Abstract
Paramutations represent directed and meiotically-heritable changes in gene regulation leading to apparent violations of Mendelian inheritance. Although the mechanism and evolutionary importance of paramutation behaviors remain largely unknown, genetic screens in maize (Zea mays) identify five components affecting 24 nucleotide RNA biogenesis as required to maintain repression of a paramutant purple plant1 (pl1) allele. Currently, the RNA polymerase IV largest subunit represents the only component also specifying proper development. Here we identify a chromodomain helicase DNA-binding 3 (CHD3) protein orthologous to Arabidopsis (Arabidopsis thaliana) PICKLE as another component maintaining both pl1 paramutation and normal somatic development but without affecting overall small RNA biogenesis. In addition, genetic tests show this protein contributes to proper male gametophyte function. The similar mutant phenotypes documented in Arabidopsis and maize implicate some evolutionarily-conserved gene regulation while developmental defects associated with the two paramutation mutants are largely distinct. Our results show that a CHD3 protein responsible for normal plant ontogeny and sperm transmission also helps maintain meiotically-heritable epigenetic regulatory variation for specific alleles. This finding implicates an intersection of RNA polymerase IV function and nucleosome positioning in the paramutation process.
Author summary
Genes are switched “on” and “off” during normal development by regulating DNA accessibility within the chromosomes. How certain gene variants permanently maintain “off” states from one generation to the next remains unclear, but studies in multiple eukaryotes implicate roles for specific types of small RNAs, some of which define cytosine methylation patterns. In corn, these RNAs come from at least two RNA polymerase II-derived complexes sharing a common catalytic subunit (RPD1). Although RPD1 both controls the normal developmental switching of many genes and permanently maintains some of these “off” states across generations, how RPD1 function defines heritable DNA accessibility is unknown. We discovered that a protein (CHD3a) belonging to a group known to alter nucleosome positioning is also required to help maintain a heritable “off” state for one particular corn gene variant controlling both plant and flower color. We also found CHD3a necessary for normal plant development and sperm transmission consistent with the idea that proper nucleosome positioning defines evolutionarily-important gene expression patterns. Because both CHD3a and RPD1 maintain the heritable “off” state of a specific gene variant, their functions appear to be mechanistically linked.
Introduction
Organismal development requires alleles to undergo controlled transitions between silent and expressed states coordinated by transcription factors, non-coding RNAs, and chromatin regulators interacting with allele-specific regulatory sequences. In many eukaryotes, small non-coding RNAs (sRNAs) such as small interfering RNAs (siRNAs) (reviewed in [1]), microRNAs (miRNAs) (reviewed in [2]), and PIWI-interacting RNAs (piRNAs) (reviewed in [3])—in complex with argonaute (AGO) proteins—can modulate chromatin structure, RNA stability, or translation to achieve developmental transitions and/or defend against foreign nucleic acids and transposable element (TE) activities (reviewed in [1]). sRNAs are typically processed from DNA-dependent RNA polymerase (RNAP) II transcripts, but in multicellular plants, additional RNAP II-related complexes [4,5] having specialized functions in transcriptional regulation [6–9] generate the majority of siRNA precursors.
RNAP II-related RNAPs IV and V collaborate to maintain repressive chromatin states through the action of RNAP IV-derived siRNAs that primarily target TEs and other repetitive sequences for de novo cytosine methylation and subsequent histone modifications via a process termed RNA-directed DNA Methylation (RdDM) [10]. Although Arabidopsis (Arabidopsis thaliana) plants lacking RNAP IV and/or V develop normally [11], misexpression of specific alleles in the absence of the maize (Zea mays) RNAP IV largest subunit (RPD1) leads to abnormal development [7,8,12–14]. Thus, in maize and likely other grasses, RNAP IV has been co-opted to define some developmental expression patterns. Unlike the eudicots typified by Arabidopsis, grasses have diversified subtypes for RNAP IV, V, and outside of maize, RNAP VI [15] defined by alternative second largest catalytic subunits [5]. The evolutionary importance of such diversity and regulatory novelty remains completely unknown.
Maize RNAP IV subtypes also establish and/or maintain meiotically heritable expression patterns of certain alleles [13,16–19] for which changes in gene regulation occur in response to trans-homolog interactions by a process known as paramutation [20–23]. Such alleles are well described at booster1 (b1) [24–27] and red1 (r1) [20,28,29]—loci encoding basic helix-loop-helix (bHLH) proteins—and at both the pericarp color1 (p1) [30–32] and purple plant1 (pl1) [33] loci encoding R2R3 Myb-type proteins. Expression patterns of these alleles can be directly visualized by pigmentation [34] because the encoded proteins are transcriptional activators of genes encoding flavonoid biosynthetic enzymes. Similar to developmental phase changes [35], certain alleles of these loci can switch from transcriptionally active to repressed states [36–38].
Characteristic of paramutation, the repressed state of a given allele appears dominant to an active one, and typically only repressed states are sexually transmitted from such heterozygotes [22,23]. Hence, active states heritably change in response to being heterozygous with a homologous allele in a repressed state. Mutation screens identify loci that function as mediators of paramutation (mop) of the B1-Intense (B1-I) allele [18,39] and factors required to maintain repression (rmr) of the Pl1-Rhoades allele [40,41]. All five known MOP and RMR proteins are either RNAP IV subunits [13,17–19] or accessory proteins [41–43] required for 24 nucleotide (24nt) RNA biogenesis [6,13,17,18,43–45].
The involvement of small RNA biogenesis components in facilitating and/or maintaining paramutations implicates a model in which regulatory landscapes are transferred between homologous alleles with differing epigenetic states via 24nt RNAs shared in trans [22,23,46]. Curiously, no potential components of an RdDM-type pathway downstream of 24nt RNAs have yet been identified in the mop and rmr screens. Two RNAP IV catalytic subunits encoded by rmr6 / mop3 / rpd1 [13,19] and rmr7 / mop2 / rpd2a [17,18] are orthologs of Arabidopsis NUCLEAR RNA POLYMERASE D1 (NRPD1) and NRPD2, respectively. Additionally, mop1 encodes a likely RNA-dependent RNA polymerase (RDR) orthologous to Arabidopsis RDR2 [42], and rmr1 encodes an SNF2-type ATP-dependent helicase similar to Arabidopsis CLASSY 3 and 4 [41]. The novel RMR2 protein is also required for full 24nt RNA biogenesis [43] but functions of any Arabidopsis orthologs remain uncharacterized. Outside of stochastic defects reported for some mop1 mutants [39], only loss of RPD1 persistently impacts plant development [12,13], indicating that RNAP IV has a role in developmental gene control independent of 24nt RNAs and any RdDM-type mechanism.
Paramutation-like behaviors in several non-plant species (reviewed in [23,47]) involve diverse sRNA-dependent mechanisms. In maize, a model that 24nt RNAs facilitate paramutations does not account for the observations that RMR1, RMR2, and RPD2a are not required to establish paramutations at Pl1-Rhoades [17,41,43]. These data indicate that although 24nt RNAs are implicated in maintaining Pl1-Rhoades repression, they may not be paramutation instigators, and the role of RNAP IV in facilitating paramutations may be independent of 24nt RNA biogenesis [6,8].
Here we describe a novel rmr locus (rmr12) where loss of function broadly affects plant development in ways mostly distinct from that of rpd1 mutants. Mutations, genetic mapping, and sequence information show rmr12 corresponds to a gene encoding a chromodomain helicase DNA-binding 3 (CHD3) protein most orthologous to Arabidopsis PICKLE (PKL). Genetic experiments show this CHD3 protein operates both somatically and in male gametophytes to ensure proper development and gamete transmission respectively. Small RNA profiling shows that RMR12 is not a component of 24nt RNA biogenesis yet genetic tests show that it specifically maintains Pl1-Rhoades repression and contributes to fidelity of the heritable feature(s) underlying its paramutagenic properties. Hence a likely nucleosome remodeler is responsible for specifying both mitotically- and meiotically-heritable epigenetic information.
Results
Mutations define the rmr12 locus
Because repressed Pl1-Rhoades states invariably condition weak pigmentation, mutations disrupting functions required to maintain this repression are easily identified by increased anthocyanin production [40]. Two distinct rmr screens using ethyl methanesulfonate (ems)-treated pollen [40,41] identified four mutations that also conferred similar developmental defects. Mutations ems98738 and ems98924 conditioned dark seedling pigmentation [40] while ems063095 and ems143190 were found with strongly pigmented anthers [41]. In all M2 and F2 progenies, dark seedling pigmentation exclusively cosegregated with narrow leaves and acute leaf angles (Fig 1). At maturity, all mutants had a dwarf stature, delayed flowering, and upright, narrow, adaxially-curled leaves (Fig 1B) having a wrinkled epidermis (Fig 1C, see S1 Fig). Inflorescences were either absent, barren, or small, with tassels having fewer secondary spikes and florets that rarely extruded anthers (Fig 1D). Grain set was rare with cobs carrying few and heterogeneous sized kernels set in disorganized rows (Fig 1E). In two F2 progenies segregating ems063095, all plants having fully-pigmented anthers diagnostic of the phenotype conferred by Pl1-Rhoades in a derepressed state (Pl-Rh) had the same developmental defects including later flowering and reduced height compared to their normal siblings displaying anther color phenotypes conditioned by a repressed Pl1-Rhoades state (Pl´) (Table 1). In these and all other examples, the function identified by these four mutations specified both proper plant development and apparent Pl1-Rhoades repression.
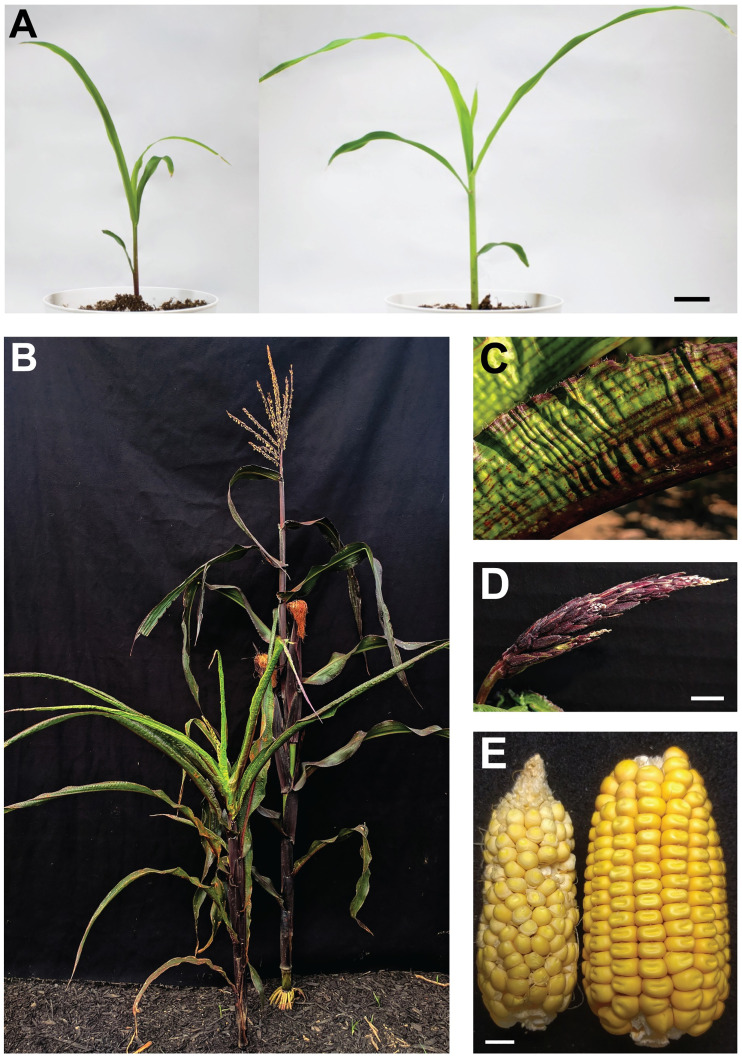
Fig 1. Mutant phenotypes.
(A) Comparison of ems063095 mutant (left) and non-mutant sibling (right) seedlings. Bar = 2cm. (B) Comparison of adult ems063095 mutant (left) and flowering non-mutant sibling (right). (C) Abaxial surface of adult ems063095 mutant leaf blade. (D) ems063095 mutant tassel at flowering. Bar = 1cm. (E) Comparison of grain set on ems98738 mutant (left) and A619 (right) generated from reciprocal crosses. Bar = 1cm.
Table 1. Characters of ems063095 F2 individuals having Pl´ and Pl-Rh anther types.
| Pl´ | Pl-Rh | |||||
|---|---|---|---|---|---|---|
| Progeny ID | DTF | Plant height | n | DTF | Plant height | n |
| 091133 | 67 ±0.6 | 184.0 ±1.7 | 92 | 90 ±1.5 | 102.5 ±2.7 | 13 |
| 091061 | 65 ±0.5 | 212.5 ±1.7 | 72 | 92 ±2.6 | 127.8 ±6.2 | 6 |
| Total | 66 ±0.4 | 196.5 ±1.6 | 164 | 90 ±1.3 | 110.5 ±3.8 | 19 |
Mean ±s.e.m.; DTF: Days to flowering
Genetic complementation test results showed all four mutations define a novel rmr locus (see S1 and S2 Tables) hereafter designated rmr12 with ems98738, ems98924, ems063095 and ems143190 mutations respectively renamed rmr12-1, -2, -3, and -4. For all four alleles, the mean frequency of mutant types was less than expected from single-locus recessive mutations (Tables 2 and 3, see S1 Methods, S3 Table). For instance, the frequency of rmr12-1 and rmr12-2 darkly-pigmented M2 seedlings was 0.10 (χ2 = 9.0, p = 0.0027) and 0.13 (χ2 = 8.6, p = 0.0033) respectively (Table 2), and the combined mutant frequency in 36 F2 progenies segregating rmr12-1, rmr12-2, or rmr12-3 was 0.18 (Table 3, see S3 Table). These observations indicate the mutations are fully recessive. Comparing the observed and expected mutant frequencies defined by a single-locus (0.25) versus a two-locus model (0.0625) generated highly significant χ2 values of 61.02 and 493.08, respectively (Table 3). Although these results support neither model, the most parsimonious interpretations are that either transmission of single-locus recessive mutant alleles is impaired or that the mutant phenotype is incompletely penetrant.
Table 2. M2 mutant seedling segregation.
| Allele | Progeny ID | No. dark seedlings | Total seedlings | Frequency | p value (χ2) |
|---|---|---|---|---|---|
| rmr12-1 | 98738 | 10 | 100 | 0.10 | 0.0027 |
| rmr12-2 | 98924 | 18 | 142 | 0.13 | 0.0033 |
Table 3. F2 rmr12 mutant frequencies.
| Progeny | No. individuals | Statistics for single locus model (0.25) | Statistics for two locus model (0.0625) | |||||
|---|---|---|---|---|---|---|---|---|
| No. | Allele | Mutant | Non-mutant | Freq. | Mutant χ2 | p value | Mutant χ2 | p value |
| 18 | rmr12-1 | 111 | 642 | 0.15 | 31.70 | 9.3e-9 | 86.86 | 5.9e-21 |
| 2 | rmr12-2 | 25 | 171 | 0.13 | 11.76 | 3.3e-4 | 13.27 | 1.4e-4 |
| 15 | rmr12-3 | 303 | 1292 | 0.19 | 22.99 | 8.5e7 | 414.66 | 1.8e-92 |
| Total | 439 | 2105 | 0.18 | 61.02 | 2.9e-15 | 493.08 | 1.5e-109 | |
Biased allele transmission is due to male gametophyte dysfunction
Because heterozygotes for rmr12 mutant alleles bear cobs with near full grain set (see S2 Fig) we inferred that mutant female gametophytes were fully functional, and therefore surmised that mutant male gametophytes were compromised. To address this idea, we reciprocally crossed rmr12-1 / rmr12-1 mutants to or by rmr12-3 / Rmr12 heterozygotes looking for paternal transmission bias. Half the offspring had developmental defects (Fig 1) when rmr12-3 / Rmr12 heterozygotes were used as females (0.49, χ2 = 0.10, p = 0.747), indicating that mutant sporophyte germination and survival are not impaired and that the mutant phenotype is fully penetrant (Table 4). In contrast, fewer mutants were observed when rmr12-3 / Rmr12 heterozygotes were used as males (0.39, χ2 = 5.28, p = 0.021) (Table 4). Because all four independent rmr12 mutant alleles show similar transmission ratio distortions (Tables 2 and 3, see S2 and S3 Tables), we hypothesized that Rmr12 is important for normal male gametophyte function.
Table 4. Mutant frequencies in progeny from reciprocal crosses.
| Parents | Progeny | |||||
|---|---|---|---|---|---|---|
| Female | Male | ID | Mutants | Non-mutants | Freq. | p value (χ2) |
| rmr12-3 / Rmr12 | rmr12-1 / rmr12-1 | 142783 | 84 | 90 | 0.483 | 0.75 |
| 142785 | 66 | 68 | 0.493 | 0.90 | ||
| rmr12-1 / rmr12-1 | rmr12-3 / Rmr12 | 142763 | 60 | 86 | 0.410 | 0.13 |
| 142850 | 21 | 42 | 0.333 | 0.06 | ||
| rmr12-3 / Rmr12 | rmr12-1 / rmr12-1 | Totals | 150 | 158 | 0.487 | 0.75 |
| rmr12-1 / rmr12-1 | rmr12-3 / Rmr12 | 81 | 128 | 0.388 | 0.02 | |
Because we observed genetic linkage of rmr12 to a mutant waxy1 (wx1) allele (see S1 Methods and S4 Table), we could test this hypothesis using 9S cell autonomous markers to monitor rmr12 allele transmissions. Because wx1 mutant pollen accumulate amylopectins that stain red with I2-KI [48] rather than amylose which stains blue, we could approximate the frequency of rmr12 alleles segregated from heterozygous plants. Fresh pollen collected from two Rmr12 wx1 / rmr12-3 Wx1 individuals were of two types (1392 blue and 1350 red) whose frequencies did not deviate from the expected 0.50 (χ2 = 0.37, p = 0.57) (Table 5). The frequency of viable pollen as assessed with fluorescein diacetate [49] was also similar between Rmr12 / rmr12-4 and Rmr12 / Rmr12 individuals (0.97 in both, p = 0.94, two-sample t-test, see S3A Fig). We then compared in vitro pollen germination frequencies from eight Rmr12 wx1 / rmr12-3 Wx1 florets. While frequencies varied from 0.3 to 0.6, the ratio of Wx1 to wx1 germinated pollen from each floret did not significantly differ from 1 (p = 0.64, one-sample t-test; see S3B Fig). These data indicate that the rmr12 mutations transmission biases are not due to meiotic errors, grain filling defects or failed pollen germination.
Table 5. Frequency of I2-KI stained pollen types from Rmr12 wx1 / rmr12-3 Wx1 individuals.
| Progeny ID | Individual | Blue pollen | Red pollen | Red frequency | p value (χ2) |
|---|---|---|---|---|---|
| 160372 | 17-62-5 | 945 | 873 | 0.48 | 0.23 |
| 160372 | 17-62-8 | 477 | 447 | 0.52 | 0.49 |
| Total | 1392 | 1350 | 0.51 | 0.57 |
We next evaluated paternal rmr12 allele transmissions via their linkage to colored aleurone1 (c1), a locus required for kernel pigmentation [50,51] located approximately 30 cM from wx1 [52,53]. We crossed Rmr12 c1 / rmr12-4 C1 males to recessive c1 testers and recorded both the frequency and distribution of colored kernels on each testcross cob. Because the mean frequency of colored kernels from all cobs (0.43) was significantly less than the expected 0.50 (p = 6.34e-07, one-sample t-test) (Table 6), we concluded that C1 transmission reflects that of rmr12-4. Furthermore, because there was no indication of aborted ovules (see S2B Fig), the transmission bias appeared to occur prior to fertilization. To test whether the bias was possibly due to differential pollen tube growth, we compared C1 transmission in the apical half of the cob to that in the basal half (Table 6) where pollen tubes would be longer. Colored kernel mean frequencies, 0.44 (apical) vs 0.42 (basal), did not differ (p = 0.22, two-sample t-test) indicating that the allele transmission bias is not due to obvious pollen tube growth competitions. Pollen tube lengths of in vitro germinated pollen from 8 Rmr12 wx1 / rmr12-4 Wx1 florets were also no different between the two stained types (p = 0.27, two-sample t-test; see S3C Fig). From these results, we conclude that rmr12 mutant pollen grains are not compromised in viability, germination, or pollen tube growth but have an unknown and incompletely penetrant male gametophyte defect.
Table 6. Cob position of testcrosses kernel phenotypes representing transmission of rmr12-4 linked c1 alleles.
| Basal half | Apical half | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Progeny ID | C1 | c1 | Freq. | C1 | c1 | Freq. | C1 | c1 | Freq. | p value (χ2) |
| 170107 | 73 | 90 | 0.45 | 68 | 99 | 0.41 | 141 | 189 | 0.42 | 0.06 |
| 170118 | 67 | 73 | 0.48 | 66 | 84 | 0.44 | 133 | 157 | 0.46 | 0.32 |
| 170122 | 63 | 86 | 0.42 | 56 | 72 | 0.43 | 119 | 158 | 0.43 | 0.10 |
| 170124 | 57 | 86 | 0.40 | 42 | 44 | 0.49 | 99 | 130 | 0.43 | 0.15 |
| 170131 | 56 | 84 | 0.40 | 50 | 54 | 0.48 | 106 | 138 | 0.43 | 0.15 |
| 170134 | 91 | 100 | 0.48 | 63 | 74 | 0.46 | 154 | 174 | 0.47 | 0.43 |
| 170323 | 100 | 193 | 0.34 | 97 | 121 | 0.44 | 197 | 314 | 0.39 | 0.0002 |
| 170324 | 59 | 74 | 0.44 | 58 | 82 | 0.41 | 117 | 156 | 0.43 | 0.09 |
| 170782 | 29 | 51 | 0.36 | 45 | 64 | 0.44 | 74 | 115 | 0.39 | 0.03 |
| Total | 595 | 837 | 0.42 | 545 | 694 | 0.44 | 1140 | 1531 | 0.43 | 4.2e-8 |
The rmr12 locus encodes a CHD3 protein
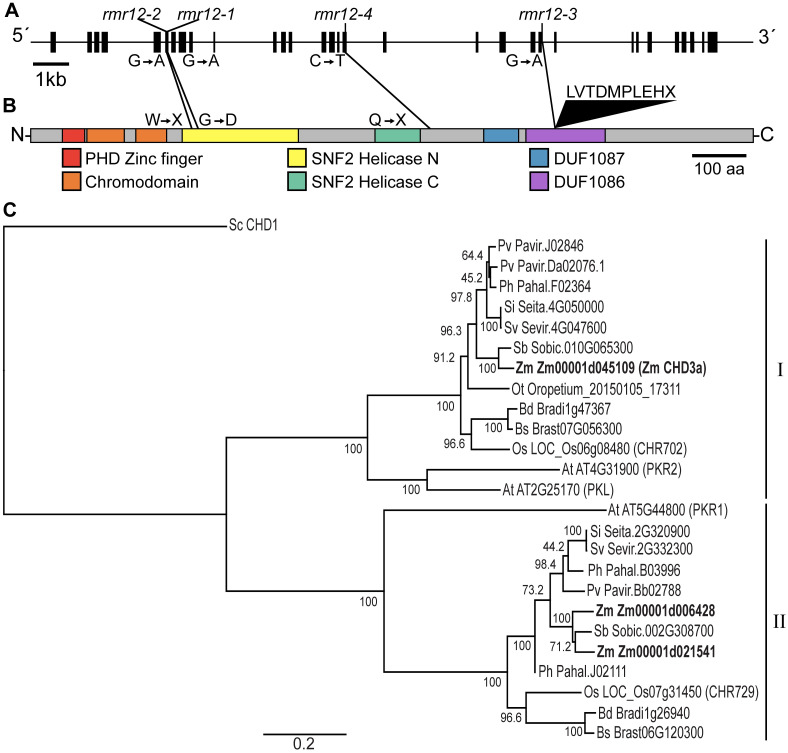
To better understand the link between development, gametophyte function and Pl1-Rhoades repression, we identified the molecular nature of rmr12 using positional information and sequence analysis. A BC3F2 mapping population was developed between the mutagenized A619 and recurrent A632 parental lines and, because wx1 and c1 linkages confirmed a 9S position, rmr12-3 mutants were genotyped with polymorphic 9S molecular markers (Table 7). This analysis narrowed the lesion to a 4Mb interval having 109 gene models (see Methods and S5 Table), none of which encode obvious RdDM-related proteins. One model, however, encodes a chromatin-related protein, a putative member of the homeodomain-like transcription factor superfamily (Zm00001d045109, chr113) composed of a plant homeodomain zinc-finger (PHD), tandem chromodomains, bipartite SNF2-type helicase, and two conserved domains of unknown function (DUF 1086 and 1087) diagnostic of chromodomain helicase DNA-binding 3 (CHD3) proteins [54].
Table 7. Recombination-based mapping of rmr12-3 from F2 mutants.
| Molecular Marker | No. individuals with specified genotype | |||||
|---|---|---|---|---|---|---|
| ID | Type | Chr 9 locationa | A619 / A619 | Heterozygotes | A632 / A632 | % A619 |
| 9_12.38 | CAPS | 12,377,322 | 66 | 2 | 0 | 99% |
| 9_16.47 | dCAPS | 16,471,870 | 25 | 9 | 1 | 84% |
| umc1586 | SSR | 25,251,065 | 16 | 16 | 3 | 69% |
| umc2128 | SSR | 25,743,586 | 4 | 4 | 1 | 67% |
| 9_50.02 | CAPS | 50,997,919 | 6 | 5 | 1 | 71% |
| 9_61.09 | dCAPS | 70,090,283 | 15 | 13 | 3 | 69% |
| 9_87.31 | CAPS | 89,637,894 | 7 | 5 | 3 | 63% |
| umc1267 | SSR | 105,956,029 | 0 | 0 | 20 | 0% |
aB73 AGPv4 genome
rmr12-3 mutant cDNA sequence (see S4 and S5 Figs) revealed a transition-type mutation in Zm00001d045109 (Fig 2A) that eliminated a canonical intron splice acceptor site and identified 67nt of retained intron sequence unique from the 37 transcript isoforms predicted in the B73 AGPv4 transcriptome [55]. All tested rmr12-3 mutants (n = 33) were homozygous for this mutation as identified with a dCAPS marker. This retained intron isoform encodes nine additional amino acids and a premature stop codon (Fig 2B; see S5 and S6 Figs). Sequences of tiled amplicons spanning the longest Zm00001d045109 transcript isoforms in all rmr12-1, rmr12-2, rmr12-3, and rmr12-4 mutant and non-mutant cDNAs supported Zm00001d045109_T004 as a predominant transcript and identified additional unique transition mutations (Fig 2A; see S5 Fig). A G to A missense in rmr12-1 changes glycine 308 to aspartic acid, and independent nonsense mutations were found in rmr12-2 and rmr12-4 (Fig 2B; see S5 and S6 Figs). Because of the rmr12-4-associated mutation, any translated protein would lack both DUF domains that in CHD3 proteins may bind DNA [56]. The G308D—occurring within an invariant GK(T/S) sequence of motif I where the adjacent lysine coordinates either a gamma or beta ATP phosphate in all SNF2-type ATPases [57]—would negatively affect ATP-binding, and any rmr12-2-encoded protein would lack all but the PHD and chromodomains (see S6 Fig). These coincident and disruptive lesions found in the defined 9S interval of plants homozygous for each of the four rmr12 mutant alleles strongly indicates that the rmr12 locus consists of a gene (Zm00001d045109) encoding a CHD3 protein, a subgroup of CHD proteins with known roles in transcriptional regulation [58].
Fig 2. rmr12 mutations disrupt a CHD3-encoding gene model.
(A) Maize gene model Zm00001d045109 highlighting transition mutations found in cDNA sequences from rmr12-1, rmr12-2, rmr12-3, and rmr12-4 mutants. (B) Predicted protein model highlighting domains diagnostic of CHD3 proteins and missense, nonsense (X), and insertion lesions corresponding to the respective transition mutations in (A). (C) Maximum likelihood tree produced from alignment of full-length maize (Zm) CHD3 protein sequences with CHD3 proteins from Arabidopsis (At) and grasses including Brachypodium distachyon (Bd), Brachypodium stacei (Bs), Oropetium thomaeum (Ot), rice (Os), Panicum hallii (Ph), Panicum virgatum (Pv), Setaria italica (Si), Setaria viridis (Sv), and Sorghum bicolor (Sb) identifies two clades (I and II). The tree is anchored with Saccharomyces cerevisiae (Sc) CHD1. Branch lengths depict substitutions per site.
A tBLASTn analysis of the B73 AGPv4 genome provided no evidence of a Zm00001d045109 duplicate but did identify closely related gene models (Zm00001d006428 and Zm00001d021541) in syntenic 2L and 7L regions. RNA-seq reads from all three chd3 genes are detected in each of 23 developmental and reproductive tissues including pollen [59] though Zm00001d045109 reads are most abundant (5–10 fold greater) in all datasets. A phylogenetic comparison of plant proteins having the DUF 1087 region, the most exclusive and conserved feature within the CHD3 clade, shows that, similar to eudicots and other grasses [60,61], maize has two distinct CHD3 subfamilies (Fig 2C). Each surveyed species has at least one subfamily I member homologous to the Zm00001d045109-encoded CHD3 that clades most closely with Arabidopsis PKL, whereas the other maize CHD3 proteins belong to the Arabidopsis PICKLE RELATED 1 (PKR1) clade II and appear to have arisen through a maize-specific whole genome duplication [62]. Zm00001d045109 is hereafter referred to as chd3a.
Rmr12 is required for normal development
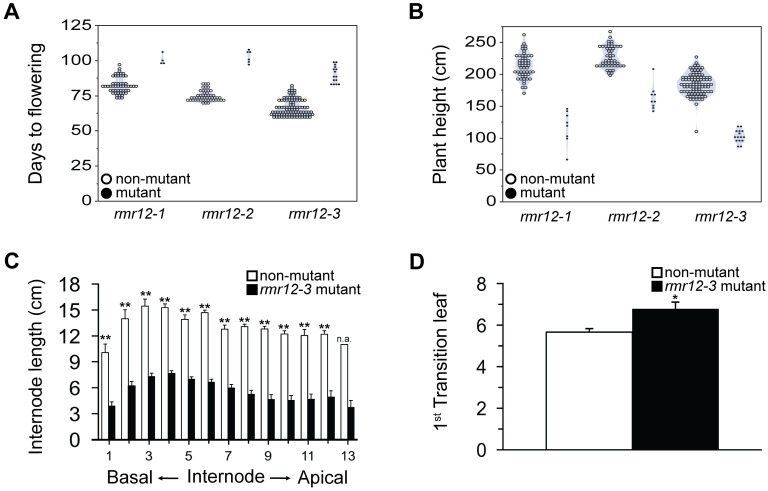
In addition to pkl mutants developing pickle-like root structures [63], they have reduced plant height [64], delayed vegetative phase change [65] and flowering [64], and reduced floral structures with aborted ovules [66]. These defects are similar to those displayed in rmr12 mutants (Fig 1) consistent with pkl and rmr12 providing orthologous functions. In contrast, many rmr12 mutant developmental defects appear distinct from those of rpd1 mutants [12]. These observations motivated a more detailed analysis to better define these similarities and differences. Quantifying days to flowering (DTF) and plant heights of independent F2 progenies segregating rmr12-1, rmr12-2, or rmr12-3 homozygotes (Fig 3A and 3B), we found all mutants flowered significantly later with a mean increase of 23.4 days (+/- 2.8 s.e.m., p = 0.0009, 5.04e-6, and 9.00e-12, two-sample t-test) and were shorter, on average 0.39 times the non-mutant sibling heights (+/- 0.06 s.e.m., p = 9.86e-6, 3.89e-6, and 5.07e-22, two-sample t-test). We conclude, therefore, that Rmr12 governs fundamental processes affecting plant growth.
Fig 3. Developmental profiles of rmr12 mutant and non-mutant F2 siblings.
(A) Days to flowering for individual progenies segregating homozygotes for rmr12-1 (mutant n = 4, non-mutant n = 53, p = 0.0009), rmr12-2 (mutant n = 6, non-mutant n = 50, p = 5.04e-6), or rmr12-3 (mutant n = 15, non-mutant n = 98, p = 9.00e-12). (B) Plant heights at flowering for progenies described in (A), rmr12-1 (mutant n = 8, non-mutant n = 54, p = 9.86e-6), rmr12-2 (mutant n = 9, non-mutant n = 50, p = 3.89e-6), or rmr12-3 (mutant n = 15, non-mutant n = 98, p = 5.07e-22). (C) Mean internode lengths (±s.e.m.) for one progeny segregating rmr12-3 homozygotes (mutant n = 12, non-mutant n = 12). ** p<0.001, n.a. = not available (single value). (D) Mean first leaf (±s.e.m.) with adult-type leaf waxes for 9 rmr12-3 mutants and 9 non-mutants from a single progeny. * p = 0.01.
Because the rpd1 mutant dwarf stature is exclusively due to reduced adult-phase internode lengths [12], we compared F2 rmr12-3 mutant and non-mutant sibling internode lengths at flowering. Although average mutant leaf number was greater than that of non-mutant siblings (12.7 versus 11.3 respectively; p = 0.003, two-sample t-test), all internode lengths, including those of the juvenile-phase, were significantly shorter (Fig 3C). Because the rpd1 mutant juvenile to adult phase transition is also delayed [12], we compared the average first leaf displaying adult leaf waxes (see Methods) between F2 rmr12-3 mutants and non-mutant siblings. Similar to rpd1 mutants, the rmr12-3 mutant transition was delayed by 1.1 leaves (leaf 5.7 vs leaf 6.8, p = 0.01, two-sample t-test) (Fig 3D).
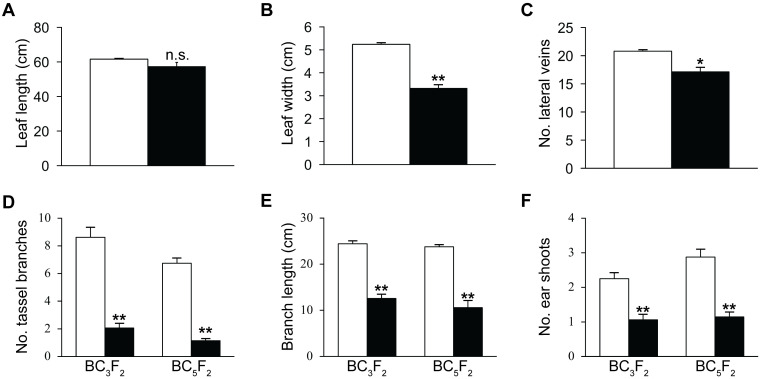
Abaxial surfaces of rpd1 mutant leaves can have adaxialized regions and occasionally exhibit ectopic outgrowths [12,13] but are generally indistinct from non-mutant leaves. In contrast, all rmr12 mutant leaves are upright (Fig 1A and 1B) like liguleless mutants [67,68], adaxially-curled (Fig 1B) like Arabidopsis polycomb-group mutants [69], and have a textured/wrinkled epidermis (Fig 1C; see S1 Fig) similar to crinkly4 mutants [70] and an RNAi knockdown of the maize BRASSINOSTEROID INSENSITIVE 1 ortholog (Zm-bri1) [71]. Leaf shape was also distinct. Among two independent progenies, mutant leaf 6 was no longer (p = 0.088, two-sample t-test), but clearly narrower (p = 5.07e-16, two-sample t-test), than the same leaf from non-mutant siblings (Fig 4A and 4B). Among three additional progenies evaluated, rmr12 mutants had fewer lateral veins, (17.2 versus 20.8 respectively, p = 0.005, two-sample t-test) at the leaf 10 midpoint (Fig 4C).
Fig 4. Morphometrics of rmr12-3 mutant and non-mutant F2 siblings.
Mean (±s.e.m.) of leaf 6 length (A) and width (B) of individuals from two progenies at flowering (mutant n = 38, non-mutant n = 236). (C) Mean number (±s.e.m.) of lateral veins at the midpoint of leaf 10 in 7-week-old plants from three progenies (mutants n = 6, non-mutants n = 10). (D) Mean tassel branch number (±s.e.m.) of individuals from eight progenies within the A632 background. BC3F2 (mutant n = 17, non-mutant n = 16). BC5F2 (mutant n = 7, non-mutant n = 8). (E) Mean primary tassel branch length (±s.e.m.) of individuals described in (D). (F) Mean number of ear shoots (±s.e.m.) at flowering of individuals described in (D). (A) to (F) solid bars: mutant, open bars: non-mutant. n.s. = not significant (p>0.05), * p<0.05, ** p<0.001.
rpd1 mutant tassels are often feminized and always have a relatively compact architecture with short internodes between more acutely upright-angled secondary spikes [12] but male flowers extrude anthers that shed pollen normally. Although secondary spikes are similarly upright, rmr12 mutant tassels rarely exhibit feminization, are severely reduced in both secondary spike numbers and primary spike lengths (1.8 vs 8 secondary spikes p = 2.07e-12, and 12 cm vs 24 cm primary spike length p = 9.5e-16, two-sample t-test for both) (Fig 4D and 4E), and the rarely-extruded anthers often fail to shed pollen. Manually extracted pollen appear visibly normal, however, and the progeny generated from mutant males indicates that at least some of their pollen is fertile (Fig 1E, Table 4; see S2 Table).
Cobs borne on rpd1 mutants are smaller with heterogeneously sized kernels set in disorganized rows diagnostic of ovule abortions [12]. Similar, yet far more extreme, defects are seen in rmr12 mutants with grain set being rare. Grain yields varied on mutant cobs with virtually none set in twelve successive Albany, CA summer nurseries compared with occasional sets on materials grown in Columbus, OH (Fig 1E). Fewer ear shoots were produced per plant (mean 1.1 vs. 2.5 ears p = 4.69e-9, two-sample t-test) in the eight F2 progenies examined (Fig 4F).
Overall, these rmr12 mutant phenotypes are mostly distinct from those of rpd1 mutants and instead mirror nearly all the known defects diagnostic of pkl loss-of-function mutants. As potential exceptions, some phenotypes including pickle-like root bulges on seedling roots and effects on light-dependent cotyledon opening [72,73] have not been adequately evaluated. Based on these apparent functional orthologies, molecular mapping data, sequence analyses, and phylogenetic relationships, we conclude that the rmr12 locus encodes the maize PKL ortholog, hereafter referred to as CHD3a.
CHD3a influences 24nt RNA biogenesis patterns
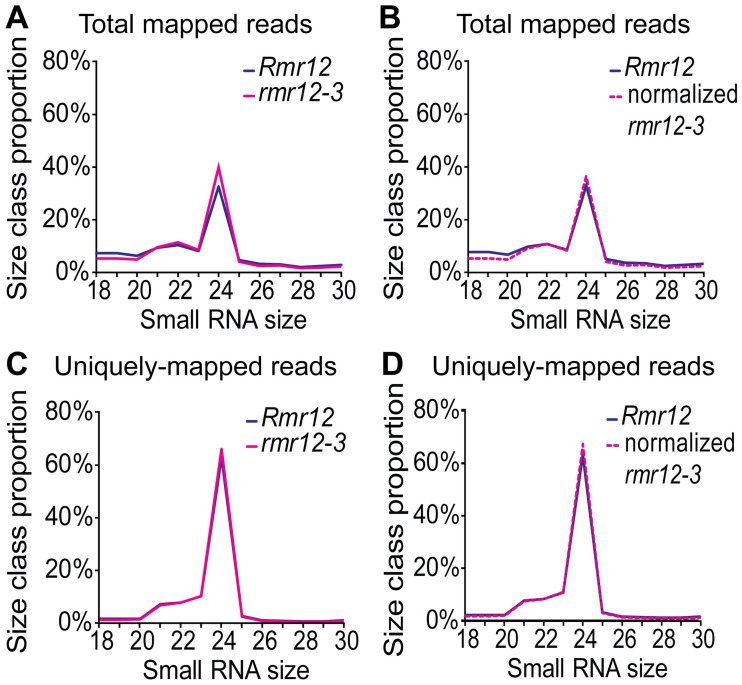
Because all known MOP and RMR proteins also maintain normal 24nt RNA levels [6,13,17,18,43–45,74], we compared PAGE-separated ethidium bromide-stained sRNAs isolated from sibling rmr12-4 / rmr12-4 and Rmr12 / rmr12-4 eight-day post-imbibition seedlings (see S7 Fig). Although similar comparisons clearly identify both RPD1- and RPD2a-dependencies [17], 24nt RNA levels appear undiminished in rmr12-4 mutants.
To more precisely compare 24nt RNA profiles, we analyzed sRNA libraries from eight-day post imbibition seedlings homozygous for either Rmr12 or rmr12-3 (two non-mutant and three mutant libraries) by mapping the reads to the B73 reference genome AGPv4 [55] using ShortStack [75]. Comparing relative percentages of all mapped 18-30nt reads, we found no significant differences in 24nt read abundances between rmr12-3 mutants and their non-mutant siblings (Fig 5A, p = 0.13, two-sample t-test). When percentages are normalized to the next most abundant size class (22nt), 24nt levels appear identical (Fig 5B). Additionally, both total and normalized 24nt read percentages are nearly identical for uniquely-mapped reads (Fig 5C and 5D) indicating CHD3a is not required for genome-wide 24nt RNA biogenesis. Of 59,127 sRNA genome clusters called on uniquely-mapped reads, 54,905 were predominantly 24nt. By DESeq2 analysis (see Methods), 14,656 (27%) of these 24nt clusters had differential read abundances of ≥2 fold change and padj (FDR) <0.05, with 7,848 increased and 6,808 decreased (see S6 Table), indicating that CHD3a influences where 24nt RNAs are produced.
Fig 5. sRNA profiles in rmr12-4 mutants.
Size class distributions for all genome-mapped 18-30nt reads in Rmr12 / Rmr12 and rmr12-3 / rmr12-3 eight-day post imbibition seedlings (A) and normalized to 22nt RNA levels (B). Distribution of uniquely-mapping 18-30nt reads (C) and normalized to 22nt RNA levels (D).
Looking for possible Pl1-Rhoades-specific sRNAs, we aligned all 18-30nt reads which either mapped uniquely or did not map to the B73 genome to a 16kb lambda clone sequence containing the Pl1-Rhoades coding region (GenBank L19494) and upstream sequence using ShortStack. None of the seven clusters called across this sequence (see S7 Table; S8 Fig), had significant differences in normalized read counts (rpm) (see S8 Table). If CHD3a regulates Pl1-Rhoades through specific targeting of 24nt RNA production, this likely occurs 3´ of the existing Pl1-Rhoades haplotype sequence where previous recombination mapping identifies a Pl1-Rhoades enhancer and sequences conferring paramutagenic properties [7].
CHD3a maintains repression of paramutant Pl1-Rhoades
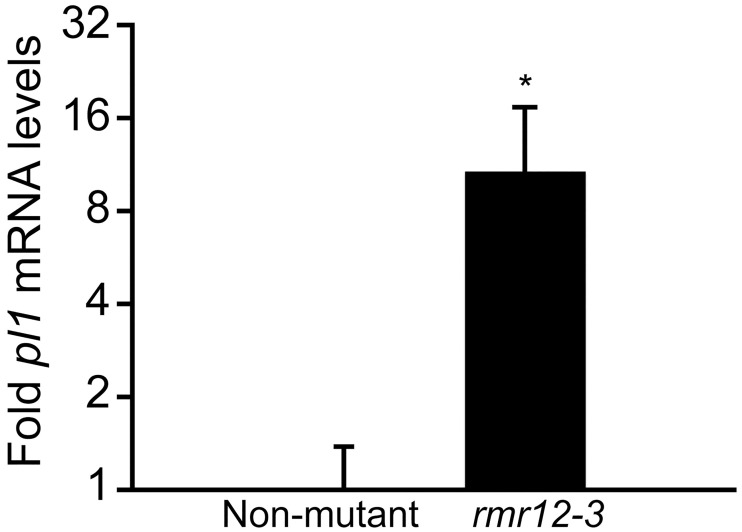
The sRNA results led us to question whether the mutants’ increased pigmentation was specifically due to Pl1-Rhoades derepression or to a general increase in anthocyanin production independent of pl1 function. To test this idea, we measured relative Pl1-Rhoades mRNA levels in rmr12-3 mutant and non-mutant siblings by qRT-PCR and found on average 10 fold more pl1 transcripts in rmr12-3 mutants (Fig 6) supporting a role for CHD3a in maintaining either the transcriptional, post-transcriptional, or co-transcriptional repression of the Pl´ state.
Fig 6. Relative pl1 mRNA levels in rmr12 mutants.
Mean fold pl1 mRNA expression (2-ΔΔCt) (±s.e.m) by qRT-PCR normalized to gapdh levels in biological triplicate non-mutant and rmr12-3 / rmr12-3 eight-day post imbibition seedlings. * p<0.05.
To address whether or not the elevation of Pl1-Rhoades mRNA was by itself sufficient to account for increased pigment, we synthesized mutants homozygous for functional yet recessive pl1 alleles and evaluated their anther pigment phenotypes. Among the twenty F2 rmr12-1 mutants segregating both pl1-B73 and Pl1-Rhoades in a Pl´ state, four had lightly-colored or near-colorless anthers typical of pl1-B73 homozygotes, six had no florets, and ten displayed darkly pigmented anthers indistinguishable from that conferred by Pl1-Rhoades in a Pl-Rh state (Table 8). Similarly, among the ten F2 rmr12-2 mutants segregating Pl´ and pl1-A632, three had lightly-colored or near-colorless anthers (Table 8). The presence of anther phenotypes typical of recessive pl1 expression in rmr12 mutant individuals indicates that CHD3a does not generally enhance pigment production and confirms that increased anther pigmentation in rmr12 mutants occurs from Pl´ derepression.
Table 8. Anther phenotypes of F2 mutants segregating recessive pl1 alleles.
| Progeny | No. mutants with given anther phenotypes | |||||
|---|---|---|---|---|---|---|
| ID | Allele | pl1 allele | No. plants | No anthers | Light/colorless | Pl-Rh-like |
| 013120 | rmr12-1 | pl1-B73 | 104 | 6a | 4 | 10 |
| 013115 | rmr12-2 | pl1-A632 | 60 | 2b | 3 | 5 |
aPlants had either no or barren tassels, or insect damaged florets.
bOne plant with a dry tassel and one plant with a vestigial tassel.
We next asked whether Pl1-Rhoades alleles could change from Pl´ to meiotically heritable Pl-Rh states in the absence of CHD3a function. Once Pl1-Rhoades changes from Pl-Rh to Pl´ it is always sexually transmitted in a Pl´ state [23], even in the absence of some proteins required to maintain Pl´ repression, including RMR2 [43] and RPD2a [17]. Pl´ can, however, heritably revert to Pl-Rh at various frequencies when either hemizygous [76,77], heterozygous with specific recessive pl1 alleles [76,77], or in rmr1 and rpd1 mutants [13,16,40,43]. To test if similar reversions occur in the absence of CHD3a, we crossed Pl´ / Pl´ rmr12 mutants by five distinct Pl-Rh / Pl-Rh testers and then evaluated the progeny anther color phenotypes. If Pl´ heritably reverts to Pl-Rh in the absence of CHD3a function, then some or all testcross progeny plants would have darkly pigmented anthers. Three of twelve progenies representing three distinct Pl-Rh / Pl-Rh testers had individuals (11 total) with intermediate or Pl-Rh-like anther colors (Table 9). These data, while relatively few in number, indicate that CHD3a contributes to maintaining meiotically-heritable information both specifying Pl1-Rhoades repression and facilitating paramutation in the subsequent generation. It remains to be evaluated whether CHD3a is also required to mediate pl1 paramutation. This evaluation will require combining Pl-Rh and Pl´ states in an rmr12 mutant and independently tracking the paramutation-inducing properties of each transmitted allele.
Table 9. Testcross rmr12 / rmr12; Pl´ / Pl´ X Rmr12 / Rmr12; Pl-Rh / Pl-Rh progeny phenotypes.
| Progeny | No. individuals with given anther color scores | ||||
|---|---|---|---|---|---|
| ID | Allele | Pl-Rh Tester | 1–4 (Pl´) | 5–6 (intermediate) | 7 (Pl-Rh) |
| 180506 | rmr12-4 | A619 | 6 | 0 | 0 |
| 180713 | rmr12-4 | A619 | 10 | 0 | 0 |
| 180787 | rmr12-4 | A619 | 6 | 0 | 3 |
| 172611 | rmr12-3 | A632 T | 1 | 0 | 0 |
| 172608 | rmr12-3 | B73 T | 2 | 0 | 0 |
| 172613 | rmr12-3 / rmr12-4 | B73 T | 56 | 0 | 0 |
| 172618 | rmr12-3 | B73 T | 26 | 0 | 0 |
| 172620 | rmr12-3 | B73 T | 3 | 0 | 0 |
| 180788 | rmr12-4 | K55 | 8 | 0 | 0 |
| 180789 | rmr12-4 | K55 | 6 | 1 | 0 |
| 180790 | rmr12-4 | K55 | 3 | 0 | 0 |
| 172609 | rmr12-3 | W23 | 14 | 0 | 0 |
| 172612 | rmr12-3 | W23 | 6 | 0 | 0 |
| 172614 | rmr12-3 | W23 | 3 | 0 | 0 |
| 172615 | rmr12-3 | W23 | 4 | 0 | 0 |
| 172619 | rmr12-3 | W23 | 1 | 0 | 0 |
| 160777 | rmr12-4 | W23 | 0 | 7a | 0 |
| 160778 | rmr12-4 | W23 | 9 | 0 | 0 |
| Total | 188 | 8 | 3 | ||
T: (Pl1-Rh carried on a T6-9 interchange).
aIndividual tassels had anther color scores ranging from 3–5.
Because not all rmr mutations similarly affect paramutant alleles at other loci [17,43], we tested whether CHD3a also maintained repression of paramutant b1 alleles by synthesizing rmr12 mutants carrying B1-I alleles of either repressed (B´) or fully expressed (B-I) states (see S1 Methods). In both mop1 and rpd1 mutants, the B´ state is derepressed such that it conditions dark leaf sheath pigmentation indistinguishable from that conferred by B-I [16,19,39]. Leaf sheath colors of the B´ and B-I rmr12 mutants were, in contrast, dissimilar (Fig 7) indicating that CHD3a is not required to maintain the B´ state. To test if CHD3a is nonetheless required to mediate b1 paramutation, B´ / B-I; rmr12-4 / rmr12-4 individuals were synthesized (see S1 Methods), and testcrossed by recessive b1 testers. All 26 individuals from three testcross progenies displayed a B´-like phenotype (Table 10) indicating that CHD3a does not mediate b1 paramutation. We conclude that CHD3a function acts to maintain locus-specific repression at Pl1-Rhoades.
Fig 7. rmr12 mutant B1-I phenotypes.
rmr12-4 mutants displaying B-I (A) and B´ (B) states.
Table 10. Phenotypes of rmr12-4 / rmr12-4; B´/ B-I X Rmr12 / Rmr12; b1 / b1 testcross progeny.
| Progeny | No. Individuals having indicated phenotype | ||
|---|---|---|---|
| ID | b1 tester | B´-like | B-I-like |
| 180787 | A619 | 11 | 0 |
| 180788 | K55 | 8 | 0 |
| 180789 | K55 | 7 | 0 |
| Total | 26 | 0 | |
Discussion
CHD3a represents the first molecular component maintaining Pl1-Rhoades paramutations seemingly outside of an RdDM-type mechanism. Because CHD3a specifically maintains repression of Pl1-Rhoades but not B1-I, these results reaffirm that paramutation behaviors occurring at distinct loci can be mechanistically distinct [17,43]. Although the RPD1 and MOP1 requirement at multiple loci [16,39] support the involvement of an RdDM-like feed-forward loop reinforcing an RNAP II-repressive chromatin state [10], the initiation, and/or maintenance of such locus-specific regulatory loops might be differentially sensitive to the actions of other factors affecting allele-specific RNAP II transcriptional control.
Identification of rmr12 as encoding a potential chromatin remodeling protein implicates the involvement of nucleosome alterations in maintaining paramutant states. In mammals, CHD3 proteins are complexed with HDACs and CpG- binding proteins [78] that facilitate transcriptional repression in hand with PRC2-mediated H3K27 methylation [79]. PKL, which might function as a monomer [80], also promotes H3K27me3 [81–84] and both positively and negatively affects mRNA levels of various H3K27me3-marked genes [81,82,84]. Despite these associations, it is unclear how PKL promotes H3K27me3 or whether it recognizes H3K27me3 in vivo, although the PHD domain from the rice CHD3, CHR729, has H3K27me3 affinity [60]. It is proposed that PKL promotes retention of H3K27me3-marked H2A.Z-containing nucleosomes through maturing prenucleosomes following transcription, an idea supported by the observation that PKL increases the size of DNase-resistant prenucleosome DNA fragments in vitro [84].
Based on the known biology of other CHD3 proteins and complexes [58,85], existing correlations between PKL function and H3K27me3 [81–84], and associations between mammalian CHD3 proteins, Histone Deacetylases (HDACs), and Polycomb Repressive Complex 2 (PRC2) [79], we envision a model in which CHD3a is required to maintain transcriptionally repressive (H3K27me3) nucleosomes that specify the Pl´ state. Because CHD3 complexes can operate at transcriptional enhancers [85], one specific hypothesis is that CHD3a acts to continually repress RNAP II transcription at the Pl1-Rhoades 3´ enhancer—a feature co-mapping with sequences required for facilitating paramutation [7]—as a prerequisite for RNAP IV to compete for such templates [6,8]. It will be critical to identify these key regulatory sequences and evaluate their nucleosome, 24nt RNA, and nascent transcription profiles to test this idea.
The Pl´ state is meiotically maintained by RNAP IV-dependent mechanisms [13,16], potentially involving 24nt RNAs and/or cytosine methylation. Because CHD3a always maintains the Pl´ state in the soma but reverted Pl-Rh states are sometimes transmitted from rmr12 mutants, it could be that other RNAP IV-dependent mechanisms deliver H3K27me3 marks to key regulatory sequences and maintain these at a certain level or location, but in the absence of CHD3a these H3K27me3 profiles are vulnerable to loss, resulting in occasional transmission of Pl1-Rhoades alleles that have reverted from Pl´ to Pl-Rh. Transmission of derepressed Pl1-Rhoades alleles from rmr12 mutants implies that a RNAP IV-dependent feature maintaining meiotic heritability must be, in part, stabilized by CHD3a function, and that feature must be capable of recruiting additional repression machinery including CHD3a in the next generation.
Although the roles of RPD1 and CHD3a in development are largely distinct, some shared mutant phenotypes, including juvenile to adult phase delays and dysregulation of Pl1-Rhoades, suggest certain alleles are co-dependent on CHD3a and RNAP IV actions. The large-scale changes in 24nt RNA distributions observed in rmr12-3 mutants indicate that CHD3a has a role in specifying where RNAP IV is recruited or functions. A similar relationship may also exist in Arabidopsis as PKL was found in a genetic screen as required to repress a luciferase (LUC) transgene driven by the RD29A promoter in a cytosine demethylase mutant (ros1) background [86], a screen that also identified several RdDM components [87]. Loss of pkl also resulted in genome-wide changes in both 24nt RNA and 5-methylcytosine (5mC) profiles but approximately half of all differentially methylated regions were hypermethylated [86] indicating that RdDM still occurs but in different locations. As the pRD29A-LUC silencing behaviors appear to share paramutation-like features [88], it is possible that RNAP IV and CHD3a co-repression is diagnostic of some paramutant alleles.
Although two C. elegans CHD3s, CHD-3 and LET-418, are required for gamete viability [89], CHD3’s roles in gamete transmission were previously unknown. CHD3a is one of only a few known proteins which when disrupted lead to male transmission ratio distortions. We found no reports in Arabidopsis that PKL-deficient gametophytes are similarly affected so it is possible that grasses have co-opted CHD3a for controlling pollen-specific genes. Our C1 transmission and in vitro germination results are inconsistent with problems in CHD3a-deficient pollen tube germination or growth and thus imply impairment of either stigma recognition and/or penetration, chemotaxis, or sperm cell delivery in rmr12 mutant gametophytes. Future pollen RNA-seq comparisons may identify the critical CHD3a targets and reveal the nature of this gametophyte dysfunction.
Maize represents a new model for understanding the role(s) of CHD3 proteins and their potential complexes. Its large physical size and abundance of staged monoecious reproductive tissues should be especially useful for understanding their functions in plant development. Investigating how CHD3a coordinates developmental phase changes as well as the phenotypic variation specified by RNAP IV and meiotically-heritable paramutations should help identify regulatory sequences of morphological significance. These sequences could be selected from existing germplasms or engineered to potentially breed adaptive or desirable traits. Identifying the genomic features that recruit CHD3a is an obvious next step in further defining the paramutation mechanism(s) and its relationship to the orderly changes in allele states occurring during development [35].
Materials and methods
Genetic materials and stock syntheses
Genetic nomenclature follows guidelines established for Zea mays and has been previously described [16]. All stocks contain functional alleles for all factors required for anthocyanin production in the anthers unless otherwise indicated. Hand pollinations were used for all stock syntheses. The rmr12 mutants were mostly used as the female parent because of their reticent anther phenotypes. The two reference alleles (ems98738 and ems98924) and two additional alleles (ems063095 and ems143190) were isolated from ems-treated pollen as previously described in [40] and [41], respectively. See S1 Methods for descriptions of specific stock syntheses. Additional pedigree information is available on request.
Phenotyping
All quantitative phenotyping was assessed on materials grown in Columbus, OH summer nurseries with the exception of the rmr12-1 and rmr12-2 height and flowering time measurements which occurred in Albany, CA summer nurseries. Transition leaves marking the juvenile to adult phase change were visually assessed. The first leaf with adult characteristics typically has dull edges (conferred by juvenile phase-specific cuticular waxes) and a glossy V-shaped section in the center. Because the transition leaf was difficult to identify in rmr12-3 mutant plants, we used toluidine blue O staining [90] to distinguish juvenile and adult waxes. Visual assignment of Pl1-Rhoades expression utilized a previously described anther color score [33] where scores 1–4 represent Pl´ states, 5–6 represent intermediate types, and 7 represents the fully expressed Pl-Rh reference state.
Pollen function
Pollen viability was assayed by fluorescein diacetate (FDA) stain as previously described [91]. Viable pollen was quantified from images taken under blue light five minutes after fresh pollen was mixed with FDA solution. In vitro pollen germination was carried out by plating fresh pollen on solid 1X pollen germination media [92] containing 10% sucrose, 0.0005% boric acid, 10mM calcium chloride, 0.05mM potassium phosphate, 6% polyethylene glycol 4000, and 0.3% noble agar. After germinating for 40 minutes at room temperature, pollen was stained with iodine potassium-iodide solution (0.1% iodine, 1% potassium iodine), imaged, and germination frequencies and pollen tube lengths of wx1 (red-brown) and Wx1 (blue) types were quantified using the image analysis software, Fiji [93]. Because iodine potassium-iodide staining can cause pollen tubes to burst, Wx1 pollen germination frequencies are reported relative to the germination frequency of wx1 types for each sample rather than as raw frequencies.
Statistics
The individual values used to generate means and graphs are available in the minimal data set (see S1 File). In cases where observed categorical variables were compared to expected frequencies, p values are based on chi-squared tests, and the chi-square values are given. Significance for comparing quantitative variables was based on two-sample t-tests assuming unequal variance (see S2 File).
Recombination mapping and candidate gene analysis
A set of rmr12-3 BC3F2 mutants was interrogated with molecular markers (see S9 Table for primer sequences and diagnostic enzymes) distinguishing parental A619 and A632 polymorphisms including simple satellite repeats (SSR) from the University of Missouri-Columbia (UMC) collection, newly designed cleaved amplified polymorphic sequences (CAPS), and derived CAPS (dCAPS), and the frequency of A619 alleles was recorded (Table 7). Results of individual mutants tested with each marker indicated single recombination events between rmr12-3 and both 9_12.38 and 9_16.47 in opposite directions indicating the rmr12 locus was between these markers. A dCAPS marker identified the rmr12-3 mutation, and rmr12-4 was genotyped with a CAPS marker (see S9 Table).
The composite sequences from PCR amplicons of cDNA from each rmr12 allele (see S5 Fig) and translated proteins were aligned to Zm00001d045109_T004 and P004, respectively, using the Geneious alignment tool ([94]; version 6.1.8) with mRNA, CDS, and protein domains predicted by simple modular architecture research tool (SMART) [95] (see S5 and S6 Figs). GenBank accessions for rmr12-A619, rmr12-1, rmr12-2, rmr12-3, and rmr12-4 complete coding sequences are MK875675-MK875679.
sRNA analysis
Low molecular weight RNA isolated from pooled eight-day post imbibition seedlings using TRIzol reagent (Invitrogen) and purified by chloroform and 5:1 acid phenol:chloroform extractions was enriched from total RNA by precipitating the majority of the high molecular weight RNAs by centrifugation (13,200 rpm for 10 minutes at 4C) in 11.5% PEG-8000, 38% formamide, 58mM NaCl. Low molecular weight RNAs were separated on a polyacrylamide gel (15% acrylamide/bis-acrylamide (19,1), 8M urea, 22.5mM Tris, 22.5 mM boric acid, 0.5mM EDTA) and stained with ethidium bromide.
sRNA libraries were made from total RNA isolated from individual eight-day post imbibition F2 sibling seedlings homozygous for either Rmr12 or rmr12-3 using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. Libraries were prepared using the gel-free size selection method of the BIOO NEXTFLEX Small RNA-Seq Kit v3 (Perkin Elmer). A pool of three mutant and non-mutant single indexed libraries was sequenced (150bp paired-end) on a single HiSeq 4000 lane by Novogene Co. Ltd. One of the non-mutant replicates produced few reads (less than 20,000) and was excluded from further analysis. The BBTools (https://jgi.doe.gov/data-and-tools/bbtools/) function bbduk was used to trim adapter sequences, remove low-quality reads, and retain 18-30nt reads with options (ktrim=r k=18 mink=11 hdist=1 tp=4 ftl=4 minlen=18 maxlength=30). The combined five libraries produced 182 million high-quality sRNA read pairs (see S10 Table). Mate 1 representing sense reads from each library was aligned to the B73 reference genome AGPv4 [55] not allowing mismatches or multiply-mapping reads, and clusters were called using ShortStack [75] with options (--mismatches 0 --mmap n --nohp --pad 75 --mincov 91). Because the ShortStack default settings for these datasets would allow a cluster to have as few as 3.4 reads per library, we set a non-default minimum coverage of 91 reads across all libraries (representing 0.5 rpm) to avoid differential cluster calls on regions with questionable biological significance. Counts from clusters defined as primarily 24nt by ShortStack were compared using DESeq2 [96], and those with log2 fold change ≥1 and padj (FDR) <0.05 were considered significant. These data are available through GEO (GSE158990).
To investigate sRNA clusters near Pl1-Rhoades, sRNA reads were first aligned to the B73 reference genome AGPv4 [55] using Bowtie (v0.12.8; options: -v 0 --best -m 1 -S) [97]. Multiply-mapping reads were excluded to ensure mapped sRNAs derived only from Pl1-Rhoades proximal sequences. Reads remaining unmapped or that mapped uniquely were subsequently aligned and clustered to the recently updated Pl1-Rhoades sequence from GenBank L19494 using ShortStack with the above parameters. The original L19494 sequence, representing the coding region and limited flanking sequences of a Pl1-Rhoades-containing lambda clone [98] was extended to include 11.6kb additional 5´ sequence with no gaps by sequencing lambda subclones and supplementing with a short genomic PCR amplicon as described [99,100]. Read counts for all seven clusters were converted to rpm 18-30nt clean reads, and differential expression was tested with 2-sample t-tests (see S8 Table).
qRT-PCR analysis
Total RNA was isolated from the first and second leaf sheaths below the lowest leaf blade of fourteen-day post imbibition Rmr12 and rmr12-3 homozygous F2 siblings using TRizol reagent (Invitrogen) as specified by the manufacturer. Tissues from two seedlings were pooled per sample. Isolated RNA was treated with DNase I (Roche), and 500ng of RNA was reverse transcribed using Protoscript II (NEB) and oligo(dT) primers. The resulting cDNA was treated with RNase A/T1, and one twenty-fifth of the RT reaction was included in technical triplicate 20μl PCR reactions with SensiMix SYBR No ROX (Bioline). Data were generated using an Eppendorf Mastercycler EP Gradient S thermocycler, and cycle threshold (Ct) values were calculated using the noiseband option in Eppendorf Mastercycler EP Realplex V2.2 software. pl1 transcripts were amplified with primers recognizing exon 1 (see S9 Table) and normalized to gapdh levels amplified using previously published primers [101] (see S9 Table).
Phylogenetic analysis
The SMART-predicted DUF1087 amino acid sequence from Zm00001d045109_P008 was used as the BLASTp query for A. thaliana and the grasses included in Phytozome v12.1 (https://phytozome.jgi.doe.gov/pz/portal.html) [102] except that maize sequences were replaced with those from B73 AGPv4 [55] obtained from Gramene (http://ensembl.gramene.org/Zea_mays/Info/Index). For each protein match from B73 AGPv4, the predicted isoform encoding the longest amino acid sequence was included for analysis. Alternative isoforms were removed from Phytozome v12 matches, and Oropetium_20150105_13389 was also removed because it lacked all other CHD domains. The full-length amino acid sequences from all protein matches, and S. cerevisiae CHD1 from Uniprot, were aligned using the MUSCLE alignment tool in Geneious ([94]; version 6.1.8) (see S3 File), and a maximum likelihood tree was created with Phyml [103] using the JTT amino acid substitution model and NNI+SPR tree topology search operation with 1000 bootstrap iterations (see S4 File). The resulting tree was oriented to display S. cerevisiae CHD1 (a founding member of the CHD clade of SNF2-ATPases) as the root using Geneious ([94]; version 6.1.8).
Supporting information
Additional mutant leaf blade phenotypes in ems063095 (A) and ems143190 (B) mutants.
(TIFF)
(A) Cob from self-pollination of an Rmr12 c1 / rmr12-4 C1 individual. (B) c1 / c1 X Rmr12 c1 / rmr12-4 C1 test cross cob holds progeny 170323.
(TIF)
(A) Frequency of viable pollen (stained with fluorescein diacetate) from four florets each from Rmr12 / Rmr12 and Rmr12 / rmr12-4 individuals. (B) Ratio of Wx1 to wx1 pollen germination frequencies from eight florets from a Rmr12 wx1 / rmr12-4 Wx1 individual. (C) wx1 and Wx1 pollen tube lengths (mm) from eight florets from a Rmr12 wx1 / rmr12-4 Wx1 individual. Boxplot whiskers encompass the range of data not including outliers (grey dots) which fall more than 1.5 X (interquartile range) above or below the box.
(TIF)
(A) Schematic representation of exons 22 and 23 in Zm00001d045109_T004 with the placement of primers (arrows) used to amplify B73 gDNA and cDNAs from Rmr12 / Rmr12 and rmr12-3 / rmr12-3 individuals (B). Hatched box represents intronic sequence retained in rmr12-3 mutants.
(TIF)
Partial mutant mRNA compiled from Sanger sequenced Rmr12-A619 and mutant cDNA amplicons aligned to a predicted reference transcript, Zm00001d045109_T004. Red = mRNA, yellow = coding sequence.
(DOCX)
rmr12 allele translations aligned to the Zm00001d045109_P004 reference protein sequence, with domains predicted by Simple Modular Architecture Research Tool (SMART).
(DOCX)
Ethidium bromide stained PAGE fractionated sRNAs from pooled Rmr12 / rmr12-4 or rmr12-4 / rmr12-4 eight-day post-imbibition seedlings. Sizes in nucleotides (nt) are shown.
(TIF)
(A) Uniquely-mapping sRNA reads from each library aligned to a lambda clone sequence containing the Pl1-Rhoades coding region. Peak heights are scaled to library depth. (B) Clusters called by ShortStack with the relative position of the Pl1-Rhoades coding region (C).
(TIF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(XLSX)
(XLSX)
(XLSX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(XLSX)
(DOCX)
(TXT)
(TXT)
Acknowledgments
We thank the Maize Genetics Cooperation Stock Center (MGCSC) (USDA-ARS, University of Illinois, Urbana, IL) and North Central Regional Plant Introduction Station (USDA-ARS, Ames, IA) for contributing valuable genetic materials. NCBI, Gramene, Phytozome, UniProt and MaizeGDB provided critical sequence and data resources. Maize nurseries were supported in part by the University of California College of Natural Resources Oxford Facilities Unit, the Ohio Agricultural Research and Development Centers Waterman Agricultural and Natural Resources Laboratory, and The Ohio State University College of Arts & Sciences Biological Sciences Greenhouse. We thank the Genomics Shared Resource at The Ohio State Comprehensive Cancer Center, Columbus Ohio for cDNA sequencing and sRNA library QC. Drs. Brian Dilkes and Charles Addo-Quaye (Purdue Univ) assisted with mapping efforts and candidate gene assessment, Matthew Warman and Dr. John Fowler (Oregon State Univ) provided advice and protocols for in vitro pollen germination experiments, and Karen Cone (Univ Missouri) generously shared the Pl1-Rhoades lambda clone. We thank members of the Hollick lab for constructive comments.
Data Availability
DNA sequences are in NCBI GenBank accessions MK875675-MK875679 and sRNA datasets are in the GEO repository under GSE158990. The updated Pl1-Rhoades lambda sequence is in GenBank accession L19494. All other relevant data are within the manuscript and its Supporting information files.
Funding Statement
Research reported in this publication was supported by The Ohio State University Comprehensive Cancer Center and the National Institutes of Health (www.nih.gov) under grant number P30 CA016058. Funding was provided by awards to J.B.H. from the National Research Initiative of the USDA Cooperative State Research, Education and Extension Service (www.usda.gov/topics/rural/cooperative-research-and-extension-services) (99-35301-7753, 2001-35301-10641, and 2006-35304-17399), the National Science Foundation (www.nsf.gov) (MCB-0419909, -0920623, -1715375) and The Ohio State Foundation. The views expressed are solely those of the authors and are not endorsed by the sponsors of this work. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Holoch D, Moazed D. RNA-mediated epigenetic regulation of gene expression. Nat Rev Genet. 2015;16: 71–84. 10.1038/nrg3863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gebert LFR, MacRae IJ. Regulation of microRNA function in animals. Nat Rev Mol Cell Biol. 2018;20: 21–37. 10.1038/s41580-018-0045-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ozata DM, Gainetdinov I, Zoch A, O’Carroll D, Zamore PD. PIWI-interacting RNAs: small RNAs with big functions. Nat Rev Genet. 2018;20: 89–108. 10.1038/s41576-018-0073-3 [DOI] [PubMed] [Google Scholar]
- 4.Ream TS, Haag JR, Wierzbicki AT, Nicora CD, Norbeck AD, Zhu J-K, et al. Subunit compositions of the RNA-silencing enzymes Pol IV and Pol V reveal their origins as specialized forms of RNA polymerase II. Mol Cell. 2009;33: 192–203. 10.1016/j.molcel.2008.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haag JR, Brower-Toland B, Krieger EK, Sidorenko L, Nicora CD, Norbeck AD, et al. Functional diversification of maize RNA Polymerase IV and V subtypes via alternative catalytic subunits. Cell Rep. 2014;9: 378–390. 10.1016/j.celrep.2014.08.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hale CJ, Erhard KF, Lisch D, Hollick JB. Production and processing of siRNA precursor transcripts from the highly repetitive maize genome. PLoS Genet. 2009;5: e1000598 10.1371/journal.pgen.1000598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erhard KF, Parkinson SE, Gross SM, Barbour J-ER, Lim JP, Hollick JB. Maize RNA Polymerase IV defines trans-generational epigenetic variation. Plant Cell. 2013;25: 808–819. 10.1105/tpc.112.107680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erhard KF, Talbot J-ERB, Deans NC, McClish AE, Hollick JB. Nascent transcription affected by RNA Polymerase IV in Zea mays. Genetics. 2015;199: 1107–1125. 10.1534/genetics.115.174714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKinlay A, Podicheti R, Wendte JM, Cocklin R, Rusch DB. RNA polymerases IV and V influence the 3′ boundaries of Polymerase II transcription units in Arabidopsis. RNA Biol. 2018;15: 269–279. 10.1080/15476286.2017.1409930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matzke MA, Kanno T, Matzke AJM. RNA-directed DNA methylation: the evolution of a complex epigenetic pathway in flowering plants. Annu Rev Plant Biol. 2015;66: 243–267. 10.1146/annurev-arplant-043014-114633 [DOI] [PubMed] [Google Scholar]
- 11.Pikaard CS, Haag JR, Ream T, Wierzbicki AT. Roles of RNA polymerase IV in gene silencing. Trends Plant Sci. 2008;13: 390–397. 10.1016/j.tplants.2008.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parkinson SE, Gross SM, Hollick JB. Maize sex determination and abaxial leaf fates are canalized by a factor that maintains repressed epigenetic states. Dev Biol. 2007;308: 462–473. 10.1016/j.ydbio.2007.06.004 [DOI] [PubMed] [Google Scholar]
- 13.Erhard KF, Stonaker JL, Parkinson SE, Lim JP, Hale CJ, Hollick JB. RNA polymerase IV functions in paramutation in Zea mays. Science. 2009;323: 1201–1205. 10.1126/science.1164508 [DOI] [PubMed] [Google Scholar]
- 14.Forestan C, Aiese Cigliano R, Farinati S, Lunardon A, Sanseverino W, Varotto S. Stress-induced and epigenetic-mediated maize transcriptome regulation study by means of transcriptome reannotation and differential expression analysis. Sci Rep. 2016;6: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trujillo JT, Seetharam AS, Hufford MB, Beilstein MA, Mosher RA. Evidence for a unique DNA-dependent RNA polymerase in cereal crops. Mol Biol Evol. 2018;35: 2454–2462. 10.1093/molbev/msy146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hollick JB, Kermicle JL, Parkinson SE. Rmr6 maintains meiotic inheritance of paramutant states in Zea mays. Genetics. 2005;171: 725–740. 10.1534/genetics.105.045260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stonaker JL, Lim JP, Erhard KF, Hollick JB. Diversity of Pol IV function is defined by mutations at the maize rmr7 locus. PLoS Genet. 2009;5: e1000706 10.1371/journal.pgen.1000706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sidorenko L, Dorweiler JE, Cigan AM, Arteaga-Vazquez M, Vyas M, Kermicle J, et al. A dominant mutation in mediator of paramutation2, one of three second-largest subunits of a plant-specific RNA polymerase, disrupts multiple siRNA silencing processes. PLoS Genet. 2009;5: e1000725 10.1371/journal.pgen.1000725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sloan AE, Sidorenko L, McGinnis KM. Diverse gene-silencing mechanisms with distinct requirements for RNA polymerase subunits in Zea mays. Genetics. 2014;198: 1031–1042. 10.1534/genetics.114.168518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brink RA. A genetic change associated with the R locus in maize which is directed and potentially reversible. Genetics. 1956;41: 872–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brink RA. Paramutation at the R locus in maize. Cold Spring Harb Symp Quant Biol. 1958;23: 379–391. 10.1101/sqb.1958.023.01.036 [DOI] [PubMed] [Google Scholar]
- 22.Chandler VL. Paramutation’s properties and puzzles. Science. 2010;330: 628–629. 10.1126/science.1191044 [DOI] [PubMed] [Google Scholar]
- 23.Hollick JB. Paramutation and related phenomena in diverse species. Nat Rev Genet. 2017;18: 5–23. 10.1038/nrg.2016.115 [DOI] [PubMed] [Google Scholar]
- 24.Coe EH. The properties, origin, and mechanism of conversion-type inheritance at the B locus in maize. Genetics. 1966;53: 1035–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stam M, Belele C, Dorweiler JE, Chandler VL. Differential chromatin structure within a tandem array 100 kb upstream of the maize b1 locus is associated with paramutation. Genes Dev. 2002;16: 1906–1918. 10.1101/gad.1006702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Louwers M, Bader R, Haring M, van Driel R, de Laat W, Stam M. Tissue- and expression level-specific chromatin looping at maize b1 epialleles. Plant Cell. 2009;21: 832–842. 10.1105/tpc.108.064329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Belele CL, Sidorenko L, Stam M, Bader R, Arteaga-Vazquez MA, Chandler VL. Specific tandem repeats are sufficient for paramutation-induced trans-generational silencing. PLoS Genet. 2013;9: e1003773 10.1371/journal.pgen.1003773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kermicle JL, Eggleston WB, Alleman M. Organization of paramutagenicity in R-stippled maize. Genetics. 1995;141: 361–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Panavas T, Weir J, Walker EL. The structure and paramutagenicity of the R-marbled haplotype of Zea mays. Genetics. 1999;153: 979–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Das OP, Messing J. Variegated phenotype and developmental methylation changes of a maize allele originating from epimutation. Genetics. 1994;136: 1121–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sidorenko L, Peterson T. Transgene-induced silencing identifies sequences involved in the establishment of paramutation of the maize p1 gene. Plant Cell. 2001;13: 319–335. 10.1105/tpc.13.2.319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goettel W, Messing J. Paramutagenicity of a p1 epiallele in maize. Theor Appl Genet. 2013;126: 159–177. 10.1007/s00122-012-1970-z [DOI] [PubMed] [Google Scholar]
- 33.Hollick JB, Patterson GI, Coe EH, Cone KC, Chandler VL. Allelic interactions heritably alter the activity of a metastable maize pl allele. Genetics. 1995;141: 709–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dooner HK, Robbins TP, Jorgensen RA. Genetic and developmental control of anthocyanin biosynthesis. Annu Rev Genet. 1991;25: 173–99. 10.1146/annurev.ge.25.120191.001133 [DOI] [PubMed] [Google Scholar]
- 35.Brink RA. Phase change in higher plants and somatic cell heredity. Q Rev Biol. 1962;37: 1–22. 10.1086/403567 [DOI] [Google Scholar]
- 36.Patterson GI, Thorpe CJ, Chandler VL. Paramutation, an allelic interaction, is associated with a stable and heritable reduction of transcription of the maize b regulatory gene. Genetics. 1993;135: 881–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hollick JB, Patterson GI, Asmundsson IM, Chandler VL. Paramutation alters regulatory control of the maize pl locus. Genetics. 2000;154: 1827–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sidorenko L, Chandler V. RNA-dependent RNA polymerase is required for enhancer-mediated transcriptional silencing associated with paramutation at the maize p1 gene. Genetics. 2008;180: 1983–1993. 10.1534/genetics.108.095281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dorweiler JE, Carey CC, Kubo KM, Hollick JB, Kermicle JL, Chandler VL. mediator of paramutation1 is required for establishment and maintenance of paramutation at multiple maize loci. Plant Cell. 2000;12: 2101–2118. 10.1105/tpc.12.11.2101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hollick JB, Chandler VL. Genetic factors required to maintain repression of a paramutagenic maize pl1 allele. Genetics. 2001;157: 369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hale CJ, Stonaker JL, Gross SM, Hollick JB. A novel Snf2 protein maintains trans-generational regulatory states established by paramutation in maize. PLoS Biol. 2007;5: 2156–2165. 10.1371/journal.pbio.0050275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alleman M, Sidorenko L, McGinnis K, Seshadri V, Dorweiler JE, White J, et al. An RNA-dependent RNA polymerase is required for paramutation in maize. Nature. 2006;442: 295–298. 10.1038/nature04884 [DOI] [PubMed] [Google Scholar]
- 43.Barbour J-ER, Liao IT, Stonaker JL, Lim JP, Lee CC, Parkinson SE, et al. required to maintain repression2 is a novel protein that facilitates locus-specific paramutation in maize. Plant Cell. 2012;24: 1761–1775. 10.1105/tpc.112.097618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nobuta K, Lu C, Shrivastava R, Pillay M, De Paoli E, Accerbi M, et al. Distinct size distribution of endogeneous siRNAs in maize: evidence from deep sequencing in the mop1-1 mutant. Proc Natl Acad Sci. 2008;105: 14958–14963. 10.1073/pnas.0808066105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arteaga-Vazquez M, Sidorenko L, Rabanal FA, Shrivistava R, Nobuta K, Green PJ, et al. RNA-mediated trans-communication can establish paramutation at the b1 locus in maize. Proc Natl Acad Sci U S A. 2010;107: 12986–12991. 10.1073/pnas.1007972107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Teixeira FK, Colot V. Repeat elements and the Arabidopsis DNA methylation landscape. Heredity. 2010;105: 14–23. 10.1038/hdy.2010.52 [DOI] [PubMed] [Google Scholar]
- 47.Rassoulzadegan M, Cuzin F. From paramutation to human disease: RNA-mediated heredity. Semin Cell Dev Biol. 2015;44: 47–50. 10.1016/j.semcdb.2015.08.007 [DOI] [PubMed] [Google Scholar]
- 48.Nelson OE. Genetic fine structure as revealed in pollen assays In: Freeling M, Walbot V, editors. The Maize Handbook. New York: Springer-Verlag; 1994. pp. 298–302. [Google Scholar]
- 49.Pinillos V, Cuevas J. Standardization of the flourochromatic reaction test to assess pollen viability. Biotech Histochem. 2008;83: 15–21. 10.1080/10520290801987204 [DOI] [PubMed] [Google Scholar]
- 50.Paz-Ares J, Ghosal D, Wienand U, Peterson PA, Saedler H. The regulatory c1 locus of Zea mays encodes a protein with homology to myb proto-oncogene products and with structural similarities to transcriptional activators. EMBO J. 1987;6: 3553–3558. 10.1002/j.1460-2075.1987.tb02684.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cone KC, Burr FA, Burr B. Molecular analysis of the maize anthocyanin regulatory locus C1. Proc Natl Acad Sci. 1986;83: 9631–9635. 10.1073/pnas.83.24.9631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Emerson RA, Beadle GW, Fraser AC. A summary of linkage studies in maize. Cornell Univ Agric Exp Stn Mem. 1935;180: 1–83. [Google Scholar]
- 53.Rhoades MM, McClintock B. The cytogenetics of maize. Bot Rev. 1935;1: 292–325. [Google Scholar]
- 54.Hu Y, Lai Y, Zhu D. Transcription regulation by CHD proteins to control plant development. Front Plant Sci. 2014;5: 1–5. 10.3389/fpls.2014.00223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jiao Y, Peluso P, Shi J, Liang T, Stitzer MC, Wang B, et al. Improved maize reference genome with single-molecule technologies. Nature. 2017;546: 524–527. 10.1038/nature22971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ryan DP, Sundaramoorthy R, Martin D, Singh V, Owen-Hughes T. The DNA-binding domain of the Chd1 chromatin-remodelling enzyme contains SANT and SLIDE domains. EMBO J. 2011;30: 2596–2609. 10.1038/emboj.2011.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Flaus A, Martin DMA, Barton GJ, Owen-Hughes T. Identification of multiple distinct Snf2 subfamilies with conserved structural motifs. Nucleic Acids Res. 2006;34: 2887–2905. 10.1093/nar/gkl295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Han SK, Wu MF, Cui S, Wagner D. Roles and activities of chromatin remodeling ATPases in plants. Plant J. 2015;83: 62–77. 10.1111/tpj.12877 [DOI] [PubMed] [Google Scholar]
- 59.Walley JW, Sartor RC, Shen Z, Schmitz RJ, Wu KJ, Urich MA, et al. Integration of omic networks in a developmental atlas of maize. Science. 2016;353: 814–818. 10.1126/science.aag1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hu Y, Liu D, Zhong X, Zhang C, Zhang Q, Zhou D-X. CHD3 protein recognizes and regulates methylated histone H3 lysines 4 and 27 over a subset of targets in the rice genome. Proc Natl Acad Sci. 2012;109: 5773–5778. 10.1073/pnas.1203148109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao C, Xu J, Chen Y, Mao C, Zhang S, Bai Y, et al. Molecular cloning and characterization of OsCHR4, a rice chromatin-remodeling factor required for early chloroplast development in adaxial mesophyll. Planta. 2012;236: 1165–1176. 10.1007/s00425-012-1667-1 [DOI] [PubMed] [Google Scholar]
- 62.Helentjaris T, Weber D, Wright S. Identification of the genomic locations of duplicate nucleotide sequences in maize by analysis of restriction fragment length polymorphisms. Genetics. 1988;118: 353–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ogas J, Cheng JC, Sung ZR, Somerville C. Cellular differentiation regulated by gibberellin in the Arabidopsis thaliana pickle mutant. Science. 1997;277: 91–94. 10.1126/science.277.5322.91 [DOI] [PubMed] [Google Scholar]
- 64.Henderson JT, Li H-C, Rider SD, Mordhorst AP, Romero-Severson J, Cheng J-C, et al. PICKLE acts throughout the plant to repress expression of embryonic traits and may play a role in gibberellin-dependent responses. Plant Physiol. 2004;134: 995–1005. 10.1104/pp.103.030148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu M, Hu T, Smith MR, Poethig RS. Epigenetic regulation of vegetative phase change in Arabidopsis. Plant Cell. 2016;28: 28–41. 10.1105/tpc.15.00854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carter B, Henderson JT, Svedin E, Fiers M, McCarthy K, Smith A, et al. Cross-talk between sporophyte and gametophyte generations is promoted by CHD3 chromatin remodelers in Arabidopsis thaliana. Genetics. 2016;203: 817–829. 10.1534/genetics.115.180141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Emerson RA. The inheritance of the ligule and auricles of corn leaves. Nebraska Agric Res Stn Annu Rep. 1912;25: 81–88. [Google Scholar]
- 68.Brink RA. Heritable characters in maize. J Hered. 1933;24: 325–326. [Google Scholar]
- 69.Goodrich J, Puangsomlee P, Martin M, Long D, Meyerowitz EM, Coupland G. A Polycomb-group gene regulates homeotic gene expression in Arabidopsis. Nature. 1997;386: 44–51. 10.1038/386044a0 [DOI] [PubMed] [Google Scholar]
- 70.Becraft PW, Stinard PS, McCarty DR. CRINKLY4: A TNFR-like receptor kinase involved in maize epidermal differentiation. Science. 1996;273: 1406–1409. 10.1126/science.273.5280.1406 [DOI] [PubMed] [Google Scholar]
- 71.Kir G, Ye H, Nelissen H, Neelakandan AK, Kusnandar AS, Luo A, et al. RNA interference knockdown of BRASSINOSTEROID INSENSITIVE1 in maize reveals novel functions for brassinosteroid signaling in controlling plant architecture. Plant Physiol. 2015;169: 826–839. 10.1104/pp.15.00367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jing Y, Lin R. PICKLE is a repressor in seedling de-etiolation pathway. Plant Signal Behav. 2013;8: 13–15. 10.4161/psb.25026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang D, Jing Y, Jiang Z, Lin R. The chromatin-remodeling factor PICKLE integrates brassinosteroid and gibberellin signaling during skotomorphogenic growth in Arabidopsis. Plant Cell. 2014;26: 2472–2485. 10.1105/tpc.113.121848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jia Y, Lisch DR, Ohtsu K, Scanlon MJ, Nettleton D, Schnable PS. Loss of RNA-dependent RNA polymerase 2 (RDR2) function causes widespread and unexpected changes in the expression of transposons, genes, and 24-nt small RNAs. PLoS Genet. 2009;5: e1000737 10.1371/journal.pgen.1000737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Johnson NR, Yeoh JM, Coruh C, Axtell MJ. Improved placement of multi-mapping small RNAs. G3 Genes, Genomes, Genet. 2016;6: 2103–2111. 10.1534/g3.116.030452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hollick JB, Chandler VL. Epigenetic allelic states of a maize transcriptional regulatory locus exhibit overdominant gene action. Genetics. 1998;150: 891–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gross SM, Hollick JB. Multiple trans-sensing interactions affect meiotically heritable epigenetic states at the maize pl1 locus. Genetics. 2007;176: 829–839. 10.1534/genetics.107.072496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wade PA, Gegonne A, Jones PL, Ballestar E, Aubry F, Wolffe AP. Mi-2 complex couples DNA methylation to chromatin remodelling and histone deacetylation. Nat Genet. 1999;23: 62–66. 10.1038/12664 [DOI] [PubMed] [Google Scholar]
- 79.Reynolds N, Salmon-Divon M, Dvinge H, Hynes-Allen A, Balasooriya G, Leaford D, et al. NuRD-mediated deacetylation of H3K27 facilitates recruitment of Polycomb Repressive Complex 2 to direct gene repression. EMBO J. 2012;31: 593–605. 10.1038/emboj.2011.431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ho KK, Zhang H, Golden BL, Ogas J. PICKLE is a CHD subfamily II ATP-dependent chromatin remodeling factor. Biochim Biophys Acta. 2013;1829: 199–210. 10.1016/j.bbagrm.2012.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Aichinger E, Villar CBR, Farrona S, Reyes JC, Hennig L, Köhler C. CHD3 proteins and Polycomb group proteins antagonistically determine cell identity in Arabidopsis. PLoS Genet. 2009;5: e1000605 10.1371/journal.pgen.1000605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang H, Rider SD, Henderson JT, Fountain M, Chuang K, Kandachar V, et al. The CHD3 remodeler PICKLE promotes trimethylation of histone H3 lysine 27. J Biol Chem. 2008;283: 22637–22648. 10.1074/jbc.M802129200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang H, Bishop B, Ringenberg W, Muir WM, Ogas J. The CHD3 remodeler PICKLE associates with genes enriched for trimethylation of histone H3 lysine 27. Plant Physiol. 2012;159: 418–432. 10.1104/pp.112.194878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Carter B, Bishop B, Ho KK, Huang R, Jia W, Zhang H, et al. The chromatin remodelers PKL and PIE1 act in an epigenetic pathway that determines H3K27me3 homeostasis in Arabidopsis. Plant Cell. 2018;30: 1337–1352. 10.1105/tpc.17.00867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hu G, Wade PA. NuRD and pluripotency: a complex balancing act. Cell Stem Cell. 2012;10: 497–503. 10.1016/j.stem.2012.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang R, Zheng Z, Chen Q, Yang L, Huang H, Miki D, et al. The developmental regulator PKL is required to maintain correct DNA methylation patterns at RNA-directed DNA methylation loci. Genome Biol. 2017;18: 103 10.1186/s13059-017-1226-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.He XJ, Hsu YF, Pontes O, Zhu J, Lu J, Bressan RA, et al. NRPD4, a protein related to the RPB4 subunit of RNA polymerase II, is a component of RNA polymerases IV and V and is required for RNA-directed DNA methylation. Genes Dev. 2009;23: 318–330. 10.1101/gad.1765209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zheng Z, Yu H, Miki D, Jin D, Zhang Q, Ren Z, et al. Involvement of multiple gene-silencing pathways in a paramutation-like phenomenon in Arabidopsis. Cell Rep. 2015;11: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Turcotte CA, Sloat SA, Rigothi JA, Rosenkranse E, Northrup AL, Andrews NP, et al. Maintenance of genome integrity by Mi2 homologs CHD-3 and LET-418 in Caenorhabditis elegans. Genetics. 2018;208: 991–1007. 10.1534/genetics.118.300686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dudley M, Poethig RS. The heterochronic Teopod1 and Teopod2 mutations of maize are expressed non-cell-autonomously. Genetics. 1993;133: 389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li X. Pollen fertility/viability assay using FDA staining. Bio-protocol. 2011;1: e75 10.21769/BioProtoc.75 [DOI] [Google Scholar]
- 92.Schreiber DN, Dresselhaus T. In vitro pollen germination and transient transformation of Zea mays and other plant species. Plant Mol Biol. 2003;21: 31–41. 10.1007/BF02773394 [DOI] [Google Scholar]
- 93.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9: 676–682. 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, et al. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28: 1647–1649. 10.1093/bioinformatics/bts199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schultz J, Milpetz F, Bork P, Ponting CP. SMART, a simple modular architecture research tool: identification of signaling domains. Proc Natl Acad Sci. 1998;95: 5857–5864. 10.1073/pnas.95.11.5857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15: 1–21. 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10: R25 10.1186/gb-2009-10-3-r25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cone KC, Cocciolone SM, Moehlenkamp CA, Weber T, Drummond BJ, Tagliani LA, et al. Role of the regulatory gene pl in the photocontrol of maize anthocyanin pigmentation. Plant Cell. 1993;5: 1807–1816. 10.1105/tpc.5.12.1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hoekenga OA. Epigenetic regulation of Pl1-Blotched [dissertation]. Columbia (MO): University of Missouri; 1998.
- 100.Gross S. Trans-sensing interactions and structural features of the maize Pl1-Rhoades allele [dissertation]. Berkeley (CA): University of California, Berkeley; 2007.
- 101.Thompson BE, Basham C, Hammond R, Ding Q, Kakrana A, Lee T-F, et al. The dicer-like1 homolog fuzzy tassel is required for the regulation of meristem determinacy in the inflorescence and vegetative growth in maize. Plant Cell. 2014;26: 4702–4717. 10.1105/tpc.114.132670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Goodstein DM, Shu S, Howson R, Neupane R, Hayes RD, Fazo J, et al. Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res. 2011;40: 1178–1186. 10.1093/nar/gkr944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol. 2010;59: 307–321. 10.1093/sysbio/syq010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional mutant leaf blade phenotypes in ems063095 (A) and ems143190 (B) mutants.
(TIFF)
(A) Cob from self-pollination of an Rmr12 c1 / rmr12-4 C1 individual. (B) c1 / c1 X Rmr12 c1 / rmr12-4 C1 test cross cob holds progeny 170323.
(TIF)
(A) Frequency of viable pollen (stained with fluorescein diacetate) from four florets each from Rmr12 / Rmr12 and Rmr12 / rmr12-4 individuals. (B) Ratio of Wx1 to wx1 pollen germination frequencies from eight florets from a Rmr12 wx1 / rmr12-4 Wx1 individual. (C) wx1 and Wx1 pollen tube lengths (mm) from eight florets from a Rmr12 wx1 / rmr12-4 Wx1 individual. Boxplot whiskers encompass the range of data not including outliers (grey dots) which fall more than 1.5 X (interquartile range) above or below the box.
(TIF)
(A) Schematic representation of exons 22 and 23 in Zm00001d045109_T004 with the placement of primers (arrows) used to amplify B73 gDNA and cDNAs from Rmr12 / Rmr12 and rmr12-3 / rmr12-3 individuals (B). Hatched box represents intronic sequence retained in rmr12-3 mutants.
(TIF)
Partial mutant mRNA compiled from Sanger sequenced Rmr12-A619 and mutant cDNA amplicons aligned to a predicted reference transcript, Zm00001d045109_T004. Red = mRNA, yellow = coding sequence.
(DOCX)
rmr12 allele translations aligned to the Zm00001d045109_P004 reference protein sequence, with domains predicted by Simple Modular Architecture Research Tool (SMART).
(DOCX)
Ethidium bromide stained PAGE fractionated sRNAs from pooled Rmr12 / rmr12-4 or rmr12-4 / rmr12-4 eight-day post-imbibition seedlings. Sizes in nucleotides (nt) are shown.
(TIF)
(A) Uniquely-mapping sRNA reads from each library aligned to a lambda clone sequence containing the Pl1-Rhoades coding region. Peak heights are scaled to library depth. (B) Clusters called by ShortStack with the relative position of the Pl1-Rhoades coding region (C).
(TIF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(XLSX)
(XLSX)
(XLSX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(XLSX)
(DOCX)
(TXT)
(TXT)
Data Availability Statement
DNA sequences are in NCBI GenBank accessions MK875675-MK875679 and sRNA datasets are in the GEO repository under GSE158990. The updated Pl1-Rhoades lambda sequence is in GenBank accession L19494. All other relevant data are within the manuscript and its Supporting information files.