Abstract
Bats are the likely zoonotic origin of several coronaviruses (CoVs) that infect humans, including SARS-CoV-1 and SARS-CoV-2, both of which have caused large-scale epidemics. The number of CoVs present in an area is strongly correlated with local bat species richness, which in turn is affected by climatic conditions that drive the geographical distributions of species. Here we show that the southern Chinese Yunnan province and neighbouring regions in Myanmar and Laos form a global hotspot of climate change-driven increase in bat richness. This region coincides with the likely spatial origin of bat-borne ancestors of SARS-CoV-1 and SARS-CoV-2. Accounting for an estimated increase in the order of 100 bat-borne CoVs across the region, climate change may have played a key role in the evolution or transmission of the two SARS CoVs.
Keywords: Coronavirus, Covid-19, Zoonoses, Species distribution modelling, Vegetation modelling, Habitat shifts
Graphical abstract
1. Introduction
Over 60% of emerging infectious disease events worldwide can be traced back to zoonoses, most of which originate in wildlife (Jones et al., 2008). Bats have a special place amongst animal pathogen hosts in that they carry the highest proportion of zoonotic viruses of all mammalian orders (Olival et al., 2017; Luis et al., 2013). Coronaviruses (CoVs) account for over a third of the sequenced bat virome (Banerjee et al., 2019), corresponding to an estimated more than 3000 different CoVs carried by the world's bats (Anthony et al., 2017a). Several CoVs known to infect humans have very likely originated in bats (Banerjee et al., 2019; Cui et al., 2019), including the three types associated with human fatalities: the Middle East respiratory syndrome (MERS) CoV (Anthony et al., 2017b; Memish et al., 2013) and severe acute respiratory syndrome (SARS) CoV 1 (Lau et al., 2005; Li et al., 2005) and 2 (Latinne et al., 2020; Zhou et al., 2020).
Strains of CoV found in bats in the southern Chinese Yunnan province currently most closely resemble both SARS-CoV-1 (Hu et al., 2017) and SARS CoV-2 (Zhou et al., 2020), suggesting this or neighbouring regions in Myanmar and Laos as plausible places of origin of the bat-borne ancestors of the two lineages (Cyranoski, 2020). These regions also comprise the native habitat of masked palm civits (Paguma larvata) and Sunda pangolins (Manis javanica) (IUCN, 2020), which are assumed to have acted as intermediate hots that eventually transmitted SARS-CoV-1 (Cui et al., 2019; Guan et al., 2003) and SARS-CoV-2 (Zhou et al., 2020; Xiao et al., 2020), respectively, to humans. Captured civets and pangolins carrying these viruses are likely to have been transported to wildlife markets in Guangdong and Wuhan, respectively (Hassanin et al., 2020), where the initial outbreaks in human populations occurred.
The number of CoVs present in an area is strongly correlated with local bat species richness (Anthony et al., 2017a). An increase in local bat richness may therefore increase the probability that a CoV with potentially harmful properties for human life is present, transmitted, or evolves in the area. Species richness, in turn, is affected by climate change, which drives the geographic distributions of species by altering the suitability of ecological habitats, forcing species to disappear from some areas whilst allowing them to expand in others (Chen et al., 2011; Hickling et al., 2006; Parmesan, 2006). These range shifts impact not only the spatial distribution of zoonoses directly by introducing their hosts to new areas, but also lead to changes in species composition and ecology, which can result in novel host-pathogen interactions that may create new transmission pathways or facilitate the evolution of harmful disease variants (Altizer et al., 2013; Carlson et al., 2020; Hoberg and Brooks, 2015; Patz et al., 1996; Retel et al., 2019). Given these mechanisms, understanding how the global distribution of bat species, and therefore of bat-borne CoVs (Anthony et al., 2017a), has shifted as the result of climate change may be an important step towards reconstructing the origin of CoV outbreaks in humans.
Here we estimated how climate change has impacted global bat species richness over the last century. Our analysis reveals a global hotspot of climate change-driven increase in bat richness in the geographical region considered as the likely origin of the bat-borne ancestors of SARS-CoV-1 and SARS-CoV-2. This provides a possible mechanistic link between climate change and the emergence of the two viruses.
2. Material and methods
We estimated species-specific geographical ranges of the world's bats based on global climatic conditions in the early 20th century and at present day following the methodology of Jetz et al. (2007) and Beyer and Manica (2020). This approach consists of first determining the global distribution of natural vegetation corresponding to a given climate, and then combining the derived vegetation maps with data on the spatial distribution and vegetation requirements of individual species. Global vegetation maps were generated based on the CRU TS v4.04 global dataset of observation-based annual reconstructions of monthly mean temperature, precipitation, cloud cover, and minimum temperature from 1901 to 2019 at a 0.5° grid resolution (Harris et al., 2020). Climatological normals (i.e., 30-year averages) of these four variables were calculated for the first and last available period of the observational data, 1901–1930 and 1990–2019, respectively. Cloud cover was converted to the percentage of possible sunshine (Doorenbos and Pruitt, 1984) and the annual minimum temperature was obtained as the minimum of the monthly minimum temperatures. The derived monthly temperature, precipitation, and sunshine, annual minimum temperature, and 1901–1930 and 1990–2019 average atmospheric CO2 concentrations (Tans and Keeling, 2020), were then used as inputs for the BIOME4 global vegetation model (Kaplan et al., 2003). BIOME4 simulates incoming solar radiation, photosynthesis, stomatal behaviour, hydrology, competition, and ecosystem dynamics to determine the dominant natural plant functional type (Kaplan et al., 2003), and has been successfully validated against empirical data (Haxeltine and Prentice, 1996; Hoogakker, 2016; Ni et al., 2000; Tang et al., 2009). In this way, we estimated the global distribution of natural vegetation based on the climatic conditions in the early 20th century and at present.
The global distribution of bats at each of the two time periods was then determined by combining the relevant vegetation map with two types of species-specific data available for all known bats: extents of occurrence and habitat requirements (IUCN, 2020). Extents of occurrence represent the outermost geographic limits of a species' observed or projected occurrence (Gaston and Fuller, 2009); these spatial envelopes do not account for the distribution of vegetation within that area and therefore generally extend substantially beyond a species' actual distribution (Gaston, 2013). Habitat requirements include one or more vegetation categories in which a species can occur. Extents of occurrence were rasterised from their spatial polygon format to a 0.5° grid, and subsequently refined by retaining only those grid cells where the previously estimated natural vegetation type, at the relevant time, was included in the species' list of habitat requirements. In this way, the geographical range of each individual bat species was estimated for the early 20th century and for the present. Finally, the total bat species richness in each grid cell was obtained as the number of species whose estimated geographic range included the grid cell at the relevant time period.
By design, our approach simulates bat distributions under the climate-derived natural vegetation, not actual land cover. Whilst anthropogenic land use change has removed the natural vegetation type in many areas, there are typically some remnants left nearby (Ramankutty and Foley, 1999), making it likely that species can indeed be found in the grid cells estimated as suitable in our analysis. Conversely, anthropogenic land use change may have led to increases in local bat richness in areas where the natural vegetation is unsuitable; however, these increases would not be attributable to the impact of climate change, which the approach used here specifically aims to assess.
3. Results and discussion
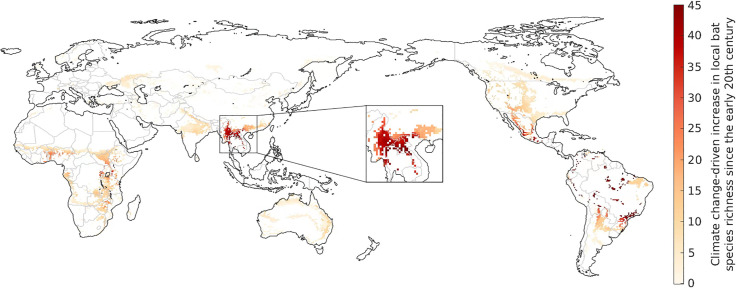
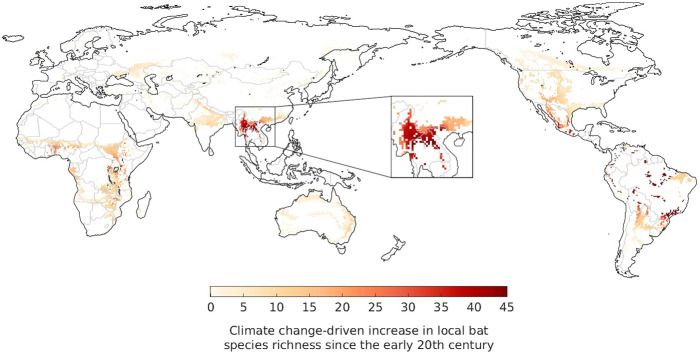
Areas estimated to have experienced significant increases in bat species richness as the result of climate change-driven range shifts include regions around Central Africa, several scattered patches in Central and South America, and notably a large spatial cluster located in the southern Chinese Yunnan province and neighbouring regions in Myanmar and Laos (Fig. 1 ). This latter hotspot coincides with the region currently considered as the most likely origin of the bat-borne ancestors of SARS-CoV-1 and SARS-CoV-2 (Cyranoski, 2020). The estimated climate change-driven increase of around 40 bat species across the region (Fig. 1) corresponds to a rise in the local number of bat-borne CoV in the order of 100 (±50) viruses, given that each bat species carries on average 2.67 (±1.38) CoVs (Anthony et al., 2017a).
Fig. 1.
Estimated increase in the local number of bat species due to shifts in their geographical ranges driven by climate change between the 1901-1930 and 1990-2019 period. The zoomed-in area represents the likely spatial origin of the bat-borne ancestors of SARS-CoV-1 and 2.
Given the inferred spatial origin of these two viral lineages, Fig. 1 provides evidence suggestive of a possible contributing role of climate change in the evolution or interspecies transmission of SARS-CoV-1 and SARS-CoV-2, by driving a substantial increase in bat, and therefore bat-borne CoV (Anthony et al., 2017a), richness across the region. This process would have likely created significant opportunities for cross-species viral transmission (Altizer et al., 2013; Carlson et al., 2020; Hoberg and Brooks, 2015; Patz et al., 1996; Retel et al., 2019) that may have facilitated the eventual spill-over to humans. Evidence of a similar potential contribution of climate change is less pronounced in regions associated with other CoVs that have infected humans, such as MERS-CoV, for which a plausible origin of the bat-borne ancestor has been placed in East Africa (Corman et al., 2014; Zumla et al., 2015), where we estimated only small increases in bat richness.
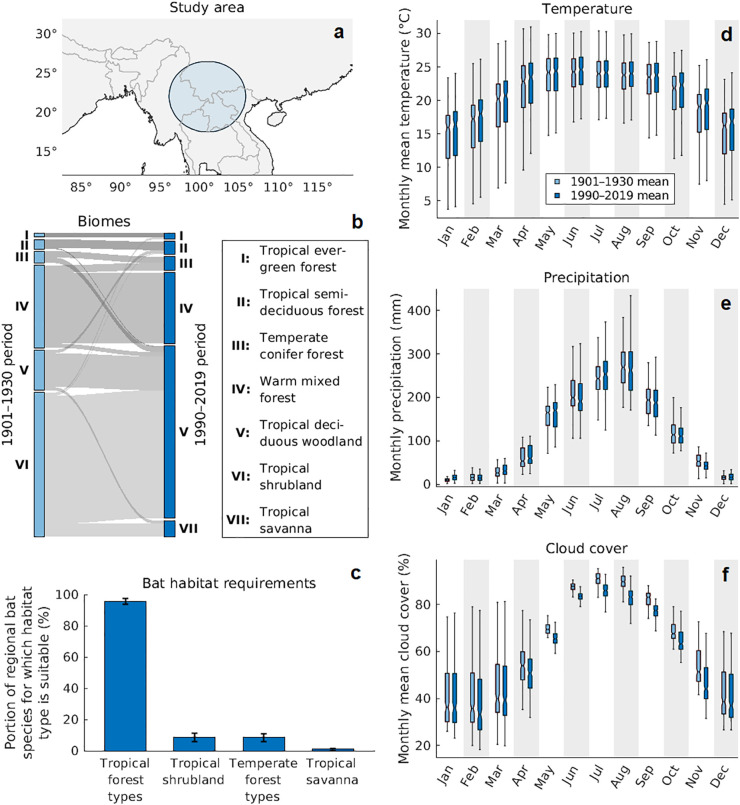
Examining the climate change-driven shifts in the distribution of natural biomes and the habitat requirements of bat species in the region around the southern Yunnan province (Fig. 2a) can provide insights into the estimated increase in the local bat richness. Whilst most forest biomes in the area have not substantially changed in total size, our data suggest a large-scale shift from tropical shrublands to tropical savannas and deciduous woodland over the past century (Fig. 2b), driven by climatic changes characterised by higher atmospheric CO2 levels, increased temperature, altered precipitation patterns, and decreased cloud cover (Fig. 2d-f). This process created a suitable environment for many bat species occurring in the region that predominantly require forest type habitats (Fig. 2c), explaining the marked increase in bat richness in Fig. 1.
Fig. 2.
Environmental conditions and bat habitats in the likely spatial origin of the bat-borne ancestors of SARS-CoV-1 and 2. (a) Area of study, defined as a 500 km radius disk centred in the southern Yunnan province (101°E, 22°N). (b) Simulated relative distribution of natural biomes in the study area in the early 20th century (light blue bars) and at present (dark blue bars). Grey flows represent shifts in biomes between the time periods. (c) Habitat requirements of bat species whose extent of occurrence (see Methods) overlaps with the study area. Uncertainty bars represent upper and lower quartiles obtained from a bootstrap approach in which habitat requirements were determined for a set of 104 random re-samples, with replacement, of these bat species. Proportions add up to more than 100% as species can have more than one suitable habitat type. (d)–(f) Boxplots representing the distribution of past and present monthly temperature, precipitation, and cloud cover normals across the study area. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Whilst the zoonotic and spatial origin of SARS-CoV-1 have been determined with some certainty (Cyranoski, 2020), the details of the origin of SARS-CoV-2 continue to be an active field of research (Mallapaty, 2020). It is therefore important to note that our inference that climate change may have played a role in the outbreak relies on future evidence remaining supportive of the suggestion made based on the genetic evidence available to date that the ancestor of SARS-CoV-2 was bat-borne and occurred in the Yunnan province or the neighbouring regions. Furthermore, the methods used here to simulate vegetation and to map bat ranges are not without uncertainties and limitations (Beyer and Manica, 2020; Hallgren and Pitman, 2000); further work using both alternative vegetation models (Sitch et al., 2008) and species distribution models (Franklin, 2010) is therefore needed to clarify the pattern suggested by our data. By mapping the geographic ranges of bats based only on climatic conditions represented by natural vegetation, we have aimed to isolate the effect of climate change on global bat species richness. Other biotic and abiotic factors not considered in our approach, such as hunting, invasive species, and pollution, can also play significant roles in determining bat habitat suitability (Frick et al., 2020) and require further study in the context of CoV outbreaks. Investigating the important role of anthropogenic land use change, in particular (Frick et al., 2020; Jones et al., 2013; Morse et al., 2012), can benefit from satellite-based land cover datasets (Liu et al., 2020; Sulla-Menashe et al., 2019), although global maps are available only for the more recent past. Finally, estimated climate change-driven shifts in the geographical distributions of pathogen-carrying species will need to be integrated into epidemiological models in order to quantitatively assess the role of climate change and the contribution of specific climatic variables on viral sharing networks (Albery et al., 2020; Carlson et al., 2020). These models also need to incorporate other impacts of climate change, such as the effect of warmer climates on the susceptibility of animal host species to pathogens (Roberts et al., 2018) and that of microbial adaptation to higher temperatures on the effectiveness of the human endothermy thermal barrier (Casadevall, 2020).
Spill-overs of CoVs and other zoonoses to humans have been shown to be closely linked to an increase in contact with pathogen-carrying wildlife, driven by the expansion and intensification of agriculture, hunting, and infrastructural development (Jones et al., 2013; Morse et al., 2012; Bloomfield et al., 2020; Han et al., 2016; Gibb et al., 2020a). To reduce the risk of future zoonotic spill-overs, it is crucial to introduce measures to protect natural habitats, impose strong regulations on wildlife hunting and trade, establish appropriate animal welfare standards on farms, markets and transport vehicles, and discourage high-zoonotic-risk dietary and medicinal customs (Nabi et al., 2020; Petrovan, 2020) whilst accommodating the socio-economic needs that drive current patterns. Sound understanding of the ecological dynamics underlying zoonotic disease emergence is essential for effective health and environmental planning (Campbell-Lendrum et al., 2015; Gibb et al., 2020b) and for eliminating dangerous and counterproductive practices such as bat persecution (Zhao, 2020). In addition to these measures, previous studies have stressed the importance of recognising the critical role of climate change in the emergence and spread of infectious diseases (Altizer et al., 2013; Carlson et al., 2020; Epstein, 2001; Harvell et al., 2002; Patz et al., 1996). Given the possibility raised by our analysis that global greenhouse gas emissions may have been a contributing factor in the SARS-CoV-1 and SARS-CoV-2 outbreaks, we echo calls for decisive climate change mitigation, including as part of Covid-19 economic recovery programmes (Rosenbloom and Markard, 2020), as a means to minimise future zoonotic spill-overs and the tremendous social and economic damage associated with them.
CRediT authorship contribution statement
C.M. and R.M.B. and designed the project. R.M.B. conducted the analysis and wrote the manuscript. R.M.B., A.M., and C.M. interpreted the results and revised the manuscript.
Funding
R.M.B. and A.M. were supported by ERC Consolidator Grant 647797 "LocalAdaptation".
Declaration of competing interest
The authors declare no competing interests.
Acknowledgements
The authors thank four anonymous reviewers for their helpful comments.
Editor: Jay Gan
References
- Albery G.F., Eskew E.A., Ross N., Olival K.J. Predicting the global mammalian viral sharing network using phylogeography. Nat. Commun. 2020;11:1–9. doi: 10.1038/s41467-020-16153-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altizer S., Ostfeld R.S., Johnson P.T.J., Kutz S., Harvell C.D. Climate change and infectious diseases: from evidence to a predictive framework. Science. 2013;341:514–519. doi: 10.1126/science.1239401. [DOI] [PubMed] [Google Scholar]
- Anthony S.J., et al. Global patterns in coronavirus diversity. Virus Evol. 2017;3:vex012. doi: 10.1093/ve/vex012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony S.J., et al. Further evidence for bats as the evolutionary source of middle east respiratory syndrome coronavirus. mBio. 2017;8 doi: 10.1128/mBio.00373-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A., Kulcsar K., Misra V., Frieman M., Mossman K. Bats and Coronaviruses. Viruses. 2019;11:41. doi: 10.3390/v11010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer R.M., Manica A. Historical and projected future range sizes of the world’s mammals, birds, and amphibians. Nat. Commun. 2020;11:5633. doi: 10.1038/s41467-020-19455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield L.S.P., McIntosh T.L., Lambin E.F. Habitat fragmentation, livelihood behaviors, and contact between people and nonhuman primates in Africa. Landsc. Ecol. 2020;35:985–1000. [Google Scholar]
- Campbell-Lendrum D., Manga L., Bagayoko M., Sommerfeld J. Climate change and vector-borne diseases: what are the implications for public health research and policy? Philos. Trans. R. Soc. B Biol. Sci. 2015;370:20130552. doi: 10.1098/rstb.2013.0552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson C.J., Albery G.F., Merow C., Trisos C.H., Zipfel C.M., Eskew E.A., Olival K.J., Ross N., Bansal S. Climate change will drive novel cross-species viral transmission. BioRxiv. 2020 doi: 10.1101/2020.01.24.918755. [DOI] [PubMed] [Google Scholar]
- Casadevall A. Climate change brings the specter of new infectious diseases. J. Clin. Invest. 2020;130:553–555. doi: 10.1172/JCI135003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen I.-C., Hill J.K., Ohlemüller R., Roy D.B., Thomas C.D. Rapid range shifts of species associated with high levels of climate warming. Science. 2011;333:1024–1026. doi: 10.1126/science.1206432. [DOI] [PubMed] [Google Scholar]
- Corman V.M., et al. Rooting the phylogenetic tree of Middle East respiratory syndrome coronavirus by characterization of a conspecific virus from an African bat. J. Virol. 2014;88:11297–11303. doi: 10.1128/JVI.01498-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J., Li F., Shi Z.-L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyranoski D. The biggest mystery: what it will take to trace the coronavirus source. Nature. 2020 doi: 10.1038/d41586-020-01541-z. [DOI] [PubMed] [Google Scholar]
- Doorenbos J., Pruitt W. 1984. Guidelines for Predicting Crop Water Requirements. (Food and Agriculture Organisation of the United Nations) [Google Scholar]
- Epstein P.R. Climate change and emerging infectious diseases. Microbes Infect. 2001;3:747–754. doi: 10.1016/s1286-4579(01)01429-0. [DOI] [PubMed] [Google Scholar]
- Franklin J. Cambridge University Press; 2010. Mapping Species Distributions: Spatial Inference and Prediction. [Google Scholar]
- Frick W.F., Kingston T., Flanders J. A review of the major threats and challenges to global bat conservation. Ann. N. Y. Acad. Sci. 2020;1469:5–25. doi: 10.1111/nyas.14045. [DOI] [PubMed] [Google Scholar]
- Gaston K. Springer; 2013. J. Rarity. [Google Scholar]
- Gaston K.J., Fuller R.A. The sizes of species’ geographic ranges. J. Appl. Ecol. 2009;46:1–9. [Google Scholar]
- Gibb R., et al. Zoonotic host diversity increases in human-dominated ecosystems. Nature. 2020;1–5 doi: 10.1038/s41586-020-2562-8. [DOI] [PubMed] [Google Scholar]
- Gibb R., Franklinos L.H.V., Redding D.W., Jones K.E. Ecosystem perspectives are needed to manage zoonotic risks in a changing climate. BMJ. 2020;371:m3389. doi: 10.1136/bmj.m3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y., et al. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science. 2003;302:276–278. doi: 10.1126/science.1087139. [DOI] [PubMed] [Google Scholar]
- Hallgren W.S., Pitman A.J. The uncertainty in simulations by a Global Biome Model (BIOME3) to alternative parameter values. Glob. Change Biol. 2000;6:483–495. [Google Scholar]
- Han B.A., Kramer A.M., Drake J.M. Global patterns of zoonotic disease in mammals. Trends Parasitol. 2016;32:565–577. doi: 10.1016/j.pt.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris I., Osborn T.J., Jones P., Lister D. Version 4 of the CRU TS monthly high-resolution gridded multivariate climate dataset. Sci. Data. 2020;7:109. doi: 10.1038/s41597-020-0453-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvell C.D., et al. Climate warming and disease risks for terrestrial and marine biota. Science. 2002;296:2158–2162. doi: 10.1126/science.1063699. [DOI] [PubMed] [Google Scholar]
- Hassanin A., Grandcolas P., Veron G. Covid-19: natural or anthropic origin? Mammalia. 2020;1 [Google Scholar]
- Haxeltine A., Prentice I.C. BIOME3: An equilibrium terrestrial biosphere model based on ecophysiological constraints, resource availability, and competition among plant functional types. Global Biogeochem. Cycles. 1996;10:693–709. [Google Scholar]
- Hickling R., Roy D.B., Hill J.K., Fox R., Thomas C.D. The distributions of a wide range of taxonomic groups are expanding polewards. Glob. Change Biol. 2006;12:450–455. [Google Scholar]
- Hoberg E.P., Brooks D.R. Evolution in action: climate change, biodiversity dynamics and emerging infectious disease. Philos. Trans. R. Soc. Lond. B Biol. 2015;370 doi: 10.1098/rstb.2013.0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogakker B. a. a. et al. terrestrial biosphere changes over the last 120 kyr. Clim. Past. 2016;12:51–73. [Google Scholar]
- Hu B., et al. Discovery of a rich gene pool of bat SARS-related coronaviruses provides new insights into the origin of SARS coronavirus. PLoS Pathog. 2017;13 doi: 10.1371/journal.ppat.1006698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IUCN The IUCN red list of threatened species. Version 2020-1. 2020. https://www.iucnredlist.org/
- Jetz W., Wilcove D.S., Dobson A.P. Projected impacts of climate and land-use change on the global diversity of birds. PLoS Biol. 2007;5:e157. doi: 10.1371/journal.pbio.0050157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K.E., et al. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B.A., et al. Zoonosis emergence linked to agricultural intensification and environmental change. Proc. Natl. Acad. Sci. 2013;110:8399–8404. doi: 10.1073/pnas.1208059110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J.O., et al. Climate change and Arctic ecosystems: 2. Modeling, paleodata-model comparisons, and future projections. J. Geophys. Res. Atmospheres. 2003;108 [Google Scholar]
- Latinne A., et al. Origin and cross-species transmission of bat coronaviruses in China. bioRxiv. 2020 doi: 10.1101/2020.05.31.116061. 2020.05.31.116061. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lau S.K.P., et al. Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats. Proc. Natl. Acad. Sci. U. S. A. 2005;102:14040–14045. doi: 10.1073/pnas.0506735102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., et al. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005;310:676–679. doi: 10.1126/science.1118391. [DOI] [PubMed] [Google Scholar]
- Liu H., et al. Annual dynamics of global land cover and its long-term changes from 1982 to 2015. Earth Syst. Sci. Data. 2020;12:1217–1243. [Google Scholar]
- Luis A.D., et al. A comparison of bats and rodents as reservoirs of zoonotic viruses: are bats special? Proc. R. Soc. B Biol. Sci. 2013;280:20122753. doi: 10.1098/rspb.2012.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallapaty S. Coronaviruses closely related to the pandemic virus discovered in Japan and Cambodia. Nature. 2020;588:15–16. doi: 10.1038/d41586-020-03217-0. [DOI] [PubMed] [Google Scholar]
- Memish Z.A., et al. Middle east respiratory syndrome coronavirus in bats. Saudi Arabia. Emerg. Infect. Dis. 2013;19:1819–1823. doi: 10.3201/eid1911.131172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S.S., et al. Prediction and prevention of the next pandemic zoonosis. Lancet Lond. Engl. 2012;380:1956–1965. doi: 10.1016/S0140-6736(12)61684-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabi G., Siddique R., Ali A., Khan S. Preventing bat-born viral outbreaks in future using ecological interventions. Environ. Res. 2020;185:109460. doi: 10.1016/j.envres.2020.109460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni J., Sykes M.T., Prentice I.C., Cramer W. Modelling the vegetation of China using the process-based equilibrium terrestrial biosphere model BIOME3. Glob. Ecol. Biogeogr. 2000;9:463–479. [Google Scholar]
- Olival K.J., et al. Host and viral traits predict zoonotic spillover from mammals. Nature. 2017;546:646–650. doi: 10.1038/nature22975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmesan C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 2006;37:637–669. [Google Scholar]
- Patz J.A., Epstein P.R., Burke T.A., Balbus J.M. Global climate change and emerging infectious diseases. JAMA. 1996;275:217–223. [PubMed] [Google Scholar]
- Petrovan S.O., et al. 2020. Post COVID-19: A Solution Scan of Options for Preventing Future Zoonotic Epidemics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramankutty N., Foley J.A. Estimating historical changes in global land cover: croplands from 1700 to 1992. Glob. Biogeochem. Cycles. 1999;13:997–1027. [Google Scholar]
- Retel C., Märkle H., Becks L., Feulner P.G. Ecological and evolutionary processes shaping viral genetic diversity. Viruses. 2019;11(3):220. doi: 10.3390/v11030220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts K.E., Hadfield J.D., Sharma M.D., Longdon B. Changes in temperature alter the potential outcomes of virus host shifts. PLoS Pathog. 2018;14 doi: 10.1371/journal.ppat.1007185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbloom D., Markard J. A COVID-19 recovery for climate. Science. 2020;368:447. doi: 10.1126/science.abc4887. [DOI] [PubMed] [Google Scholar]
- Sitch S., et al. Evaluation of the terrestrial carbon cycle, future plant geography and climate-carbon cycle feedbacks using five Dynamic Global Vegetation Models (DGVMs) Glob. Change Biol. 2008;14:2015–2039. [Google Scholar]
- Sulla-Menashe D., Gray J.M., Abercrombie S.P., Friedl M.A. Hierarchical mapping of annual global land cover 2001 to present: the MODIS collection 6 land cover product. Remote Sens. Environ. 2019;222:183–194. [Google Scholar]
- Tang G., Shafer S.L., Bartlein P.J., Holman J.O. Effects of experimental protocol on global vegetation model accuracy: a comparison of simulated and observed vegetation patterns for Asia. Ecol. Model. 2009;220:1481–1491. [Google Scholar]
- Tans P., Keeling R. 2020. Trends in Atmospheric Carbon Dioxide. https://www.esrl.noaa.gov/gmd/ccgg/trends/data.html. [Google Scholar]
- Xiao K., et al. Isolation of SARS-CoV-2-related coronavirus from Malayan pangolins. Nature. 2020;1–4 doi: 10.1038/s41586-020-2313-x. [DOI] [PubMed] [Google Scholar]
- Zhao H. COVID-19 drives new threat to bats in China. Science. 2020;367:1436. doi: 10.1126/science.abb3088. [DOI] [PubMed] [Google Scholar]
- Zhou P., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumla A., Hui D.S., Perlman S. Middle East respiratory syndrome. Lancet. 2015;386:995–1007. doi: 10.1016/S0140-6736(15)60454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]