Abstract
Objective
Literature describing use of clozapine by children and adolescents is limited. The primary study objective was to assess the patterns of clozapine use in an inpatient child and adolescent population.
Methods
A retrospective review of child and adolescent inpatients receiving clozapine at a Canadian children’s hospital from January 2000 through December 2014 was conducted. Interdisciplinary comprehensive data collection was conducted by experienced clinicians. Baseline population characteristics and psychiatric illness risk factors were captured. Illness symptoms and severity were assessed retrospectively using validated measures including the Brief Psychiatric Rating Scale (BPRS), Children’s Global Assessment Scale (CGAS) and Clinical Global Impressions (CGI) scales. Estimated clozapine dosing requirements for each patient to achieve a serum level associated with response was calculated. Clozapine-related adverse events were captured.
Results
Twenty-eight inpatients (64% female) receiving clozapine during the study period were identified. Mean age at clozapine initiation was 15.8 years. Twenty-three patients (82%) were taking clozapine at discharge, and of these, 22 patients (96%) experienced at least minimal improvement in BPRS and CGAS scores. Patients took a mean of 33.1 days from clozapine start to reach their maximum clozapine dosage, a mean maximum of 57% of their estimated clozapine dose requirement. Mean length of stay following clozapine initiation was 60.7 days. We observed a high rate of benign hematological adverse events, but no episodes of severe neutropenia. The majority of patients were of ethnicity associated with high risk for metabolic adverse events.
Conclusion
Most hospitalized, treatment-refractory children requiring clozapine clinically improve despite experiencing high, but largely manageable, adverse event rates.
Keywords: clozapine, child, adolescent, early-onset, schizophrenia spectrum disorders
Mots clés: clozapine, enfant, adolescent, début hâtif, troubles du spectre de la schizophrénie
Résumé
Objectif
La littérature décrivant l’utilisation de la clozapine par les enfants et les adolescents est limitée. Le premier objectif de l’étude était d’évaluer les modèles de l’utilisation de la clozapine dans une population hospitalisée d’enfants et d’adolescents.
Méthodes
Une revue rétrospective d’enfants et d’adolescents hospitalisés recevant de la clozapine dans un hôpital canadien pour enfants de janvier 2000 à décembre 2014 a été menée. Une collection de données interdisciplinaires détaillées a été menée par des cliniciens d’expérience. Les caractéristiques de la population au départ et les facteurs de risque de maladie psychiatrique ont été captés. Les symptômes et la gravité de la maladie ont été évalués rétrospectivement à l’aide de mesures validées notamment l’échelle abrégée d’évaluation psychiatrique (BPRS), l’échelle d’évaluation globale des enfants (CGAS) et les échelles d’évaluation clinique (CGI) Les besoins de dosage estimés de clozapine pour que chaque patient obtienne un niveau sérique associé à la réponse ont été calculés. Les effets indésirables liés à la clozapine ont été captés.
Résultats
Vingt-huit patients hospitalisés (64% de sexe féminin) recevant de la clozapine durant la période de l’étude ont été identifiés. L’âge moyen lors de l’initiation de la clozapine était de 15,8 ans. Vingt-trois patients (82%) prenaient de la clozapine au congé, et sur ceux-là, 22 patients (96%) connaissaient au moins un minimum d’amélioration aux scores de BPRS et CGAS. Les patients ont pris une moyenne de 33,1 jours à partir du début de la clozapine pour apprendre à connaître leur dosage maximum de clozapine, un maximum moyen de 57% de leur besoin estimé, de dose de clozapine. La durée de séjour moyenne suivant l’initiation de clozapine était de 60,7 jours. Nous avons observé un taux élevé d’effets indésirables d’hématologie bénigne mais aucun épisode de neutropénie grave. La majorité des patients étaient d’une ethnicité associée à un risque élevé d’événements métaboliques indésirables.
Conclusion
La plupart des enfants réfractaires au traitement hospitalisés nécessitant de la clozapine s’améliorent cliniquement même s’ils connaissent des effets indésirables élevés mais largement traitables.
Introduction
Clinical guidelines and an emerging body of evidence support the use of clozapine in children and adolescents with refractory psychosis, but it is underutilized (Abidi et al., 2017; Driver, Thomas, Gogtay & Rapoport, 2020). Early onset psychosis is a complex phenomenon that can be understood as a dimensional construct. Reflecting mild and transitory symptoms with no associated functional impairment through to pervasive, unremitting symptoms with profound functional impairment (van Os, Linscott, Myin-Germeys, Delespaul & Krabbendam, 2009). In the absence of a contributory and reversible comorbidity (e.g., medical condition or active substance use), children and youth with the most severe forms of psychosis commonly evolve to develop a primary and severe mental illness sharing the same clinical symptoms and neurobiology of adult schizophrenia, schizoaffective, or bipolar disorder (Rapoport, Addington & Frangou, 2005; Gochman, Miller & Rapoport, 2011). Mild psychotic or psychotic-like symptoms in children and adolescents are relatively common, with prevalence rates as high as 17% (Kelleher et al., 2012). Prevalence of the more severe forms of psychosis, however, that meet criteria for schizophrenia and schizoaffective disorders, are much less common, with adult lifetime prevalence rates at approximately 0.5% and early childhood onset rates at approximately 0.041% (Simeone, Ward, Rotella, Collins & Windisch, 2012; Driver et al., 2020).
Although rare, individuals with early onset schizophrenia (EOS, <18 years of age), schizoaffective, or bipolar disorder tend to have a more complex etiology, an insidious onset with associated diagnostic confusion, more pervasive premorbid abnormalities including cognitive delays and neuro-atypicalities, and poorer long-term outcomes including residual symptoms, physical morbidities, and higher dependency (Clemmensen, Vernal & Steinhausen, 2012; McClellan, Stock & AACAP Committee on Quality Issues, 2013). Further, less than half of individuals with EOS tend to respond to first- or second-generation US Food & Drug Administration (FDA) approved antipsychotic treatments (Sikich et al., 2008). The atypical antipsychotic, clozapine, has demonstrated effectiveness in treatment-refractory schizophrenia, schizoaffective disorder, and bipolar disorder in adults (HLS Therapeutics, Inc., 2019; Calabrese et al., 1996). An emerging body of evidence supports the use of clozapine with children. For purposes of this study, children are defined as persons between the ages of 10 and 19 years of age. Schizophrenia spectrum illness is used here to include schizophrenia and schizoaffective disorder. Case reports of the successful use of clozapine for treatment of schizophrenia in children were first published in 1992 (Birmaher, Baker, Kapur, Quintana & Ganguli, 1992). Subsequently, small, double-blind trials of clozapine in patients with childhood-onset schizophrenia (COS, onset <13 years of age) versus haloperidol and olanzapine as well as a trial in patients with EOS versus olanzapine demonstrated superior efficacy of clozapine over standard treatments (Kumra et al, 1996; Shaw et al., 2006; Kumra et al., 2008). A correlation between clinical improvement on the Scale for the Assessment of Negative Symptoms (SANS) and mean clozapine serum concentration has been identified in children taking clozapine (Frazier et al., 2003). Additionally, in children taking clozapine, clinical improvement on the Brief Psychiatric Rating Scale (BPRS) and the Scale for Assessment of Positive Symptoms (SAPS) is associated with the norclozapine (n-desmethylclozapine, NDMC) to clozapine ratio (Sporn et al., 2007).
The Canadian Schizophrenia Guidelines and the British National Institute for Health Care Excellence (NICE) guidelines on psychosis and schizophrenia in children and young people state that clozapine treatment should be offered to children with schizophrenia spectrum illness who have not responded adequately to pharmacological treatment despite the use of adequate doses of at least two different antipsychotic drugs sequentially administered for 6 to 8 weeks each (Abidi et al, 2017; National Institute for Health and Care Excellence, 2016). Despite these guidelines, the demonstrated efficacy of clozapine in adults with refractory schizophrenia spectrum illnesses, and the emerging evidence supporting the use of clozapine in children with refractory schizophrenia spectrum illnesses, clozapine remains underutilized (Abidi et al., 2017). Further, based on adult bipolar disorder treatment guidelines, clozapine (monotherapy or adjunctive) may be used as a third-line treatment for refractory bipolar symptoms (Yatham et al., 2018).
Barriers to clozapine utilization described in the literature are medical risk and concern among families and healthcare providers regarding potential clozapine-related adverse events (Kelly, Freudenreich, Sayer & Love, 2018). Higher incidences of leukopenia and dose-related adverse events with clozapine treatment are reported in children compared to adults (Gerbino-Rosen, et al., 2005). This is possibly due to children’s achievement of relatively higher proportional NDMC concentrations (Frazier, et al., 2003). Despite higher rates of leukopenia and neutropenia, the incidence of agranulocytosis remains uncommon and does not appear higher in children versus adults (Gerbino-Rosen et al., 2005). Myocarditis during clozapine treatment is rarely reported in children (Mudra, Luedecke, Grafmann & Schulte-Markwort, 2018). Clozapine is associated with a dose-related increased risk of metabolic syndrome and a lowering of the seizure threshold (Pringsheim, Panagiotopoulous, Davidson & Ho, 2011; Wong & Delva, 2007).
Although the population of children requiring clozapine is relatively small in number, the resource allocation to support these complex children is significant. Given the challenge of long-term poor prognosis for children with EOS, it is imperative to strive to enhance outcomes (Clemmensen et al., 2012). To better understand this exceptionally vulnerable population, we retrospectively evaluated inpatient clozapine treatment response and adverse events.
Methods
The study was approved by the University of British Columbia – Children’s & Women’s Health Centre of BC Research Ethics Board. All children admitted to the inpatient mental health programs at BC Children’s Hospital (BCCH) between January 2000 and December 2014 for whom clozapine was dispensed during their admission were identified via the inpatient pharmacy database. When multiple admissions for the same individual were identified, the inpatient admission during which clozapine was first dispensed was evaluated. Data collection was performed via retrospective chart review. Captured parameters included: demographics, anthropometric data, prior individual and family medical and psychiatric history, prior medication trials, substance use history, hematological monitoring, vital signs, electrocardiogram readings, chemistry and metabolic lab tests, physical and mental status examination, illness severity and clinical response.
Illness symptoms, severity ratings, and clinical response were assessed retrospectively using validated measures by an experienced psychologist. Assessments were completed by detailed review of interdisciplinary chart notes and all available collateral records. Adhering to descriptive item anchors, ratings on five clinical response scales were completed at three time points: admission, immediately prior to clozapine start, and discharge. Specifically, the 18 item BPRS anchored version with 1–7 item ratings; Clinical Global Impression (CGI) scales: Severity of Illness (CGI-S), Global Improvement (CGI-I), and Efficacy Index (CGI-EI); and the Children’s Global Assessment Scale (CGAS) were used (Woerner, Manuzza & Kane, 1988; Busner & Targum, 2007, Shaffer et al., 1983). All measures are well established clinical symptom and treatment response assessment tools with acceptable reliability and validity evidence (Woerner et al., 1988). Descriptive statistics are provided for all measures. To test for change in clinical symptoms and clinical response across time, repeated measures ANOVA with Greenhouse-Geisser correction for lack of sphericity was used for the continuous outcome variables assessed at three time points (BPRS, CGI-S, and CGAS). For the continuous outcome variables assessed at two time points (CGI-I and CGI-EI), paired-sample t-tests were run to compare clinical improvement plus adverse events over time.
Rostami-Hodjegan et al., (2004) (RH) developed nomograms for estimating clozapine dosing requirements in adults via multiple logistic regression analyses. The RH nomograms incorporate patient gender, age, weight, and smoking status to estimate clozapine daily dosage required to achieve target serum clozapine concentration (Rostami-Hodjegan et al., 2004). The RH nomograms form the basis of the clozapine dose estimators published in the British National Health Service clozapine guidelines (Medical Director, 2014). We are unaware of a published comparable clozapine dosing estimator for use in children. There is known fluctuation of hormone regulation during puberty and adolescence but it remains unclear whether or how this physiological change may impact clozapine dosing or level (Kennedy, 2008). Using the adult RH nomograms for gender and smoking status, with adjustment for age and weight as measured at the time of clozapine initiation, we calculated the maximally achieved daily clozapine dose during the inpatient admission as a percentage of their RH-estimated daily clozapine dose requirement.
Descriptive statistics were computed using Microsoft Excel 2010. Inferential statistical analyses were computed using the Statistical Package for the Social Sciences (SPSS) version 25.
Results
Twenty-eight patients were identified during the 15-year study period. Five patients discontinued clozapine during their admission, with four of the five discontinuing after less than two weeks. Clozapine was discontinued for the following reasons (one each): increased aggression, cardiovascular adverse events (hypertension, tachycardia, chest pain), neutropenia/physician discontinuation, neutropenia/withdrawal of consent by parent, planned discontinuation (no indication for clozapine following diagnostic clarification). Consequently, symptom severity and clinical response ratings post-clozapine trial, at time of discharge were assessed for 23 patients (14 females, 9 males).
Baseline patient characteristics and discharge diagnoses are summarized in Table 1. Patients had a mean age of 15.8 years at clozapine start, reflecting EOS. Females comprised 64% of patients. The evaluated admission represented the first presentation for psychiatric treatment in 25% of patients. The majority (71.4%) of patients were of ethnicity associated with a high risk for metabolic adverse events. Nearly half (46.4%) of patients had an affective illness (comorbid or primary) present. There was a mean of 2.9 documented prior antipsychotic trials per patient prior to initiating clozapine.
Table 1.
Baseline patient characteristics and discharge diagnoses
| Males (n=10, 36%) | Females (n=18, 64%) | All patients (n=28) | |
|---|---|---|---|
| Age at admission (years) | 16 ± 1.5 | 15.5 ± 2.3 | 15.7 ± 2 |
| Mean ± SD (min–max) | (13.3–17.7) | (10.7–18.9) | (10.7–13.9) |
| Age at clozapine start (years) | 16.1 ± 1.5 | 15.7 ± 2.3 | 15.8 ± 2 |
| Mean ± SD (min–max) | (13.6–17.9) | (11.1–19.3) | (11.1–19.3) |
| Weight (kg) at admission, Mean ± SD | 84.3 ± 22.8 | 49.1 ± 11.4 | 61.7 ± 23.5 |
| n = 10 | n = 18 | n = 28 | |
|
|
|||
| Race/Ethnicity | |||
| White/Caucasian | 3 | 5 | 8 |
| Chinese | 3 | 3 | 6 |
| Filipino | - | 3 | 3 |
| Southeast Asian | 3 | - | 3 |
| Aboriginal | - | 2 | 2 |
| Black/African | - | 2 | 2 |
| South Asian | 1 | 1 | 2 |
| Latin American | - | 1 | 1 |
| Japanese | - | 1 | 1 |
| n (%) | n (%) | n (%) | |
|
|
|||
| History of cannabis use | 5 (50) | 5 (27.7) | 10 (35.7) |
| Tobacco smoking at clozapine initiation | 1 (3.6) | 1 (3.6) | 2 (7.1) |
| Primary psychotic illness | 10 (100) | 15 (83.3) | 25 (89.3) |
| Affective illness present | 4 (40) | 9 (50) | 13 (46.4) |
| First mental health presentation | 2 (20) | 5 (27.8) | 7 (25) |
| Antipsychotic drug trials prior to clozapine initiation, mean ± SD | 2.8 ± 1.2 | 3.0 ± 1.5 | 2.9 ± 1.3 |
| Discharge diagnoses (per treating psychiatrist / non-retrospective): | |||
| Schizophreniform | 1 (10) | 2 (11.1) | 3 (10.7) |
| Schizophrenia | 5 (50) | 6 (33.3) | 11 (39.3) |
| Schizoaffective | 4 (40) | 7 (38.9) | 11 (39.3) |
| Bipolar disorder | 0 (0) | 2 (11.1) | 2 (7.1) |
| Non-psychotic non-bipolar illness | 0 (0) | 1 (5.6) | 1 (3.6) |
Of the documented family psychiatric history, among first-degree relatives, psychotic and affective illnesses were present in 3 (10.7%) and 9 (32.1%) families, respectively. Among second-degree relatives, psychotic and affective illnesses were present in 17.9% and 14.3% of families, respectively. In addition, several first-degree relatives were identified as having a suspected or undiagnosed mental illness.
Six patients (21%) had a positive family history of diabetes (1 patient with a first-degree relative, 5 with a second-degree relative). Seven patients (25%) had a positive family history of hypertension (2 with a first-degree relative, 3 with a second-degree relative). One patient (3.6%) had an unspecified family member with seizure disorder.
In our review, ten patients (35.7%) had a history of cannabis use. Of those with prior cannabis use, three patients (10.7%) used cannabis on a regular or daily basis at time of hospitalization. Overall, the rate of substance use beyond nicotine and cannabis was low in our cohort. Two of the 28 patients (7.1%) reported a history of polysubstance use, however they were abstinent during their hospital admission. Several other patients reported historical one-time or occasional use of substances such as cocaine, stimulants or hallucinogens.
Retrospective ratings of illness symptoms, severity, and clinical response across the five outcome measures at each time point are summarized in Table 2.
Table 2.
Symptom Profile and Clinical Change Over Time (n=23)
| Ratings by Time | |||
|---|---|---|---|
|
|
|||
| Time 1 | Time 2 | Time 3 | |
|
|
|
|
|
| Measure | Admission Mean (SD) |
Clozapine Start Mean (SD) |
Discharge Mean (SD) |
| BPRS (total scale score) | 68.8 (11.8) | 69.8 (12.0) | 51.2 (13.6) |
| CGAS | 28.1 (7.5) | 26.9 (9.3) | 44.3 (10.1) |
| CGI-Severity | 6.6 (0.7) | 6.6 (0.6) | 5.0 (1.0) |
| CGI-Improvement | n/a | 4.5 (1.1) | 2.4 (0.7) |
| CGI-Efficacy Index | n/a | 11.8 (3.4) | 6.6 (2.3) |
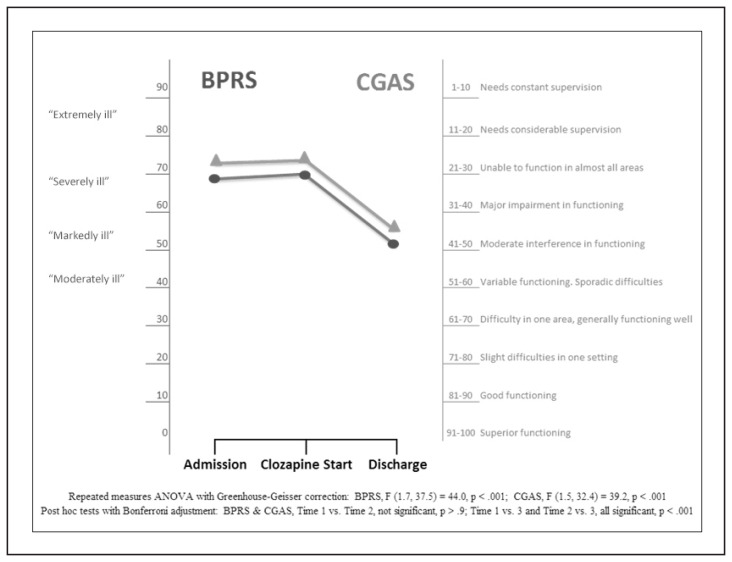
Outcome response as assessed via BPRS and CGAS scores measured at admission, pre-clozapine start, and discharge are shown in Figure 1, which also includes a detailed explanation of scale scores and associated clinical symptoms/response.
Figure 1.
Clinical Outcome Response by Time: BPRS† and CGAS Scores
Circle (●) = BPRS score; triangle (▲) = CGAS score
† Designations of moderately, markedly, severely or extremely ill are based on a study linking BPRS scores to CGI-S designations (Leucht et al., 2006).
As outlined in Table 2 and Figure 1, the patients requiring clozapine treatment are among the most severely ill. They also fail to clinically improve despite prior antipsychotic trials, wrap-around milieu therapy, and abstinence from substance use due to inpatient admission. Patients also experienced anosognosia (lack of insight of their illness) and secondary problems were observed during their inpatient stay, such as increased aggression, agitation, and increased flight risk.
CGI-S scores in the range of 6 to 7 reflect severe to extreme illness, while a CGI-S score of 5 reflects marked illness. CGI-I scores in the range of 4 or greater reflect no clinical change to minimally worse. Scores in the range of 2 to 3 reflect minimal improvement to much improvement. CGI-EI scores in the range of 11–12 reflect minimal to no improvement plus interfering adverse events. CGI-EI scores in the range of 6–7 reflect partial remission of symptoms plus adverse events that minimally interfere with functioning. Twenty-two of 23 patients (95.7%) taking clozapine at discharge demonstrated functional improvement and a CGI-I score of 3 or less.
To test for statistically significant change across time, repeated measures ANOVA with Greenhouse-Geisser correction for violation of sphericity found that mean BPRS, CGAS, and CGI-S scores improved significantly across time, F (1.7, 37.5) = 44.04, p < .001; F (1.5, 32.4) = 39.2, p < .001; F (1.3, 28.1) = 47.6, p < .001, respectively. Post hoc tests using the Bonferroni correction to control for inflated Type I error, revealed that treatment efforts prior to clozapine start elicited minimal to no clinical gains (Time 1 vs. Time 2; all insignificant at p > .9 across BPRS, CGAS, CGI-S). Whereas treatment response after clozapine start reveals significant improvement by discharge across measures (Time 2 vs. Time 3, all significant at p < .001 across BPRS, CGAS, and CGI-S).
Paired-sample t-tests compared clinical improvement plus adverse events over time on the CGI-I and CGI-EI. There was significant improvement on both measures from clozapine start to discharge; t (21, 21) = 7.5, 6.1, p < .001, respectively.
Length of admission and clozapine dosing is summarized in Table 3. In females, length of admission (126.8 days, min-max: 15–246 days) and length of stay after clozapine initiation (68 days, min-max: 6–194 days) were longer than in males (99.3 days and 49.2 days respectively). Time required to achieve maximal clozapine dosage was also longer in females than in males (37.6 days compared to 26 days). Mean maximal clozapine daily doses were higher in males than females (173.8 mg/day vs. 143.4 mg/day) though percentage of RH-estimated clozapine dosing requirement achieved was lower in males than females (51% vs. 58%. respectively).
Table 3.
Length of admission and clozapine dosing
| Males (n = 10, 36%) | Females (n = 18, 64%) | All patients (n = 28) | |
|---|---|---|---|
| Length of admission (days), mean ± SD | 99.3 ± 54.6 | 126.8 ± 64.4 | 117 ± 61.5 |
| Length of stay from clozapine start to discharge (days), mean ± SD | 49.2 ± 17.2 | 68 ± 47.9 | 60.7 ± 40.7 |
| (n=9) | (n=14) | (n=23) | |
|
|
|||
| Time from clozapine start to maximal achieved clozapine dose (days), mean ± SD (min–max) | 26 ± 13.2 (9–47) | 37.6 ± 25.6 (3–87) | 33.1 ± 22.1 (3–87) |
| (n=10) | (n=16) | (n=26) | |
|
|
|||
| Smoked tobacco use at date of clozapine initiation | 1 (10%) | 1 (5.6%) | 2 (7.1%) |
| Maximal achieved clozapine dose (mg/day), mean ± SD (min–max) | 173.8 ± 90.6 (62.5–300) | 143.4 ± 25.6 (18.75–400) | 154.2 ± 87 (18.75–400) |
| RH-estimated† clozapine daily dose requirement (mg/day), mean ± SD (min–max) | 356.4 ± 87.6 (290.6–581.9) | 234.7 ± 44.9 (201.2–406) | 278.2 ± 85.8 (201.2–581.9) |
| Maximally achieved dose as % of RH-estimated† clozapine daily dose requirement, mean ± SD | 51% ± 30% | 58% ± 29% | 57% ± 29% |
Per the method of Rostami-Hodjegan et al., 2004,23
A number of findings emerged that illuminate the complexity of this population. At the time of clozapine start, 61% of patients displayed low mood symptoms in addition to psychosis, with 48% demonstrating evidence of probable depression, regardless of formal diagnosis of schizoaffective disorder or bipolar illness. Of those with a formal diagnosis of schizoaffective disorder or bipolar illness, 9 of 12 patients (75%) received concurrent pharmacotherapy targeting mood; 7 of 12 patients (58.3%) received lithium, 1 of 12 patients (8.3%) received lamotrigine and 1 of 12 patients (8.3%) received escitalopram.
Using criteria of CGI-EI <7, 61% were positive responders while by criteria of CGI-I ≤2, 52% were positive responders.
Adverse events are summarized in table 4. Stated adverse event rates reflect any documented occurrence of an adverse event of any severity, and does not imply an adverse event was sustained or severe in nature.
Table 4.
Clozapine-related adverse events
| Adverse event | Males (n=10) n (%) |
Females (n=18) n (%) |
All patients (n=28) n (%) |
|---|---|---|---|
| Tachycardiaa | 9 (90) | 16 (88.9) | 25 (89.3) |
| Increased appetite | 7 (70) | 11 (61.1) | 18 (64.3) |
| Abnormal movements and/or extrapyramidal symptoms (EPS) | 7 (70) | 11 (61.1) | 18 (64.3) |
| Hypertensionb | 8 (80) | 9 (50) | 17 (60.7) |
| Sedation/fatigue | 7 (70) | 8 (44.4) | 15 (53.6) |
| Hematology ‘yellow alert’c,d | 5 (50) | 8 (44.4) | 13 (46.4) |
| Dizziness and/or hypotensione | 4 (40) | 8 (44.4) | 12 (42.9) |
| Sialorrhea | 5 (50) | 7 (38.9) | 12 (42.9) |
| Enuresis | 3 (30) | 5 (27.8) | 8 (28.6) |
| Constipationf | 3 (30) | 4 (22.2) | 7 (25) |
| QTc prolongationg | 2 (20) | 3 (16.7) | 5 (17.9) |
| Headache | 3 (30) | 2 (11.1) | 5 (17.9) |
| Eosinophiliah | 3 (30) | 1 (5.6) | 4 (14.3) |
| Seizure-like activity | 0 (0) | 2 (11.1) | 2 (7.1) |
| Abnormal EEG | 0 (0) | 1 (5.6) | 1 (3.6) |
| Abnormal metabolic parametersi | 2 (20) | 1 (5.6) | 3 (10.7) |
| Feverj | 1 (3.6) | 1 (3.6) | 2 (7.1) |
EEG = electroencephalogram
Pulse > 100 beats/minute
Systolic blood pressure (SBP) > 140 mm Hg or diastolic blood pressure (DBP) > 90 mm Hg
Yellow alert definition: absolute WBC value of 2–3.5 × 109/L or absolute neutrophil count (ANC) of 1.5–2 × 109/L, or falling WBC (decrease of 3 × 109/L or greater in the last 4 weeks reaching a value of <4 × 109/L) or ANC (decrease of 1.5 × 109/L or greater in the last 4 weeks reaching a value of <2.5 × 109/L) values.
In patients experiencing a ‘yellow alert’ mean time to first alert was 16.6 days in females and 26.4 days in males. Mean maximal ANC reduction from baseline was 1.61 × 109/L in females, and 0.58 × 109/L in males.
Hypotension: SBP < 90 mm Hg or DBP < 60 mm Hg
Subjective report of constipation or documented laxative administration
QTc value (Bazett correction) > 440 msec (males) or > 450 msec (females)
Eosinophil value > 0.6 × 109/L
Any of the following: elevated fasting glucose, elevated fasting insulin, elevated fasting triglycerides, abnormal low fasting HDL
Oral temperature of ≥ 38°C
Five of 28 patients (18%) had bloodwork for myocarditis monitoring (troponin and c-reactive protein (CRP)). No abnormalities in troponin values were detected, while three patients experienced transient rises in CRP post-clozapine initiation that were not concerning for myocarditis.
Discussion
Almost all of the patients in our study who were taking clozapine at discharge demonstrated clinically meaningful functional improvement. The changes in BPRS and CGAS scores from pre-clozapine to discharge were clinically meaningful. At discharge, on average, moderate functional impairment remains consistent with persistent symptoms attenuated in breadth or depth. CGI-S scores at discharge reflect residual intrusive and distressing or impairing symptoms, but improved independence and functioning.
With respect to individual symptoms as assessed on BPRS ratings, greatest improvement (greater than 1.4 point reduction) over time was found on items that capture positive symptoms of psychosis: suspiciousness, unusual thoughts, disorganization, and hallucinations. Items with moderate improvement (0.8 to 1.4 point reduction) included: anxiety, hostility, excitement, uncooperativeness, grandiosity, tension, and disorientation.
A significant family history of psychiatric illness and metabolic risk factors was present in our patient cohort. Use of atypical antipsychotic medications, in particular, clozapine is associated with increased risk of metabolic syndrome and lowering of seizure threshold (Pringsheim et al., 2011; Wong et al., 2007). Identification of relevant family history can help identify patients at high risk for drug-induced adverse events such as weight gain, diabetes, hypercholesterolemia and increased risk of seizure activity.
In British Columbia (BC), 25% of all children under the age of 18 surveyed reported prior cannabis use (Smith et al., 2019). Comparatively, in our study, we observed a higher rate (35.7%) of prior cannabis use. Cannabis use is associated with a higher incidence of psychotic illness (D’Souza, et al., 2016). Adolescents with EOS and comorbid cannabis use have also been shown to have poorer functional outcomes (Bagot, Milin & Kaminer, 2015).
The percentage of children in BC who have smoked tobacco declined from 26% in 2008 to 18% in 2018, however 21% of children had vaped nicotine within the past month (Smith et al., 2019). In contrast to BC provincial adolescent data, we observed a much lower incidence of tobacco smoking at the time of clozapine initiation in our study. Clozapine dose requirements appear to be 2–3 fold higher in cigarette smokers compared to non-smokers, due to induction of cytochrome P450 (CYP) 1A2 enzyme activity by polycyclic aromatic hydrocarbons in cigarette smoke (Tsuda, Saruwatari, & Yasui-Furukori, 2014). Due to this clinically significant drug interaction with clozapine, clinicians should inquire regarding tobacco use at start of treatment, and throughout the course of clozapine treatment, as ability and/or desire to smoke may change over time. Use of nicotine replacement therapy (NRT) or vaping of nicotine does not induce CYP 1A2 activity (Lucas & Martin, 2013; Berm et al., 2015). Inpatient smoking policies may precipitate a significant reduction in cigarette smoking, or a switch to use of vaped nicotine or use of NRT, all of which may significantly alter clozapine dosing requirements. A theoretical interaction also exists for induction of CYP 1A2 activity by patients who smoke dried cannabis, however currently there is no evidence demonstrating a need for significant clozapine dose adjustment. Close clinical monitoring may be prudent for patients with known heavy chronic cannabis use.
In our study, patients had a higher mean number of antipsychotic trials prior to clozapine initiation than recommended by Canadian schizophrenia treatment guidelines for children, which recommend sequential use of adequate doses of at least 2 different antipsychotic drugs, each for 6 to 8 weeks (Abidi et al., 2017). In our study, the most common prior second generation antipsychotic (SGA) trials were: risperidone (n=22), quetiapine (n=16), olanzapine (n=15), and aripiprazole (n=12). Of note, aripiprazole was first marketed in Canada in 2009, midway through our study period (Bristol-Myers Squibb Canada, 2017). Psychiatrists in both our inpatient and community settings have expressed reluctance to initiate clozapine, particularly if they do not have an on-site multidisciplinary team to support clinical monitoring (personal communication with Ardelle Komaryk, September 2019). This phenomenon is well-recognized in literature as a barrier to treatment of refractory schizophrenia (Kelly, et al., 2018). In a 2005 UK clozapine prescribing practices survey in child and adolescent psychiatry, the main barriers to clozapine initiation identified included prescriber unfamiliarity with clozapine and need for intensive monitoring which includes the requirement for frequent bloodwork (Cirulli, 2005). Unfamiliarity and discomfort with clozapine treatment amongst clinicians and families can impact patients and lead to delays in both clozapine initiation and treatment continuation.
In adults, treatment response to clozapine is correlated with pre-dose serum clozapine concentrations above 350 ng/mL (SI units, 1070 nmol/L) (Spina et al., 2000). The RH nomograms were developed with the goal of estimating clozapine daily dosage requirements for individual patients to achieve a clozapine serum level above 350 ng/mL (Rostami-Hodjegan et al, 2004). On average, our study patients reached a maximum of 57% of their RH-estimated clozapine dose during their inpatient admission. It took a mean of 33.1 days from clozapine start to maximal clozapine dosage, and this time was longer in females than in males. In our patients, the time required for clozapine dose titration was much longer than adult clozapine titration recommendations (about 14 days), with a possible factor limiting titration rate being symptomatic orthostatic tachycardia (HLS Therapeutics, Inc., 2019). Given the trepidation amongst community providers regarding clozapine titration and monitoring, the inpatient admission represents the best opportunity to achieve a clozapine dosage associated with significant treatment response. A level of comfort and knowledge of care staff and family psychoeducation in managing clozapine-related adverse events helps support clozapine dose titration in the inpatient setting. Detailed suggestions for clozapine titration in the acute care patient discharge summary provide helpful guidance for community psychiatrists that may have limited experience with clozapine treatment in children.
Yellow alert status warnings occurred commonly in our patients and should be anticipated when prescribing clozapine. These alerts may be distressing for patients, families and care providers. We observed at least one family withdraw consent for clozapine treatment in the face of their child experiencing a yellow alert. In our study, sampling times for all CBCs resulting in yellow alerts were drawn between 0730–1030h. There is a documented diurnal variation in neutrophil count (Souto-Filho, Portugal & Nucci, 2019). We have found an effective strategy to reduce the frequency of yellow alerts is to schedule CBC testing in the afternoon hours when neutrophil counts are higher, rather than at the typical morning blood collection time. Females in our study had fewer hematological alerts than males, which is consistent with prior published literature (Palominao, Kukoyi & Xiong, 2010). However, when females in the study cohort experienced a decrease in ANC, this occurred earlier during treatment and was more severe than in males. One patient had adjusted hematological alert cutoffs due to the presence of benign ethnic neutropenia (HLS Therapeutics, Inc., 2019).
Most of the CBC sampling in our patients occurred during the first 26 weeks of clozapine treatment, the time in the treatment course which is associated with greater risk of hematologic abnormalities compared to subsequent weeks (HLS Therapeutics, Inc., 2019).
No hematological ‘red alert’ (defined as WBC <2×109/L or ANC count<1.5×109/L) was identified in the study patients during hospital admission. This is a lower rate of hematological adverse events compared with published literature (Gerbino-Rosen et al., 2005). This should be reassuring for prescribers and families, and will hopefully alleviate concerns regarding clozapine induced hematological adverse events in children and adolescents.
During the first three weeks of clozapine therapy, a transient rise in oral temperature above 38°C may occur and in rare cases may be associated with WBC fluctuation (HLS Therapeutics, Inc., 2019). One study patient had a self-limiting temperature greater than 38°C with no significant change in WBC on day 21 post-clozapine initiation. One additional study patient had a temperature greater than 38°C and elevated WBC on day 38 post-clozapine initiation who was subsequently diagnosed with left lower lobe pneumonia. This patient had been initiated on lithium prior to clozapine start which may have also contributed to leukocytosis (Palominao et al., 2010).
Tachycardia was the most common adverse effect documented in our study. At baseline, children have a higher heart rate compared to adults (Ostchega, Porter, Hughes, Dillon & Nwankwo, 2011). In adults, clozapine treatment may result in a 10–15 bpm heart rate elevation (HLS Therapeutics, Inc., 2019). We observed a number of patients in our study with significantly higher (50–60 bpm) orthostatic pulse shifts during clozapine dose titration. Tachycardia may be secondary to hypotension however the compensatory ability for children and adolescents is greater compared to adults and can result in larger degrees of symptomatic orthostatic tachycardia (Singer et al., 2012). Observed orthostatic pulse and blood pressure shifts, or reports of associated symptoms may necessitate a slower pace of clozapine dose titration. In some cases, the severity of mental illness may limit patients’ ability to subjectively describe physical symptoms. The range of pulse and blood pressure shifts can be mitigated by medication administration of three daily divided doses and if not contraindicated, fluid augmentation.
Due to evaluation of adverse effects being limited to the timeline of inpatient treatment during clozapine dosage titration, it is not known if tachycardia was sustained with continued long-term treatment in this cohort.
Antipsychotic medications including clozapine carry risk of QTc interval prolongation on 12-lead electrocardiogram (ECG) (Shah, Aftab & Coverdale, 2014). Eight of 28 study patients (29%) had an abnormal QTc measurement (>440 msec in males or >450 msec in females). Both increased and decreased QTc intervals from baseline were observed in our study patients taking clozapine. For example, one patient taking escitalopram was then started on clozapine (escitalopram was continued) and had a decrease in QTc interval from baseline. The largest QTc interval shift was an increase of 78 msec from baseline in a patient who was already taking risperidone prior to clozapine initiation. Limitations include: lack of consensus on definition of QTc interval prolongation in children and adolescents and ECG machine QTc values which use the Bazett correction formula. Causal attribution of any observed QTc prolongation with clozapine may be complicated by use of other first or second-generation antipsychotics for breakthrough symptoms that also have QTc prolonging effects.
Extrapyramidal symptoms (EPS) occurred at a relatively high rate (64%) in patients receiving clozapine in our study. No incidence of withdrawal dyskinesia was observed. Although clozapine is associated with a lower rate of EPS than first-generation antipsychotics (FGAs), it is not absent and clinical observation suggests that children may be more sensitive to EPS induced by SGAs (Hong & Bishop, 2010). We were unable to delineate the timing of when EPS occurred, and the rate may be higher than expected if other SGAs were administered earlier in the admission during the switch to clozapine. In addition, as needed use of FGAs and SGAs for breakthrough agitation and psychotic symptoms may have led to the higher apparent EPS rate during clozapine treatment. Consistent use of standardized assessment tools for abnormal involuntary movements is an important consideration for multidisciplinary teams.
Clozapine-induced myocarditis is rarely reported to occur in children and adolescents (Mudra et al., 2018). Since 2012, our institution has completed baseline echocardiogram and baseline and weekly troponin and CRP testing for 4 weeks after clozapine initiation as per literature recommendations (Ronaldson, Fitzgerald, Taylor, Topliss & McNeil, 2011). Nevertheless, such monitoring is not currently required by the clozapine product monograph (HLS Therapeutics, Inc., 2019).
Two study patients experienced seizure-like activity which did not preclude continuation of clozapine treatment. One of these patients had a diagnosis of epilepsy prior to starting clozapine treatment, and was managed by addition of valproate. Anticonvulsant treatment was not required for the other patient.
Seventy-five percent of study patients were of high-risk ethnicity for developing insulin resistance, diabetes, obesity and hypercholesterolemia (Pringsheim et al., 2011). A significant increase in appetite was observed in more than half of the patients during clozapine treatment. Increased appetite may manifest in weight gain over time during longitudinal follow-up. Due to inconsistent documentation of height, we were unable to calculate BMI or BMI percentiles for most patients. Changes in weight, lipid profile and fasting blood glucose can occur early in clozapine treatment. In our study, limited follow-up testing occurred after clozapine initiation, as the mean duration of hospitalization from clozapine start to discharge was shorter than the duration between follow-up intervals recommended in guidelines (Pringsheim et al, 2011). There is a risk that ongoing metabolic monitoring may lapse after discharge due to lack of coordination between the antipsychotic prescribers and primary care clinicians (Hager et al., 2019). Dedicated mental health metabolic monitoring programs may help ensure consistently appropriate metabolic follow-up occurs for patients taking clozapine.
Similar to a recently published retrospective review of clozapine use in children and adolescents in a US and Australian-based cohort, we observed a high rate of adverse events that require diligent clinician monitoring (Steinauer et al., 2018).
Study data collection was performed exclusively by experienced interdisciplinary care team members (family nurse practitioner, clinical pharmacy specialist, clinical psychologist) to improve interpretation and capture of relevant data from large volumes of charting.
One observation from conducting this retrospective review is that there are inherent gaps in care and communication when documentation by clinicians of varying disciplines occurs separately, leading to fragmentation of clinically relevant observations. An interdisciplinary guideline and documentation framework for clozapine monitoring specific to children and adolescents would decrease risk and better integrate complex interdisciplinary care.
Limitations of this retrospective review include: small sample size, single institution, non-blinded retrospective ratings of clinical symptoms, and incomplete, inconsistent or missing chart documentation.
Conclusion
In summary, children with treatment-resistant schizophrenia spectrum disorders or bipolar disorder requiring clozapine treatment have a profound degree of functional impairment. Given the vulnerability of children with early onset psychosis, close clinical monitoring and avoidance of treatment delay are imperative to mitigate worsening clinical, functional and cognitive outcomes (Diaz-Caneja, 2015). Despite an identified high incidence of manageable adverse events secondary to clozapine, based on the findings of this review, the benefit of functional improvement appears to outweigh the risks.
Footnotes
Conflicts of Interest
The authors have no financial relationships to disclose. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- Abidi S, Mian I, Garcia-Ortega I, Lecomte T, Raedler T, Jackson K, …Addington D. Canadian Guidelines for the Pharmacological Treatment of Schizophrenia Spectrum and Other Psychotic Disorders in Children and Youth. Canadian Journal of Psychiatry. 2017;62(9):635–647. doi: 10.1177/0706743717720197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagot KS, Milin R, Kaminer Y. Adolescent Initiation of cannabis use and early-onset psychosis. Substance Abuse. 2015;36(4):524–533. doi: 10.1080/08897077.2014.995332. [DOI] [PubMed] [Google Scholar]
- Berm EJ, Ruijsbroek R, Loonen AJ, Goethals KR, Wilffert B, van Hasselt F. Switching to e-cigarettes affects drug concentration [Abstract] Netherlands Tijdschrift Voor Geneeskunde. 2015;159:A9090. [PubMed] [Google Scholar]
- Birmaher B, Baker R, Kapur S, Quintana H, Ganguli R. Clozapine for the treatment of adolescents with schizophrenia. Journal of the American Academy of Child and Adolescent Psychiatry. 1992;31(1):160–164. doi: 10.1097/00004583-199201000-00024. [DOI] [PubMed] [Google Scholar]
- Bristol-Myers Squibb Canada. Product Monograph: Abilify®. Bristol-Myers Squibb Canada. 2017. [Accessed October 14, 2019]. from https://pdf.hres.ca/dpd_pm/00038225.PDF.
- Busner J, Targum SD. The clinical global impressions scale: Applying a research tool in clinical practice. Psychiatry (Edgmont) 2007;4(7):28–37. [PMC free article] [PubMed] [Google Scholar]
- Calabrese JR, Kimmel SE, Woyshville MJ, Rapport DJ, Faust CJ, Thompson PA, Meltzer HY. Clozapine for treatment-refractory mania. American Journal of Psychiatry. 1996;153(6):759–764. doi: 10.1176/ajp.153.6.759. [DOI] [PubMed] [Google Scholar]
- Cirulli G. Clozapine prescribing in adolescent psychiatry: Survey of prescribing practice in in-patient units. Psychiatric Bulletin. 2005;29(10):377–380. doi: 10.1192/pb.29.10.377. [DOI] [Google Scholar]
- Clemmensen L, Vernal DL, Steinhausen HC. A systematic review of the long-term outcome of early onset schizophrenia. BMC Psychiatry. 2012;12:150. doi: 10.1186/1471-244X-12-150.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Caneja CM, Pina-Camacho L, Rodriguez-Quiroga A, Fraguas D, Parellada M, Arango C. Predictors of outcome in early-onset psychosis: A systematic review. NPJ Schizophrenia. 2015;1:14005. doi: 10.1038/npjschz.2014.5.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver DI, Thomas S, Gogtay N, Rapoport JL. Childhood-onset schizophrenia and early-onset schizophrenia spectrum disorders: An update. Child & Adolescent Psychiatric Clinics of North America. 2020;29(1):71–90. doi: 10.1016/j.chc.2019.08.017. [DOI] [PubMed] [Google Scholar]
- D’Souza D, Radhakrishnan R, Sherif M, Cortes-Briones J, Cahill J, Gupta S, … &, Ranganathan M. Cannabinoids and psychosis. Current Pharmaceutical Design. 2016;22(42):6380–6391. doi: 10.2174/1381612822666160826105628.. [DOI] [PubMed] [Google Scholar]
- Frazier JA, Cohen LG, Jacobsen L, Grothe D, Flood J, Baldessarini RJ, … &, Rapoport JL. Clozapine pharmacokinetics in children and adolescents with childhood-onset schizophrenia. Journal of Clinical Psychopharmacology. 2003;23(1):87–91. doi: 10.1097/00004714-200302000-00012.. [DOI] [PubMed] [Google Scholar]
- Gerbino-Rosen G, Roofeh D, Tompkins DA, Feryo D, Nusser L, Kranzler H, … &, Kumra S. Hematological adverse events in clozapine-treated children and adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44(10):1024–1031. doi: 10.1097/01.chi.0000171904.23947.54.. [DOI] [PubMed] [Google Scholar]
- Gochman P, Miller R, Rapoport JL. Childhood-onset schizophrenia: The challenge of diagnosis. Current Psychiatry Reports. 2011;13(5):321–322. doi: 10.1007/s11920-011-0212-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager K, Kading M, O’Donnell C, Yapel A, MacDonald D, Albee JN, Schneiderhan M. Bridging community mental health and primary care to improve medication monitoring and outcomes for patients with mental illness taking second-generation antipsychotics-HDC/DFMC bridge project, phase 1: Group concept mapping. The Primary Care Companion CNS Disorders. 2019;21(4) doi: 10.4088/PCC.19m02452.. pii: 19m02452. [DOI] [PubMed] [Google Scholar]
- HLS Therapeutics Inc. Clozaril Product Monograph. HLS Therapeutics. 2019. [Accessed January 4 2019]. from http://www.hlstherapeutics.com/wp-content/uploads/monograph_pdf/HLS-Clozaril-PM-E.pdf.
- Hong IS, Bishop JR. Anticholinergic use in children and adolescents after initiation of antipsychotic therapy. Annals of Pharmacotherapy. 2010;44(7–8):1171–1180. doi: 10.1345/aph.1M643.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher I, Connor D, Clarke MC, Devlin N, Harley M, Cannon M. Prevalence of psychotic symptoms in childhood and adolescence: A systematic review and meta-analysis of population-based studies. Psychological Medicine. 2012;42(9):1857–1863. doi: 10.1017/S0033291711002960. [DOI] [PubMed] [Google Scholar]
- Kelly DL, Freudenreich O, Sayer MA, Love RC. Addressing barriers to clozapine underutilization: A national effort. Psychiatric Services. 2018;69(2):224–227. doi: 10.1176/appi.ps.201700162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MJ. Hormonal regulation of hepatic drug-metabolizing enzyme activity during adolescence. Clinical Pharmacology and Therapeutics. 2008;84(6):662–673. doi: 10.1038/clpt.2008.202.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumra S, Frazier JA, Jacobsen LK, McKenna K, Gordon CT, Lenane MC, … &, Rapoport JL. Childhood-onset schizophrenia. A double-blind clozapine-haloperidol comparison. Archives of General Psychiatry. 1996;53(12):1090–1097. doi: 10.1001/archpsyc.1996.01830120020005.. [DOI] [PubMed] [Google Scholar]
- Kumra S, Kranzler H, Gerbino-Rosen G, Kester HM, De Thomas C, Kafantaris V, … &, Kane JM. Clozapine and “high-dose” olanzapine in refractory early-onset schizophrenia: A 12-week randomized and double-blind comparison. Biological Psychiatry. 2008;63(5):524–529. doi: 10.1016/j.biopsych.2007.04.043. [DOI] [PubMed] [Google Scholar]
- Leucht S, Kane JM, Etschel E, Kissling W, Hamann J, Engel RR. Linking the PANSS, BPRS, and CGI: Clinical implications. Neuropsychopharmacology. 2006;31(10):2318–2325. doi: 10.1038/sj.npp.1301147.. [DOI] [PubMed] [Google Scholar]
- Lucas C, Martin J. Smoking and drug interactions. Australian Prescriber. 2013;36(3):102–104. doi: 10.18773/austprescr.2013.037. [DOI] [Google Scholar]
- McClellan J, Stock S. Practice parameters for the assessment and treatment of children and adolescents with schizophrenia. Journal of the American Academy of Child and Adolescent Psychiatry. 2013;52(9):976–990. doi: 10.1016/j.jaac.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Medical Director. Clozapine Guideline - Community. Greater Manchester Mental Health. 2014. [Accessed October 14, 2019]. from https://www.gmmh.nhs.uk/download.cfm?doc=docm93jijm4n2857.pdf&ver=4593.
- Mudra S, Luedecke D, Grafmann M, Schulte-Markwort M. Myocarditis during treatment with clozapine in 2 adolescent cases. Journal of Clinical Psychopharmacology. 2018;38(6):639–640. doi: 10.1097/JCP.0000000000000967.. [DOI] [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence. Psychosis and schizophrenia in children and young people: Recognition and management. Clinical guideline number 155. 2016. [Accessed January 4, 2019]. from http://guidance.nice.org.uk/CG155. [PubMed]
- Ostchega Y, Porter KS, Hughes J, Dillon CF, Nwankwo T. Resting pulse rate reference data for children, adolescents, and adults: United States, 1999–2008. National Health Statistics Report. 2011;41:1–16. [PubMed] [Google Scholar]
- Palominao A, Kukoyi O, Xiong G. Leukocytosis after lithium and clozapine combination therapy. Annals of Clinical Psychiatry. 2010;22(3):205–206. [PubMed] [Google Scholar]
- Pringsheim T, Panagiotopoulos C, Davidson J, Ho J. Evidence-based recommendations for monitoring safety of second generation antipsychotics in children and youth. Journal of the Canadian Academy of Child and Adolescent Psychiatry. 2011;20(3):218–233. [PMC free article] [PubMed] [Google Scholar]
- Rapoport JL, Addington AM, Frangou S. The neurodevelopmental model of schizophrenia: Update 2005. Molecular Psychiatry. 2005;10(5):434–449. doi: 10.1038/sj.mp.4001642. [DOI] [PubMed] [Google Scholar]
- Ronaldson KJ, Fitzgerald PB, Taylor AJ, Topliss DJ, McNeil JJ. A new monitoring protocol for clozapine-induced myocarditis based on an analysis of 75 cases and 94 controls. Australian and New Zealand Journal of Psychiatry. 2011;45(6):458–465. doi: 10.3109/00048674.2011.572852. [DOI] [PubMed] [Google Scholar]
- Rostami-Hodjegan A, Amin AM, Spencer EP, Lennard MS, Tucker GT, Flanagan RJ. Influence of dose, cigarette smoking, age, sex, and metabolic activity on plasma clozapine concentrations: A predictive model and nomograms to aid clozapine dose adjustment and to assess compliance in individual patients. Journal of Clinical Psychopharmacology. 2004;24(1):70–78. doi: 10.1097/01.jcp.0000106221.36344.4d.. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Gould MS, Brasic J, Ambrosini P, Fisher P, Bird H, Aluwahlia S. A children’s global assessment scale (CGAS) Archives of General Psychiatry. 1983;40(11):1228–1231. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- Shah AA, Aftab A, Coverdale J. QTc prolongation with antipsychotics: Is routine ECG monitoring recommended? Journal of Psychiatric Practice. 2014;20(3):196–206. doi: 10.1097/01.pra.0000450319.21859.6d.. [DOI] [PubMed] [Google Scholar]
- Shaw P, Sporn A, Gogtay N, Overman GP, Greenstein D, Gochman P, … &, Rapoport JL. Childhood-onset schizophrenia: A double-blind, randomized clozapine-olanzapine comparison. Archives of General Psychiatry. 2006;63(7):721–730. doi: 10.1001/archpsyc.63.7.721. [DOI] [PubMed] [Google Scholar]
- Sikich L, Frazier JA, McClellan J, Findling RL, Vitiello B, Ritz L, … &, Lieberman JA. Double-blind comparison of first- and second-generation antipsychotics in early-onset schizophrenia and schizo-affective disorder: Findings from the treatment of early-onset schizophrenia spectrum disorders (TEOSS) study. American Journal of Psychiatry. 2008;165(11):1420–1431. doi: 10.1176/appi.ajp.2008.08050756. [DOI] [PubMed] [Google Scholar]
- Simeone JC, Ward AJ, Rotella P, Collins J, Windisch R. An evaluation of variation in pusblished estimates of schizophrenia prevalence from 1990–2013: A systematic literature review. BMC Psychiatry. 2012;15:193. doi: 10.1186/s12888-015-0578-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W, Sletten DM, Opfer-Gehrking TL, Brands CK, Fischer PR, Low PA. Postural tachycardia in children and adolescents: What is abnormal? Journal of Pediatrics. 2012;160(2):222–226. doi: 10.1016/j.jpeds.2011.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A, Forsyth K, Poon C, Peled M, Saewyc E McCreary Centre Society. [Accessed October 14, 2019];Balance and connection in BC: The health and well-being of our youth. 2019 from https://www.mcs.bc.ca/pdf/balance_and_connection.pdf. [Google Scholar]
- Souto-Filho JTD, Portugal RD, Nucci M. Effect of circadian variation on neutrophil mobilization to the peripheral blood in benign constitutional neutropenia. Experimental Hematology. 2019;69:22–26. doi: 10.1016/j.exphem.2018.10.007. [DOI] [PubMed] [Google Scholar]
- Spina E, Avenoso A, Facciola G, Scordo MG, Ancione M, Madia AG, … &, Perucca E. Relationship between plasma concentrations of clozapine and norclozapine and therapeutic response in patients with schizophrenia resistant to conventional neuroleptics. Psychopharmacology. 2000;148(1):83–89. doi: 10.1007/s002130050028. [DOI] [PubMed] [Google Scholar]
- Sporn AL, Vermani A, Greenstein DK, Bobb AJ, Spencer EP, Clasen LS, … &, Gogtay Nl. Clozapine treatment of childhood-onset schizophrenia: Evaluation of effectiveness, adverse effects, and long-term outcome. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46(10):1349–1356. doi: 10.1097/chi.0b013e31812eed10. [DOI] [PubMed] [Google Scholar]
- Steinauer LM, Leung JG, Burkey BW, McGrane IR, Letts V, Goren JL, … &, Vande Voort JL. A retrospective multicenter evaluation of clozapine use in pediatric patients admitted for acute psychiatric hospitalization. Journal of Child and Adolescent Psychopharmacology. 2018;28(9):615–619. doi: 10.1089/cap.2018.0036. [DOI] [PubMed] [Google Scholar]
- Tsuda Y, Saruwatari J, Yasui-Furukori N. Meta-analysis: The effects of smoking on the disposition of two commonly used antipsychotic agents, olanzapine and clozapine. [Accessed October 14, 2019];British Medical Journal. 2014 4(3):e004216. doi: 10.1136/bmjopen-2013-004216. from https://bmjopen.bmj.com/content/4/3/e004216.long. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Os J, Linscott RJ, Myin-Germeys I, Delespaul P, Krabbendam L. A systematic review and meta-analysis of the psychosis continuum: Evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychological Medicine. 2009;39(2):179–195. doi: 10.1017/S0033291708003814. [DOI] [PubMed] [Google Scholar]
- Woerner MG, Mannuzza S, Kane JM. Anchoring the BPRS: An aid to improved reliability. Psychopharmacology Bulletin. 1988;24(1):112–117. [PubMed] [Google Scholar]
- Wong J, Delva N. Clozapine-induced seizures: Recognition and treatment. Canadian Journal of Psychiatry. 2007;52(7):457–463. doi: 10.1177/070674370705200708. [DOI] [PubMed] [Google Scholar]
- Yatham LN, Kennedy SH, Parikh SV, Schaffer A, Bond DJ, Frey BN, … &, Berk M. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disorders. 2018;20(2):97–170. doi: 10.1111/bdi.12609. [DOI] [PMC free article] [PubMed] [Google Scholar]