Abstract
Objective
To define the prevalence and characteristics of spinal cord transient ischemic attack (sTIA) in a large retrospective series of patients who met diagnostic criteria for spontaneous spinal cord infarction (SCI).
Methods
An institution-based search tool was used to identify patients evaluated at the Mayo Clinic in Rochester, MN, from 1997 to 2017 with spontaneous SCI (n = 133). Cases were subsequently reviewed for transient myelopathic symptoms preceding infarction that were suspected ischemic in nature. We performed a descriptive analysis of patients with sTIA before SCI.
Results
Of 133 patients with a diagnosis of spontaneous SCI, we identified 4 patients (3%) who experienced sTIA before SCI. The median age at presentation was 61.5 years (range 46–75 years), 2 (50%) were women, and 3 (75%) had traditional vascular risk factors. Localization was cervical cord in 2 cases (50%) and thoracic cord in 2 cases (50%); all patients developed SCI in the same distribution as their preceding sTIA symptoms. All patients experienced recurrent sTIA before SCI. Symptoms ranged from seconds to a few minutes before returning to baseline. No patients had pain as a feature of sTIA.
Conclusions
sTIAs are possible but rare in patients who subsequently have a SCI. Clinical features are similar to those of SCI, with rapid onset of severe myelopathic deficits, followed by prompt resolution. Vascular risk factors are common in these patients. Thus, recognition of a sTIA may represent a valuable opportunity for vascular risk factor modification and stroke prevention. However, given the rarity, physicians should explore other possible explanations when sTIA is considered.
Spinal cord infarction (SCI) is an uncommon but underrecognized cause of acute myelopathy. New guidelines for the diagnosis of spontaneous SCI have been proposed with emphasis on severe nontraumatic myelopathic deficits within 12 hours.1 Based on our understanding of mechanisms underlying cerebral ischemia, we anticipate some patients with SCI have transient symptoms analogous to a transient ischemic attack (TIA). Although stuttering symptoms involving fluctuating myelopathic deficits without full resolution were seen in 23% of patients in a recent study of spontaneous SCI, an assessment for patients qualifying as a true TIA was not evaluated.1 Spinal cord TIA (sTIA) is a diagnosis proposed at times for patients with transient bilateral neurologic symptoms, but with trace literature support and a poor understanding of characteristics. We sought to evaluate the prevalence and characteristics of sTIA in a large series of patients who ultimately met diagnostic criteria for SCI.
Methods
We sought to evaluate the prevalence and characteristics of sTIA in a large series of patients who ultimately met diagnostic criteria for SCI.
We used an institution-based search tool to identify patients with spontaneous SCI at Mayo Clinic, Rochester, MN, from January 1, 1997, to December 1, 2017. We searched clinical notes for the terms spinal cord infarction, spinal cord stroke, anterior spinal artery, posterior spinal artery, and vascular myelopathy. We subsequently reviewed the data of all patients to verify the diagnosis. None of the included cases had spinal cord trauma, compression, or a recent procedure within 1 month. Inclusion criteria were final diagnosis of spontaneous SCI and adequate clinical and radiologic data to exclude other etiologies. Patients with periprocedural SCI were excluded.
Two neurologists (S.W.E. and N.L.Z.) reviewed the medical records of patients who met the inclusion criteria to assess for transient myelopathic symptoms before SCI.1 sTIA was defined as acute myelopathic neurologic deficits lasting less than 24 hours with full recovery before subsequent infarction, not due to alternative identifiable etiology. Patients with incomplete recovery, patients with a more likely explanation, and patients with pain alone were excluded. We only included patients diagnosed with SCI, given the purpose of including a well-defined population for this study. All patients included have been reported in the literature previously, without a focus on TIA.1
sTIA was defined as acute myelopathic neurologic deficits lasting less than 24 hours with full recovery before subsequent infarction, not due to alternative identifiable etiology.
Standard protocol approvals, registrations, and patient consent
This study was approved by the Institutional Review Board of Mayo Clinic, Rochester, MN. All patients provided written consent to use their medical records for research.
Data availability
Anonymized data and statistical analysis will be shared by request from any qualified investigator for 5 years after the date of publication.
Results
Patient characteristics
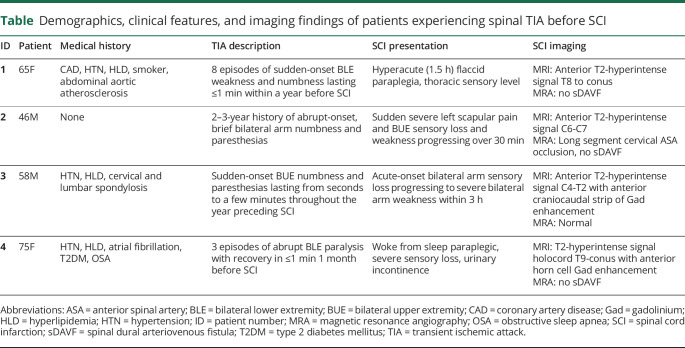
Of 133 patients 18 years or older with a diagnosis of spontaneous SCI,1 we identified 4 patients (3%) with sTIA (table). The median age at presentation was 61.5 years (range 46–75 years), 2 (50%) were women, and 3 (75%) had traditional vascular risk factors. Risk factors that were not adequately controlled at the time of sTIA included patient 1 without antiplatelet medication for coronary artery disease, patient 3 diagnosed with hyperlipidemia at SCI presentation, and patient 4 had subtherapeutic international normalized ratio on multiple occasions in months preceding her neurologic presentation. Notably, patient 1 had severe abdominal aortic atherosclerosis and patient 3 had extensive cervical and lumbar spondylosis. No patients had a history of cerebral TIA or stroke. However, 2 patients (50%) had a cerebral infarction after SCI: patient 4 had an incidentally discovered ischemic stroke as part of a delirium evaluation during her initial SCI hospitalization (warfarin was held in the setting of gastrointestinal bleed), and patient 1 had a posterior circulation ischemic stroke 2 years after SCI.
Table.
Demographics, clinical features, and imaging findings of patients experiencing spinal TIA before SCI
Clinical features
Ischemia localization was cervical spinal cord in 2 cases (50%) and thoracic spinal cord in 2 cases (50%); all patients eventually developed SCI in the same distribution as their sTIA symptoms. Symptoms consisted of abrupt bilateral upper extremity numbness and paresthesias in 2 patients (50%), paraparesis in 1 patient (25%), and paraparesis with sensory loss in 1 patient (25%). Recurrent sTIA occurred in all patients before SCI; the frequency was specified in 2 patients, reporting 3 and 8 recurrent spells. Duration ranged from seconds to a few minutes before returning to baseline. One patient had recurrent symptoms for 3 years before SCI (long cervical segment ASA occlusion), 2 endorsed symptoms over the year preceding SCI, and 1 developed symptoms in the month before SCI. All 4 patients experienced sTIA within the year of SCI. No patients reported pain as a feature of sTIA. No patients had documentation of evaluation or imaging performed at the time of sTIA.
Discussion
We report sTIA before infarction in 4 of 133 patients (3%) from a large series of SCI. In each case, the transient symptoms were similar in character and localized to the same vascular distribution as the subsequent SCI. Symptoms were abrupt, brief, and reached maximal intensity almost immediately, followed by resolution within minutes. Although back and/or limb pain are common features of SCI,1 no patients endorsed pain as a component of their sTIA.
There is a paucity of literature regarding sTIA, but other studies of SCI have reported similarly low rates of the phenomenon. A series from 1996 describing 44 patients with vascular myelopathy found 3 cases (7%) of isolated sTIA. All 3 cases had ASA syndrome with deficits lasting 1 minute to 1 day, followed by complete resolution. Etiologies were reported as aortic atheroembolic, cardioembolic, and unknown.2 Another series analyzed 27 patients with spontaneous SCI from 1990 to 2003 and found 2 (7%) patients with sTIA preceding the diagnosis of SCI. One patient experienced recurrent spells for 6 years preceding SCI with cervical ASA occlusion, similar to our patient with 3 years of recurrent symptoms with ASA occlusion.3 Several other case series of spontaneous SCI do not report any patients with preceding sTIA.4,5 Although back pain has been previously reported with sTIA,6 most of the cases in the literature do not describe pain as a major feature.2,3,7,8
The relative infrequency of spinal cord ischemia has been previously explored. Anatomical studies of the spinal cord vasculature have demonstrated a rich collateral network of segmental arteries at each vertebral level that help reinforce the anterior and posterior spinal arteries. Furthermore, there is a strong network of anastomoses from nearby paraspinal muscles and other paravertebral tissues, supporting the notion that spinal cord circulation is a longitudinally connected, flexible system with intrinsic autoregulatory and anastomotic mechanisms that can compensate in the case of hypoperfusion.9 In addition, several common mechanisms of cerebral ischemia seem less likely to affect the spinal cord vasculature, including large vessel atherosclerotic disease with increased exposure to shear forces with turbulence, and cardioembolism with a more likely route of embolism to the cerebral vasculature.
Although these studies suggest that sTIA is rare, the true incidence of spinal cord ischemia is unclear. SCI itself has been reported to represent 1% of all strokes, but recent evidence suggests that it is frequently misdiagnosed as transverse myelitis.10 A large series of SCI has highlighted clinical, laboratory, and MRI data with proposed diagnostic criteria to help differentiate SCI from alternative etiologies such as inflammatory myelitis.1 Specific imaging features to confirm the diagnosis of SCI in the right clinical setting include focal diffusion restriction (diffusion-weighted imaging/apparent diffusion coefficient) of the spinal cord, associated vertebral body infarction, and/or adjacent arterial dissection/occlusion. Several supportive but less specific T2-hyperintensity patterns include “owl eyes,” anterior “pencil-like” hyperintensity, anteromedial spot, anterior U/V, and holocord or hologrey signal among other features. Lesions are frequently longitudinally extensive (≥3 vertebral segments), noncontiguous, and at times respect a clear arterial territory (ASA or posterior spinal artery). Gadolinium enhancement is frequently present subacutely, highlighting the predominant area of ischemia, typically in a craniocaudal linear strip/arterial territory.1 By contrast, the appearance of spinal cord lesions in inflammatory myelopathies is generally different (e.g., T2-hyperintensity features and gadolinium enhancement patterns).11,12 However, it is important to note that at times, imaging features can overlap with nonspecific T2-hyperintense imaging patterns in SCI, and thus, critical clinical details should always be taken into consideration to help differentiate etiologies. An extensive review of inflammatory myelopathies and imaging findings to help differentiate from SCI has been previously highlighted.1,13
Although many patients with SCI present with stuttering myelopathy deficits (23%),1 our data suggest that a true sTIA is a very rare clinical syndrome, even within a large series of spontaneous SCI. Therefore, clinicians need to carefully consider differential diagnoses in patients with suspected transient acute myelopathy symptoms including spinal dural arteriovenous fistula, spondylosis with dynamic compression, and demyelination with the Lhermitte phenomenon. Other diagnoses that may cause similar symptoms include lumbar stenosis, compressive radiculopathy or neuropathy, cerebral ischemia, presyncope, cataplexy, functional spells, drop attack with seizure or hydrocephalus, and periodic paralysis.
Limitations of our study include the retrospective design and lack of criterion standard for the diagnosis of sTIA. We only identified patients with sTIA who eventually had SCI; our findings may not be applicable to patients with sTIA not followed by SCI. However, the fact that we identified our sTIA cases from a large series of patients with confirmed SCI confers greater reliability to our case identification. The cases identified were highly dependent on carefully delineated history, and it is possible that other cases were overlooked. Conversely, the study design is subject to recall bias after SCI and symptoms may have been attributable to other possible etiologies, although none were identified on careful review.
This study indicates that sTIAs occurred infrequently among patients who ultimately had a SCI. Patients report similar clinical features as those described in SCI,1 with rapid onset of severe myelopathic deficits, followed by prompt resolution. Given the rarity, physicians should explore other possible explanations for the symptoms. However, typical features should raise suspicion for sTIA because prompt recognition of sTIA can offer a valuable opportunity for vascular risk factor modification and possible stroke prevention.
TAKE-HOME POINTS
→ sTIA is a rare clinical entity, and alternate etiologies should be strongly considered.
→ sTIA typically presents with rapid onset of severe myelopathic deficits, followed by prompt symptom resolution.
→ Typical features should raise suspicion of vascular etiology because early recognition provides a valuable opportunity for risk factor modification.
Appendix. Authors
Footnotes
Editorial, page 469
Study funding
No targeted funding reported.
Disclosure
The authors report no disclosures relevant to the manuscript. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Zalewski NL, Rabinstein AA, Krecke KN, et al. Characteristics of spontaneous spinal cord infarction and proposed diagnostic criteria. JAMA Neurol 2019;76:56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheshire WP, Santos CC, Massey EW, Howard JF Jr. Spinal cord infarction: etiology and outcome. Neurology 1996;47:321–330. [DOI] [PubMed] [Google Scholar]
- 3.Novy J, Carruzzo A, Maeder P, Bogousslavsky J. Spinal cord ischemia: clinical and imaging patterns, pathogenesis, and outcomes in 27 patients. Arch Neurol 2006;63:1113–1120. [DOI] [PubMed] [Google Scholar]
- 4.Sandson TA, Friedman JH. Spinal cord infarction. Report of 8 cases and review of the literature. Medicine (Baltimore) 1989;68:282–292. [PubMed] [Google Scholar]
- 5.Weidauer S, Nichtweiss M, Lanfermann H, Zanella FE. Spinal cord infarction: MR imaging and clinical features in 16 cases. Neuroradiology 2002;44:851–857. [DOI] [PubMed] [Google Scholar]
- 6.Newcombe DS. Intermittent spinal ischemia. A reversible cause of neurologic dysfunction and back pain. Arthritis Rheum 1994;37:142–144. [DOI] [PubMed] [Google Scholar]
- 7.Hussain MS, Shuaib A, Siddiqi ZA. Spinal cord transient ischemic attacks: a possible role for abciximab. Neurology 2005;64:761–762. [DOI] [PubMed] [Google Scholar]
- 8.Ates I, Kaplan M, Özçalık M, Yılmaz N. Transient ischemic attacks of spinal cord due to abdominal aortic aneurysm thrombus. Ann Vasc Surg 2016;30:307.e7-307.e9. [DOI] [PubMed] [Google Scholar]
- 9.Etz CD, Kari FA, Mueller CS, et al. The collateral network concept: a reassessment of the anatomy of spinal cord perfusion. J Thorac Cardiovasc Surg 2011;141:1020–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zalewski NL, Flanagan EP, Keegan BM. Evaluation of idiopathic transverse myelitis revealing specific myelopathy diagnoses. Neurology 2018;90:e96–e102. [DOI] [PubMed] [Google Scholar]
- 11.Zalewski NL, Rabinstein AA, Brinjikji W, et al. Unique gadolinium enhancement pattern in spinal dural arteriovenous fistulas. JAMA Neurol 2018;75:1542–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zalewski NL, Morris PP, Weinshenker BG, et al. Ring-enhancing spinal cord lesions in neuromyelitis optica spectrum disorders. J Neurol Neurosurg Psychiatry 2017;88:218–225. [DOI] [PubMed] [Google Scholar]
- 13.Zalewski NL, Flanagan EP. Autoimmune and paraneoplastic myelopathies. Semin Neurol 2018;38:278–289. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data and statistical analysis will be shared by request from any qualified investigator for 5 years after the date of publication.