Abstract
Background
Totally laparoscopic total gastrectomy (TLTG) using the overlap reconstruction method is associated with fewer postoperative complications and fast recovery than laparoscopic-assisted radical total gastrectomy (LATG). However, evidence on the safety and feasibility of TLTG (overlap reconstruction) in patients with advanced Siewert III esophagogastric junction cancer and gastric cancer of the upper and middle third of the stomach is scarce.
Methods
This study is a prospective, single-center, single-blind, two-arm randomized controlled trial designed to include 292 patients with advanced Siewert III esophagogastric junction cancer and gastric cancer of the upper and middle third of the stomach who will be randomly assigned to two groups: a TLTG overlap group (n=146) and an LATG group (n=146). The patients’ demographics, pathological characteristics, intraoperative variables, postoperative complications, postoperative recovery variables, 3-year disease-free survival and 3-year overall survival will be collected and analyzed. The primary outcome is the postoperative complications within 30 days after surgery including intra-abdominal hemorrhage, anastomotic leakage, duodenal stump fistula, pancreatic fistula, chyle leakage, abdominal infection, intestinal obstruction, wound complications, pulmonary infection, pleural effusion, pulmonary embolism, cardiovascular and cerebrovascular complications, and deep vein thrombosis. The secondary outcomes are the 3-year disease-free survival and 3-year overall survival.
Discussion
This trial will provide high-level evidence for the safety and feasibility of TLTG (overlap reconstruction) compared with LATG in advanced Siewert III esophagogastric junction cancer and the upper and middle third of gastric cancer.
Trial Registration
This trial has been registered at the Chinese Clinical Trial Registry: ChiCTR1900025667 (registration date: September 4, 2019).
Keywords: totally laparoscopic total gastrectomy, laparoscopic-assisted total gastrectomy, overlap reconstruction, Siewert III esophagogastric junction cancer, gastric cancer, randomized controlled trial
Background
Since Kitano et al1 reported the world’s first laparoscopic-assisted distal gastrectomy in 1994, the surgical techniques have greatly evolved and the surgical indications have expanded from early gastric cancer to locally advanced gastric cancer.2 With further improvements in the techniques and equipment, laparoscopic assisted surgery has progressed towards totally laparoscopic surgery.3 Compared with traditional open surgery, laparoscopic surgery provides better visualization of the operative field and is associated with less postoperative pain, fast recovery, fewer complications, and a shorter hospital stay.3 Meanwhile, the long-term survival outcomes of laparoscopic surgery are comparable to those of open surgery.4 A multicenter randomized controlled trial (CLASS-01) systematically evaluated the use of total laparoscopic distal gastrectomy in advanced gastric cancer. The study concluded that there was no significant difference in postoperative complications and long-term survival in total laparoscopic distal gastrectomy compared with open surgery.4 This study provides a reference and theoretical basis for evaluating the application of laparoscopic surgery in advanced gastric cancer. Currently, complete laparoscopic distal gastrectomy is recommended by the guidelines and has been proven to be safe and feasible.5 However, totally laparoscopic total gastrectomy (TLTG) is not as extensively used as totally laparoscopic distal gastrectomy.6
At present, laparoscopic total gastrectomy is still mainly laparoscopically assisted. In laparoscopic-assisted radical total gastrectomy (LATG), the digestive tract reconstruction is performed through an auxiliary incision. However, it is difficult to expose the surgical field in patients with obesity, small angle of the rib arch, or high tumor position. In these cases, the incision has to be extended and the risk of anastomosis complications is increased coupled with fewer advantages for minimally invasive surgery.7 In contrast, with TLTG, the surgeon has a better view of the operative field, which is advantageous for lymph node dissection of the lower esophagus and more suitable for patients with larger lesions, higher tumor position and obesity.8,9 The only problem is that it requires advanced laparoscopic suturing skills to perform the esophagojejunal anastomosis.
It was reported that the incidence of complications after laparoscopic total gastrectomy is as high as 10–40%,6 and the incidence of esophageal jejunal anastomotic leakage is 1.7–6.5%.10 Esophageal jejunal anastomotic leakage is a serious complication, accounting for 0.9–8.5% of the overall complications, and once it occurs, the mortality rate can be as high as 50%.11 Excessive tension at the anastomotic site, leading to bleeding and disruption of the esophageal mucosa, causes anastomotic leakage.12 Therefore, the development of a safe, simple, and secure anastomotic method is very crucial for the widespread use of TLTG. However, there is no consensus on the best method for reconstruction during TLTG.
In recent years, many studies have recognized the advantages of linear anastomosis in total laparoscopic esophagojejunostomy.13 Linear anastomosis with laparoscopy is relatively simple and can be performed in deeper and narrower areas like the esophageal hiatus. The diameter of the linear anastomosis is not affected by the diameter of the esophagus and jejunum. Hence, the diameter of the anastomosis is large, and the probability of anastomotic stenosis is relatively low.14 Schneider et al15 reported that the diameter of the anastomosis obtained with a linear stapler was significantly larger than that obtained with a circular stapler. In addition, the blood supply to the stapler after linear anastomosis does not fall to a critical level,16 and the risk of anastomotic leakage is low.13
The overlap method for total laparoscopic esophageal jejunostomy was proposed by Inaba et al17 in 2010. In the overlap method, the position of the esophagus and jejunum is parallel to the direction of the intestinal peristalsis. The anastomosis is wide with lower mesenteric tension. Most of the current studies show that the incidence of anastomotic-related complications after overlap anastomosis is low and the short-term results are satisfactory.18 Kawamura et al19 retrospectively reported that the incidence of postoperative anastomotic complication was 0.7% in the overlap group and another study from the United States also reported the feasibility and safety of the overlap method in advanced gastric cancer.20
However, the safety and effectiveness of the overlap method is still controversial. To date, there are no large-scale clinical randomized controlled trials comparing TLTG using the overlap method with LATG for advanced Siewert III esophagogastric junction cancer and cancer of the upper and middle third of the stomach in terms of safety and efficacy. Here, we designed a study protocol to conduct a single-center, prospective randomized controlled trial to compare the efficacy and safety of TLTG using the overlap method with LATG for advanced Siewert III esophagogastric junction cancer and upper and middle third gastric cancer.
Methods and Design
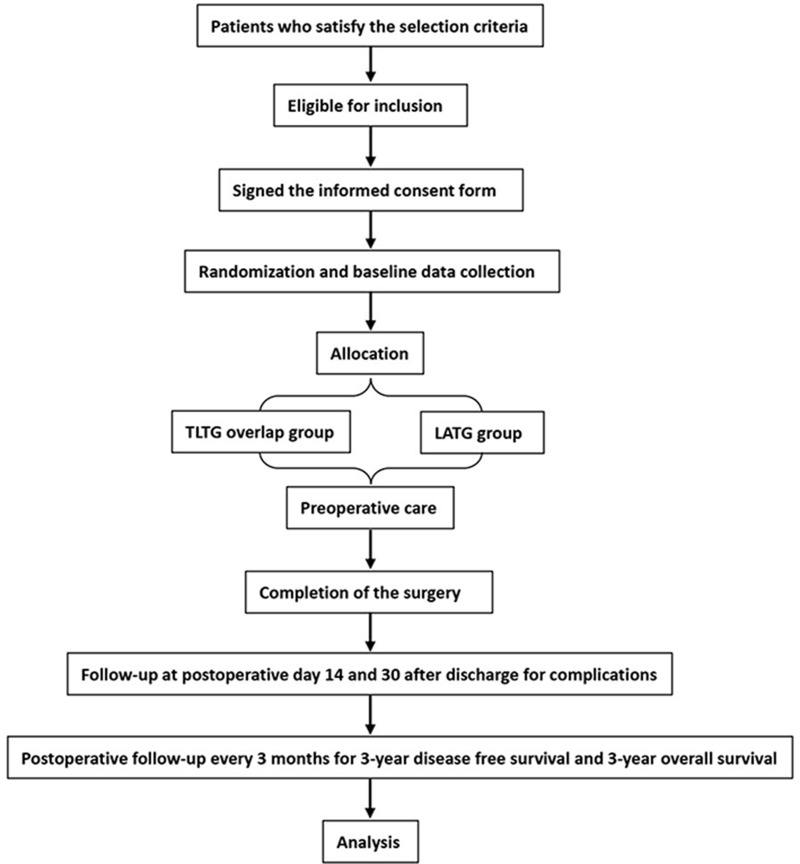
This is a prospective, single center, single-blind, two-arm randomized controlled trial (RCT) in which 292 patients will be rolled from the Department of Digestive Surgery, Xi Jing Hospital, The Fourth Military Medical University, China from October 2019 to October 2024. They will be randomly assigned by computer to two groups. The experimental group will receive TLTG (overlap reconstruction) and the control group will receive LATG. The trial will compare the safety and efficacy of TLTG with overlap reconstruction with that of LATG for the treatment of advanced Siewert III esophagogastric junction cancer and upper and middle third gastric cancer. A complete checklist of items according to the Standardized Protocol Items: Recommendations for Intervention Trials (2013) 13 is provided. Figure 1 shows the flow chart for the trial.
Figure 1.
Flow chart for the trial.
Participant Selection
Patients diagnosed with advanced Siewert III esophagogastric junction cancer or gastric cancer of the upper and middle part of the stomach who will undergo laparoscopic radical total gastrectomy at Xijing hospital will be recruited. In total, 292 eligible patients will be identified and randomized (1:1) to the TLTG (overlap) and LATG groups. The inclusion criteria are as follows: 1) age between 18 and 65 years; 2) Siewert type III adenocarcinoma of the esophagogastric junction or gastric cancer of the upper and middle part of the stomach; 3) no neoadjuvant chemotherapy received; 4) feasible to perform D2 (lymph node dissection according to the Japanese gastric cancer guidelines 2010 third edition recommendation) radical total gastrectomy (R0 resection), 5) absence of distant metastases and surrounding adjacent organ invasion on preoperative contrast-enhanced computed tomography (CT) chest and abdomen; 6) preoperative staging cT2-4aN0-3M0 (according to AJCC-7th TNM tumor staging); 7) voluntary participation in the study and the provision of informed consent; 8) preoperative Eastern Cooperative Oncology Group (ECOG) score of 0 to 2; 9) preoperative white blood cells (WBC) ≥ 3 × 109/L, absolute neutrophil count (NEU) ≥ 1.5 × 109/L, platelet (PLT) ≥100 × 109/L; and 10) preoperative American Society of Anesthesiologists (ASA) score of I–III.
The exclusion criteria are as follows: 1) contraindications for laparoscopic surgery; 2) uncontrolled severe medical diseases, including unstable angina or myocardial infarction within 6 months, congestive heart failure greater than New York Heart Association (NYHA) 2, arrhythmia requiring antiarrhythmic drugs, thrombosis or embolic events within 6 months, severe central nervous system or blood system disease, heart, lung, liver, or kidney dysfunction, immunodeficiency, liver cirrhosis, portal hypertension, or splenomegaly; 3) trauma, fracture, or serious surgery within 6 weeks before the start of the study; 4) autologous bone marrow transplantation or stem cell rescue treatment within 4 months before the start of the study; 5) history of allogeneic organ transplantation; 6) pregnant or lactating women; 7) serious mental illness; 8) history of previous esophageal or gastric surgery; 9) history of other malignant tumors within 5 years; 10) emergency surgery; 11) participating in other clinical trials.
The elimination criteria are as follows: 1) inability to perform R0 resection during surgery; 2) combined organ resection due to invasion of surrounding organs intraoperatively; 3) presence of distant metastasis on intraoperative exploration or development of intraoperative severe cardiovascular and cerebrovascular complications; 4) conversion to laparotomy; 5) conversion to thoracotomy to perform anastomosis; 6) postoperative histopathological findings of coexistent gastric neuroendocrine tumors, squamous cell carcinoma, lymphoma, stromal tumors, and other types of tumors; 7) participant request to withdraw from the study after enrollment.
Surgical Intervention/Treatment Protocols
Surgery will be performed by an experienced surgeon who has performed more than 50 cases of TLTG (overlap) and more than 100 cases of LATG with an annual case load >200. Patients will be anesthetized and placed in a flat position. The head will be raised by 30°. The operator will stand on the left side of the patient, the first assistant on the right side of the patient, and the camera assistant between the legs of the patient. A 10 mm trocar will be placed as the first port at the lower edge of the umbilicus and a pneumoperitoneum will be established. The pneumoperitoneum pressure will be maintained at 12–14 mmHg (1 mmHg = 0.133 kpa). In all, five trocars will be placed in a “V” pattern.
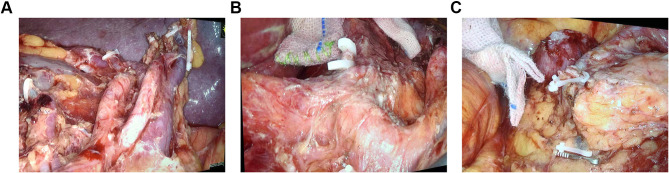
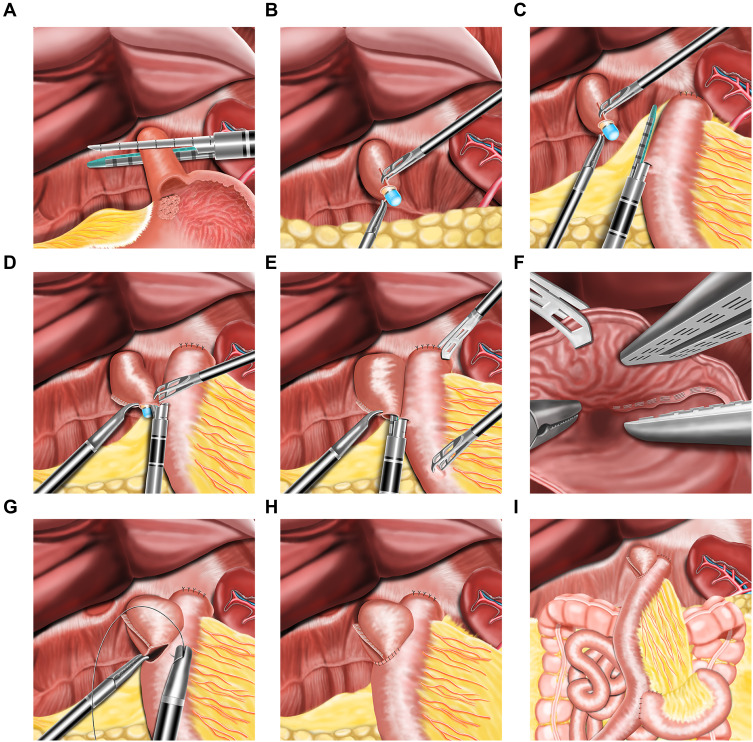
The D2 lymph node dissection will be performed according to the Japanese Gastric Cancer treatment protocol and the whole stomach and the lower esophagus will be fully mobilized (Figure 2A–C). The esophagus and the duodenum will be transected using a 60-mm linear stapler. The resected specimen will be removed through the umbilical port site by extending the incision. The right diaphragmatic crus and the ventral side of the esophageal hiatus will be partially divided to widen the surgical field for reconstruction, if necessary. The experimental group will receive TLTG. For TLTG, all the anastomoses will be performed laparoscopically. Jejunojejunostomy (Y anastomosis) will be performed before esophagojejunostomy. The jejunum will be intracorporeally transected at a point 20 cm distal to the ligament of Treitz using a 60-mm Endo-GIA linear stapler. At approximately 55 cm distal to the planned site for esophagojejunostomy, a side-to-side jejunojejunostomy will be performed using a 60-mm linear stapler. The entry point will be closed with an extracorporeal interrupted hand-sewn technique using absorbable monofilament sutures. The jejunal mesenteric defect will be closed to prevent internal herniation. The duodenal and distal jejunal staple lines will be reinforced with interrupted 4–0 absorbable sutures. A small hole will be made 6 cm distal to the stapler line on the antimesenteric side of the jejunal limb, while another small hole will be made on the left wall of the esophageal stump. One limb of the 45-mm linear stapler will be inserted into the esophageal stump using the nasogastric tube as a guide and another limb of the liner stapler will be placed in the jejunal limb. The forks of the stapler will be closed and fired to construct a Roux-en-Y side-to-side esophagojejunal anastomosis. After the hemostasis at the anastomosis site is confirmed, the entry hole will be closed by continuous full-thickness hand suturing using a 3–0 barbed suture. The main process of the overlap method is shown in Figure 3A–I.
Figure 2.
Intraoperative images showing the radical D2 lymphadenectomy during total gastrectomy. (A) Splenic hilar lymph node dissection. (B) The lymph nodal dissection near the origin of the common hepatic artery, left gastric artery and the splenic artery. (C) The lymph nodal dissection near the right gastroepiploic artery, right gastroepiploic vein, and removal of the subpyloric lymph nodes.
Figure 3.
Schematic representation of the technique of esophagojejunostomy after totally laparoscopic total gastrectomy by overlap method. (A) Division of the distal esophagus with a linear cutting closure device. (B) The gastric tube is passed through the cut end of the esophagus. (C) One arm of the linear staple is inserted into the proximal jejunum. (D) Under the guidance of nasogastric tube, the other arm of linear stapler is inserted into the stump of the esophagus. (E) The staples were fired and the esophagojejunal linear anastomosis is performed. (F) Hemostasis along the staple line is confirmed. (G) Closure of the common opening with barbed suture. (H) Completion of the esophagojejunal anastomosis. (I) The overview of all the anastomoses after the completion of the surgery.
The patients in the control group will receive LATG. The procedure for radical D2 lymphadenectomy and the complete mobilization of the stomach will be the same as that used in the overlap group. The esophagojejunal anastomosis will be performed with the traditional open Roux-en-Y anastomosis technique using a circular stapler. An 8–10 cm incision will be made in the upper abdomen. Purse-string sutures will be placed 2 to 3 cm away from the upper edge of the tumor and the esophagus and the duodenum will be routinely transected. The anvil of the circular stapler will be inserted into the esophageal stump. The jejunum will be extracorporeally transected 25 cm distal to the ligament of Treitz. Then, a Roux-en-Y end-to-side esophagojejunal anastomosis will be constructed with the circular staplers. Finally, a jejunojejunal side-to-side anastomosis will be made about 55 cm below the esophagojejunostomy using a hand sewn method extracorporeally.
Sample Size Estimation
The sample size was estimated using PASS11.0 software. The incidence of postoperative complications in our central database among 2268 patients who underwent radical total gastrectomy was 19.9%. We estimated that the postoperative 30-day overall complication rate will be 8% in the experimental group. The parameters were designed for a noninferiority test with a noninferiority margin of 20% (a=0.05, b=0.20, and a power of 80%). Patients will be assigned to the experimental and control groups with a 1:1 ratio. The optimal sample size for each group was determined to be 132, and 264 participants will be needed. Allowing for a 10% drop-out rate, 292 participants (146 participants in each group) will be recruited.
Study Endpoints
The primary outcome is the complications within 30 days after surgery. The complications that will be analyzed in this study include intra-abdominal hemorrhage, gastrointestinal bleeding, anastomotic leakage, duodenal stump fistula, pancreatic fistula, chyle leakage, abdominal infection or abscess, intestinal obstruction, incision-related complications (infection, hydration, cracking, bleeding, poor healing, etc.), pulmonary infection, pleural effusion, pulmonary embolism, cardiovascular and cerebrovascular complications (including thrombosis, embolism, etc.), and deep vein thrombosis. Postoperative surgical complications will be classified according to the Clavien-Dindo classification.21 The secondary outcomes are 3-year disease-free survival and 3-year overall survival. Disease-free survival is defined as the time between the date of surgery and the first detection of local recurrence or distant metastasis. Overall survival is defined as the time between the date of surgery and death.
Data Collection
The preoperative baseline data (gender, age, BMI, ASA classification, ECOG score, blood tests [Hb, RBC, WBC, NEU%, PLT, AST, ALT, albumin, prealbumin, total bilirubin, creatinine, urea nitrogen, potassium ion, sodium ion, chloride ion, calcium ion], tumor markers [CEA, AFP, CA19-9] and comorbidities [heart disease, hypertension, diabetes, etc.]) will be recorded. Intraoperative variables including digestive tract reconstruction time (min), operation time, intraoperative blood loss, length of incision, intraoperative blood transfusion, and intraoperative complications will be documented. Postoperative pathology (tumor location, tumor size, margin, distance of upper margin, number of lymph nodes dissected, lymph node metastasis rate, TNM clinical stage, pathological type [adenocarcinoma, tubular adenocarcinoma, mucinous adenocarcinoma, signet ring cell carcinoma], Lauren classification [intestinal, diffuse, or mixed], and differentiation [well, moderate, or poor]) will be recorded. Postoperative complications (pulmonary infection, anastomotic leakage, intestinal obstruction, intra-abdominal hemorrhage, wound infection, chyle leakage, deep vein thrombosis of the lower extremity, anastomotic stenosis), postoperative overall complication rate, reoperation rate, readmission rate, mortality, and postoperative recovery variables (including the time to the first passage of flatus or feces, postoperative hospital stay, visual analog scale score at POD 1–3 and on the day of discharge, maximum body temperature at POD 1–3, WBC and NEU% at POD 1 and 3, and nutritional status [hemoglobin, albumin, and prealbumin at POD 1 and 3]) will be documented. The 3-year disease-free survival and 3-year overall survival will be collected and analyzed.
Perioperative Management
Participants in both groups will receive perioperative care in line with the enhanced recovery after surgery (ERAS) strategy. Perioperative management will be carried out by a team of researchers in accordance with clinical routine methods and accumulated experience. Briefly, before surgery, patients with nutritional risk will be given parenteral/enteral nutrition support. High-risk patients with advanced age, smoking, obesity, diabetes, chronic cardiovascular and cerebrovascular diseases, or previous history of thromboembolism will receive respiratory function training, low molecular weight heparin prophylaxis, lower extremity antithrombotic stockings, and active lower extremity massage to reduce postoperative complications.
After surgery, the abdominal drainage tube will be routinely placed on the dorsal side of the esophagojejunal anastomosis and will be removed within 48 hours if the drainage is non-bilious and low volume. The gastric tube and the Foley’s catheter can be removed before leaving the operating room. The nasojejunal tube will be placed nasally at the distal end of the jejunum-jejunum side anastomosis. Water intake will be started from postoperative day (POD) 1 and fluid intake from POD 2. The postoperative diet will be gradually upgraded from water (liquid) to thin porridge (semi-liquid) to soft food. Postoperative fluid replacement (including glucose, insulin, electrolytes, vitamins, etc.) or nutritional support (intestinal/parenteral) will be given according to the patients’ clinical condition. If the patients can drink up to 1000 mL per day, the nutrient tube will be removed, and the fluid/nutrition support will be stopped or gradually reduced. Early ambulation will be started at 8 hours after the operation and patients will be recommended to stay active for more than 8 hours per day from POD 1. The treating surgeon will determine whether or not to use anticoagulants after surgery based on the clinical conditions. Local anesthesia with bupivacaine or ropivacaine will be given before and during the operation, and an analgesic pump combined with non-steroidal anti-inflammatory drugs will also be given. Prophylactic antibiotics will be used for no more than 48 hours. Patients will be discharged if they are eating food without discomfort, no fever, no signs of infection, and no complications. In the absence of contraindications, adjuvant chemotherapy will be started 3 weeks after surgery. All patients will receive fluorouracil-based chemotherapy after surgery and will be followed up as recommended in the recent National Comprehensive Cancer Network (NCCN) guidelines.
Follow-Up
The patients will be followed up after discharge with the first follow-up on the 14th day after surgery and the second follow-up on the 30th day after surgery. During the first two follow-up visits, physical examination and blood tests including complete hemogram, liver, and kidney function tests will be conducted. Additionally, post-hospital complications will be recorded and the plan for postoperative chemotherapy will be made. If the postoperative hospital stay is >14 or 30 days or the patient is re-admitted, chemotherapy will be delayed and the cause will be recorded.
Afterward, follow-up will be performed every 3 months, during which blood tests including liver and kidney function tests will be conducted and electrolytes, tumor markers, and contrast enhanced CT (layer thickness 10 mm or less, contrast agent allergy, changed to MRI) of the chest and abdomen will be routinely evaluated. If the CT indicates distant metastasis, PET/CT will be performed if necessary. If the CT suggests local recurrence, gastroscopy will be performed. The results of each examination will be combined, and the patient’s recurrence or metastasis and its date will be recorded. The chemotherapy regimen administered to the patients will be recorded. If the patient dies, the cause and date of death will be recorded. The follow-up content is listed in Table 1.
Table 1.
Postoperative Follow-Up
| Item | 14d | 30d | 3m | 6m | 9m | 12m | 15m | 18m | 21m | 24m | 27m | 30m | 33m | 36m |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hb | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ |
| RBC | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ |
| WBC | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ |
| NEU% | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ |
| PLT | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ |
| AST | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ |
| ALT | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ |
| Albumin | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ |
| Prealbumin | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ |
| TB | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ |
| DBil | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ |
| IBIL | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ |
| Creatinine | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ |
| UN | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ |
| K+ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ |
| Na+ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ |
| Cl− | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ |
| Ca2+ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ |
| CEA | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ||
| AFP | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ||
| CA19-9 | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ||
| Complication | ※ | ※ | ||||||||||||
| C-D | ※ | ※ | ||||||||||||
| CT | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ||
| Gastroscopy* | ||||||||||||||
| Other test* | ||||||||||||||
| Chemotherapy | ||||||||||||||
| Recurrence | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ||
| Metastasis | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ||
| Survival | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ | ※ |
Note: *When necessary.
Abbreviations: TB, total bilirubin; DBil, direct bilirubin; IBIL, indirect bilirubin; UN, urea nitrogen; C-D, Clavien-Dindo classification.
Randomization and Blinding Technique
Patients who meet the selection criteria will be randomly assigned to the trial group and the control group at a ratio of 1:1. Allocation concealment and block randomization will be used. First, the SAS software will be used to set a certain number of seeds to generate 292 random numbers. All random numbers will be assigned to the two groups at a ratio of 1:1 and loaded into 292 envelopes. Each group requires two random numbers to form a block and a total of 73 blocks will be generated. The 73 blocks will be randomly sorted, and the envelopes in the block will be randomly selected according to the block order to obtain the results of the group. The surgeon cannot be blinded in this study. Additionally, because the length of the incision in the two groups of patients will be significantly different, the patients will also not be blinded. However, the nursing staff, investigators, evaluators, and statistical analysts will be blinded.
Statistical Analysis
Statistical Analysis Population
Full Analysis Set (FAS)
For all patients who are randomized and undergo the assigned surgery but lack complete follow-up data, the findings at the last follow-up will be used to carry out the analysis. The FAS population will be the primary analysis population for the analysis of the efficacy of this trial.
Per-Protocol Set
All patients who undergo surgery per the randomization protocol with complete follow-up data will be included in the statistical analysis for the efficacy of the procedures.
Intentionality Analysis
All patients assigned to a group will be included in intentionality analysis if the surgical plan is changed due to the condition of the patient, the surgeon decides to change the surgical plan according to the actual situation, or the patient receives other unplanned treatments for any reason, such as conversion to open surgery, combined organ resection, inability to perform radical total gastrectomy due to tumor invasion to local peripheral organs or distant metastases, jejunal ostomy alone, or voluntary withdrawal from the study.
Statistical Methods
All statistical analyses will be performed using SAS 9.4 (or higher version) statistical software. All P values will be two-sided and a P value < 0.05 will be considered to be statistically significant.
Subject Distribution
In this section, the number of subjects enrolled, excluded, completed, and lost to follow-up will be evaluated, and the status of each group in the analysis data set will be summarized.
Comparability Analysis
Comparability analysis will be performed on the baseline data, intraoperative conditions, postoperative pathology, etc., to determine whether the two groups are comparable. The number of cases, mean, standard deviation, median, maximum, and minimum will be listed. T-tests will be performed to compare the continuous variables (demographic characteristics such as age, height, etc.) between the groups. Laboratory indicators (such as blood test, liver function, tumor biomarkers, etc.), intraoperative indicators (operation time, blood transfusion volume, etc.), and postoperative pathology (tumor size, number of lymph node dissections, etc.) will be compared using the Wilcoxon rank sum test. Categorical variables will be expressed as number and percentage and the two groups will be compared using the Fisher exact test or χ2 tests.
Efficacy Analysis
The postoperative 30-day complications and the severity grades of the two groups will be described and the differences in the total complication rate between the two groups will be compared with the chi-square test. Survival analysis will be performed using the Kaplan-Meier method and the Log Rank test will be used to test the difference in survival rates between the two groups.
Strengths and Limitations of This Study
The feasibility of TLTG using the overlap method has not been established in prospective randomized studies.
This trial will be the first RCT to assess TLTG with the overlap method for patients with advanced Siewert III esophagogastric junction cancer and gastric cancer of the upper and middle third of the stomach.
The limitations of this study are that it is a single-center study and there is no control arm for TLTG with anastomosis using a circular stapler.
Funding Statement
This work is supported by grants from the National Natural Science Foundation of China (Key Program 81502401 and 31670828) by JW and GJ. This work is also supported by the Ethicon Excellence in Surgery Grant (EESG) (Key Program HZB-20190528-7). Our study protocol has already undergone peer-review by the funding body of the Ethicon Excellence in Surgery Grant (EESG).
Abbreviations
TLTG, totally laparoscopic total gastrectomy; LATG, laparoscopic-assisted radical total gastrectomy; RCT, randomized controlled trial; CT, computed tomography; ECOG, Eastern Cooperative Oncology Group; WBC, white blood cells; NEU, neutrophil count; ASA, American Society of Anesthesiologists; NYHA, New York Heart Association; ERAS, enhanced recovery after surgery; POD, postoperative day; FAS, Full Analysis Set.
Data Sharing Statement
The datasets used in this study will be available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
Ethics approval has been obtained from the Ethics Committee at the First Affiliated Hospital (Xijing Hospital) of the Fourth Military Medical University (KY20192093-X-1). This trial will be conducted in accordance with the Declaration of Helsinki. Before allowing participation in the study, written informed consent will be obtained from each participant and their guardians. The present study protocol was prepared in accordance with the Standardized Protocol Items: Recommendations for Intervention Trials statement.
Consent to Publication
All authors agree to publish.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Although the work is supported by the Ethicon Excellence in Surgery Grant (EESG), the authors declare that they have no competing interests.
References
- 1.Kitano S, Iso Y, Moriyama M, Sugimachi K. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc. 1994;4(2):146–148. [PubMed] [Google Scholar]
- 2.Kim HH, Hyung WJ, Cho GS, et al. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report–a Phase III multicenter, prospective, randomized Trial (KLASS Trial). Ann Surg. 2010;251(3):417–420. doi: 10.1097/SLA.0b013e3181cc8f6b [DOI] [PubMed] [Google Scholar]
- 3.Okabe H, Obama K, Tsunoda S, Tanaka E, Sakai Y. Advantage of completely laparoscopic gastrectomy with linear stapled reconstruction: a long-term follow-up study. Ann Surg. 2014;259(1):109–116. doi: 10.1097/SLA.0b013e31828dfa5d [DOI] [PubMed] [Google Scholar]
- 4.Hu Y, Huang C, Sun Y, et al. Morbidity and mortality of laparoscopic versus open d2 distal gastrectomy for advanced gastric cancer: a randomized controlled trial. J Clin Oncol. 2016;34(12):1350–1357. doi: 10.1200/JCO.2015.63.7215 [DOI] [PubMed] [Google Scholar]
- 5.Oki E, Sakaguchi Y, Ohgaki K, et al. Surgical complications and the risk factors of totally laparoscopic distal gastrectomy. Surg Laparosc Endosc Percutan Tech. 2011;21(3):146–150. doi: 10.1097/SLE.0b013e318219a66b [DOI] [PubMed] [Google Scholar]
- 6.Kunisaki C, Makino H, Takagawa R, et al. A systematic review of laparoscopic total gastrectomy for gastric cancer. Gastric Cancer. 2015;18(2):218–226. doi: 10.1007/s10120-015-0474-3 [DOI] [PubMed] [Google Scholar]
- 7.Kim MG, Kim BS, Kim TH, Kim KC, Yook JH, Kim BS. The effects of laparoscopic assisted total gastrectomy on surgical outcomes in the treatment of gastric cancer. J Korean Surg Soc. 2011;80(4):245–250. doi: 10.4174/jkss.2011.80.4.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim HS, Kim BS, Lee IS, Lee S, Yook JH, Kim BS. Comparison of totally laparoscopic total gastrectomy and open total gastrectomy for gastric cancer. J Laparoendosc Adv Surg Tech A. 2013;23(4):323–331. doi: 10.1089/lap.2012.0389 [DOI] [PubMed] [Google Scholar]
- 9.Nakauchi M, Suda K, Kadoya S, Inaba K, Ishida Y, Uyama I. Technical aspects and short- and long-term outcomes of totally laparoscopic total gastrectomy for advanced gastric cancer: a single-institution retrospective study. Surg Endosc. 2016;30(10):4632–4639. doi: 10.1007/s00464-015-4726-4 [DOI] [PubMed] [Google Scholar]
- 10.Inokuchi M, Otsuki S, Fujimori Y, Sato Y, Nakagawa M, Kojima K. Systematic review of anastomotic complications of esophagojejunostomy after laparoscopic total gastrectomy. World J Gastroenterol. 2015;21(32):9656–9665. doi: 10.3748/wjg.v21.i32.9656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LaFemina J, Viñuela EF, Schattner MA, Gerdes H, Strong VE. Esophagojejunal reconstruction after total gastrectomy for gastric cancer using a transorally inserted anvil delivery system. Ann Surg Oncol. 2013;20(9):2975–2983. doi: 10.1245/s10434-013-2978-6 [DOI] [PubMed] [Google Scholar]
- 12.Zuiki T, Hosoya Y, Kaneda Y, et al. Stenosis after use of the double-stapling technique for reconstruction after laparoscopy-assisted total gastrectomy. Surg Endosc. 2013;27(10):3683–3689. doi: 10.1007/s00464-013-2945-0 [DOI] [PubMed] [Google Scholar]
- 13.Kitagami H, Morimoto M, Nakamura K, et al. Technique of Roux-en-Y reconstruction using overlap method after laparoscopic total gastrectomy for gastric cancer: 100 consecutively successful cases. Surg Endosc. 2016;30(9):4086–4091. doi: 10.1007/s00464-015-4724-6 [DOI] [PubMed] [Google Scholar]
- 14.Hiyoshi Y, Oki E, Ando K, et al. Outcome of esophagojejunostomy during totally laparoscopic total gastrectomy: a single-center retrospective study. Anticancer Res. 2014;34(12):7227–7232. [PubMed] [Google Scholar]
- 15.Schneider R, Gass JM, Kern B, et al. Linear compared to circular stapler anastomosis in laparoscopic Roux-en-Y gastric bypass leads to comparable weight loss with fewer complications: a matched pair study. Langenbecks Arch Surg. 2016;401(3):307–313. doi: 10.1007/s00423-016-1397-0 [DOI] [PubMed] [Google Scholar]
- 16.Zilling TL, Walther BS, Ranstam J. Intersecting staple lines and blood flow in oesophagojejunal anastomoses. Br J Surg. 1990;77(12):1375–1378. doi: 10.1002/bjs.1800771218 [DOI] [PubMed] [Google Scholar]
- 17.Inaba K, Satoh S, Ishida Y, et al. Overlap method: novel intracorporeal esophagojejunostomy after laparoscopic total gastrectomy. J Am Coll Surg. 2010;211(6):e25–9. doi: 10.1016/j.jamcollsurg.2010.09.005 [DOI] [PubMed] [Google Scholar]
- 18.Morimoto M, Kitagami H, Hayakawa T, Tanaka M, Matsuo Y, Takeyama H. The overlap method is a safe and feasible for esophagojejunostomy after laparoscopic-assisted total gastrectomy. World J Surg Oncol. 2014;12(1):392. doi: 10.1186/1477-7819-12-392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawamura H, Ohno Y, Ichikawa N, et al. Anastomotic complications after laparoscopic total gastrectomy with esophagojejunostomy constructed by circular stapler (OrVil™) versus linear stapler (overlap method). Surg Endosc. 2017;31(12):5175–5182. doi: 10.1007/s00464-017-5584-z [DOI] [PubMed] [Google Scholar]
- 20.Treitl D, Hochwald SN, Bao PQ, Unger JM, Ben-David K. Laparoscopic total gastrectomy with D2 lymphadenectomy and side-to-side stapled esophagojejunostomy. J Gastrointest Surg. 2016;20(8):1523–1529. doi: 10.1007/s11605-016-3162-7 [DOI] [PubMed] [Google Scholar]
- 21.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]