Abstract
Objective
The aim of the present study was to investigate the possible correlation between the percentage of daily energy intake from fat (PEF) with insulin resistance (IR) in women with polycystic ovary syndrome (PCOS).
Methods
In this cross-sectional study, a total of 186 females with PCOS were screened. Daily dietary intake data were collected by a trained nutritionist using the 24-h dietary recall method over three consecutive days. A total of 111 subjects who had complete data were divided into two groups based on the percentage of daily energy intake from fat (PEF): the normal PEF (NPEF) group (PEF < 30%) and the high PEF (HPEF) group (PEF ≥ 30%). Pearson’s correlation analysis and stepwise multivariate linear regression analysis were used to analyze the correlation of PEF with homeostasis model assessment of insulin resistance (HOMA-IR).
Results
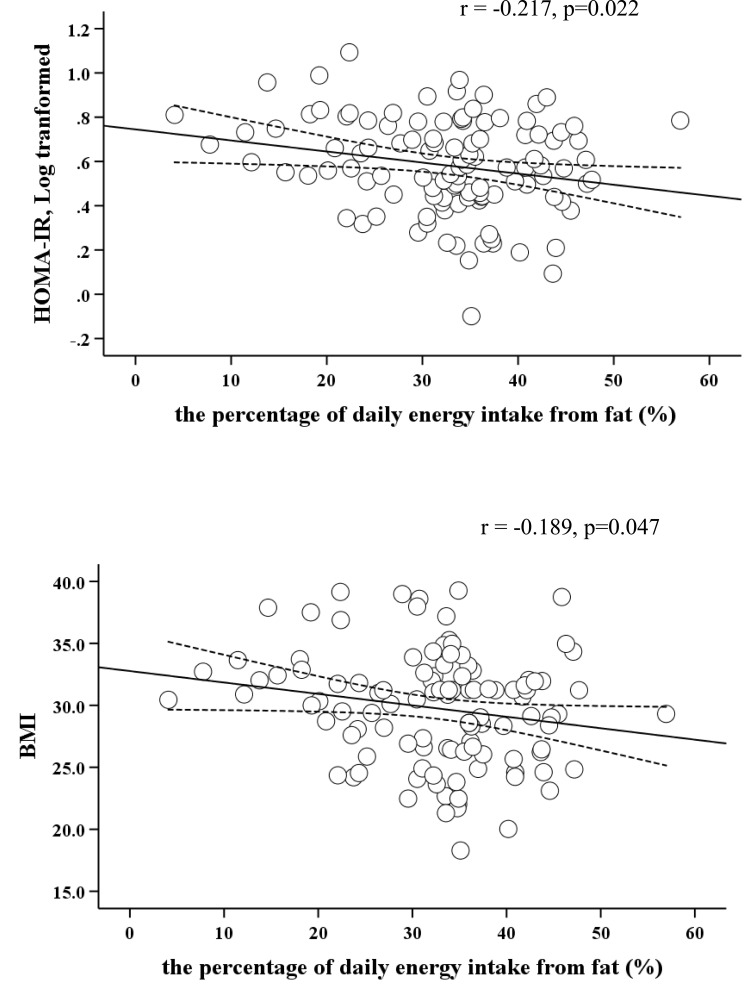
The total prevalence rate of overweight/obesity was 80.2%. There were significant differences in waist circumference (WC), body mass index (BMI), fasting insulin, and HOMA-IR (P < 0.001) among the normal weight, the overweight, and the obese groups, but no significant differences were observed in total energy and dietary macronutrients intake in the three groups. The daily intake of fat and protein, fasting insulin, and HOMA-IR in the NPEF group were significantly higher than those in the HPEF group. Pearson’s correlation analysis showed PEF in PCOS women was negatively correlated with BMI (r= −0.189, p=0.047) and HOMA-IR (log-transformed) (r= −0.217, p=0.022). Further, stepwise multivariate linear regression analysis showed PEF was negatively correlated with HOMA-IR (p<0.05).
Conclusion
The percentage of daily energy intake from fat is negatively correlated with IR in women with PCOS.
Keywords: the percentage of daily energy intake from fat, polycystic ovary syndrome, insulin resistance, daily dietary intake, obesity
Introduction
Polycystic ovary syndrome (PCOS) is the most common cause of anovulatory infertility in women of reproductive age, which is also one of the most common endocrine disorders.1 Apart from impaired reproduction, PCOS is associated with obesity, glucolipid metabolism disorders and hypertension, which increases the risk of cardiovascular diseases (CVD) and the long-term CVD-related morbidity and mortality.2 Obesity is one of the common features of PCOS, approximately 30–75% of PCOS patients are overweight or obese.3 It is reported that overweight or obese women with PCOS are at two- or three-fold higher risk compared with their non-PCOS counterparts, respectively.4,5 Moreover, obesity can aggravate IR and hyperinsulinemia, even worsen the clinical, endocrine and metabolic characteristics of PCOS.6
Insulin resistance (IR) refers to the body’s inability to utilize insulin for various reasons in the presence of elevated blood glucose concentrations, and then the body compensates to secrete excess insulin to maintain the stability of blood glucose. IR is prevalent in PCOS patients and affects approximately 75% of lean women and 95% of overweight women.7 The levels of IR and secondary hyperinsulinemia in overweight and obese PCOS women are significantly higher than those in nonobese PCOS women, and insulin sensitivity would worsen with increasing weight gain.8,9 Studies revealed that IR can increase the risk of developing diabetes and exhibit significantly higher androgens, eventually exacerbating metabolic and reproductive abnormalities in patients with PCOS.10,11
Dietary factors are speculated to be the risk factors for PCOS. Unhealthy lifestyles, especially inappropriate diets, result in obesity and IR, and may even exacerbate the metabolic and reproductive disorders of PCOS.12,13 Increased mono/polyunsaturated fat diet yields statistically significant reductions in hyperinsulinemia in subjects with PCOS.14 Decreased trans fatty acid intake predicts reduced insulinogenic index in overweight/obese women with PCOS.15 A recent population-based case-control study found a lower energy percentage supplied by protein and carbohydrates and higher energy percentage supplied by fat in PCOS patients than in controls.16 However, there are limited data on the relationship between daily energy from dietary components with IR in subjects with PCOS. So, the aim of the present study was to explore the possible associations between the consumption of fat and IR in PCOS patients.
Patients and Methods
Participants
In the current cross-sectional study, a total of 186 female patients with PCOS from the Department of Endocrinology and Diabetes, the First Affiliated Hospital of Xiamen University (Xiamen, China) were screened. PCOS was diagnosed according to the Rotterdam definition, which has been described in our previous publication.17 Face-to-face interviews were conducted to collect subjects’ health information and daily dietary intake assessment data. Of 186 patients, 111 subjects who had complete data on clinical and daily dietary intake assessment were left for the present analysis. This study was approved by the Human Research Ethics Committee of the First Affiliated Hospital of Xiamen University (Xiamen, China). Written informed consent was obtained from each participant.
Data Collection
The anthropometric indices were recorded including height, weight, and waist circumference (WC) measured by using a calibrated scale. Blood pressure was measured with OMRON electronic sphygmomanometer after 15 minutes rest in sitting position. WC was measured at the midpoint between the lowest rib and the iliac crest. Body mass index (BMI) was calculated as the weight in kilograms divided by the square of the height in meters. We defined a BMI of 25–30 kg/m2 as overweight and BMI greater than 30 kg/m2 as obese according to the World Health Organization’s criteria.18
Fasting blood samples were used to measure hormonal and biochemical parameters. Lipid profiles including triglyceride (TG), total cholesterol (TC), and high-density lipoprotein cholesterol (HDL-c) were determined on HITACHI 7450 analyzer (HITACHI, Tokyo, Japan). Low-density lipoprotein cholesterol (LDL-c) was calculated by Friedewald’s formula.19 Fasting blood glucose (FBG) was measured by the hexokinase method. Serum fasting insulin (Roche Elecsys Insulin Test, Roche Diagnostics, Mannheim, Germany), luteinizing hormone (LH), and follicle-stimulating hormone (FSH), and testosterone (T) (Siemens Healthcare Diagnostics Inc, Massachusetts, USA) were measured by electrochemiluminescence immunoassay. Oral glucose tolerance test with 75 g of glucose (OGTT) was performed in all subjects. After a fasting blood sample was obtained, subjects ingested 250 mL solution containing 75 g of dextrose within five minutes, and then venous blood samples were obtained at 120 min for determination of plasma glucose and plasma insulin, respectively. Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated using the following formula: (fasting insulin (μIU/mL) × fasting glucose (mmol/L))/22.5. Subjects were considered as having IR when the HOMA-IR index was ≥ 2.6×10−6 mol × U/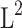 .20
.20
Dietary Intake Assessment
Daily dietary intake data were collected by a trained nutritionist using the 24-h dietary recall method over three consecutive days.21 The trained nutritionist used standard forms for the dietary recalls. To ensure that the subjects accurately reported their dietary intake, visual aids were used to assist with portion size descriptions such as food models and photographs. The trained nutritionist did face-to-face interviews with the participants to collect all food consumption information including type, amounts, type of meal, and place of consumption during the preceding 24 hours. Subjects were divided into two groups based on the percentage of daily energy intake from fat: the normal percentage of daily energy intake from fat (less than 30% of total daily energy intake) (NPEF) group and the higher percentage of daily energy intake from fat (more than or equal to 30% of total daily energy intake) (HPEF) group.
Statistical Analyses
Data were presented as the mean ± standard deviation (SD) for continuous variables or as median (inter-quartile range, IQR) for number and percentage for categorical variables. Skewness and kurtosis tests for normality found that fasting glucose, fasting insulin, 2-h glucose, 2-h insulin, TG, LDL-c, testosterone, LH/FSH ratio, the percentage of dietary fiber intake and HOMA-IR did not follow normal distributions. Differences between two groups were analyzed on continuous variables using the Student’s t-test for those with normal distribution and Kruskal–Wallis test for those with skewed distribution. Pearson’s correlation analysis was used to analyze the correlation of the percentage of daily energy intake from fat (PEF) with HOMA-IR (log-transformed), BMI and total daily energy intake. Univariate and stepwise linear regression analyses were used to explore the association of PEF with HOMA-IR. For multivariable linear regression analyses, age, systolic blood pressure (SBP), diastolic blood pressure (DBP), TG, TC, LDL-c, and HDL-c were adjusted. All statistical analyses were performed using SPSS version 21.0 software (IBM Corporation, Armonk, NY). All p-values were two-sided and p-value<0.05 was considered statistically significant.
Results
Among the 111 PCOS patients whose mean ages (±SD) were 28.3 ± 4.9 years, 30 (27.0%) were overweight and 59 (53.2%) were obese, respectively. The total prevalence rate of overweight/obesity was 80.2%.
Clinical Characteristics and Daily Dietary Intake Categorized by BMI
The clinical characteristics and energy intake of the studied groups were shown in Table 1. There were no significant differences in age, SBP, DBP, fasting glucose, 2-h glucose, 2-h insulin, lipid profiles (TG, TC, HDL-c, and LDL-c), testosterone, LH/FSH ratio, total energy intake, and the amount of daily dietary macronutrients intake (protein, fat, and carbohydrates, respectively) among the normal weight, the overweight and the obese groups. But there were statistically significant differences in BMI (23.2 ± 1.7 kg/m2, 27.7 ± 1.2 kg/m2, 33.2 ± 2.6 kg/m2, respectively, p<0.001), WC (79.9 ± 5.5 cm, 90.3 ± 4.6 cm, 101.7 ± 9.0 cm, respectively, p<0.001), fasting insulin (82.6 (55.7–105.2) pmol/L, 110.6 (91.4–141.4) pmol/L, 145.4 (107.0–190.4) pmol/L, respectively, p<0.001) and HOMA-IR (2.4 (1.7–3.2), 3.4 (2.8–4.7), 4.8 (3.6–6.4), respectively, p<0.001) among these three groups. However, PEF in the obese group was lowest (34.6 ± 6.6%, 35.3 ± 8.3%, 30.6 ± 10.2%, respectively, p=0.040) among the three groups, and the percentages of daily energy from carbohydrates in the same group were highest (46.2 ± 7.1%, 46.2 ± 9.9%, 52.3 ±11.5%, respectively, p=0.010).
Table 1.
Clinical Characteristics and Daily Dietary Intake of Patients with Polycystic Ovary Syndrome by BMI Category
| Normal Weight | Overweight | Obese | P value | |
|---|---|---|---|---|
| N | 22(19.8%) | 30(27.0%) | 59(53.2%) | |
| Age (years) | 28.3 ± 4.9 | 27.8 ± 5.5 | 26.1 ± 4.9 | 0.184 |
| BMI (kg/m2) | 23.2 ± 1.7 | 27.7 ± 1.2 | 33.2 ±2.6 | <0.001 |
| WC (cm) | 79.9 ± 5.5 | 90.3 ± 4.6 | 101.7 ± 9.0 | <0.001 |
| SBP (mmHg) | 115.4 ±10.6 | 121.8 ± 12.1 | 122.7 ±13.1 | 0.085 |
| DBP (mmHg) | 78.3± 12.0 | 81.6± 7.4 | 82.9 ± 12.0 | 0.288 |
| Fasting glucose (mmol/L) | 4.7(4.6–5.3) | 4.9(4.6–5.4) | 5.1(4.8–5.4) | 0.113 |
| Fasting insulin (pmol/L) | 82.6(55.7–105.2) | 110.6(91.4–141.4) | 145.4(107.0–190.4) | <0.001 |
| 2-h glucose (mmol/L) | 7.7(6.1–8.9) | 6.8(6.2–8.5) | 6.9(5.9–8.5) | 0.839 |
| 2-h insulin (pmol/L) | 658.1(461.8–1143.5) | 650.5(427.1–1025.7) | 823.9(539.7–1191.9) | 0.278 |
| HOMA-IR | 2.4(1.7–3.2) | 3.4(2.8–4.7) | 4.8(3.6–6.4) | <0.001 |
| TG (mmol/L) | 1.2(0.9–1.8) | 1.4(1.1–2.1) | 1.5(1.1–2.0) | 0.378 |
| TC (mmol/L) | 4.9±0.8 | 5.3± 1.0 | 5.2±0.7 | 0.351 |
| HDL-c (mmol/L) | 1.3±0.3 | 1.2±0.2 | 1.2±0.2 | 0.148 |
| LDL-c (mmol/L) | 2.6(2.1–2.8) | 2.8(2.3–3.4) | 2.9(2.6–3.4) | 0.084 |
| Testosterone (ng/dL) | 43.7(30.9–81.3) | 53.4(40.7–71.8) | 52.1(43.6–77.6) | 0.563 |
| LH/FSH ratio | 1.6(0.8–2.6) | 1.3(0.7–2.1) | 1.4(1.0–1.9) | 0.899 |
| Total energy (Kcal) | 1710.9±379.2 | 1900.4±522.9 | 1744.3±606.5 | 0.361 |
| Carbohydrate intake (g/day) | 197.0±47.0 | 216.7±65.2 | 223.6±84.5 | 0.355 |
| Daily energy intake from carbohydrates (%) | 46.2±7.1 | 46.2±9.9 | 52.3±11.5 | 0.010 |
| Protein intake (g/day) | 82.5±26.3 | 85.9±27.7 | 73.9±28.9 | 0.135 |
| Daily energy intake from protein (%) | 19.1±3.7 | 18.5±5.2 | 17.1±4.1 | 0.124 |
| Daily fat intake (g/day) | 65.8±19.3 | 76.7±32.4 | 61.6±33.3 | 0.097 |
| PEF (%) | 34.6±6.6 | 35.3±8.3 | 30.6±10.2 | 0.040 |
| Daily dietary fiber intake (g/day) | 9.0(5.9–12.2) | 8.7(5.3–11.4) | 8.4(5.9–11.0) | 0.929 |
Note: Values are expressed as mean ± SD or median (inter-quartile range).
Abbreviations: BMI, body mass index; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; HOMA-IR, homeostasis model assessment of insulin resistance; TG, triglycerides; TC, total cholesterol; HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol; LH, luteinizing hormone, FSH, follicle stimulating hormone; PEF, the percentage of daily energy intake from fat.
Data Categorized by the Percentage Energy Intake from Fat
Clinical practice guidelines recommend the percentage energy from dietary fat should not exceed 30% for the management of obesity.22 In order to further explore the effect of PEF on women with PCOS, the subjects were divided into the NPEF group and the HPEF group (Table 2). In the current cohort, 79 (71.2%) PCOS women had more than 30% energy intake from fat with the average fat intake of 78.9± 26.4 g/day in the HPEF group. However, the mean fasting insulin (142.7 (108.2–190.0) vs 108.6 (87.5–147.3), p=0.005) and HOMA-IR (4.8 (3.5–6.4) vs 3.4 (2.7–5.0), p=0.008) were significantly higher in the NPEF group than that in the HPEF group. Regarding the other dietary macronutrient intakes, patients in the NPEF group had significantly higher daily intake of protein (66.1±28.7 vs 84.0±26.7 g/day, p=0.002), but there was no significant difference in daily carbohydrate intake (233.0± 96.2 vs 209.7±61.5 g/day, p=0.131) and dietary fiber intake (8.1 (5.8–10.6) vs 8.8 (6.2–12.1) g/day, p=0.264). In terms of the energy ratio from macronutrients, there were significant differences between two groups in carbohydrates (61.1±9.3 vs 44.7± 6.9%, p<0.001), and fat (21.1±6.3 vs 37.4±5.3%, p<0.001) but not in protein (17.8± 5.4 vs 17.9± 4.0%, p=0.893).
Table 2.
Clinical Characteristics and Daily Dietary Intake of Patients with Polycystic Ovary Syndrome by the Percentage of Daily Energy Intake from Fat
| NPEF Group | HPEF Group | P value | |
|---|---|---|---|
| n | 32(28.8%) | 79 (71.2%) | |
| Age (years) | 26.9 ± 4.7 | 27.08 ± 5.294 | 0.660 |
| BMI (kg/m2) | 30.8 ± 4.3 | 29.4 ± 4.6 | 0.154 |
| WC (cm) | 95.4 ± 10.4 | 94.0 ± 11.7 | 0.556 |
| SBP (mmHg) | 122.6 ± 14.8 | 120.7 ± 11.6 | 0.509 |
| DBP (mmHg) | 83.0± 13.7 | 81.2± 9.9 | 0.5475 |
| Fasting glucose (mmol/L) | 5.0(4.6–5.2) | 4.9(4.7–5.4) | 0.997 |
| Fasting insulin (pmol/L) | 142.7(108.2–190.0) | 108.6(87.5–147.3) | 0.005 |
| 2-h glucose (mmol/L) | 7.1(6.2–8.6) | 6.9(6.0–8.6) | 0.735 |
| 2-h insulin (pmol/L) | 834.0(549.3–1198.6) | 707.6(454.1–1048.2) | 0.303 |
| HOMA-IR | 4.8(3.5–6.4) | 3.4(2.7–5.0) | 0.008 |
| TG (mmol/L) | 1.3(1.1–2.0) | 1.4(1.1–2.0) | 0.524 |
| TC (mmol/L) | 5.15 ± 0.77 | 5.15 ± 0.80 | 0.805 |
| HDL-c (mmol/L) | 1.14 ± 0.18 | 1.23 ± 0.22 | 0.054 |
| LDL-c (mmol/L) | 2.8(2.6–3.4) | 2.8(2.3–3.4) | 0.614 |
| Testosterone (ng/dL) | 56.2(40.1–82.4) | 51.9(41.3–75.0) | 0.615 |
| LH/FSH ratio | 1.3(0.9–1.4) | 1.4(0.9–2.2) | 0.123 |
| Total energy (Kcal) | 1519.2±587.1 | 1885.4± 495.6 | 0.001 |
| Daily carbohydrate intake (g/day) | 233.0± 96.2 | 209.7±61.5 | 0.131 |
| Daily energy intake from carbohydrates (%) | 61.1± 9.3 | 44.7± 6.9 | <0.001 |
| Daily protein intake (g/day) | 66.1±28.7 | 84.0±26.7 | 0.002 |
| Daily energy intake from protein (%) | 17.8± 5.4 | 17.9± 4.0 | 0.893 |
| Daily fat intake (g/day) | 35.9±18.3 | 78.9± 26.4 | <0.001 |
| PEF (%) | 21.1±6.3 | 37.4±5.3 | <0.001 |
| Daily dietary fiber intake (g/day) | 8.1(5.8–10.6) | 8.8(6.2–12.1) | 0.264 |
Note: Values are expressed as mean ± SD or median (inter-quartile range).
Abbreviations: BMI, body mass index; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; HOMA-IR, homeostasis model assessment of insulin resistance; TG, triglycerides; TC, total cholesterol; HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol; LH, luteinizing hormone; FSH, follicle stimulating hormone; PEF, the percentage of daily energy intake from fat; NPEF, within normal percentage of daily energy intake from fat (<30%); HPEF, the group with higher percentage of daily energy intake from fat (≥30%).
Correlation of Percentage of Dairy Fat Intake with Insulin Resistance
Pearson’s correlation analysis was performed to explore the correlation of PEF with HOMA-IR (log-transformed), BMI and total energy intake in Figure 1. PEF in PCOS patients was negatively correlated with BMI (r= −0.189, p=0.047) and HOMA-IR (log-transformed) (r= −0.217, p=0.022). We performed univariate and stepwise multivariate linear regression analysis to determine if PEF was still correlated with HOMA-IR (log-transformed) (Table 3). In the univariate linear regression analysis, the coefficients (95% CI) of per 1 mmHg increase of SBP and 1% of PEF for HOMA-IR (log-transformed) were 0.003 (0.0002–0.007, p= 0.039) and −0.005 (−0.009 - −0.001, p= 0.022) respectively, and the coefficients (95% CI) of per 1 mmol/L increase of TC, TG and LDL-c for HOMA-IR (log-transformed) were 0.074 (0.026–0.123, p=0.003), 0.090 (0.051–0.129, p<0.001) and 0.088 (0.033–0.142, p=0.002), respectively. Further stepwise multivariate linear regression analysis showed the standardized OR of per 1 SD increase of PEF for HOMA-IR (log-transformed) was −0.248 (P=0.008).
Figure 1.
The correlation of the percentage of daily energy intake from fat with HOMA-IR (log-transformed) and BMI.
Table 3.
Correlation of the Percentage of Daily Energy Intake from Fat with HOMA-IR (Log Transformed) Using Univariate and Stepwise Linear Regression Analysis
| Univariate | Stepwise | |||
|---|---|---|---|---|
| Unadjusted Coefficient (95% CI) | P value | Standardized Coefficients | P value | |
| Age | −0.004 (−0.012 to 0.004) | 0.312 | – | – |
| SBP | 0.003 (0.0002 to 0.007) | 0.039 | 0.191 | 0.041 |
| DBP | 0.003 (−0.001 to 0.007) | 0.153 | – | – |
| TC | 0.074 (0.026 to 0.123) | 0.003 | – | – |
| TG | 0.090 (0.051 to 0.129) | <0.001 | 0.346 | <0.001 |
| LDL-c | 0.088 (0.033 to 0.142) | 0.002 | 0.242 | 0.011 |
| HDL-c | −0.117 (−0.300 to 0.065) | 0.206 | – | – |
| PEF | −0.005 (−0.009 to −0.001) | 0.022 | −0.248 | 0.008 |
Abbreviations: SBP, systolic blood pressure; DBP, diastolic blood pressure; HOMA-IR, homeostasis model assessment of insulin resistance; TG, triglycerides; TC, total cholesterol; HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol; PEF, the percentage of daily energy intake from fat.
Discussion
In the current study, we found the prevalence rate of overweight and obesity of 80.2% in women with PCOS, and 71.2% of PCOS women had more than 30% daily energy intake from fat. Stratification by BMI revealed there were significant differences in WC, fasting insulin, and HOMA-IR, the percentage of daily energy intake from fat and carbohydrates, while other clinical characteristics were similar among the three groups. The levels of mean fasting insulin and HOMA-IR were significantly higher in the NPEF group than that in the HPEF group. Pearson’s correlation analysis demonstrated that PEF in women with PCOS was negatively correlated with BMI and HOMA-IR. Furthermore, stepwise multivariate linear regression analysis showed an independently negative correlation between PEF and HOMA-IR.
The reported prevalence of overweight and obesity in PCOS women has been described previously, and briefly, women with PCOS are generally more obese and more likely to have IR than age-matched control women.5,23 In our cohort, the prevalence rates of overweight and obesity were also higher and the severities of IR increased along with BMI (Table 1). Nutritional and dietary factors including dietary habits and pattens play an important role in prevention and treatment of PCOS.24 As the first-line treatment of PCOS management,25 adjusting macronutrient composition is an important part of dietary intervention. Although the optimal dietary composition in PCOS is unknown now, many previous studies focused on adjusting the proportion of protein and carbohydrates such as high-protein diet and low-carbohydrate diet26 and low glycemic index (GI) diet,27 which are effective in improving IR and blood glucose metabolism in PCOS women. Moreover, Nybacka et al15 demonstrated that an increase of dietary fiber intake in PCOS women was negatively correlated with increase in BMI, and contributed to the improvement of insulin sensitivity and glucose metabolism. However, there are limited studies exploring the relationship between fat intake and endocrine abnormalities in women with PCOS.
There are few studies that identify percentage of fat intake as a significant factor of IR in women with PCOS. Some epidemiological studies revealed there was an association between higher fat intake and reduced insulin sensitivity.28 Ahmadi et al29 observed that PCOS women had a diet higher in fat intake compared to the healthy controls. In the present study, approximately 71.2% of subjects’ daily energy percentage from fat intake exceeded the 30% recommended by the American Heart Association, which was similar to previous studies.23 However, the percentage of daily fat intake was negatively correlated to IR and BMI, which meant the more obese PCOS women consumed less energy intake from fat per day even their daily percentages energy from dietary fat had exceeded the recommended value. We speculate that these results may be related to the following factors. First of all, it is necessary to take into account that overweight/obese patients tend to restrict their energy intake and avoid high-fat products in order to control weight, which would result in a reduction in the percentage of fat intake and daily energy intake.30 Another possibility is that the improvement of IR in obese or overweight PCOS women may be related to polyunsaturated fatty acids. Although fat is considered to be a dietary component associated with obesity,31 in fact, fat is a blend of different fatty acids and different fatty acids have different effects on IR. Riserus32 et al found that polyunsaturated fatty acids and monounsaturated fatty acids had benefits in improving insulin sensitivity and preventing type 2 diabetes, which was consistent with the experimental animal studies which showed that polyunsaturated fatty acids had a positive effect on insulin sensitivity.33 In contrast, saturated and trans fatty acids will worsen IR. In addition, daily dietary intake data using standard forms for dietary recall for obese/overweight patients may underestimate the dietary intake information.
Our study has some limitations. First, the sample size in this study was relatively small, and further prospective studies with large sample size are needed to evaluate the relationship between PEF with IR. Second, we used dietary recall to reflect dietary intake evaluation in the present study. This assessment cannot avoid the risk of underestimation, and subjects with higher BMI are more likely to underreport their food intake.30 Also, there may be great personal differences in the methods of diet recall. In a future study, diet photos or mobile application (APP) for 7-day weighed food diaries could be used to record and analyze diet more accurately. Third, the diagnosis of IR was based on HOMA-IR rather than hyperinsulinemic euglycemic clamp. Fourth, in the multivariate linear regression analysis, we did not use variables such as BMI, WC, and the percentage of daily energy intake from other macronutrients as confounding factors, which may have reduced the study’s power. Fifth, in the present study there were also no healthy women with regular diet as a control, which could help guide the significance of PCOS patients whether they are lean or obese. In addition, this was a cross-sectional study and we did not analyze the proportion of various fatty acids in fat. Therefore, the causality between PEF and IR in PCOS cannot be drawn. Further research is needed to explore the true relationship of daily energy intake from fat with IR in patients with PCOS in the future.
Conclusion
In summary, the current results indicate that higher percentage of daily energy intake from fat was negatively correlated with IR in women with PCOS. Further studies are needed to clarify the mechanisms underlying the association between the percentage of fat intake and metabolic abnormalities in PCOS in order to provide more information on dietary intake and dietary composition in women with PCOS to improve fertility and weight loss.
Acknowledgments
We are grateful to all the patients for their participation.
We thank Dr. Hongyi Yang, who worked in Department of Obstetrics and Gynecology, the First Affiliated Hospital of Xiamen University and referred PCOS patients to us.
Funding Statement
CL was funded by National Natural Science Foundation of China (No. 81870611) and Natural Science Foundation of Fujian Province grant (No. 2020J011242).
Statement of Ethics
All procedures performed in this study were in accordance with the Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the Human Research Ethics Committee of the First Affiliated Hospital of Xiamen University (Xiamen, China) (Reference number: KYH2018-017).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
- 1.Jin P, Xie Y Treatment strategies for women with polycystic ovary syndrome. Gynecol Endocrinol. 2018;34(4):272–277. doi: 10.1080/09513590.2017.1395841 [DOI] [PubMed] [Google Scholar]
- 2.Teede HJ, Misso ML, Costello MF, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril. 2018;110(3):364–379. doi: 10.1016/j.fertnstert.2018.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toscani MK, Mario FM, Radavelli-Bagatini S, et al. Insulin resistance is not strictly associated with energy intake or dietary macronutrient composition in women with polycystic ovary syndrome. Nutr Res. 2011;31(2):97–103. doi: 10.1016/j.nutres.2011.01.009 [DOI] [PubMed] [Google Scholar]
- 4.Kaczmarek C, Haller DM, Yaron M. Health-related quality of life in adolescents and young adults with polycystic ovary syndrome: a systematic review. J Pediatr Adolesc Gynecol. 2016;29(6):551–557. doi: 10.1016/j.jpag.2016.05.006 [DOI] [PubMed] [Google Scholar]
- 5.Lim SS, Davies MJ, Norman RJ, et al. Overweight, obesity and central obesity in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2012;18(6):618–637. doi: 10.1093/humupd/dms030 [DOI] [PubMed] [Google Scholar]
- 6.Escobar-Morreale HF Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol. 2018;14(5):270–284. doi: 10.1038/nrendo.2018.24 [DOI] [PubMed] [Google Scholar]
- 7.Stepto NK, Cassar S, Joham AE, et al. Women with polycystic ovary syndrome have intrinsic insulin resistance on euglycaemic-hyperinsulaemic clamp. Hum Reprod. 2013;28(3):777–784. doi: 10.1093/humrep/des463 [DOI] [PubMed] [Google Scholar]
- 8.Lim SS, Norman RJ, Davies MJ, et al. The effect of obesity on polycystic ovary syndrome: a systematic review and meta-analysis. Obes Rev. 2013;14(2):95–109. doi: 10.1111/j.1467-789X.2012.01053.x [DOI] [PubMed] [Google Scholar]
- 9.Dunaif A Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev. 1997;18(6):774–800. doi: 10.1210/edrv.18.6.0318 [DOI] [PubMed] [Google Scholar]
- 10.Chen L, Xu WM, Zhang D Association of abdominal obesity, insulin resistance, and oxidative stress in adipose tissue in women with polycystic ovary syndrome. Fertil Steril. 2014;102(4):1167–1174.e4. doi: 10.1016/j.fertnstert.2014.06.027 [DOI] [PubMed] [Google Scholar]
- 11.Jena D, Choudhury AK, Mangaraj S, et al. Study of visceral and subcutaneous abdominal fat thickness and its correlation with cardiometabolic risk factors and hormonal parameters in polycystic ovary syndrome. Indian J Endocrinol Metab. 2018;22(3):321–327. doi: 10.4103/ijem.IJEM_646_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Norman RJ, Davies MJ, Lord J, et al. The role of lifestyle modification in polycystic ovary syndrome. Trends Endocrinol Metab. 2002;13(6):251–257. doi: 10.1016/S1043-2760(02)00612-4 [DOI] [PubMed] [Google Scholar]
- 13.Panagiotakos DB, Pitsavos C, Chrysohoou C, et al. Impact of lifestyle habits on the prevalence of the metabolic syndrome among Greek adults from the ATTICA study. Am Heart J. 2004;147(1):106–112. doi: 10.1016/S0002-8703(03)00442-3 [DOI] [PubMed] [Google Scholar]
- 14.Perelman D, Coghlan N, Lamendola C, et al. Substituting poly- and mono-unsaturated fat for dietary carbohydrate reduces hyperinsulinemia in women with polycystic ovary syndrome. Gynecol Endocrinol. 2017;33(4):324–327. doi: 10.1080/09513590.2016.1259407 [DOI] [PubMed] [Google Scholar]
- 15.Nybacka Å, Hellstrom PM, Hirschberg AL Increased fibre and reduced trans fatty acid intake are primary predictors of metabolic improvement in overweight polycystic ovary syndrome-Substudy of randomized trial between diet, exercise and diet plus exercise for weight control. Clin Endocrinol (Oxf). 2017;87(6):680–688. doi: 10.1111/cen.13427 [DOI] [PubMed] [Google Scholar]
- 16.Zhang J, Liu Y, Liu X, et al. High intake of energy and fat in southwest chinese women with PCOS: a population-based case-control study. PLoS One. 2015;10(5):e0127094. doi: 10.1371/journal.pone.0127094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng S, Tong M, Dong L, et al. Lipid accumulation product independently correlate with hepatic steatosis quantified by controlled attenuation parameter in women with polycystic ovary syndrome. Endocr Connect. 2020;9(2):154–162. doi: 10.1530/EC-19-0559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii,1–253. [PubMed] [Google Scholar]
- 19.Jellinger PS, Handelsman Y, Rosenblit PD, et al. American association of clinical endocrinologists and american college of endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease - executive summary complete appendix to guidelines. Endocr Pract. 2017;23(4):479–497. doi: 10.4158/EP171764.GL [DOI] [PubMed] [Google Scholar]
- 20.Ascaso JF, Pardo S, Real JT, et al. Diagnosing insulin resistance by simple quantitative methods in subjects with normal glucose metabolism. Diabetes Care. 2003;26(12):3320–3325. doi: 10.2337/diacare.26.12.3320 [DOI] [PubMed] [Google Scholar]
- 21.Whitton C, Ho JCY, Tay Z, et al. Relative validity and reproducibility of a food frequency questionnaire for assessing dietary intakes in a multi-ethnic asian population using 24-h dietary recalls and biomarkers. Nutrients. 2017;9(10):1059. doi: 10.3390/nu9101059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moran LJ, Brinkworth GD, Norman RJ Dietary therapy in polycystic ovary syndrome. Semin Reprod Med. 2008;26(1):85–92. doi: 10.1055/s-2007-992928 [DOI] [PubMed] [Google Scholar]
- 23.Douglas CC, Norris LE, Oster RA, et al. Difference in dietary intake between women with polycystic ovary syndrome and healthy controls. Fertil Steril. 2006;86(2):411–417. doi: 10.1016/j.fertnstert.2005.12.054 [DOI] [PubMed] [Google Scholar]
- 24.Faghfoori Z, Fazelian S, Shadnoush M, et al. Nutritional management in women with polycystic ovary syndrome: a review study. Diabetes Metab Syndr. 2017;11 (Suppl 1):S429–S432. doi: 10.1016/j.dsx.2017.03.030 [DOI] [PubMed] [Google Scholar]
- 25.Thomson RL, Buckley JD, Noakes M, et al. The effect of a hypocaloric diet with and without exercise training on body composition, cardiometabolic risk profile, and reproductive function in overweight and obese women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93(9):3373–3380. doi: 10.1210/jc.2008-0751 [DOI] [PubMed] [Google Scholar]
- 26.Farshchi H, Rane A, Love A, et al. Diet and nutrition in polycystic ovary syndrome (PCOS): pointers for nutritional management. J Obstet Gynaecol. 2007;27(8):762–773. doi: 10.1080/01443610701667338 [DOI] [PubMed] [Google Scholar]
- 27.Diamanti-Kandarakis E, Paterakis T, Alexandraki K, et al. Indices of low-grade chronic inflammation in polycystic ovary syndrome and the beneficial effect of metformin. Hum Reprod. 2006;21(6):1426–1431. doi: 10.1093/humrep/del003 [DOI] [PubMed] [Google Scholar]
- 28.Marsh K, Brand-Miller J The optimal diet for women with polycystic ovary syndrome? Br J Nutr. 2005;94(2):154–165. doi: 10.1079/BJN20051475 [DOI] [PubMed] [Google Scholar]
- 29.Ahmadi A, Akbarzadeh M, Mohammadi F, et al. Anthropometric characteristics and dietary pattern of women with polycystic ovary syndrome. Indian J Endocrinol Metab. 2013;17(4):672–676. doi: 10.4103/2230-8210.113759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gemming L, Jiang Y, Swinburn B, et al. Under-reporting remains a key limitation of self-reported dietary intake: an analysis of the 2008/09 New Zealand adult nutrition survey. Eur J Clin Nutr. 2014;68(2):259–264. doi: 10.1038/ejcn.2013.242 [DOI] [PubMed] [Google Scholar]
- 31.Bray GA, Popkin BM Dietary fat intake does affect obesity! Am J Clin Nutr. 1998;68(6):1157–1173. doi: 10.1093/ajcn/68.6.1157 [DOI] [PubMed] [Google Scholar]
- 32.Risérus U Fatty acids and insulin sensitivity. Curr Opin Clin Nutr Metab Care. 2008;11(2):100–105. doi: 10.1097/MCO.0b013e3282f52708 [DOI] [PubMed] [Google Scholar]
- 33.Astrup A Healthy lifestyles in Europe: prevention of obesity and type II diabetes by diet and physical activity. Public Health Nutr. 2001;4(2b):499–515. doi: 10.1079/PHN2001136 [DOI] [PubMed] [Google Scholar]