Abstract
Background
Migraine is a common and often refractory feature for individuals with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) without consensus guidelines for treatment. Migraine treatment poses a theoretical risk within this unique population with precarious cerebrovascular autoregulation, given the vasomodulatory influence of many antimigraine medications. In this systematic review and meta-analysis, we evaluate the frequency and efficacy of treatments for migraine in individuals with CADASIL.
Methods
A search protocol was designed to include all available publications reporting antimigraine therapies for CADASIL. Individual responses to medications were categorized as unfavorable, neutral, or favorable. Responses across medication classes were compared using the Mann-Whitney U test.
Results
Thirteen studies were included, yielding a cohort of 123 individuals with a median age of 53 years (range: 23–83 years), with 61% (75/123) being women. No controlled trials were identified. Simple analgesics (35.8%, 44/123) and beta-blockers (22.0%, 27/123) were the most common abortive and prophylactic strategies, respectively. Over half (54.4%) of all patients had used more than 1 medication sequentially or concomitantly. Beta-blockers were significantly associated with a neutral or unfavorable response (13.5%, 22/163, p = 0.004). We found no significant associations among other medication categories.
Conclusions
Migraine in CADASIL remains a formidable therapeutic challenge, with patients often tried on several medications. Antimigraine prophylaxis with beta-blockers may be contraindicated relative to other common therapies in CADASIL. Controlled studies are needed to rigorously evaluate the safety and efficacy of antimigraine therapies in this population.
Migraine with aura and progressive subcortical microangiopathy are hallmark features of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL). This syndrome serves as a monogenic stroke model stemming from a pathogenic missense mutation of the NOTCH3 gene on chromosome 19.1 Migraine, almost exclusively with aura, is an inaugural symptom in half of afflicted individuals; however, data on optimal migraine treatment are limited.2,3 Anecdotal reports suggest that acetazolamide and valproate have therapeutic potential, although the vast majority of common migraine therapeutics affect cerebral vasoreactivity and are of unknown clinical detriment in this unique population that has precarious vascular autoregulation.4–6
To date, no randomized trials investigating the efficacy or safety of specific migraine therapies in patients with CADASIL are available. The present review examines adults with CADASIL who have received any form of treatment for migraines so as to compare efficacy among medications used in this population. Outcomes, in this case, refer to changes in migraine frequency, severity, or disability related to migraine. We present a systematic, registered review of reported migraine therapeutic strategies and their efficacy in adults with CADASIL.
Methods
Systematic review protocol
This review adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.7 The protocol for this review can be found on the PROSPERO International Prospective Register of Systematic Reviews (registration number: CRD42019125962).
Search strategy
A medical librarian (T.J.B.) developed and performed searches in the MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, PsycINFO (all via the Ovid interface), Scopus, Web of Science, Epistemonikos databases, and several grey literature sources, as defined by the Twelfth International Conference on Grey Literature in Prague in 2010.8 A unique search strategy was created for each database or resource and was peer reviewed by another experienced librarian. Both subject headings and text-word terms for “CADASIL,” “migraine,” and “headache” were used, as well as related and exploded terms, including MeSH and EMBASE terms in combination with keyword searching. There were no search limitations regarding language or publication date. A full search strategy is presented in appendix 2 (links.lww.com/CPJ/A148). The searches were performed on March 6, 2019, and March 7, 2019, for all aforementioned databases and grey literature resources, respectively. The risk for bias of each included report was independently assessed by P.A.G. and M.K.B. The Risk Of Bias In Non-randomized Studies-1 criteria were used to assess article quality and potential for bias for case series.9 This tool provides a qualitative, categorical assessment of risk of bias for pre- and post-intervention domains. For the purposes of this study, we deemed 3 domains to be most important in comparing study quality: bias through confounding, bias in participant selection, and bias in outcome measurements (table e-1, links.lww.com/CPJ/A147). We generated subscores for patient selection, ascertainment of results, and causality using the Murad system for each individual case report (table e-2).10
Search parameters
The search parameters were intentionally broad so as to minimize the risk of unintentional exclusion of potentially relevant sources. Inclusion criteria consisted of original research on adult human subjects with a confirmed diagnosis of CADASIL and a description of migraine therapeutic strategies used. Following the initial search and removal of duplicate articles, P.A.G. and M.K.B. reviewed the title and abstracts of all publications. There were no limits to language or publication date. If the suitability and relevance of a particular study were unclear from the title and abstract alone, the full article was reviewed by both P.A.G. and M.K.B. If required information was not readily available in the article, P.A.G. or J.F.M. requested supplemental data from the reviewed article's authors.
Data and outcome measurements
Data extracted (if available) included demographics, migraine characteristics, and migraine therapeutic strategies. Only patients with documented antimigraine medication use were included in the analysis. Subjects' responses to medications were classified into 3 outcome categories with respect to migraine intensity, migraine frequency, migraine duration, or patient-reported adverse drug reactions (ADRs). Unfavorable outcomes were defined as worsening of at least 1 migraine characteristic or ADR after receiving a drug compared with predrug status. Neutral outcomes were defined as no substantial change in migraine characteristics and no clinically substantial ADR after receiving a drug compared with predrug status. Favorable outcomes were defined as an improvement in at least 1 migraine characteristic and no ADR reported.
Author discretion was used to manually classify ambiguous or cursory descriptions of responses to medication, such as “Ibuprofen ineffective. Since starting aspirin, frequency reduced.” In this case, we would classify the ibuprofen exposure as neutral and the aspirin exposure as favorable. We considered a patient's listed medications in their report or subject log to be a comprehensive list of all potential antimigraine medications to which they were exposed.
Statistical analysis
Continuous variables were summarized with median and range, whereas categorical variables were summarized with frequency and percent. The number of medication exposures was defined as the number of unique patients who were reported to have taken a certain medication. Patient outcomes were reassigned numerical values where unfavorable responses were counted as 0, neutral responses were counted as 1, and favorable responses were counted as 2 to maintain hierarchical order. The Mann-Whitney U test was then used to compare the numerical distributions of patient responses across medication categories. For each response category, we sorted and ranked the responses of all exposures to a specific medication category and then compared the response distribution with the ranked responses of all other medications, excluding the exposures to the current category. The difference in mean ranks between the “yes” and “no” groups allowed us to determine the strength of observed differences in responses. Because of the exploratory nature of this study, recorded patient responses to individual treatments were considered to satisfy the assumption of independence, and adjustment for multiple comparisons was not implemented. All tests were 2 sided, and p values <0.05 were statistically significant. All statistical analyses were performed in R Statistical Software (version 3.4.2; R Foundation for Statistical Computing, Vienna, Austria).
Data availability
Anonymized data not published within this article will be made available by request from any qualified investigator.
Results
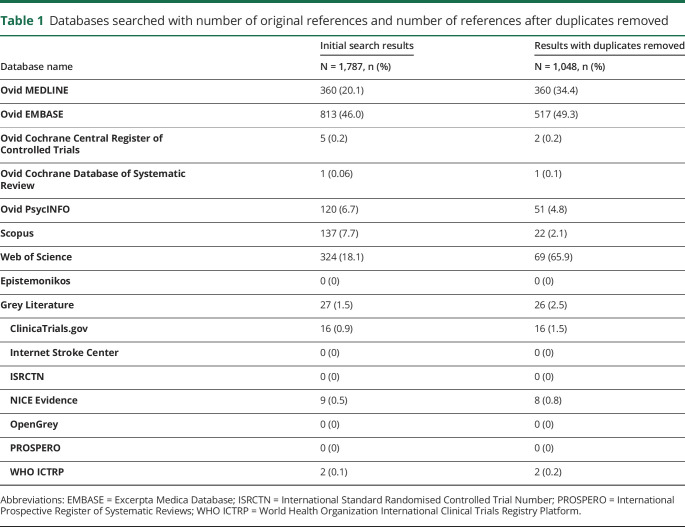
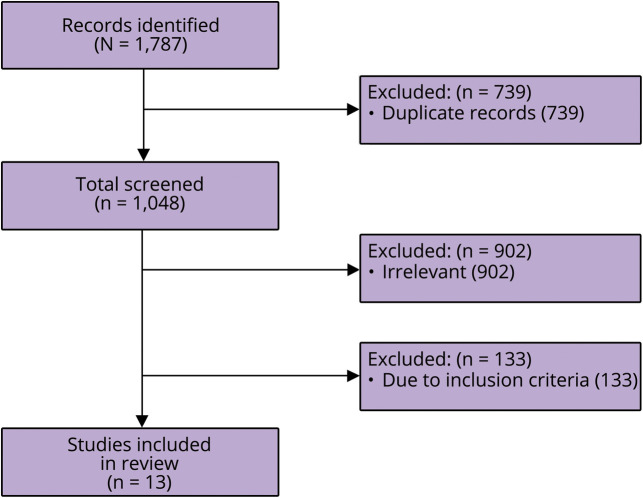
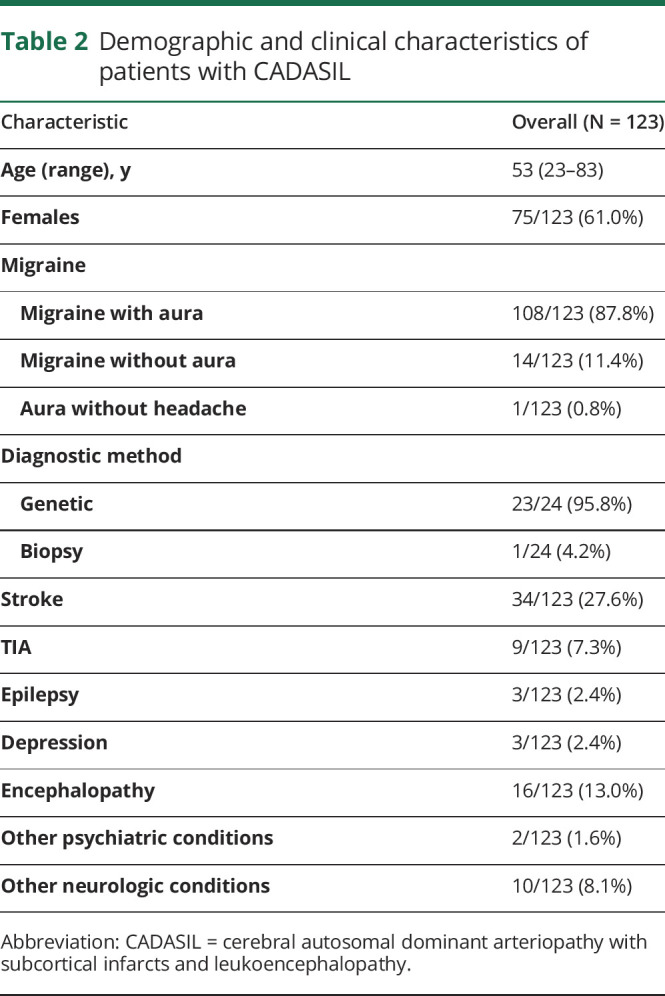
A total of 1,787 studies were identified across 8 databases, and 7 grey literature resources, of which 739 were interdatabase duplicates, table 1. After evaluation of relevance, bias, quality, and adherence to inclusion criteria, 13 studies were included in the final analysis, figure. From the 13 articles, patient characteristics and clinical information on the 123 included individuals are summarized in table 2. The population had a median age of 53 years (range, 23–83 years), with 61.0% (75/123) being women. Reports for 19.5% (24/123) of individuals in this review specified the precise means with which the CADASIL was diagnosed for each individual. It should be noted that the largest CADASIL cohort captured in this review reported that 98.7% (296/300) of their subjects were diagnosed genetically, although individual patient-level data were not reported.11 A total of 87.8% (108/123) subjects had a diagnosis of migraine with aura. Roughly 11.4% of patients (14/123) experienced migraine without aura, and a singular patient experienced aura without headache. In addition, we observed somewhat more favorable responses in older population (median age 55 years [26–83], p = 0.062), although this difference was not statistically significant. There were no sex differences across response levels. Further migraine characteristics including aura semiology, frequency, duration, and age at onset were not consistently reported. Although baseline cognitive and functional characteristics were not consistently reported, 27.6% (34/123) reported history of “stroke” and 13.0% (16/123) reported history of “encephalopathy.” A summary table of articles captured, number of patients included, and medications described are available in table e-3, links.lww.com/CPJ/A147.
Table 1.
Databases searched with number of original references and number of references after duplicates removed
Figure. PRISMA flow diagram showing how the 13 studies included in the present review were identified from the initial search.
Table 2.
Demographic and clinical characteristics of patients with CADASIL
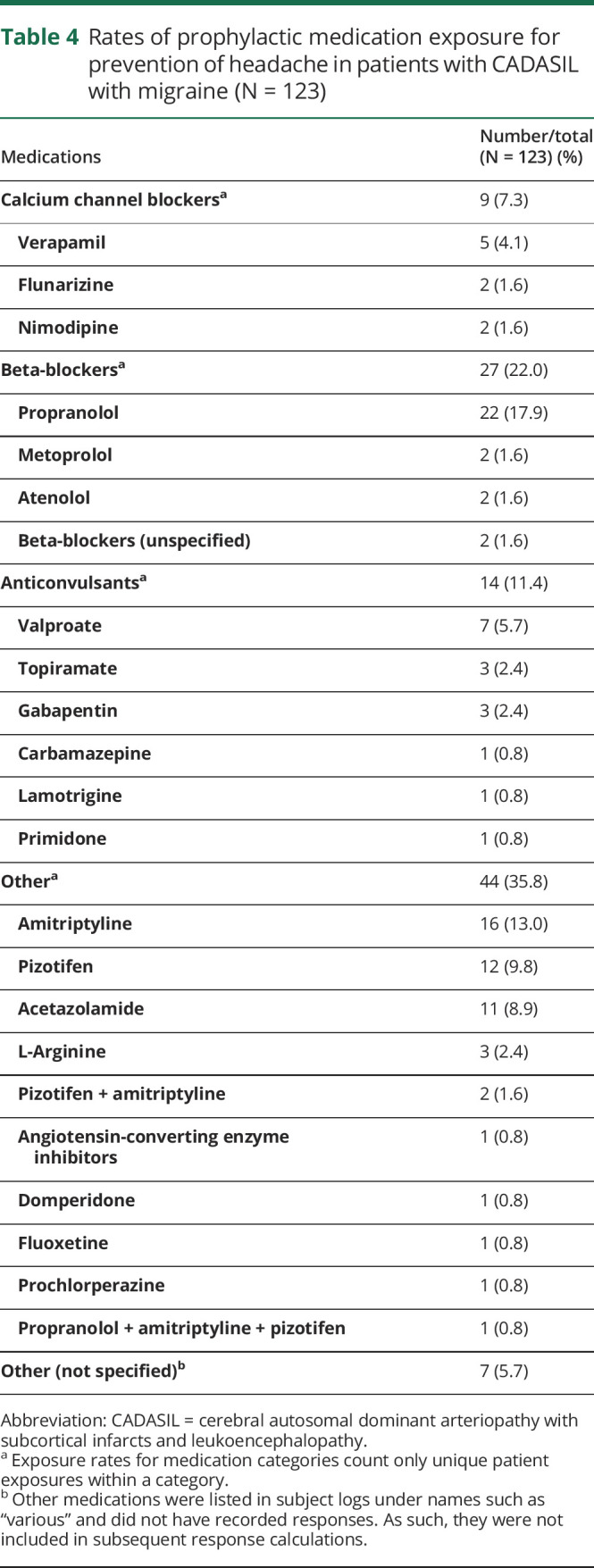
Rates of medication exposure are summarized in tables 3 and 4. Overall, simple analgesics were used most commonly for acute migraine therapy (35.7%, 44/123), whereas beta-blockers were the largest prophylactic medication class (22.0%, 27/123). The combination medication paracetamol with codeine (22.0%, 27/123), sumatriptan (15.4%, 19/123), and aspirin (13.8%, 17/123) were the most frequently used migraine-specific medications. Propranolol (17.9%, 22/123), amitriptyline (13.0%, 16/123), pizotifen (9.8%, 12/123), and acetazolamide (8.9%, 11/123) were commonly used for prophylaxis. Of the 123 patients, 45.5% (56/123) reported antimigraine medication monotherapy. The remainder reported multiple medication exposures, either sequentially or concomitantly, where 37.4% (46/123) had 2 treatments, 8.1% (10/123) had 3 treatments, and 6.5% (8/123) had 4 treatments, and 2.4% (3/123) had undergone 5 unique treatments. There were no reports of interventional approaches such as onobotulinumtoxin A injections or use of antimigraine wearable devices. Use of calcitonin gene-related peptide (CGRP) monoclonal antibody modulators was not reported. In addition, pharmacotherapy dosage, frequency, and route of administration were infrequently reported.
Table 3.
Rates of abortive medication exposure for acute treatment of headache in patients with CADASIL with migraine (N = 123)
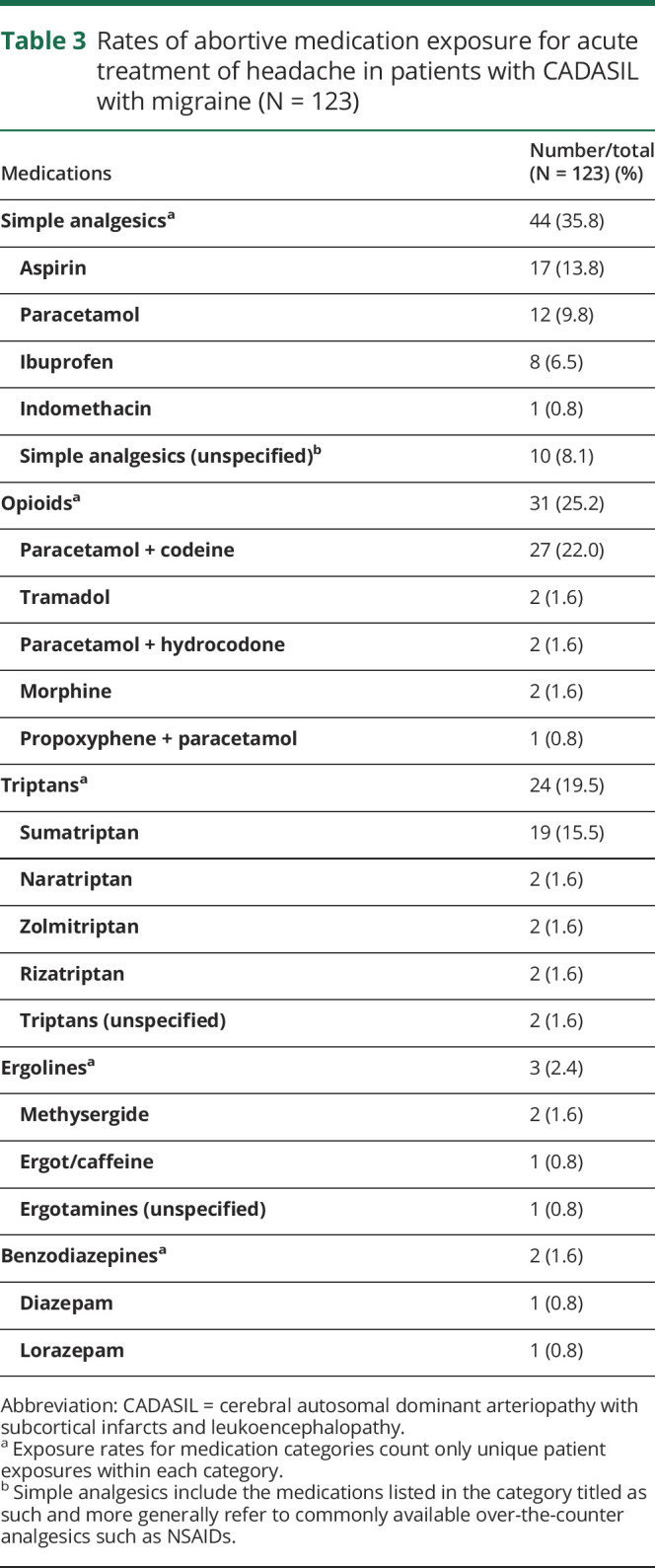
Table 4.
Rates of prophylactic medication exposure for prevention of headache in patients with CADASIL with migraine (N = 123)
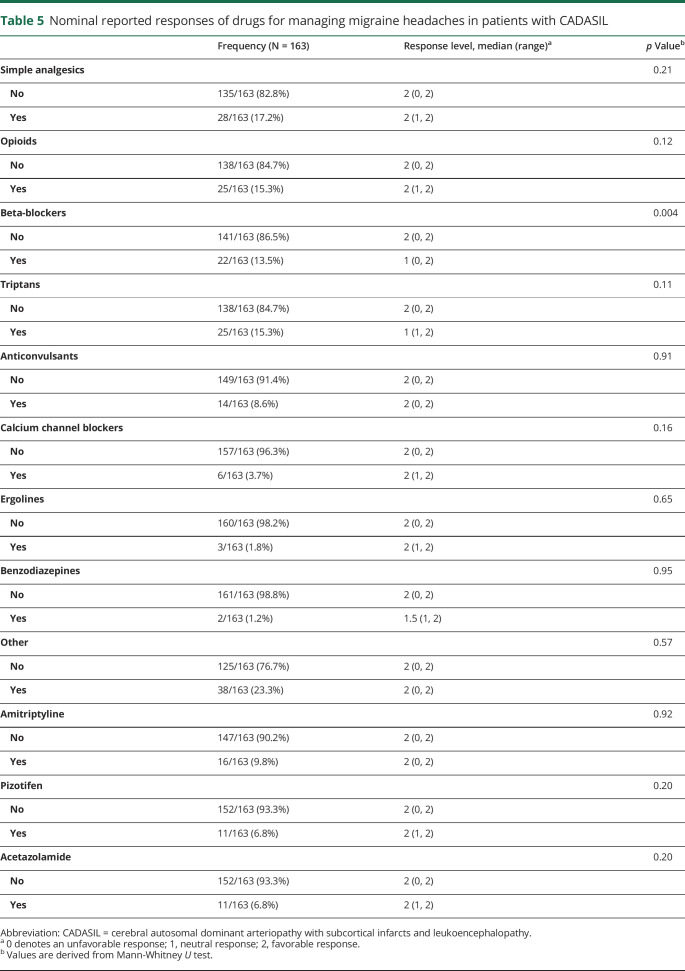
The distribution of patient responses across medication categories is summarized in table 5. A total of 225 exposures were documented in our 123 patients, but ambiguous or incomplete data were found in 27.6% (62/225) of medication exposures. The remaining 163 reported responses to individual drug exposures were used to calculate response rates. Patients who received beta-blockers had significantly less favorable outcomes compared with patients who did not (p = 0.004). Four of these 5 unfavorable responses were in response to propranolol, with the fifth being an unspecified beta-blocker. Among these unfavorable responses, 3/5 (60%) were due to worsened fatigue, 1/5 (20%) was due to intolerable nightmares, and the remaining 1/5 (20%) was unspecified. There were no reported unfavorable responses to either atenolol or metoprolol.
Table 5.
Nominal reported responses of drugs for managing migraine headaches in patients with CADASIL
Those treated with simple analgesics, opioids, calcium channel blockers, ergolines, benzodiazepines, and other selected medications such as pizotifen and acetazolamide reported no unfavorable responses. These medications also did not have a significantly different response distribution when compared individually with the responses to other medication exposures.
Discussion
Through our meta-analysis and systematic review, we identified 123 cases of adult individuals with CADASIL and coexistent migraine. The analysis revealed a cohort of primarily middle-aged adults with a majority being women. Migraine with aura was reported in the vast majority of individuals, although further characteristics of the aura are unknown. Nearly 1 in 10 individuals reported “encephalopathy” as a coexistent feature; however, it is unknown whether this represented an underlying cognitive impairment, encephalopathic migraine aura, or a milder presentation of the so-called CADASIL coma. 12 In addition, advancing age seemed to be associated with favorable responses to antimigraine medications. Although speculative, this finding may be related to the natural disease course of CADASIL or migraine in general, both of which typically improve with advancing age.
We identified considerable variation within migraine abortive and prophylactic techniques. More than half of the cohort reported being treated either concurrently or sequentially with 2 or more antimigraine medications, reflecting the difficulty in treating migraines in this population. Simple analgesics such as ibuprofen, aspirin, and acetaminophen were among the most commonly used abortive medications. Patients treated with acetazolamide reported no unfavorable responses, although the response distribution was not significantly different than all other medications.
Beta-blockers are commonly considered to be an effective strategy for migraine headaches in the general migraineur population.13,14 However, we found that beta-blockers may be detrimental in the management of migraine in patients with CADASIL. Beta-blockers showed a disproportionately high rate of unfavorable responses, accounting for 5 of the 12 total unfavorable responses. Cardioselective beta-blockers may be better tolerated than nonselective beta-blockers in this population. The aforementioned unfavorable responses included fatigue (60%, 3/5) and nightmares (20%, 1/5). Nightmares are a known side effect of beta-blockers, but it is unclear whether patients with CADASIL have an increased susceptibility to these effects.15,16
We noted that agents that have prominent and direct influence on vasoreactivity through receptor-mediated action typically resulted in a neutral response rate. Given the underlying vascular smooth muscle cell (VSMC) degeneration in CADASIL, one may question whether the vasodilatory action of these medications is dampened. In addition, given the influence of VSMCs on angiogenesis and endothelial maturation, aberrant functioning of VSMCs may lead to an immature endothelium that is less responsive to circulating receptor-mediated factors.17
Uniquely, acetazolamide, a vasodilator anecdotally reported to yield favorable responses in CADASIL, yielded no unfavorable responses in our cohort.18 Statistical analysis described above showed that the overall response to acetazolamide was not significantly different than the average response distribution of the total exposures. Unfortunately, no controlled studies exist that would allow for a more rigorous evaluation of efficacy. Acetazolamide itself possesses a mechanism of action that is not entirely understood.18,19 It is known that those with CADASIL have a significant reduction in the function of myosin light-chain kinase (MLCK), which serves as the primary mediator of VSMC contraction through modulation by the NOTCH receptor signaling cascade.20 Recent evidence suggests that acetazolamide leads to increased phosphorylation of MLCK, increasing its inherent activity leading to vasodilation.21 We postulate that if acetazolamide is truly effective in CADASIL migraine, it may be bypassing the defective NOTCH signaling cascade and immature vascular endothelium to produce vasodilation through modulation of an intrinsically preserved MLCK.
As with acetazolamide, sodium valproate is of particular interest, given its pleiotropic effects and purported cytoprotective effect on VSMCs. Valproate is theorized to facilitate headache relief in patients with CADASIL through indirect modulation of the interaction between NOTCH3 and the cellular Fas-associated death domain–like interleukin-1 beta-converting enzyme–inhibitory protein pathway, which is a regulatory pathway for VSMC survival.22 Case reports captured in this meta-analysis describe sodium valproate rapidly improving status migrainosus in 1 patient and fully abolishing migraines after receiving the drug for treatment of a hypomanic episode in another.6,23 Controlled trials of valproate in this population may be warranted.
Our meta-analysis may assist clinicians facing the therapeutic complexity of migraines associated with CADASIL. We found minimal to no ADRs associated with antimigraine medications, both for abortive and prophylactic indications, regardless of therapeutic mechanism of action. However, our findings suggest that beta-blockers should not be used for management of migraine in CADASIL because of the significantly increased risk of unfavorable responses. There are no guideline-based treatment strategies for managing migraine in this population, and general migraine treatment guidelines may be misleading. Future studies should aim to provide objective comparative data using standardized and validated clinical metrics such as the Migraine Disability Assessment Score and Headache Impact Test-6.24,25 In addition, data regarding the safety and efficacy of CGRP-modulatory monoclonal antibodies and onabotulinumtoxin A are lacking.
The conclusions of our systematic review are limited by the possibility of publication and selection bias and the subjective nature of outcomes. Beyond demographic information and medications used, there was inconsistent information regarding migraine characteristics or dosage regimens. Despite finding statistically significant differences among responses and medication classes, randomized controlled trials would be needed to definitively establish the effects of medications on migraines in patients with CADASIL.
Migraine with aura is a common, often refractory, feature of CADASIL with no evidence-based guidelines for optimal treatment. Our registered systematic review and meta-analysis suggests that beta-blockers should be avoided for migraine prophylaxis in CADASIL, as it seems to demonstrate worse outcomes compared with other common therapies. No medication included in this review consistently treated or prevented migraines. Further studies are warranted to investigate the safety and efficacy of presently available acute and prophylactic migraine therapies.
Acknowledgment
The authors thank Dr. Rhea Y.Y. Tan for her kindness and timely response to our request for supplementary information. They also thank Dr. Vazquez Do Campo for providing a full text copy of her case series.
Appendix. Authors
Study funding
J.F. Meschia receives funding for the CREST-2 Trial from the NINDS U01 NS080168 and from the Mayo Clinic through the Earl & Nyda Swanson Neurosciences Research Fund & the Harley N. and Rebecca N. Hotchkiss Endowed Fund in Neuroscience Research, Honoring Ken and Marietta.
Disclosure
The authors report no disclosures relevant to the manuscript. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
TAKE-HOME POINTS
→ There is considerable variability in the therapeutic approaches to migraine in CADASIL, with no clear standard of care.
→ No adverse responses were reported in patients using triptans, despite prevailing opinions against the use of vasoactive medications in patients with CADASIL.
→ Beta-blockers seem to be a poor choice for controlling migraine in this patient population.
References
- 1.Chabriat H, Joutel A, Dichgans M, Tournier-Lasserve E, Bousser MG. CADASIL. Lancet Neurol 2009;8:643–653. [DOI] [PubMed] [Google Scholar]
- 2.Guey S, Mawet J, Hervé D, et al. Prevalence and characteristics of migraine in CADASIL. Cephalalgia 2015;36:1038–1047. [DOI] [PubMed] [Google Scholar]
- 3.Liem MK, Oberstein SAJL, van der Grond J, Ferrari MD, Haan J. CADASIL and migraine: a narrative review. Cephalalgia 2010;30:1284–1289. [DOI] [PubMed] [Google Scholar]
- 4.Dash S, Bogdanova O, Xavier A, Farkas J, Pullicino P. Cerebral vasospasm from sumatriptan. Neurology 2004;63:2128. [DOI] [PubMed] [Google Scholar]
- 5.Mueller L, Gallagher RM, Ciervo CA. Vasospasm-lnduced myocardial infarction with sumatriptan. Headache 1996;36:329–331. [DOI] [PubMed] [Google Scholar]
- 6.Martikainen MH, Roine S. Rapid improvement of a complex migrainous episode with sodium valproate in a patient with CADASIL. J Headache Pain 2012;13:95–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moher D, Liberati A, Tetzlaff J, Altman DG, The PG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLOS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schöpfel J. Towards a Prague definition of grey literature. Paper presented at the Twelfth International Conference on Grey Literature: Transparency in Grey Literature. Grey Tech Approaches to High Tech Issues. Prague; December 6–7, 2010; Czech Republic.
- 9.Sterne JAC, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evidence Based Med 2018;23:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan RYY, Markus HS. CADASIL: migraine, encephalopathy, stroke and their inter-relationships. PLoS One 2016;11:e0157613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eswaradass VP, Ramasamy B, Kalidoss R, Gnanagurusamy G. Cadasil coma: unusual cause for acute encephalopathy. Ann Indian Acad Neurol 2015;18:483–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology 2012;78:1337–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jackson JL, Kuriyama A, Kuwatsuka Y, et al. Beta-blockers for the prevention of headache in adults, a systematic review and meta-analysis. PLoS One 2019;14:e0212785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldner JA. Metoprolol-induced visual hallucinations: a case series. J Med Case Rep 2012;6:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson DF, Pierce DR. Drug-induced nightmares. Ann Pharmacother 1999;33:93–98. [DOI] [PubMed] [Google Scholar]
- 17.Yang K, Proweller A. Vascular smooth muscle notch signals regulate endothelial cell sensitivity to angiogenic stimulation. J Biol Chem 2011;286:13741–13753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forteza AM, Brozman B, Rabinstein AA, Romano JG, Bradley WG. Acetazolamide for the treatment of migraine with aura in CADASIL. Neurology 2001;57:2144–2145. [DOI] [PubMed] [Google Scholar]
- 19.Huang L, Yang Q, Zhang L, Chen X, Huang Q, Wang H. Acetazolamide improves cerebral hemodynamics in CADASIL. J Neurol Sci 2010;292:77–80. [DOI] [PubMed] [Google Scholar]
- 20.Basu S, Srinivasan DK, Yang K, et al. Notch transcriptional control of vascular smooth muscle regulatory gene expression and function. J Biol Chem 2013;288:11191–11202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bertone NI, Groisman AI, Mazzone GL, Cano R, Tabares L, Uchitel OD. Carbonic anhydrase inhibitor acetazolamide shifts synaptic vesicle recycling to a fast mode at the mouse neuromuscular junction. Synapse 2017;71:e22009. [DOI] [PubMed] [Google Scholar]
- 22.Yuan P, Salvadore G, Li X, et al. Valproate activates the Notch3/c-FLIP signaling cascade: a strategy to attenuate white matter hyperintensities in bipolar disorder in late life? Bipolar Disord 2009;11:256–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J, Li J, Kong F, Lv H, Guo Z. Bipolar II disorder as the initial presentation of CADASIL: an underdiagnosed manifestation. Neuropsychiatr Dis Treat 2017;13:2175–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stewart WF, Lipton RB, Kolodner K. Migraine disability assessment (MIDAS) score: relation to headache frequency, pain intensity, and headache symptoms. Headache 2003;43:258–265. [DOI] [PubMed] [Google Scholar]
- 25.Shin HE, Park JW, Kim YI, Lee KS. Headache impact test-6 (HIT-6) scores for migraine patients: their relation to disability as measured from a headache diary. J Clin Neurol 2008;4:158–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigator.