosomes for degradation. It is also intriguing to think if a similar recruitment mechanism, through LIR motifs and LIR docking sites, also applies at the PM for endocytosis, especially the NCE pathway that Rtn3L has been involved in. This way, Rtn3L could coordinate the whole endocytic pathway from endocytosis, to endosome maturation, to autophagic turnover of cargoes and organelles.
Tubular ER has evolved to perform specialized and complex functions in mammalian cells. We discovered that a paralog of the conserved class of tubular ER membrane proteins—the reticulons—localizes to ER-endosome MCSs to regulate endosome maturation and cargo sorting. Rtn3L-mediated endosome maturation further highlights the prevalence and functional importance of ER tubules at MCSs.
STAR Methods
RESOURCE AVAILABILITY
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Gia Voeltz (Gia.Voeltz@colorado.edu).
Materials Availability
Plasmids in this study will be available upon request with a complete Material Transfer Agreement and will be deposited to Addgene.
Data and Code Availability
This study did not generate/analyze any dataset, and MATLAB code for quantifying three-way junctions is available in method details.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Cell culture
COS-7 cells (ATCC), 293T cells (ATCC) and HeLa cells (ATCC, CCL-2) were cultured in DMEM (Gibco 12430-054) containing 10% FBS and 50 units/mL Penicillin, 50 µg/mL Streptomycin (Invitrogen, 15070063). U-2 OS cells (ATCC) were cultured in McCoy’s 5A (Invitrogen, 16600-108) with 10% FBS and 50 units/mL Penicillin, 50 µg/mL Streptomycin (Invitrogen, 15070063). Cells were cultured at 37°C with 5% CO2.
METHOD DETAILS
Plasmids and reagents
mNeonGreen (mNG)-Sec61β was generated by PCR amplifying human Sec61β from AcGFP-Sec61β (Shibata et al., 2008) and inserted into mNG-C1 (purchased from Allele Biotechnology) using HindIII/KpnI sites. mCherry-Sec61β (Zurek et al., 2011) was subcloned from AcGFP-Sec61β (Shibata et al., 2008) into mCherry-C1 using BglII/EcoRI sites. BFP-Sec61β (Zurek et al., 2011) was subcloned from AcGFP-Sec61β (Shibata et al., 2008) into mTagBFP-C1 using BglII/EcoRI sites. mCh-Climp63 was a gift from J. Friedman and was described previously (English and Voeltz, 2013). Mouse Climp63 was PCR amplified and cloned into AcGFP-C1 using XhoI/EcoRI sites to generate AcGFP-Climp63. AcGFP was then substituted with mCherry using NheI/XhoI sites to generate mCh-Climp63. Rtn4a-GFP was described before (Shibata et al., 2008). Full length Rtn4 was PCR amplified from Rtn4a-myc (Voeltz et al., 2006) and inserted into AcGFP-N1 with XhoI/KpnI sites. Rtn4a-mNG was subcloned from Rtn4a-AcGFP into mNG-N1 (purchased from Allele Biotechnology) with XhoI/KpnI sites. Rtn3L-GFP was a gift from P. Chitwood. Full length Rtn3 (Rtn3L_NM_201428.3) was PCR amplified from human HeLa cDNA and cloned into AcGFP-N1 XhoI/BamHI sites. Rtn3L-mNG was subcloned from Rtn3L-AcGFP into mNG-N1 using XhoI/BamHI sites. Rtn3LΔ413-824-mNG was generated with Gibson assembly following manufacturer’s protocol (NEB). Template was Rtn3L-mNG, and primers were HWO510, HWO511, HWO512, HWO513 (Table S1). Rtn3LΔ1-412-mNG was generated with Gibson assembly following manufacturer’s protocol (NEB). Template was Rtn3L-mNG, and primers were HWO514, HWO515, HWO516, HWO517 (Table S1). Rtn3LΔ5LIR-mNG was generated with five rounds of site-directed mutagenesis from Rtn3L-mNG with primers: HWO520, HWO521, HWO522, HWO523, HWO524, HWO525, HWO526, HWO527, HWO528, HWO529 (Table S1). Rtn3LΔ6LIR-mNG was generated with six rounds of site-directed mutagenesis from Rtn3L-mNG with primers: HWO520, HWO521, HWO522, HWO523, HWO524, HWO525, HWO526, HWO527, HWO528, HWO529, HWO530, and HWO531 (Table S1). Rtn3S-GFP was a gift from P. Chitwood. Short isoform of Rtn3 (Rtn3S_ NM_006054.4) was PCR amplified from human HeLa cDNA and cloned into AcGFP-N1 with XhoI/BamHI sites. Rtn3cytoRtn4RHD-mNG and Rtn4cytoRtn3RHD-mNG were generated with Gibson assembly following manufacturer’s protocol (NEB). Templates were Rtn3L-mNeonGreen and Rtn4a-mNeonGreen. Primers used to generate these chimeric constructs are: HWO55, HWO56, HWO57, HWO58 for Rtn3cyto-Rtn4RHD; HWO134, HWO135, HWO145, HWO146 for Rtn4cyto-Rtn3RHD (Table S1). mCh-Tubulin was a gift from J. Friedman and was described before (Friedman et al., 2010). EYFP in EYFP-α-Tubulin (Takara Bio Inc.) was substituted with mCherry. BFP-Rab5 was a gift from J. Friedman and was described before (Friedman et al., 2013). Human Rab5b was PCR amplified from human cDNA and cloned into mTagBFP-C1 with XhoI/BamHI sites. mRuby-SKL was a gift from Michael Davidson (Addgene plasmid # 54840). LAMP1-mCh was a gift from Amy Palmer (Addgene plasmid # 45147) (Van Engelenburg and Palmer, 2010). GFP-LC3 was a gift from Toren Finkel (Addgene plasmid # 24920) (Lee et al., 2008). GFP-Rab7 and mCh-Rab7 were gifts from P. Chitwood and was described before (Rowland et al., 2014). Human Rab7a was PCR amplified from human cDNA and cloned into AcGFP/mCherry-C1 with XhoI/HindIII sites. GFP-Rab7Q67L was a gift from J. Friedman and was generated by site-directed mutagenesis from GFP-Rab7. SNAP-C1 vector was a gift from Michael Davidson (Addgene plasmid #58186). SNAP-Ensconsin was subcloned from mEmerald-Ensconsin-C-18 into SNAP-C1 backbone using EcoRI/BamHI sites. mEmerald-Ensconsin-C-18 was a gift from Michael Davidson (Addgene plasmid # 62753). BFP-MAVS was generated by PCR amplifying BFP containing the MAVS transmembrane domain sequence on the reverse primer and inserted back into BFP-C1 backbone with AgeI/NotI sites. mNG-Rtn3cyto-MAVS was generated by PCR amplifying human Rtn3L cytoplasmic domain containing the MAVS transmembrane domain sequence on the reverse primer. Primers were HWO116, HWO118 (Table S1). The PCR product was cloned into mNG-C1 with XhoI/BamHI sites. mScarlet-EEA1 was subcloned from GFP-EEA1 (Friedman et al., 2013) into pmScarlet-C1 vector [a gift from Dorus Gadella (Addgene plasmid #85042) (Bindels et al., 2017)] with XhoI/BamHI sites. mScarlet-Rab7 was subcloned from mCh-Rab7 (Rowland et al., 2014) into pmScarlet-C1 vector with XhoI/HindIII sites. SNAP-Rab5 was subcloned from BFP-Rab5 (Friedman et al., 2013) into SNAP-C1 vector with XhoI/BamHI sites. mScarlet-Rab9a was PCR amplified from human cDNA (NM_004251.5) and cloned into pmScarlet-C1 vector with XhoI/BamHI sites. GFP-Rab9aQ66L was generated by site-directed mutagenesis from GFP-Rab9a (subcloned from mScarlet-Rab9a into GFP-C1 vector with with XhoI/BamHI sites) using primers HWO439, HWO440 (Table S1). mScarlet-Rab9aS21N was generated by site-directed mutagenesis from mScarlet-Rab9a using primers HWO437, HWO438 (Table S1). mScarlet-Rab9aΔFSV (F87AS88AV89A) was generated by site-directed mutagenesis from mScarlet-Rab9a using primers HWO545, HWO546 (Table S1). mScarlet-V5-TurboID-Rab9a and mScarlet-V5-TurboID-Rab9aΔFSV (F87AS88AV89A) were generated with Gibson assembly following manufacturer’s protocol (NEB). Templates are mScarlet-Rab9a, mScarlet-Rab9aΔFSV. Primers used to generate these chimeric constructs are: HWO458, HWO459, HWO460, HWO461 (Table S1). V5-TurboID-NES was a gift from Alice Ting (Addgene plasmid # 107169) (Branon et al., 2018). mNG-VAPB was PCR amplified from human cDNA (NM_004738.4) and cloned into mNG-C1 vector with EcoRI/BamHI sites.
Rabbit Rtn3L antibody (MilliporeSigma, HPA015650) was used at 1:2000 for immunoblotting. Mouse Rtn3 antibody (Santa Cruz, sc-374599, detects both long and short isoforms of Rtn3) was used at 1:2000 for immunoblotting. Rabbit EGFR antibody (CST, 4267T) was used at 1:1000 for immunoblotting. Rabbit GAPDH antibody (MilliporeSigma, G9545) was used at 1: 100000 for immunoblotting. Rabbit Tubulin antibody (Abcam, ab18251) was used at 1: 100000 immunoblotting. Rabbit Rab9a antibody (CST, 5133) was used at 1:1000 for immunoblotting. Rabbit ERM antibody (CST, 3142) was used at 1:1000 for immunoblotting. Mouse V5 antibody (Invitrogen, R960-25) was used at 1:2000 for immunoblotting. Mouse CI-MPR antibody (Invitrogen, MA1-066) was used at 1:1000 for uptake assay (see below). Rabbit Giantin antibody (BioLegend, PRB-114C) was used at 1:500 for immunofluorescence. Anti-Mouse IgG (whole molecule)-Peroxidase antibody produced in goat (Sigma, A4416) was used at 1:3000 for immunoblotting. Anti-Rabbit IgG (whole molecule)-Peroxidase antibody produced in goat (Sigma, A6154) was used at 1:3000 for immunoblotting. Goat anti-Rabbit IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 405 (Thermo Fisher Scientific Cat# A-31556) was used at 1:300 for immunofluorescence. Donkey anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 488 (Thermo Fisher Scientific Cat# A-21206) was used at 1:300 for immunofluorescence. Donkey Anti-Mouse IgG (H+L) Antibody, Alexa Fluor 594 Conjugated (Thermo Fisher Scientific Cat# A-21203) was used at 1:300 for immunofluorescence. Donkey Anti-Mouse IgG (H+L) Polyclonal Antibody, Alexa Fluor 647 Conjugated (Thermo Fisher Scientific Cat# A-31571) was used at 1:300 for immunofluorescence. Donkey anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 594 (Thermo Fisher Scientific Cat# A-21207) was used at 1:300 for immunofluorescence. Donkey anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 647 (Thermo Fisher Scientific Cat# A-31573) was used at 1:300 for immunofluorescence. Donkey Anti-Mouse IgG (H+L) Antibody, Alexa Fluor 488 Conjugated (Thermo Fisher Scientific Cat# A-21202) was used at 1:300 for immunofluorescence.
Transfection
For imaging, cells were plated on 35mm imaging dishes (MatTek) for 16hrs, then transfected with intended plasmids with Lipofectamine 3000 following manufacturers protocol. Briefly, two separate 250 µL transfection mixes were assembled in Opti-MEM (Invitrogen, 31985-088) with 2 µL P3000 reagents per µg of plasmid and 2.5 µL/mL Lipofectamine 3000, respectively. Plasmids and P3000 are mixed with Lipofectamine reagent after 5 minutes incubation at room temperature. Mixed reactions were incubated at room temperature for 20 minutes, after which transfection mix was added to cells for 5hrs. Cells were imaged 16hrs later in FluoroBrite DMEM (Gibco, Cat. #A18967-01) containing 10% FBS, 50 units/mL Penicillin, 50 µg/mL Streptomycin, 1 mM sodium pyruvate, and 1x GlutaMAX (Gibco, 35050061). SNAP constructs were imaged by incubating cells with Janelia Fluor® 646, SE (1.5 µM) for 30min in serum free FluoroBrite DMEM. Janelia Fluor® 646 prior to imaging, SE was a gift from Luke Lavis lab in Janelia.
The following concentrations were used for each plasmid: 200 ng/mL mNG-Sec61β; 200 ng/mL mCherry-Sec61β; 375 ng/mL BFP-Sec61β 150 ng/mL mCh-Climp63; 150 ng/mL Rtn4a-mNG/GFP (overexpression in Figures 1C and 1E); 25 ng/mL Rtn4a-mNG/GFP (localization in Figures 1I and S3G); 150 ng/mL Rtn3L-mNG/GFP (overexpression in Figures 1C and 1E); 25 ng/mL Rtn3L-mNG/GFP (localization in everywhere else except for Figures 1C and 1E); 25 ng/mL Rtn3LΔ6LIR-mNG; 25 ng/mL Rtn3LΔ1-412-mNG; 25 ng/mL Rtn3LΔ413-824-mNG; 25 ng/mL Rtn3LΔ5LIR-mNG; 25 ng/mL Rtn3S-mNG/GFP (localization in Figures 1J and S3F); 25 ng/mL Rtn3cytoRtn4RHD-mNeonGreen (localization in Figure 1K); 150ng/mL Rtn3cytoRtn4RHD-mNeonGreen (rescue in Figure 7B); 25 ng/mL Rtn4cytoRtn3RHD-mNeonGreen (localization in Figure 1L); 63 ng/mL mCh-Tubulin; 50 ng/mL BFP-Rab5; 2 ng/mL mRuby-SKL; 150 ng/mL LAMP1-mCh; 50 ng/mL GFP-LC3; 25 ng/mL mCh-Rab7; 25 ng/mL GFP-Rab7; 150 ng/mL GFP-Rab7Q67L (rescue in Figure 7B); 50 ng/mL mScarlet-Rab9a; 150 ng/mL GFP-Rab9Q66L (rescue in Figure 7B); 150 ng/mL mScarlet-V5-TurboID-Rab9a; 250 ng/mL mScarlet-Rab9aS21N; 300 ng/mL mScarlet-Rab9aΔFSV; 900 ng/mL mScarlet-V5-TurboID-Rab9aΔFSV; 50 ng/mL mScarlet-EEA1; 25 ng/mL mNG-VAPB; 150 ng/mL SNAP-Ensconsin; 250 ng/mL mNG-Rtn3cyto-MAVS (in RTN3Δ cells).
Generating knockout cell lines
All knockout cell lines were generated with CRISPR-Cas9 systems following published protocol (Ran et al., 2013). Briefly, guide RNAs were cloned into Lenti-CRISPRv2 (a gift from Feng Zhang, Addgene plasmid # 52961) (Sanjana et al., 2014), which was then transfected into cells (U-2 OS and HeLa) for 16hrs. Cells were recovered for 24hrs then selected with puromycin for 72hrs. Surviving cells were expanded and diluted into single clones. Single clones were expanded and verified for deletion with PCR and immunoblot. Targeting sequences used for RTN3 KO (targeting the RHD) were: TTACATGAAAATGAGTCCGG and AATTGACTTGGTCTGATCTC; for RTN4 KO (targeting the first two exons) were: CGTTCAAGTACCAGTTCGTG & GGCGCGCCCCTGATGGACTT. Note that in RTN3Δ cells the RHD is targeted and resulted in a KO product which should not be functional in the absence of the RHD.
Knockdown with siRNA
For RNAi experiments, cells were transfected with negative control siRNA (Ambion AM4635) or 25nM siRNA against RTN3 (Dharmacon ON-TARGETplus SMARTPool L-020088-01) or RAB9A (Dharmacon ON-TARGETplus SMARTPool L-004177-00). ERM knockdown was achieved according to (Chirivino et al., 2011). Briefly, cells were treated with 10nM each of the three synthesized Dharmacon ON-TARGETplus siRNA: ezrin GCGCGGAGCUGUCUAGUGA; radixin GGCAUUAAGUUCAGAAUUA; moesin UCGCAAGCCUGAUACCAUU. siRNA were transfected using DharmaFECT 1 transfection reagent for 6hrs. 32hrs later, same concentrations of siRNAs together with florescent markers for imaging were transfected together into cells with Lipofectamine 3000 without P3000 in Opti-MEM.
Microscopy
Cells were imaged using a spinning disk confocal microscope. The spinning disk is composed a Nikon Ti2E body with PSF; CSU-X1 Yokogawa confocal scanner unit; OBIS 405, 488, 561, 640 nm lasers and an ANDOR iXON Ultra 512X512 EMCCD camera. Images were acquired with Nikon Plan apo λ 100X oil objective (1.45 N.A) and Nikon Plan apo 63X oil objective (1.4 N.A). Images were acquired with micro-manager 2.0. Images were processed in Fiji (Schindelin et al., 2012) and Adobe Illustrator (Adobe). Figure 2B and 2D were acquired on Zeiss LSM880 with Airyscan detector and a 63x1.4-NA Plan Apo objective.
Endosome maturation assay and quantification
To study endosome maturation, HeLa cells were transfected with BFP-Rab5 and GFP-Rab7 to marked early and late endosomes, respectively. 16hrs after transfection, cells were starved for 1hr and treated with 100ng/mL EGF-647 (Invitrogen E35351) (correspond to 10ng/mL unlabeled EGF) for 10min and 20min and then immediately fixed with 4% paraformaldehyde. EGF-positive endosomes (newly formed) in the cell periphery were marked, and binned into Rab5+, Rab7+ or Rab5+/Rab7+.
EGFR degradation assay
EGFR degradation assay carried out according to (Jongsma et al., 2016). Briefly, RTN3/RAB9A knockdown or RTN3 knockout cells were starved for 16hrs in DMEM containing 0.1% FBS. Cells were treated with 20ng/mL EGF (PeproTech AF-100-15-100UG) for 0, 30, 60, 120 minutes and were immediately harvested for immunoblotting. The same amount of whole cell lysates were loaded on SDS-PAGE gels and EGFR was probed with GAPDH as loading control.
CI-MPR sorting assay
CI-MPR localization was quantified as previously described (Hoyer et al., 2018). Briefly, RTN3/RAB9A knockdown or RTN3 knockout cells were incubated for 1hr with CI-MPR antibody at 1:1000 and then fixed with 4% paraformaldehyde in 1X PBS. To quantify CI-MPR distribution, cells were also stained with Giantin (1:500) for the Golgi. An ROI was taken near the nucleus/Golgi and the florescence intensity of the ROI (Golgi) and outside of the ROI (vesicle) were calculated. The ratio between vesicle/Golgi was plotted. More peripheral distribution of CI-MPR should have a lager vesicle/Golgi ratio.
TurboID proximity labeling experiments
HeLa cells were transfected with designated concentrations of V5-TurboID constructs (see transfection section). 16hrs after transfection, cells were treated with 500 µM biotin for 3hrs. After biotin treatment, approximately 107 HeLa cells were washed once with cold 1x PBS, trypsinized, and pelleted. Cell pellets were washed twice with cold 1X PBS and then lysed in 1mL lysis buffer (50mM Tris-HCl pH=7.4, 150mM NaCl, 0.5mM EDTA, 10% glycerol, 1% Triton™ X-100) at 4°C for 1hr (nutating). Lysed cells were centrifuged at max speed at 4°C to remove cell debris. 5% of the supernatant (0.5% was loaded on SDS-PAGE gel) was saved as “load” while 95% of supernatant was loaded on biotin antibody agarose beads (Immunechem, ICP0615) for binding overnight at 4°C. Cell lysate bound beads were washed three times in cold lysis buffer and eluted with 2x Laemmli sample buffer (with 355mM 2-mercaptoethanol). Both load and elute samples were loaded on SDS-PAGE gels and blotted for V5 (TurboID) and Rtn3L (endogenous) following standard immunoblotting protocols. For quantification, pulldown levels of endogenous Rtn3L were calculated by Rtn3L band intensity in “elute” divided by Rtn3L band intensity in “load” and then divided by V5 band intensity in “elute”. All numbers normalized to V5-TurboID-Rab9aWT in Figure 5D.
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistics
All data presented in all figures are from at least three biological replications. All numbers mentioned in texts are medians and plotted as boxplots in figures. All statistical analyses were performed in GraphPad Prism 8. When comparing two samples, two-tailed student t-tests were used. When analyzing significance for more than two samples, one-way ANOVA tests were performed and p values were derived from Tukey’s test. ns=not significant, **p<0.01, ***p<0.001, ****p<0.0001. Details of statistical analysis and exact values of n (numbers of cells) quantified in each experiment can be found in the figure legends.
Image quantification
Number of peripheral three-way junctions (Figure 1A–1D) was quantified using a customized MatLab script in a 10 µm x 10 µm box. Three-way junctions in Rtn4a OE cells were counted manually.
T = imread(‘filename.tif’);
level = graythresh(T);
Threshold = imbinarize(T,level);
BW_skel=bwmorph(Threshold,’skel’,Inf);
BW_3WJ=bwmorph(BW_skel,’branchpoints’);
[L,NUM] = bwlabel(BW_3WJ)
BW_Merge=imfuse(BW_skel,BW_3WJ,’falsecolor’,’ColorChannels’,[1 2 0]);
imshow(BW_Merge)
ER morphology (Figure 1E–1F) was quantified according to (Zurek et al., 2011). Briefly, different categories of cells containing either peripheral ER sheets or ER tubules were binned, and percent cells containing expanded ER sheets are calculated. Rtns ER coverage (Figure 1H–1M) is scored by calculating colocalization between different Rtns and a general ER marker (Sec61β). Briefly, colocalized signals are calculated using colocalization plugin in Fiji. Total ER signals are calculated with thresholded general ER marker (Sec61β). The Rtns percent ER coverage is calculated with colocalized signal divided by total ER signal. Percent Rtn3L puncta on MTs (Figure S2C) were calculated by Rtn3L puncta on MTs divided by total number of Rtn3L puncta. Percent ER tubule and MTs crossing (Figure S2C) were scored by total overlapping signal divided by total ER signal, similar to Rtns and ER coverage. Rtn3L’s speed (Figure S2D) was calculated by tracking the distance and duration of dynamic Rtn3L puncta in time-lapse movies (5 second per frame, anterograde or retrograde). Rtn3L (WT and mutant)/organelle association (Figure 2; Figure 3; Figure 4C–4D; Figure 5A and Figure S5A) was quantified by measuring the duration of time of individual dynamic organelle (endosomes marked by Rab5, EEA1 or Rab7; lysosomes marked by LAMP1; peroxisomes marked by SKL; autophagosomes marked by LC3) that was associated with Rtn3L in 2-min movies with 5 seconds intervals. Rab9a recruitment (Figure S5C) was scored by percent of Rab9a signal that was also positive for Rab7 signal. ER dynamics (Figure S2E) were scored by overlapping the first frame (t=0s) and last frame (t=60s) of peripheral ER network (10 µm x 10 µm ROIs) and Pearson correlation coefficient was calculated (Rowland et al., 2014). Levels of WT and mutant Rtn3L/Rab9a wrapping (Figure 5A–5B; Figure 5F–5H) were quantified by marking the percent of Rab9a endosomes that are circumscribed by both Rtn3L and ER (Sec61β) signals.
Supplementary Material
COS-7 cell expressing Rtn3L-GFP (green) and mCh-Sec61β (ER, red). Cell imaged every 5 seconds.
COS-7 cell expressing Rtn3L-GFP (green), BFP-Sec61β (ER, not shown), and mCh-Tubulin (MTs, grey). Cell imaged every 5 seconds.
COS-7 cell expressing Rtn3L-mNG (green), BFP-Sec61β (ER, blue), and mCh-Rab7 (LEs, red). Cell imaged every 5 seconds.
COS-7 cell expressing Rtn3L-mNG (green), BFP-Sec61β (ER, not shown), SNAP-Rab5 (EE marker, blue) and mScarlet-EEA1 (late EE marker, magenta). Cell imaged every 5 seconds.
COS-7 cells expressing Rtn3L-mNG (green), BFP-Sec61β (ER, not shown), SNAP-Ensconsin (grey, MTs) and mCh-Rab7 (LEs, red). Cell imaged every 5 seconds.
U-2 OS RTN3Δ cells were transfected with mNG-Rtn3cyto-MAVS (green) and mCh-Rab7 (LEs, red). Cell imaged every 5 seconds.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-RTN3 antibody produced in rabbit | Sigma-Aldrich | Cat# HPA015650, RRID:AB_1856499 |
| Rtn-3 Antibody (F-6) | Santa Cruz Biotechnology | Cat# sc-374599, RRID:AB_10986405 |
| EGF Receptor (D38B1) XP Rabbit mAb antibody | Cell Signaling Technology | Cat# 4267, RRID:AB_2246311 |
| Anti-GAPDH antibody produced in rabbit | Sigma-Aldrich | Cat# G9545, RRID:AB_796208 |
| Alpha Tubulin antibody | Abcam | Cat# ab18251, RRID:AB_2210057 |
| Rab9 (D22A6) Rabbit mAb antibody | Cell Signaling Technology | Cat# 5133, RRID:AB_10634754 |
| Ezrin/Radixin/Moesin Antibody | Cell Signaling Technology | Cat# 3142, RRID:AB_2100313 |
| V5 Tag Monoclonal Antibody | Thermo Fisher Scientific | Cat# R960-25, RRID:AB_2556564 |
| IGF2R Monoclonal Antibody (2G11) (CI-MPR) | Thermo Fisher Scientific | Cat# MA1-066, RRID:AB_2264554 |
| Rabbit Anti-Giantin Polyclonal Antibody, Unconjugated | BioLegend | Cat# PRB-114C-200, RRID:AB_291560 |
| Anti-Mouse IgG (whole molecule)-Peroxidase antibody produced in goat | Sigma-Aldrich | Cat# A4416, RRID:AB_258167 |
| Anti-Rabbit IgG (whole molecule)-Peroxidase antibody produced in goat | Sigma-Aldrich | Cat# A6154, RRID:AB_258284 |
| Goat anti-Rabbit IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 405 | Thermo Fisher Scientific | Cat# A-31556, RRID:AB_221605 |
| Donkey anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 488 | Thermo Fisher Scientific | Cat# A-21206, RRID:AB_2535792 |
| Donkey anti-Mouse IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 594 | Thermo Fisher Scientific | Cat# A-21203, RRID:AB_141633 |
| Donkey anti-Mouse IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 647 | Thermo Fisher Scientific | Cat# A-31571, RRID:AB_162542 |
| Donkey anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 594 | Thermo Fisher Scientific | Cat# A-21207, RRID:AB_141637 |
| Donkey anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 647 | Thermo Fisher Scientific | Cat# A-31573, RRID:AB_2536183 |
| Donkey anti-Mouse IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 488 | Thermo Fisher Scientific | Cat# A-21202, RRID:AB_141607 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Lipofectamine™ 3000 Transfection Reagent | Thermo Fisher Scientific | Cat# L3000150 |
| DharmaFECT 1 Transfection Reagent | Horizon Discovery | Cat# T-2001-02 |
| Biotin Antibody Agarose | ImmuneChem Pharmaceuticals Inc. | Cat# ICP0615 |
| Animal-Free Recombinant Human EGF | PeproTech | Cat# AF-100-15 |
| Epidermal Growth Factor, Biotinylated, complexed to Alexa Fluor™ 647 Streptavidin (Alexa Fluor™ 647 EGF complex) | Thermo Fisher Scientific | Cat# E35351 |
| Critical Commercial Assays | ||
| Gibson Assembly® Master Mix | New England Biolabs | Cat# E2611L |
| SuperSignal™ West Pico PLUS Chemiluminescent Substrate | Thermo Fisher Scientific | Cat# 34578 |
| SuperSignal™ West Femto Maximum Sensitivity Substrate | Thermo Fisher Scientific | Cat# 34096 |
| ZymoPURE - Express Plasmid Midiprep Kit | Zymo Research | Cat# D4213 |
| ZymoPURE Plasmid Miniprep Kit | Zymo Research | Cat# D4212 |
| Experimental Models: Cell Lines | ||
| COS-7 | ATCC | Cat# CRL-1651, RRID:CVCL_0224 |
| 293T | ATCC | Cat# CRL-3216, RRID:CVCL_0063 |
| HeLa | ATCC | Cat# CCL-2, RRID:CVCL_0030 |
| U-2 OS | ATCC | Cat# HTB-96, RRID:CVCL_0042 |
| Oligonucleotides | ||
| Silencer™ Negative Control No. 1 siRNA | Thermo Fisher Scientific | Cat# AM4635 |
| ON-TARGETplus Human RTN3 siRNA | Horizon Discovery | Cat# L-020088-01-0020 |
| ON-TARGETplus Human RAB9A siRNA | Horizon Discovery | Cat# L-004177-00-0020 |
| ON-TARGETplus human EZRIN siRNA: GCGCGGAGCUGUCUAGUGA | Horizon Discovery | (Chirivino et al., 2011) |
| ON-TARGETplus human RADIXIN siRNA: GGCAUUAAGUUCAGAAUUA |
Horizon Discovery | (Chirivino et al., 2011) |
| ON-TARGETplus human MOESIN siRNA: UCGCAAGCCUGAUACCAUU |
Horizon Discovery | (Chirivino et al., 2011) |
| Targeting sequence #1 used for RTN3 KO (targeting the RHD): TTACATGAAAATGAGTCCGG |
IDT | N/A |
| Targeting sequence #2 used for RTN3 KO (targeting the RHD): AATTGACTTGGTCTGATCTC |
IDT | N/A |
| Targeting sequence #1 used for RTN4 KO (targeting the first two exons): CGTTCAAGTACCAGTTCGTG |
IDT | N/A |
| Targeting sequence #2 used for RTN4 KO (targeting the first two exons): GGCGCGCCCCTGATGGACTT. |
IDT | N/A |
| Gibson assembly primers used in this paper see Table S1 | IDT | N/A |
| Recombinant DNA | ||
| mCherry-Sec61β | (Zurek et al., 2011) | N/A |
| BFP-Sec61β | (Zurek et al., 2011) | N/A |
| mCh-Climp63 | (English and Voeltz, 2013) | N/A |
| Rtn4a-GFP | (Shibata et al., 2008) | N/A |
| mCh-Tubulin | (Friedman et al., 2010) | N/A |
| BFP-Rab5 | (Friedman et al., 2013) | N/A |
| GFP-Rab7 | (Rowland et al., 2014) | N/A |
| mCh-Rab7 | (Rowland et al., 2014) | N/A |
| GFP-Rab7Q67L | Jonathan Friedman | N/A |
| mRuby-SKL | Michael Davidson | Addgene plasmid # 54840 |
| LAMP1-mCh | (Van Engelenburg and Palmer, 2010) | Addgene plasmid # 45147 |
| GFP-LC3 | (Lee et al., 2008) | Addgene plasmid # 24920 |
| SNAP-C1 | Michael Davidson | Addgene plasmid #58186 |
| pmScarlet-C1 | (Bindels et al., 2017) | Addgene plasmid #85042 |
| V5-TurboID-NES | (Branon et al., 2018) | Addgene plasmid # 107169 |
| mNeonGreen (mNG)-Sec61β | This Paper | N/A |
| Rtn4L-mNG | This Paper | N/A |
| Rtn3L-GFP | This Paper | N/A |
| Rtn3L-mNG | This Paper | N/A |
| Rtn3LΔ413-824-mNG | This Paper | N/A |
| Rtn3LΔ1-412-mNG | This Paper | N/A |
| Rtn3LΔ5LIR-mNG | This Paper | N/A |
| Rtn3LΔ6LIR-mNG | This Paper | N/A |
| Rtn3S-GFP | This Paper | N/A |
| Rtn3cytoRtn4RHD-mNG | This Paper | N/A |
| Rtn4cytoRtn3RHD-mNG | This Paper | N/A |
| SNAP-Ensconsin | This Paper | N/A |
| BFP-MAVS | This Paper | N/A |
| This Paper | This Paper | N/A |
| mScarlet-EEA1 | This Paper | N/A |
| mScarlet-Rab7 | This Paper | N/A |
| SNAP-Rab5 | This Paper | N/A |
| mScarlet-Rab9a | This Paper | N/A |
| GFP-Rab9aQ66L | This Paper | N/A |
| mScarlet-Rab9aS21N | This Paper | N/A |
| mScarlet-Rab9aΔFSV | This Paper | N/A |
| mScarlet-V5-TurboID-Rab9a | This Paper | N/A |
| mScarlet-V5-TurboID-Rab9aΔFSV | This Paper | N/A |
| mNG-VAPB | This Paper | N/A |
| Software and Algorithms | ||
| Fiji | (Schindelin et al., 2012) | https://fiji.sc/ |
| Adobe Illustrator | Adobe | https://www.adobe.com/products/illustrator.html |
| MATLAB | MathWorks | https://www.mathworks.com/products/matlab.html |
| GraphPad Prism 8 | GraphPad | www.graphpad.com |
Highlights.
Rtn3L is not functionally redundant with Rtn4a
Rtn3L localizes to junctions between ER tubules, endosomes, and microtubules
Rtn3L is recruited to ER-endosome membrane contact sites as endosomes mature
Rtn3L/Rab9a contact sites are required for endosome maturation and cargo sorting
Acknowledgments:
We thank Jessie Bacha, Patrick Chitwood, Jason Lee, Eric Sawyer, Jonathan Striepen and Tricia Nguyen for insight and helpful discussions.
Funding:
G.K.V. is an Investigator of the Howard Hughes Medical Institute; H.W. was supported by a graduate training grant in Signaling and Cellular Regulation (NIH T32 GM008759) and by NIH grant R01 GM120998 to G.K.V.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests: The authors declare no competing interests.
References
- Audhya A, Desai A, and Oegema K (2007). A role for Rab5 in structuring the endoplasmic reticulum. J. Cell Biol 178, 43–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer N, Schober D, Prchla E, Murphy RF, Blaas D, and Fuchs R (1998). Effect of Bafilomycin A1 and Nocodazole on Endocytic Transport in HeLa Cells: Implications for Viral Uncoating and Infection. J. Virol 72, 9645–9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancourt-Solis MA, Desai T, and McNew JA (2018). The atlastin membrane anchor forms an intramembrane hairpin that does not span the phospholipid bilayer. J. Biol. Chem 293, 18514–18524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindels DS, Haarbosch L, van Weeren L, Postma M, Wiese KE, Mastop M, Aumonier S, Gotthard G, Royant A, Hink MA, et al. (2017). mScarlet: a bright monomeric red fluorescent protein for cellular imaging. Nat. Methods 14, 53–56. [DOI] [PubMed] [Google Scholar]
- Branon TC, Bosch JA, Sanchez AD, Udeshi ND, Svinkina T, Carr SA, Feldman JL, Perrimon N, and Ting AY (2018). Efficient proximity labeling in living cells and organisms with TurboID. Nat. Biotechnol 36, 880–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldieri G, Barbieri E, Nappo G, Raimondi A, Bonora M, Conte A, Verhoef LGGC, Confalonieri S, Malabarba MG, Bianchi F, et al. (2017). Reticulon 3–dependent ER-PM contact sites control EGFR nonclathrin endocytosis. Science (80−. ). 356, 617–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirivino D, Del Maestro L, Formstecher E, Hupé P, Raposo G, Louvard D, and Arpin M (2011). The ERM proteins interact with the HOPS complex to regulate the maturation of endosomes. Mol. Biol. Cell 22, 375–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinet C, Stöter M, Bradshaw CR, Samusik N, Rink JC, Kenski D, Habermann B, Buchholz F, Henschel R, Mueller MS, et al. (2010). Systems survey of endocytosis by multiparametric image analysis. Nature 464, 243–249. [DOI] [PubMed] [Google Scholar]
- Dong R, Saheki Y, Swarup S, Lucast L, Harper JW, and De Camilli P (2016). Endosome-ER Contacts Control Actin Nucleation and Retromer Function through VAP-Dependent Regulation of PI4P. Cell 166, 408–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driskell OJ, Mironov A, Allan VJ, and Woodman PG (2007). Dynein is required for receptor sorting and the morphogenesis of early endosomes. Nat. Cell Biol 9, 113–120. [DOI] [PubMed] [Google Scholar]
- Elbaz-Alon Y, Guo Y, Segev N, Harel M, Quinnell DE, Geiger T, Avinoam O, Li D, and Nunnari J (2020). PDZD8 interacts with Protrudin and Rab7 at ER-late endosome membrane contact sites associated with mitochondria. Nat. Commun 11, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Engelenburg SB, and Palmer AE (2010). Imaging type-III secretion reveals dynamics and spatial segregation of Salmonella effectors. Nat. Methods 7, 325–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English AR, and Voeltz GK (2013). Rab10 GTPase regulates ER dynamics and morphology. Nat. Cell Biol 15, 169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa EJ, Calero M, Sridevi K, and Pfeffer SR (2009). RhoBTB3: A Rho GTPase-Family ATPase Required for Endosome to Golgi Transport. Cell 137, 938–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JR, Webster BM, Mastronarde DN, Verhey KJ, and Voeltz GK (2010). ER sliding dynamics and ER-mitochondrial contacts occur on acetylated microtubules. J Cell Biol 190, 363–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JR, Dibenedetto JR, West M, Rowland AA, and Voeltz GK (2013). Endoplasmic reticulum-endosome contact increases as endosomes traffic and mature. Mol. Biol. Cell 24, 1030–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano F, Saheki Y, Idevall-Hagren O, Colombo SF, Pirruccello M, Milosevic I, Gracheva EO, Bagriantsev SN, Borgese N, and De Camilli P (2013). PI(4,5)P2-Dependent and Ca2+-Regulated ER-PM Interactions Mediated by the Extended Synaptotagmins. Cell 153, 1494–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gougeon PY, Prosser DC, Da-Silva LF, and Ngsee JK (2002). Disruption of Golgi morphology and trafficking in cells expressing mutant prenylated Rab acceptor-1. J. Biol. Chem 277, 36408–36414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumati P, Morozzi G, Hölper S, Mari M, Harwardt MLIE, Yan R, Müller S, Reggiori F, Heilemann M, and Dikic I (2017). Full length RTN3 regulates turnover of tubular endoplasmic reticulum via selective autophagy. Elife 6, e25555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillén-Samander A, Bian X, and De Camilli P (2019). PDZD8 mediates a Rab7-dependent interaction of the ER with late endosomes and lysosomes. Proc. Natl. Acad. Sci. U. S. A 116, 22619–22623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi H, Lippé R, McBride HM, Rubino M, Woodman P, Stenmark H, Rybin V, Wilm M, Ashman K, Mann M, et al. (1997). A novel Rab5 GDP/GTP exchange factor complexed to Rabaptin-5 links nucleotide exchange to effector recruitment and function. Cell 90, 1149–1159. [DOI] [PubMed] [Google Scholar]
- Hoyer MJ, Chitwood PJ, Ebmeier CC, Striepen JF, Qi RZ, Old WM, and Voeltz GK (2018). A Novel Class of ER Membrane Proteins Regulates ER-Associated Endosome Fission. Cell 175, 254–265.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Shibata Y, Voss C, Shemesh T, Li Z, Coughlin M, Kozlov MM, Rapoport TA, and Prinz WA (2008). Membrane proteins of the endoplasmic reticulum induce high-curvature tubules. Science 319, 1247–1250. [DOI] [PubMed] [Google Scholar]
- Hu J, Shibata Y, Zhu P-P, Voss C, Rismanchi N, Prinz WA, Rapoport TA, and Blackstone C (2009). A class of dynamin-like GTPases involved in the generation of the tubular ER network. Cell 138, 549–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huotari J, and Helenius A (2011). Endosome maturation. EMBO J 30, 3481–3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutt DM, Da-Silva LF, Chang LH, Prosser DC, and Ngsee JK (2000). PRA1 inhibits the extraction of membrane-bound Rab GTPase by GDI1. J. Biol. Chem 275, 18511–18519. [DOI] [PubMed] [Google Scholar]
- Johansson M, Rocha N, Zwart W, Jordens I, Janssen L, Kuijl C, Olkkonen VM, and Neefjes J (2007). Activation of endosomal dynein motors by stepwise assembly of Rab7-RILP-p150Glued, ORP1L, and the receptor betalll spectrin. J. Cell Biol 176, 459–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongsma MLM, Berlin I, Wijdeven RHM, Janssen L, Janssen GMC, Garstka MA, Janssen H, Mensink M, van Veelen PA, Spaapen RM, et al. (2016). An ER-Associated Pathway Defines Endosomal Architecture for Controlled Cargo Transport. Cell 166, 152–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jozsef L, Tashiro K, Kuo A, Park E, Skoura A, Albinsson S, Rivera-Molina F, Harrison KD, Iwakiri Y, Toomre D, et al. (2014). Reticulon 4 is necessary for endoplasmic reticulum tubulation, STIM1-Orai1 coupling, and store-operated calcium entry. J. Biol. Chem 289, 9380–9395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaminets A, Heinrich T, Mari M, Grumati P, Huebner AK, Akutsu M, Liebmann L, Stolz A, Nietzsche S, Koch N, et al. (2015). Regulation of endoplasmic reticulum turnover by selective autophagy. Nature 522, 354–358. [DOI] [PubMed] [Google Scholar]
- Kinchen JM, and Ravichandran KS (2010). Identification of two evolutionarily conserved genes regulating processing of engulfed apoptotic cells. Nature 464, 778–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld S, and Mellman I (1989). The Biogenesis of Lysosomes. Annu. Rev. Cell Biol 5, 483–525. [DOI] [PubMed] [Google Scholar]
- Kucera A, Borg Distefano M, Berg-Larsen A, Skjeldal F, Repnik U, Bakke O, and Progida C (2016). Spatiotemporal Resolution of Rab9 and CI-MPR Dynamics in the Endocytic Pathway. Traffic 17, 211–229. [DOI] [PubMed] [Google Scholar]
- Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE, Tsokos M, Alt FW, and Finkel T (2008). A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc. Natl. Acad. Sci 105, 3374–3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Cathey PI, Wu H, Parker R, and Voeltz GK (2020). Endoplasmic reticulum contact sites regulate the dynamics of membraneless organelles. Science (80−. ). 367, eaay7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi D, Soldati T, Riederer MA, Goda Y, Zerial M, and Pfeffer SR (1993). Rab9 functions in transport between late endosomes and the trans Golgi network. EMBO J 12, 677–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson DP, Brett CL, and Merz AJ (2009). Vps-C complexes: gatekeepers of endolysosomal traffic. Curr. Opin. Cell Biol 21, 543–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan NC, Jahn TR, Reid E, and O’Kane CJ (2012). Reticulon-like-1, the Drosophila orthologue of the Hereditary Spastic Paraplegia gene reticulon 2, is required for organization of endoplasmic reticulum and of distal motor axons. Hum. Mol. Genet 21, 3356–3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orso G, Pendin D, Liu S, Tosetto J, Moss TJ, Faust JE, Micaroni M, Egorova A, Martinuzzi A, McNew JA, et al. (2009). Homotypic fusion of ER membranes requires the dynamin-like GTPase atlastin. Nature 460, 978–983. [DOI] [PubMed] [Google Scholar]
- Phillips MJ, and Voeltz GK (2015). Structure and function of ER membrane contact sites with other organelles. Nat. Rev. Mol. Cell Biol 17, 69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poteryaev D, Fares H, Bowerman B, and Spang A (2007). Caenorhabditis elegans SAND-1 is essential for RAB-7 function in endosomal traffic. EMBO J 26, 301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poteryaev D, Datta S, Ackema K, Zerial M, and Spang A (2010). Identification of the switch in early-to-late endosome transition. Cell 141, 497–508. [DOI] [PubMed] [Google Scholar]
- Raiborg C, Wenzel EM, Pedersen NM, Olsvik H, Schink KO, Schultz SW, Vietri M, Nisi V, Bucci C, Brech A, et al. (2015). Repeated ER–endosome contacts promote endosome translocation and neurite outgrowth. Nature 520, 234–238. [DOI] [PubMed] [Google Scholar]
- Rämö O, Kumar D, Gucciardo E, Joensuu M, Saarekas M, Vihinen H, Belevich I, Smolander O-P, Qian K, Auvinen P, et al. (2016). NOGO-A/RTN4A and NOGO-B/RTN4B are simultaneously expressed in epithelial, fibroblast and neuronal cells and maintain ER morphology. Sci. Rep 6, 35969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, and Zhang F (2013). Genome engineering using the CRISPR-Cas9 system. Nat. Protoc 8, 2281–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riederer MA, Soldati T, Shapiro AD, Lin J, and Pfeffer SR (1994). Lysosome biogenesis requires Rab9 function and receptor recycling from endosomes to the trans-Golgi network. J. Cell Biol 125, 573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha N, Kuijl C, Van Der Kant R, Janssen L, Houben D, Janssen H, Zwart W, and Neefjes J (2009). Cholesterol sensor ORP1L contacts the ER protein VAP to control Rab7-RILP-p150Glued and late endosome positioning. J. Cell Biol 185, 1209–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland AA, Chitwood PJ, Phillips MJ, and Voeltz GK (2014). ER contact sites define the position and timing of endosome fission. Cell 159, 1027–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjana NE, Shalem O, and Zhang F (2014). Improved vectors and genome-wide libraries for CRISPR screening. Nat. Methods 11, 783–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. (2012). Fiji: An open-source platform for biological-image analysis. Nat. Methods 9, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid SL, Fuchs R, Male P, and Mellman I (1988). Two distinct subpopulations of endosomes involved in membrane recycling and transport to lysosomes. Cell 52, 73–83. [DOI] [PubMed] [Google Scholar]
- Shibata Y, Voss C, Rist JM, Hu J, Rapoport TA, Prinz WA, and Voeltz GK (2008). The reticulon and DP1/Yop1p proteins form immobile oligomers in the tubular endoplasmic reticulum. J Biol Chem 283, 18892–18904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata Y, Shemesh T, Prinz WA, Palazzo AF, Kozlov MM, and Rapoport TA (2010). Mechanisms determining the morphology of the peripheral ER. Cell 143, 774–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivars U, Aivazian D, and Pfeffer SR (2003). Yip3 catalyses the dissociation of endosomal Rab-GDI complexes. Nature 425, 856–859. [DOI] [PubMed] [Google Scholar]
- Söding J (2005). Protein homology detection by HMM-HMM comparison. Bioinformatics 21, 951–960. [DOI] [PubMed] [Google Scholar]
- Tolley N, Sparkes IA, Hunter PR, Craddock CP, Nuttall J, Roberts LM, Hawes C, Pedrazzini E, and Frigerio L (2008). Overexpression of a Plant Reticulon Remodels the Lumen of the Cortical Endoplasmic Reticulum but Does not Perturb Protein Transport. Traffic 9, 94–102. [DOI] [PubMed] [Google Scholar]
- Voeltz GK, Prinz WA, Shibata Y, Rist JM, and Rapoport TA (2006). A class of membrane proteins shaping the tubular endoplasmic reticulum. Cell 124, 573–586. [DOI] [PubMed] [Google Scholar]
- Vonderheit A, and Helenius A (2005). Rab7 associates with early endosomes to mediate sorting and transport of Semliki forest virus to late endosomes. PLoS Biol 3, 1225–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CW, Stromhaug PE, Kauffman EJ, Weisman LS, and Klionsky DJ (2003). Yeast homotypic vacuole fusion requires the Ccz1-Mon1 complex during the tethering/docking stage. J. Cell Biol 163, 973–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman-Storer CM, and Salmon EDD (1998). Endoplasmic reticulum membrane tubules are distributed by microtubules in living cells using three distinct mechanisms. Curr. Biol 8, 798–807. [DOI] [PubMed] [Google Scholar]
- Westrate LM, Lee JE, Prinz WA, and Voeltz GK (2015). Form follows function: the importance of endoplasmic reticulum shape. Annu. Rev. Biochem 84, 791–811. [DOI] [PubMed] [Google Scholar]
- Wu H, Carvalho P, and Voeltz GK (2018). Here, there, and everywhere: The importance of ER membrane contact sites. Science (80−. ). 361, eaan5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoncu R, Perera RM, Balkin DM, Pirruccello M, Toomre D, and De Camilli P (2009). A Phosphoinositide Switch Controls the Maturation and Signaling Properties of APPL Endosomes. Cell 136, 1110–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurek N, Sparks L, and Voeltz G (2011). Reticulon short hairpin transmembrane domains are used to shape ER tubules. Traffic 12, 28–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
COS-7 cell expressing Rtn3L-GFP (green) and mCh-Sec61β (ER, red). Cell imaged every 5 seconds.
COS-7 cell expressing Rtn3L-GFP (green), BFP-Sec61β (ER, not shown), and mCh-Tubulin (MTs, grey). Cell imaged every 5 seconds.
COS-7 cell expressing Rtn3L-mNG (green), BFP-Sec61β (ER, blue), and mCh-Rab7 (LEs, red). Cell imaged every 5 seconds.
COS-7 cell expressing Rtn3L-mNG (green), BFP-Sec61β (ER, not shown), SNAP-Rab5 (EE marker, blue) and mScarlet-EEA1 (late EE marker, magenta). Cell imaged every 5 seconds.
COS-7 cells expressing Rtn3L-mNG (green), BFP-Sec61β (ER, not shown), SNAP-Ensconsin (grey, MTs) and mCh-Rab7 (LEs, red). Cell imaged every 5 seconds.
U-2 OS RTN3Δ cells were transfected with mNG-Rtn3cyto-MAVS (green) and mCh-Rab7 (LEs, red). Cell imaged every 5 seconds.
Data Availability Statement
This study did not generate/analyze any dataset, and MATLAB code for quantifying three-way junctions is available in method details.


