Abstract
Periodontal diseases, including gingivitis and periodontitis, are a global oral health problem. Porphyromonas gingivalis, a key pathogen involved in the onset of periodontitis, is able to colonize the subgingival epithelium and invade the underlying connective tissue due to the contribution of cysteine proteases known as gingipains. In this study, we investigated the effects of a phenolic extract prepared from tart cherry (Prunus cerasus L.) juice on the growth, adherence, and protease activity of P. gingivalis. We also assessed the protective effect of the tart cherry extract on the disruption of the oral epithelial barrier induced by P. gingivalis. The tart cherry extract that contains procyanidins and quercetin and its derivatives (rutinoside, glucoside) as the most important phenolic compounds attenuated P. gingivalis growth, reduced adherence to an experimental basement membrane matrix model, and decreased the protease activities of P. gingivalis. The tart cherry extract also exerted a protective effect on the integrity of the oral epithelial barrier in an in vitro model infected with P. gingivalis. More specifically, the extract prevented a decrease in transepithelial electrical resistance as well as the destruction of tight junction proteins (zonula occludens-1 and occludin). These results suggest that the tart cherry phenolic extract may be a promising natural product for the treatment of periodontitis through its ability to attenuate the virulence properties of P. gingivalis and curtail the ability of this pathogen to impair the oral epithelial barrier.
Introduction
Over 700 microbial species have been detected in the oral cavity, the majority of which maintain commensal relationships with the host [1]. However, when oral hygiene is deficient, dental biofilm accumulates and develops into a complex microbial community with synergistic interactions leading to metabolic collaborations, nutritional interdependence, and oxygen consumption [1]. This enables the establishment of obligate anaerobic bacteria, some of which have the ability to modulate the host inflammatory response [2]. The disruption of oral homeostasis induces a dysbiosis in the microbial community that triggers the onset of periodontitis, an inflammatory disease of bacterial origin characterized by the destruction of the underlying structures of the periodontium [3]. If left untreated, this can lead to extensive destruction of connective tissue, resorption of alveolar bone, and tooth loss. It is estimated that nearly 10% of the adult population worldwide is afflicted by severe periodontitis [4]. Over the last decade, epidemiologic evidence has accumulated suggesting that periodontal disease is a risk factor for more serious systemic diseases, including cardiovascular disease, type 2 diabetes, and rheumatoid arthritis [5].
The most documented periodontal pathogen is likely Porphyromonas gingivalis, a Gram-negative anaerobic bacterium that produces a broad array of virulence factors, including proteases [6]. P. gingivalis expresses three different cysteine proteases (Arg-gingipains A and B and Lys-gingipain) in cell membrane-bound and secreted forms [7, 8]. These proteases are involved in nutrient acquisition, host colonization, inactivation of host defense mechanisms, and tissue destruction [7, 8]. Therefore, gingipains are likely to be critical for bacterial survival and multiplication in vivo. In addition, P. gingivalis lipopolysaccharides (LPS) stimulate cytokine and matrix metalloproteinase production by resident and immune cells in the periodontium [9]. These inflammatory mediators play a prominent role in the pathogenesis of periodontitis by mediating periodontal attachment loss and alveolar bone destruction [10–13].
There has been a growing interest in tart cherry (Prunus cerasus L.) research over the last decade due to accumulating evidence that it is a functional food. Studies have suggested that whole fruits as well as extracts can lower the risk of cardiovascular disease, decrease low-density lipoprotein (LDL) cholesterol levels, help manage type 2 diabetes, and reduce inflammatory disorders such as arthritis [14–17]. Consuming tart cherry juice also reduces recovery time and soreness after resistance or endurance training by athletes by lowering oxidative stress and muscle inflammation [18]. These health benefits have been strongly linked to its high polyphenol (mainly flavonoid) content [19]. The potential of polyphenols from berry fruits for the prevention and treatment of periodontal diseases has been widely explored more recently [20–22]. Proanthocyanidins from blueberry and cranberry have been shown to inhibit biofilm formation and the adherence of major periodontal pathogens, exert anti-inflammatory properties, and reinforce epithelial barrier integrity [20–22]. Recent work in our laboratory showed that two tart cherry phenolic extracts exhibit anti-adherence properties and impede biofilm formation by the major oral pathogens Streptococcus mutans, Candida albicans, and Fusobacterium nucleatum [23]. The aim of the present study was to investigate the effects of a phenolic extract prepared from tart cherry (P. cesarus L.) juice on the growth, adherence, and protease activity of P. gingivalis. We also assessed the ability of the tart cherry extract to protect against the P. gingivalis-induced disruption of the oral epithelial barrier.
Materials and methods
Preparation of the tart cherry phenolic extract
Montmorency tart cherry (P. cerasus L.) juice concentrate kindly provided by King Orchards (Central Lake, MI, USA) underwent extensive dialysis (3 days/4°C) through a membrane with a 1-kDa molecular weight cut-off. Non-dialyzable material was freeze-dried and stored at 4°C in the dark. The phenolic composition of the tart cherry extract determined by chromatographic and mass spectrometry analyses has been reported previously [23]. Quercetin and its derivatives (rutinoside, glucoside) as well as procyanidins are the main constituents of the extract.
Bacteria and growth conditions
P. gingivalis ATCC 33277 was grown under anaerobic conditions (80% N2, 10% CO2, 10% H2) at 37°C in Todd-Hewitt Broth (THB; BBL Microbiology Systems, Cockeysville, MD, USA) supplemented with 0.001% hemin (Sigma-Aldrich Canada Co., Oakville, ON, Canada) and 0.0001% vitamin K (Sigma-Aldrich Canada Co.) (THB-HK).
Effect of the tart cherry extract on P. gingivalis growth
A 24-h culture (early stationary growth phase) of P. gingivalis was diluted in fresh culture medium to an optical density at 660 nm (OD660) of 0.1. Equal volumes (100 μL) of diluted bacterial culture and two-fold serial dilutions of the tart cherry extract (ranging from 0.49 to 2000 μg/mL) in THB-HK were added to the wells of a 96-well tissue culture microplate (Sarstedt Inc., St-Leonard, QC, Canada). The microplate was incubated at 37°C for 48 h under anaerobic conditions prior to assessing bacterial growth by recording the OD660 using a Synergy 2 microplate reader (BioTek Instruments, Winooski, VT, USA). Wells with no tart cherry extract or P. gingivalis were used as controls. Triplicate assays in two independent experiments were performed.
Iron-chelating activity of the tart cherry extract
The capacity of the tart cherry extract to chelate iron was determined using a universal siderophore assay, with chrome azurol S and hexadecyltrimethylammonium bromide as indicators, as per the protocol described by Schwyn and Neilands [24]. Ferrichrome (Sigma-Aldrich Canada Co.), a siderophore produced by Ustilago sphaerogena, served as a positive control. Triplicate assays in two independent experiments were performed.
Effect of the tart cherry extract on the adherence of P. gingivalis to a basement membrane matrix model
To determine the effect of the tart cherry extract on the adherence of P. gingivalis to an experimental basement membrane matrix model, the bacterial cells were first labeled with fluorescein isothiocyanate (FITC) as described previously [25]. Matrigel™ (BD Biosciences, San Jose, CA, USA), a solubilized basement membrane preparation extracted from the Engelbreth-Holm-Swarm mouse sarcoma composed of several extracellular matrix proteins, including laminin, type IV collagen, heparin sulfate proteoglycans, and entactin, was diluted 1/10 in ice-cold PBS and was added (50 μL) to the wells of a 96-well clear bottom black microplate (Greiner Bio-One North America, Monroe, NC, USA). After gelification (room temperature for 2 h), the Matrigel™ was washed twice with PBS, and two-fold serial dilutions of the tart cherry extract (100 μL; 31.25, 62.5, 125, or 250 μg/mL in PBS) were added on top of the gel. After 30 min, 100 μL of FITC-labeled P. gingivalis cells (OD660 = 0.5) were added to the wells, and the plate was incubated for a further 4 h at 37°C. Unbound bacteria were then removed by aspiration, and the wells were washed twice with PBS. Relative fluorescence units (RFU; excitation wavelength 495 nm; emission wavelength 525 nm) corresponding to the level of bacterial adherence were determined using a Synergy 2 microplate reader. Wells with no bacteria were used as controls to measure basal auto-fluorescence. Control wells without tart cherry extract were used to determine 100% adherence values. Triplicate assays in two independent experiments were performed.
Effect of the tart cherry extract on the protease activities of P. gingivalis
The effect of the tart cherry extract on collagen degradation by P. gingivalis was first investigated. A 48-h cell-free culture supernatant was mixed with the tart cherry extract (final concentration of 31.25, 62.5, 125, or 250 μg/mL) and fluorescent substrate type I collagen DQ (Molecular Probes, Eugene, OR, USA) (100 μg/mL). Leupeptin (1 μM; Sigma-Aldrich Canada Co.) served as a positive inhibitor control. The mixtures were incubated at 37°C, and fluorescence was measured every 30 min for 2 h using a Synergy 2 multi-mode microplate reader with the excitation and emission wavelengths set at 495 nm and 525 nm, respectively. The effect of the tart cherry extract on P. gingivalis Arg- and Lys-gingipains was also assessed. Briefly, P. gingivalis cells suspended in 50 mM phosphate-buffered saline (PBS, pH 7.2) at an OD660 of 0.1 were mixed with the tart cherry extract (final concentration of 31.25, 62.5, 125, or 250 μg/mL), 10 mM dithiothreitol, and either 5 mM N-α-benzoyl-DL-arginine-p-nitroanilide (Arg-gingipain substrate; Sigma-Aldrich Canada Co.) or N-p-tosyl-glycine-proline-lysine-p-nitroanilide (Lys-gingipain substrate; Sigma-Aldrich Canada Co.). Nα-p-tosyl-L-lysine chloromethyl ketone hydrochloride (8 mM; Sigma-Aldrich Canada Co.) served as a positive inhibitor control. After a 60-min incubation at 37°C, the hydrolysis of the chromogenic substrates was determined by recording the absorbance at 405 nm (A405) with a Synergy 2 multi-mode microplate reader. Triplicate assays in two independent experiments were performed.
Protective effect of the tart cherry extract against the P. gingivalis-induced disruption of oral epithelial barrier integrity in an in vitro model
The immortalized oral epithelial cell line B11, previously characterized by Groeger, Michel & Meyle [26], was cultured at 37°C in a 5% CO2 atmosphere in keratinocyte-serum free medium (K-SFM) supplemented with growth factors (50 μg/mL of bovine pituitary extract and 5 ng/mL of human epidermal growth factor) and 100 μg/mL of penicillin G-streptomycin. The ability of the tart cherry extract to preserve the integrity of the oral epithelial barrier of an in vitro model infected with P. gingivalis was investigated by monitoring the transepithelial electrical resistance (TEER). B11 cells (3 × 105 cells per insert) were seeded on Costar Transwell clear polyester membrane inserts (6.5-mm diameter; 0.4-μm pore size; Corning Co., Cambridge, MA, USA). The apical and basolateral compartments were filled with 100 μL and 600 μL of complete K-SFM, respectively. Following a 72-h incubation (37°C, 5% CO2 atmosphere), the conditioned medium was replaced with fresh antibiotic-free K-SFM, and the cells were incubated for a further 16 h. Non-cytotoxic concentrations of the tart cherry extract (7.813, 15.625, 31.25, 62.5, or 125 μg/mL in culture medium), determined in a preliminary analysis using a 3-(4,5-dimethylthiazol-2-yl)-2,5diphenyltetrazolium bromide (MTT) assay and a 48-h exposure (Roche Diagnostics, Laval, QC, Canada), were added to the apical compartment along with P. gingivalis at a multiplicity of infection (MOI) of 104. TEER values were measured with an ohm/voltmeter (EVOM2; World Precision Instruments, Sarasota, FL, USA) after a 1, 2, 4, 6, 8, 24, and 48-h incubation. Measurements were recorded in triplicate for each treatment. The results were converted to Ohms (Ω)/cm2 by multiplying the resistance by the membrane surface area. Each value was compared to the initial resistance of the well at time 0 (100% value). The integrity of the oral epithelial barrier in the presence of P. gingivalis and the tart cherry extract was also investigated by tracking the paracellular transport of FITC-conjugated dextran (FD-4; 4.4 kDa; Sigma-Aldrich Canada Co.). Briefly, B11 cells were seeded as described above, and FD-4 (1 mg/mL), P. gingivalis (MOI of 104), and the tart cherry extract (15.625, 31.25, 62.5, or 125 μg/mL) were added to the apical compartment. Fluorescence in the basolateral compartment was measured using a Synergy 2 multi-mode microplate reader after a 6, 24, and 48-h incubation. All conditions were tested in triplicate.
Immunofluorescence staining of zonula occludens-1 and occludin
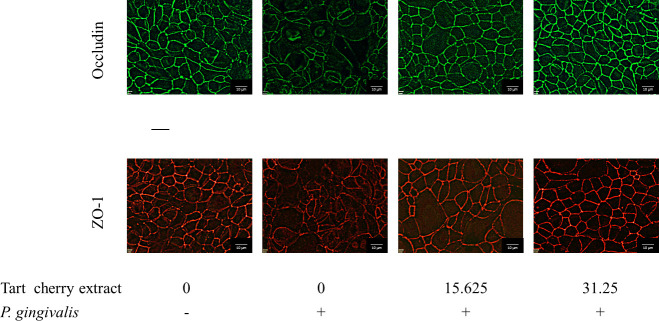
The effect of the tart cherry extract on P. gingivalis-mediated damage to the tight junction proteins zonula occludens-1 (ZO-1) and occludin was monitored by immunofluorescence staining. B11 cells were treated (24 h) with P. gingivalis (MOI of 104). The tight junction proteins were immunostained with either occludin antibody-Alexa Fluor 488 conjugate or ZO-1 antibody-Alexa Fluor 594 conjugate (Thermo Fisher Scientific, Waltham, MA, USA), as described previously [23]. An Olympus FSX100 fluorescence microscope and FSX-BSW imaging software (Olympus, Tokyo, Japan) were used to observe the immunostaining. Treatments were performed in triplicate and a representative set of data is presented.
Statistical analysis
Results are expressed as means ± standard deviations (SD). The statistical analyses were performed using a one-way analysis of variance with a post hoc Bonferroni multiple comparison test (GraphPad Software Inc., La Jolla, CA, USA). The level of significance was set at p < 0.01.
Results
The antibacterial activity of the tart cherry extract against P. gingivalis was determined by assessing bacterial growth in a broth microdilution assay following a 48-h incubation. As shown in Fig 1, although the tart cherry extract did not completely inhibit growth, a significant reduction in growth was observed starting with a concentration of 7.81 μg/mL of the tart cherry extract. At the highest concentration tested (2 mg/mL), the tart cherry extract reduced the growth of P. gingivalis by 57.3%. We then used a universal siderophore assay to assess the iron-chelating activity of the tart cherry extract, a property that may contribute to its ability to reduce the growth of P. gingivalis. Fig 2 shows that the tart cherry extract dose-dependently chelated iron as did ferrichrome, which was used as a positive control.
Fig 1. Effect of the tart cherry extract on the growth of P. gingivalis.
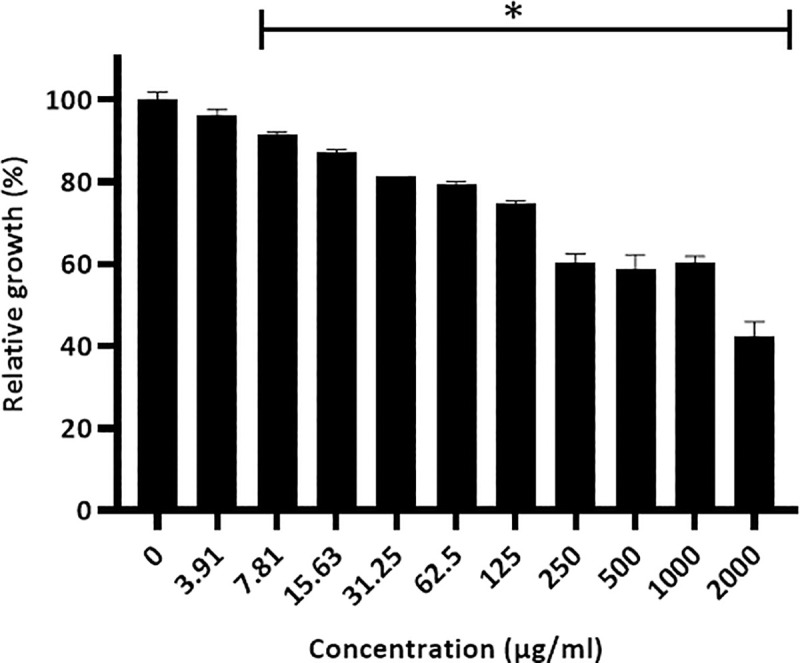
A 100% value was attributed to the control (no tart cherry extract). Results are expressed as the means ± SD of triplicate assays from two independent experiments. *, Significantly different (p < 0.01) from the control (no tart cherry extract).
Fig 2. Iron-chelating activity of the tart cherry extract assessed using a universal siderophore colorimetric assay.
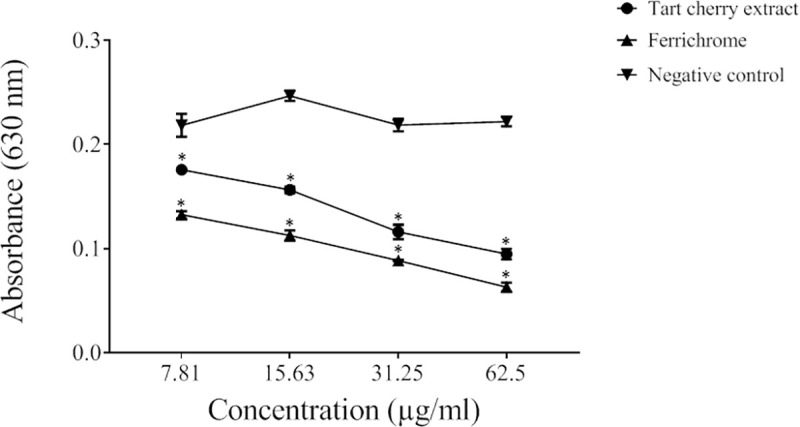
Results are expressed as the means ± SD of triplicate assays from two independent experiments. A decrease in A630 occurs when a strong chelator removes the iron from the chrome azurol S dye. Ferrichrome, a siderophore produced by U. sphaerogena, was used as positive control. All values are significantly different (p < 0.01) from the negative control (no tart cherry extract).
The effect of the tart cherry extract on the adherence of FITC-labeled P. gingivalis to a polystyrene surface coated with Matrigel®, used as an experimental basement membrane matrix model, was investigated. The tart cherry extract dose-dependently inhibited the adherence of P. gingivalis to the Matrigel®. More specifically, at a concentration of 250 μg/mL, the extract reduced adherence by 35.0% (Table 1).
Table 1. Effect of the tart cherry extract on the adherence of P. gingivalis to Matrigel®, a basement membrane matrix model.
| Tart cherry extract (μg/mL) | Relative adherence (%) |
|---|---|
| 0 | 100 ± 7.8 |
| 31.25 | 94.6 ± 4.5 |
| 62.5 | 99.3 ± 15.6 |
| 125 | 67.7 ± 14.3 * |
| 250 | 65.0 ± 10.1 * |
Results are expressed as the means ± SD of triplicate assays from two independent experiments.
*, Significantly different (p < 0.01) from the control (no tart cherry extract).
P. gingivalis can mediate the destruction of periodontal connective tissue by breaking down type I collagen. We showed that the tart cherry extract inhibits, in a dose-and time-dependent manner, the degradation of collagen by P. gingivalis. After a 2-h incubation, the lowest concentration (31.25 μg/mL) of the extract inhibited collagen degradation by 33.3% while the highest concentration (250 μg/mL) inhibited degradation by 82.1% (Fig 3). The effect of the tart cherry extract on the cell-associated Arg- and Lys-gingipain activities of P. gingivalis was also evaluated. As reported in Table 2, the extract dose-dependently inhibited both activities. More specifically, at a concentration of 250 μg/mL, the extract inhibited the Arg- and Lys-gingipains by 62.5% and 37.5%, respectively.
Fig 3. Effect of the Tart Cherry Extract (TCE) on collagen degradation by P. gingivalis.
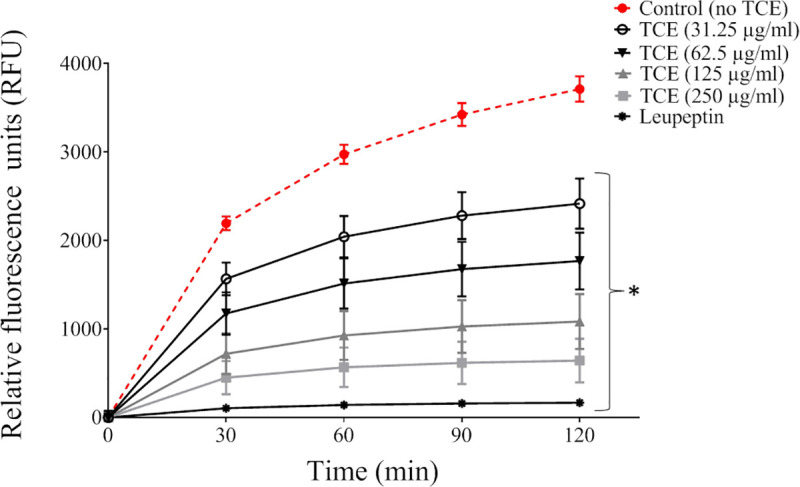
Collagen degradation was assayed using a fluorescent substrate. Results are expressed as the means ± SD of triplicate assays.
Table 2. Effect of the tart cherry extract on the Arg- and Lys-gingipain activities of P. gingivalis.
| Compounds | Arg-gingipain activity (%) | Lys-gingipain activity (%) |
|---|---|---|
| None | 100 | 100 |
| Tart cherry extract (μg/mL) | ||
| 31.25 | 100 ± 12.54 | 97.94 ± 5.51 |
| 62.5 | 86.73 ± 4.55 * | 92.03 ± 2.51 |
| 125 | 58.41 ± 4.54 * | 83.29 ± 5.55 * |
| 250 | 37.47 ± 11.81 * | 62.47 ± 7.40 * |
| TLCK (commercial inhibitor) | 0 * | 0 * |
Results are expressed as the means ± SD of triplicate assays from two independent experiments.
*, Significantly different (p < 0.01) from the control (no tart cherry extract).
As shown in Fig 4, for an incubation time ≥ 8 h, P. gingivalis (MOI of 104) caused a significant time-dependent decrease in TEER in an in vitro model of the oral epithelial barrier using a double-chamber system. The increase in TEER observed for shorter incubation periods (1, 2, 4, and 6 h) may be an epithelial cell barrier defense response to the initial bacterial challenge. The TEER values decreased by 21.8%, 32.5%, and 75.6% after a 8, 24, and 48-h exposure to P. gingivalis, respectively. However, the addition of the tart cherry extract to the apical compartment together with P. gingivalis resulted in a significant protective effect with as little as 7.9 μg/mL following a 8 and 24-h incubation, as indicated by the smaller decrease in TEER values. Concentrations of the tart cherry extract ≥ 15.625 μg/mL were required to prevent the P. gingivalis-mediated decrease in TEER following a 48-h incubation.
Fig 4. Effect of the tart cherry extract on the disruption of epithelial barrier integrity by P. gingivalis (MOI of 104) assessed by monitoring TEER over a period of 48 h.
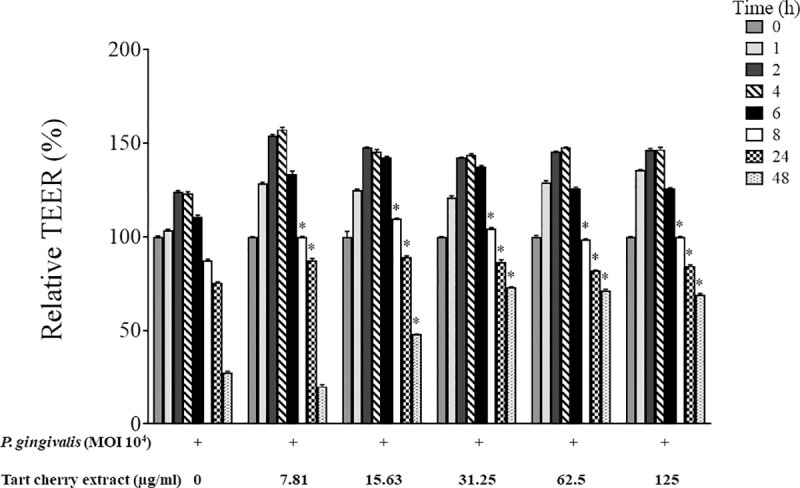
A 100% value was attributed to the TEER value at time 0. Results are expressed as the means ± SD of triplicate assays. *, Significantly different (p < 0.01) from the control (no tart cherry extract).
P. gingivalis-mediated damage to the oral epithelial barrier and the protective effect of the tart cherry extract were further investigated by monitoring FD-4 transport across the in vitro model. No significant differences for all the concentrations of the extract tested were observed after a 24-h challenge with P. gingivalis (Fig 5). However, after a 48-h challenge, the tart cherry extract decreased the P. gingivalis-mediated flux of FD-4 across the oral epithelial barrier. At a concentration of 125 μg/mL, the tart cherry extract reduced the P. gingivalis-mediated flux of FD-4 by 4.2-fold.
Fig 5. Effect of the tart cherry extract on the disruption of epithelial barrier integrity by P. gingivalis (MOI of 104) assessed by measuring FD-4 transepithelial transport over a period of 48 h.
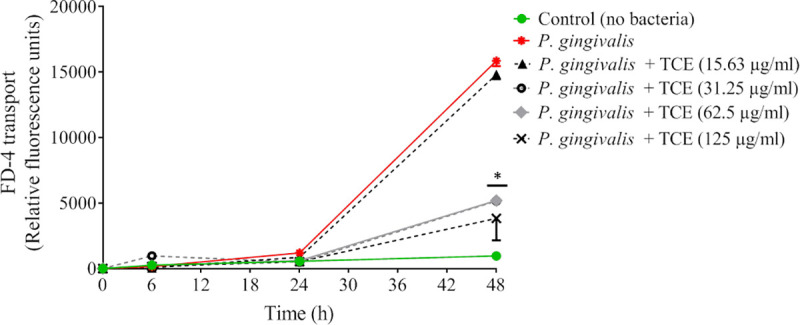
Results are expressed as the means ± SD of triplicate assays. *, Significantly different (p < 0.01) from the control (no tart cherry extract).
ZO‐1 and occludin in the P. gingivalis-treated oral epithelial barrier model were immunostained to investigate the effect of the tart cherry extract on these two tight junction proteins. Treating the oral epithelial barrier model with P. gingivalis resulted in discontinuous, weak labeling of ZO‐1 and occludin compared to untreated control cells (Fig 6). However, the addition of ≥ 31.25 μg/mL of the tart cherry extract together with P. gingivalis prevented the degradation of ZO‐1 and occludin.
Fig 6. Effect of the tart cherry extract on the immunofluorescence staining of zonula-occludens 1 (ZO-1) and occludin of oral epithelial cell monolayers treated with P. gingivalis (MOI of 104) for 24 h.
Discussion
P. gingivalis is a key member of the pathogenic subgingival biofilm that induces chronic inflammation and that, in turn, leads to destructive periodontitis [6]. It has been detected in 75% of active sites and in 59.7% of inactive sites in 96% of patients with progressive adult periodontitis [27]. Given its critical role in the pathogenesis of periodontitis, P. gingivalis may be a potential target for developing a therapeutic approach to prevent and treat this disease. In this study, we investigated the potential of a tart cherry phenolic extract, containing as main components procyanidins as well as quercetin and its derivatives (rutinoside, glucoside), to help restore periodontal health by focusing on its effect on P. gingivalis.
We first showed that the growth of P. gingivalis is attenuated by the tart cherry extract, which may be associated, at least in part, with the fact that the extract can chelate iron. Iron is an essential nutrient for most bacteria and, as such, is an important factor during infections [28]. Hemin was added to the culture medium as a source of iron and protoporphyrin IX in order to support the in vitro growth of P. gingivalis. Ohya et al. have reported that the growth of P. gingivalis is negatively affected under hemin-limited conditions [29]. Given this, the curtailment of iron available to P. gingivalis due to the iron-chelating property of the tart cherry extract could explain the significant reduction in growth. Interestingly, other studies have shown that plant polyphenols act as natural antimicrobial compounds because of their ability to chelate iron ions [30, 31] and that the iron-chelating efficiency of phenolic compounds depends on the number of binding sites they possess [32].
The establishment of a dysbiotic periodontal community, of which P. gingivalis is a key member, is largely dependent on its ability to obtain nutrients (peptides, amino acids, iron) from the proteolytic degradation of host proteins such as tissue constituents (collagen, fibronectin, etc.) and hemoproteins (hemoglobin, hemopexin, etc.) [4]. We thus explored the ability of the tart cherry extract to inhibit the proteases produced by P. gingivalis. The tart cherry extract acted as an effective inhibitor of Arg-gingipains and, to a lesser extent, of Lys-gingipain. In addition to its iron-scavenging property, the capacity of the tart cherry extract to inhibit Arg- and Lys-gingipain activities may also contribute to attenuating the growth of P. gingivalis. The capacity of other plant polyphenols, such as those in black tea, green tea, blueberry, and cranberry, to inhibit P. gingivalis gingipains has been previously reported [25, 33, 34].
A variety of pathogenic roles have been associated with Arg-gingipains A and B and Lys-gingipain produced by P. gingivalis [7, 8]. Gingipains enable bacteria to evade the innate immunity response in periodontal pockets by reducing macrophage phagocytosis by decreasing the surface expression of CD14 [35]. Moreover, gingipains may play a key role in allowing P. gingivalis to survive complement-dependent killing [36, 37]. The inhibition of gingipains by the tart cherry extract may thus prevent P. gingivalis, along with other periodontal pathogens, to escape the host defense system and thus enhance its survival in periodontal pockets.
As P. gingivalis gingipains are considered to play a central role in the pathogenesis of periodontitis, there is considerable interest in the possibility of identifying and developing gingipain inhibitors as potential therapeutics [38]. Such inhibitors may have applications beyond periodontal diseases. Indeed, P. gingivalis gingipains were recently detected in the brains of Alzheimer's patients where they may exert a neurotoxic effect associated with neuronal dysfunction [39]. In addition, it has been suggested that the blockage of this neurotoxicity using small-molecule inhibitors that target gingipains may reduce neurodegeneration in Alzheimer's disease [39].
The collagenase activity of P. gingivalis may also be an important virulence factor since it may participate in gingival tissue breakdown. Indeed, type I collagen makes up approximately 60% of the tissue volume of the periodontium. In this study, we showed that the tart cherry extract inhibits collagen degradation by P. gingivalis, suggesting that it may contribute to reducing the tissue destructive process. In addition to the action of proteases produced by P. gingivalis, matrix metalloproteinases secreted by resident and immune cells also participate in this process [40]. We are currently investigating whether the tart cherry extract is also effective in inhibiting the catalytic activity of matrix metalloproteinases.
The adherence of P. gingivalis to the oral mucosa is the initial step in the invasion of tissues. The ability of P. gingivalis to adhere to several extracellular matrix proteins, including laminin and type IV collagen, has been previously reported [41]. The present study showed that the tart cherry extract can decrease the adherence of P. gingivalis to Matrigel®, a basement membrane matrix model. Such property may alter the ability of P. gingivalis to colonize the subgingival sites and consequently reduce their numbers.
The oral epithelial barrier plays a key role in protecting the host against microbial invasion. Tight junction proteins ensure the structural integrity of the barrier by keeping epithelial cells closely attached to one another. They also possess a gate function that modulates the passage of ions and molecules through the paracellular pathway [42]. Groeger et al. used specific gingipain inhibitors and gingipain-deficient mutants to show that these P. gingivalis proteases are involved in the degradation of cell-to-cell junctions and the disruption of the epithelial barrier [43]. Moreover, Andrian et al. studied an engineered human oral mucosa model composed of primary epithelial cells and fibroblasts and reported that gingipains are involved in the ability of P. gingivalis to infiltrate multilayered epithelial cell structures, migrate through the basement membrane, and reach the underlying connective tissue [44]. In a previous study, we found that the integrity of the oral epithelial barrier is strengthened in the presence of tart cherry extracts, as shown by an increase in TEER values and an overexpression of the tight junction proteins ZO-1 and occluding [23]. These two proteins play a key role in establishing cell-to-cell contacts and in maintaining the function of the epithelial barrier and the permeability of the paracellular pathway. Katz et al. reported that P. gingivalis gingipains can degrade epithelial junction transmembrane proteins, including occludin [45]. Given that the tart cherry extract inhibits the proteolytic activity of P. gingivalis, we hypothesized that it may protect the oral epithelial barrier against bacteria-induced damage. We used an in vitro model to show that there is an increase in TEER following a short incubation (≤ 6 h) with P. gingivalis followed by a decline in TEER. This may reflect a tightening of the host defense barrier until it succumbs to the proteolytic activity of the bacteria. Our results showed that the tart cherry extract can protect the integrity of the oral epithelial barrier despite a challenge with P. gingivalis by maintaining transepithelial resistance and reducing the flux of FD-4. The protective effect was confirmed by monitoring the expression and distribution of ZO-1 and occludin. This is in agreement with several studies that have reported that quercetin, a major component of the tart cherry extract, has a protective effect on the intestinal epithelial barrier by its ability to induce an increase in TEER values and the overexpression of tight junction proteins [46–48]. Part of the protective effect is also likely related to the ability of the tart cherry fraction to inhibit the Arg- and Lys-gingipains of P. gingivalis, which can degrade junction proteins.
Conclusions
Polyphenols have attracted attention in the medical field as potential therapeutic molecules for reducing the use of antibiotics and, as a result, limiting antibiotic resistance among bacteria [49]. The results obtained in the present study suggest that a phenolic extract of tart cherry juice exerts antibacterial activity against P. gingivalis by reducing its growth and weakening its virulence factors, mostly by inhibiting gingipain activities. Clinical studies are needed to determine whether tart cherry juice consumption or the incorporation of a tart cherry phenolic extract in oral hygiene products (mouthrinses and chewing gums) or slow periodontal-release devices (inserted in diseased periodontal sites) may be potentially used for the treatment of periodontitis.
Acknowledgments
The technical assistance of Marie-Pierre Morin is acknowledged. We wish to thank S. Groeger and J. Meyle (Department of Periodontology, Justus-Liebig-University Giessen, Germany) for kindly providing the B11 keratinocyte cell line.
Data Availability
All relevant data are within the manuscript.
Funding Statement
DG; 2020-11; Laboratoire de Contrôle Microbiologique de l'Université Laval; The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Lamont RJ, Koo H, Hajishengallis G. The oral microbiota: dynamic communities and host interactions. Nat Rev Microbiol. 2018;16: 745–759. 10.1038/s41579-018-0089-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hajishengallis G, Krauss JL, Liang S, Mcintosh ML, Lambris JD. Pathogenic microbes and community service through manipulation of innate immunity. Adv Exp Med Biol. 2012;946: 69–85. 10.1007/978-1-4614-0106-3_5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hajishengallis G. Immuno-microbial pathogenesis of periodontitis: Keystones, pathobionts, and the host response. Trends Immunol. 2014;35: 3–11. 10.1016/j.it.2013.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frencken J, Sharma P, Stenhouse L, Green D, Laverty D, Dietrich T. Global epidemiology of dental caries and severe periodontitis–a comprehensive review. J Clin Periodontol. 2017;44: S94–S105. 10.1111/jcpe.12677 [DOI] [PubMed] [Google Scholar]
- 5.Konkel JE, Boyle CO, Krishnan S. Distal consequences of oral inflammation. Front Immunol. 2019;10 10.3389/fimmu.2019.01403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.How KY, Song KP, Chan KG, Caldwell CC. Porphyromonas gingivalis: An overview of periodontopathic pathogen below the gum line. Front Microbiol. 2016;7 10.3389/fmicb.2016.00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fitzpatrick R, Wijeyewickrema L, Pike R. The gingipains: scissors and glue of the periodontal pathogen, Porphyromonas gingivalis. Future Microbiol. 2009;4: 471–478. 10.2217/fmb.09.18 [DOI] [PubMed] [Google Scholar]
- 8.Grenier D, La V. Proteases of Porphyromonas gingivalis as important virulence factors in periodontal disease and potential targets for plant-derived compounds: A review article. Curr Drug Targets. 2011;12: 322–331. 10.2174/138945011794815310 [DOI] [PubMed] [Google Scholar]
- 9.Jain S, Darveau RP. Contribution of Porphyromonas gingivalis lipopolysaccharide to periodontitis. Periodontol 2000. 2010;54: 53–70. 10.1111/j.1600-0757.2009.00333.x.Contribution [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mysak J, Podzimek S, Sommerova P, Lyuya-Mi Y, Bartova J, Janatova T, et al. Porphyromonas gingivalis: Major periodontopathic pathogen overview. J Immunol Res. 2014;2014 10.1155/2014/476068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakayama M, Ohara N. Molecular mechanisms of Porphyromonas gingivalis-host cell interaction on periodontal diseases. Jpn Dent Sci Rev. 2017;53: 134–140. 10.1016/j.jdsr.2017.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cekici A, Kantarci A, Hasturk H, Van Dyke TE. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontol 2000. 2014;64: 57–80. 10.1111/prd.12002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan W, Wang Q, Chen Q. The cytokine network involved in the host immune response to periodontitis. Int J Oral Sci. 2019;11 10.1038/s41368-019-0064-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chai SC, Davis K, Wright RS, Kuczmarski MF, Zugui Z. Function pressure and low-density lipoprotein cholesterol in older adults: a randomized controlled trial. Food Funct. 2018;9: 3185–3194. 10.1039/c8fo00468d [DOI] [PubMed] [Google Scholar]
- 15.Kelley DS, Adkins Y, Laugero KD. A review of the health benefits of cherries. Nutrients. 2018;10: 1–22. 10.3390/nu10030368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirakosyan A, Gutierrez E, Ramos B, Seymour EM, Bolling SF. The inhibitory potential of Montmorency tart cherry on key enzymes relevant to type 2 diabetes and cardiovascular disease. Food Chem. 2018;252: 142–146. 10.1016/j.foodchem.2018.01.084 [DOI] [PubMed] [Google Scholar]
- 17.McCune L, Kubota C, Stendell-Hollis N, Thamson C. Cherries and health: A review. Crit Rev Food Sci Nutr. 2011;51: 1–12. 10.1080/10408390903001719 [DOI] [PubMed] [Google Scholar]
- 18.Vitale KC, Hueglin S, Broad E. Tart cherry juice in athletes: A literature review and commentary. Nutr Ergogenic Aids. 2017;16: 230–239. 10.1249/JSR.0000000000000385 [DOI] [PubMed] [Google Scholar]
- 19.Mayta-Apaza AC, Marasini D, Carbonero F. Tart cherries and health: Current knowledge and need for a better understanding of the fate of phytochemicals in the human gastrointestinal tract. Crit Rev Food Sci Nutr. 2019;59: 626–638. 10.1080/10408398.2017.1384918 [DOI] [PubMed] [Google Scholar]
- 20.Ben Lagha A, Desjardins Y, Grenier D. Wild blueberry (Vaccinium angustifolium Ait.) polyphenols target Fusobacterium nucleatum and the host inflammatory response: Potential innovative molecules for treating periodontal diseases. J Agric Food Chem. 2015;63: 6999–7008. 10.1021/acs.jafc.5b01525 [DOI] [PubMed] [Google Scholar]
- 21.Ben Lagha A, LeBel G, Grenier D. Dual action of highbush blueberry proanthocyanidins on Aggregatibacter actinomycetemcomitans and the host inflammatory response. BMC Complement Altern Med. 2018;18: 1–14. 10.1186/s12906-017-2057-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feghali K, Feldman M, La VD, Santos J, Grenier D. Cranberry proanthocyanidins: Natural weapons against periodontal diseases. J Agric Food Chem. 2012; 5728–5735. 10.1021/jf203304v [DOI] [PubMed] [Google Scholar]
- 23.Ben Lagha A, LeBel G, Grenier D. Tart cherry (Prunus cerasus L.) fractions inhibit biofilm formation and adherence properties of oral pathogens and enhance oral epithelial barrier function. Phyther Res. 2020;34: 886–895. 10.1002/ptr.6574 [DOI] [PubMed] [Google Scholar]
- 24.Schwyn B, Neilands J. Universal chemical assay for the detection determination of siderophores. Anal Biochem. 1987;160: 47–56. 10.1016/0003-2697(87)90612-9 [DOI] [PubMed] [Google Scholar]
- 25.Ben Lagha A, Groeger S, Meyle J, Grenier D. Green tea polyphenols enhance gingival keratinocyte integrity and protect against invasion by Porphyromonas gingivalis. Pathog Dis. 2018;76: 1–9. 10.1093/femspd/fty030 [DOI] [PubMed] [Google Scholar]
- 26.Groeger S, Michel J, Meyle J. Establishment and characterization of immortalized human gingival keratinocyte cell lines. J Periodontal Res. 2008;43: 604–614. 10.1111/j.1600-0765.2007.01019.x [DOI] [PubMed] [Google Scholar]
- 27.López NJ. Occurrence of Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis, and Prevotella intermedia in progressive adult periodontitis. J Periodontol. 2000;71: 948–954. 10.1902/jop.2000.71.6.948 [DOI] [PubMed] [Google Scholar]
- 28.Wooldridge KG, Williams PH. Iron uptake mechanisms of pathogenic bacteria. FEMS Microbiol Rev. 1993;12: 325–348. 10.1111/j.1574-6976.1993.tb00026.x [DOI] [PubMed] [Google Scholar]
- 29.Ohya M, Cueno ME, Tamura M, Ochiai K. Varying hemin concentrations affect Porphyromonas gingivalis strains differently. Microb Pathog. 2016;94: 54–59. 10.1016/j.micpath.2015.10.016 [DOI] [PubMed] [Google Scholar]
- 30.Lemanceau P, Expert D, Gaymard F, Bourgogne D, Dijon F, Bernard C, et al. Role of iron in plant-microbe interactions 1st ed Advances in Botanical Research. Elsevier Ltd; 2009. 10.1016/S0065-2296(09)51012-9 [DOI] [Google Scholar]
- 31.Mila I, Scalbert A, Expert D. Iron withholding by plant polyphenols and resistance to pathogens and rots. Phytochemistry. 1996;42: 1551–1555. [Google Scholar]
- 32.Khokhar S, Apenten RKO. Iron binding characteristics of phenolic compounds: some tentative structure-activity relations. Food Chem. 2003;81: 133–140. [Google Scholar]
- 33.Bodet C, Chandad F, Grenier D. Anti-inflammatory activity of a high-molecular-weight cranberry fraction on macrophages stimulated by lipopolysaccharides from periodontopathogens. J Dent Res. 2006;85: 235–239. 10.1177/154405910608500306 [DOI] [PubMed] [Google Scholar]
- 34.Ben Lagha A, Howell A, Grenier D. Highbush blueberry proanthocyanidins alleviate Porphyromonas gingivalis-induced deleterious effects on oral mucosal cells. Anaerobe. 2020;65: 102266 10.1016/j.anaerobe.2020.102266 [DOI] [PubMed] [Google Scholar]
- 35.Wilensky A, Tzach-Nahman R, Potempa J, Shapira L, Nussbaum G. Porphyromonas gingivalis gingipains selectively reduce CD14 expression, leading to macrophage hyporesponsiveness to bacterial infection. J Innate Immun. 2015;7: 127–135. 10.1159/000365970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grenier D, Roy S, Chandad F, Plamondon P, Yoshioka M, Nakayama K, et al. Effect of inactivation of the Arg- and/or Lys-gingipain gene on selected virulence and physiological properties of Porphyromonas gingivalis. Infect Immun. 2003;71: 4742–4748. 10.1128/iai.71.8.4742-4748.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Popadiak K, Potempa J, Riesbeck K, Blom AM, Popadiak K, Potempa J, et al. Biphasic effect of gingipains from Porphyromonas gingivalis on the human complement system. J Immunol. 2020;178: 7242–7250. 10.4049/jimmunol.178.11.7242 [DOI] [PubMed] [Google Scholar]
- 38.Allaker RP, Douglas CWI. Non-conventional therapeutics for oral infections. Virulence. 2015;6: 196–207. 10.4161/21505594.2014.983783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dominy SS, Lynch C, Ermini F, Benedyk M, Marczyk A, Konradi A, et al. Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci Adv. 2019;5: eaau3333 10.1126/sciadv.aau3333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Checchi V, Maravic T, Bellini P, Generali L, Consolo U, Breschi L, et al. The role of matrix metalloproteinases in periodontal disease. Int J Environ Res Public Health. 2020;17: 4923 10.3390/ijerph17144923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amano A. Molecular interaction of Porphyromonas gingivalis with host cells: Implication for the microbial pathogenesis of periodontal disease. J Periodontol. 2003;74: 90–96. 10.1902/jop.2003.74.1.90 [DOI] [PubMed] [Google Scholar]
- 42.Wang S, Pang X, Zheng M, Tang Y. The maintenance of an oral epithelial barrier. Life Sci. 2019;227: 129–136. 10.1016/j.lfs.2019.04.029 [DOI] [PubMed] [Google Scholar]
- 43.Groeger S, Doman E, Chakraborty T, Meyle J. Effects of Porphyromonas gingivalis infection on human gingival epithelial barrier function in vitro. J Oral Sci. 2010;118: 582–589. 10.1111/j.1600-0722.2010.00782.x [DOI] [PubMed] [Google Scholar]
- 44.Andrian E, Grenier D, Rouabhia M. In vitro models of tissue penetration and destruction by Porphyromonas gingivalis. Infect Immun. 2004;72: 4689–4698. 10.1128/IAI.72.8.4689-4698.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Katz J, Sambandam V, Wu JH, Michalek SM, Balkovetz DF. Characterization of Porphyromonas gingivalis-induced degradation of epithelial cell junctional complexes. Infect Immun. 2000;68: 1441–1449. 10.1128/iai.68.3.1441-1449.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amasheh M, Schlichter S, Amasheh S, Mankertz J, Zeitz M, Fromm M, et al. Quercetin enhances epithelial barrier function and increases claudin-4 expression. J Nutr. 2008; 1067–1073. 10.1093/jn/138.6.1067 [DOI] [PubMed] [Google Scholar]
- 47.Carrasco-Pozo C, Morales P, Gotteland M. Polyphenols protect the epithelial barrier function of Caco-2 cells exposed to indomethacin through the modulation of occludin and zonula occludens-1 expression. J Agric Food Chem. 2013;61: 5291–5297. 10.1021/jf400150p [DOI] [PubMed] [Google Scholar]
- 48.Suzuki T, Hara H. Quercetin enhances intestinal barrier function through the assembly of zonula occludens-2, occludin, and claudin-1 and the expression of claudin-4 in Caco-2 cells. J Nutr. 2009; 965–974. 10.3945/jn.108.100867 [DOI] [PubMed] [Google Scholar]
- 49.Daglia M. Polyphenols as antimicrobial agents. Curr Opin Biotechnol. 2012;23: 174–181. 10.1016/j.copbio.2011.08.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript.