Abstract
Background
Coronavirus disease 2019 (COVID-19) has become a global health threat, and thus, an early and effective set of predictors is needed to manage the course of the disease.
Objectives
We aim to determine the effect of SARS-CoV-2 on lipid profile and to evaluate whether the atherogenic index of plasma (AIP) could be used to predict in-hospital mortality in COVID-19 patients.
Methods
In this retrospective chart review study, a total of 139 confirmed COVID-19 patients, whose diagnoses are confirmed by PCR and computerized tomography results, are enrolled. The study population is divided into two groups: the deceased patient group and the survivor group. For each patient, fasting total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C) and the triglyceride values are obtained from the laboratory tests required at the admission to hospital. Finally, the AIP is calculated as the base 10 logarithm of the triglyceride to HDL-C ratio. Distributional normality of the data is checked and depending on the normality of the data, either T test or Mann Whithey U test is employed to compare the two aforementioned study groups.
Results
Mean age of the study population is 49.2 ± 20.8 and 61.2% (n = 85) is male. Out of the 139 patients 26 have deceased and the remaining 113 patients survived the disease. Mean age of the deceased patients was 71.8*8.9 and mean age of the survivor patients is 44.0*19.2 (p < 0.001). The deceased group had more patients with hypertension (50.0% vs. 23.0, p = 0.006), diabetes mellitus (35.6% vs. 10.6%, p = 0.002), cardiovascular diseases (23.1% vs. 4.4%, p = 0.001), chronic renal insufficiency (11.5% vs. 0.9%, p = 0.003) and atrial fibrillation (7.7% vs 0%, p = 0.003).
The AIP values in the deceased group are found to be statistically higher (p < 0.001) than the survivor group. As a measure of mortality, the area under the operating characteristic curve for the AIP is calculated as 0.850 (95% confidence interval: 0.772–0.928) along with the optimal cut-off value of 0.6285 (78.6% sensitivity and 80.5% specificity). Furthermore, the AIP value is observed to be elevated in patients with pneumonia, intubation history, and intensive care admission during hospital stay (p = 0.002, p < 0.001 and p < 0.001, respectively). Finally, compared to the survivor group, total cholesterol, HDL-C, LDL-C values are lower (p = 0.004, p < 0.001 and p < 0.001, respectively) and triglyceride levels are higher (p < 0.001) in deceased patients.
Conclusion
In this study, we show that the AIP levels higher than 0.6285 can predict in-hospital mortality for COVID-19 patients. Moreover, the AIP emerges as a good candidate to be used as an early biomarker to predict pneumonia, intubation and intensive care need. Hence, regular check of the AIP levels in COVID-19 patients can improve management of these patients and prevent deterioration of the disease.
Keywords: SARS-CoV-2, COVID-19, Mortality, Atherogenic index of plasma, Lipids
Introduction
After its emergence in Wuhan City, Hubei Province, China, SARS-CoV-2 has gained entry to many countries across the globe, causing a global pandemic.1, 2, 3 As a consequence, many scientists investigated the disease with intense efforts. Numerous studies have been published, that investigate the mechanism of the virus, its’ spread, symptoms, its’ effects on the body, and the possible treatment methods.4, 5, 6, 7, 8, 9 One of the prominent results in these studies is that some hematological and biochemical markers can predict the prognosis of the disease.10 As an extension of this finding, availability of indicators to predict the prognosis of the disease and whether it will end in mortality for the patient at the beginning of the disease, would make it possible to identify the risky patients and indicate which patients do not need a close follow-up.
Such an indicator stems from the lipids, which are the basic building blocks of the cell and the virus membranes.11 Viral infections can cause changes in the lipid profile of the host organism. This occurs because the virus controls the host metabolism in order to meet the requirements for its replication.12 Previously published studies, especially on RNA viruses, indicated the effects of these viruses on lipid metabolism. It has been shown that LDL-C level decreases and HDL-C level increases in HIV-infected patients.13 In addition, it was found that the triglyceride level increased in the advanced stages of the disease.14
Triglyceride and HDL-C, which measure cardiovascular mortality risk, are two lipids that is examined routinely. Furthermore, the logarithmic transformation of triglyceride to HDL-C ratio is known as the Atherogenic Index of Plasma (AIP).15 The AIP is associated with chronic diseases such as diabetes, hypertension and cardiovascular disease.16, 17, 18, 19, 20 Additionally, the relationship between the severity of viral infections and the AIP has been identified.21 Moreover, there are studies which discuss the impact of SARS-CoV on the disorders of patients’ lipid profile in long-term, while their short term effects are not clear.22 In this study, our aim is to investigate the effect of SARS-CoV-2 on lipid profile and the effect of the AIP on survival in COVID-19.
Methods
Study design and participants
For this study, 561 patients who were admitted to our center and were diagnosed with laboratory or computerized tomography confirmed COVID-19 are evaluated. This study is designed to be retrospective; as a consequence, 419 out of the original 561 patients who did not have their lipid profile evaluated at their hospitalization had to be excluded. 3 patients out of the remaining 142 patients were under statin and/or fenofibrate treatment, thus, also excluded from the study. The remaining 139 patients are enrolled in this study.
Data collection and analysis
The data set is from the confirmed COVID-19 patients who had been hospitalized at our health center from 20.03.2020 to 26.05.2020. It is collected and evaluated by two physicians and independently reviewed by the researchers to double-check collected data. Demographic and clinical data, as well as previous medication histories are collected from hospital medical records. Fasting total cholesterol, HDL-C, LDL-C, and triglyceride values are obtained from the results of laboratory tests at the time of admission to the hospital. Finally, the AIP is calculated as the base 10 logarithm of the triglyceride to HDL-C ratio. Only the laboratory values measured on the first day of hospitalization and before treatment were used in the study. The values checked in the following days were definitely not used for the study. Patients without a lipid profile assessment in their blood parameters on the first day were excluded from the study.
The data is summarized in the form of “mean ± standard deviation” or “median (interquartile range)” for continuous variables and as proportions for categorical variables. Distribution of the data for normality is tested by the Shapiro–Wilk test and homogeneity of group variances are tested by the Levene test. Categorical variables are examined using Chi-square test. Normally distributed continuous variables are tested with t-test, and variables for the parameters which are not normally distributed, Mann Whitney U test is used. Receiver operating characteristic (ROC) curve analysis is used to calculate the optimal cut-off values, sensitivity, and specificity of the AIP. p-values < 0.05 were considered as statistically significant. Finally, the explanatory power of the AIP, demographic and clinical variables on mortality is evaluated by logistic regression. The aforementioned statistical analysis is conducted via SPSS 22.0 (IBM SPSS Ver. 22.0, IBM Corp, Armonk NY, USA)
Results
A total of 139 confirmed COVID-19 patients are included for the study. Mean age of the study population is 49.2 ± 20.8 and 61.2% (n = 85) of the sample is male. From, mortality rate of the patients is 18.7% (n = 26). Mean age of the deceased patients was 71.8 ± 8.9 and mean age of the survivor patients is 44.0 ± 19.2 and the difference is statistically significant (p < 0.001). Deceased group had more patients with hypertension (50.0% vs. 23.0, p = 0.006), diabetes mellitus (35.6% vs. 10.6%, p = 0.002), cardiovascular diseases (23.1% vs. 4.4%, p = 0.001), chronic renal insufficiency (11.5% vs. 0.9%, p = 0.003) and atrial fibrillation (7.7% vs 0%, p = 0.003). The baseline characteristics of the study population is shown in Table 1 .
Table 1.
Baseline characteristics of the patients with COVID-19
| Variable | Total | Deceased | Survivors | p-value |
|---|---|---|---|---|
| Patients | 139 | 26 | 113 | |
| Age years | 49.2 ± 20.8 | 71.8 ± 8.9 | 44.0 ± 19.2 | < 0.001 |
| Gender | ||||
| Male | 85 (61.2) | 19 (73.1) | 66 (58.4) | |
| Female | 54 (38.8) | 7 (26.9) | 47 (41.6) | |
| Underlying diseases | ||||
| Hypertension | 39 (28.1) | 13 (50.0) | 26 (23.0) | 0.006 |
| Diabetes mellitus | 21 (15.1) | 9 (34.6) | 12 (10.6) | 0.002 |
| Cardiovascular diseases | 11 (7.9) | 6 (23.1) | 5 (4.4) | 0.001 |
| COPD/Asthma | 10 (7.2) | 3 (11.5) | 7 (6.2) | 0.342 |
| Hypotiroidism | 3 (2.2) | 1 (3.8) | 2 (1.8) | 0.511 |
| Chronic renal insufficiency | 3 (2.2) | 3 (11.5) | 1 (0.9) | 0.003 |
| Atrial fibrillation | 2 (1.4) | 2 (7.7) | 0 (0) | 0.003 |
| Malignancy | 4 (2.9) | 2 (7.7) | 2 (1.8) | 0.103 |
| Cerebrovascular diseases | 1 (0.7) | 0 (0) | 1 (0.9) | 0.630 |
Data are presented as n, mean±standart deviation or n (%) unless otherwise stated.
Total cholesterol (121.0 ± 46.6 vs. 150.7 ± 33.7; p = 0.004), HDL-C (28.5 (21.5–32.0) vs. 44.0 (32.5–77.0); p < 0.001) and LDL-C values (63.2 ± 39.8 vs. 118.1 ± 52.1; p < 0.001) are higher in survivor group. Triglyceride (136.0 (113.0–198.0) vs. 64.0 (38.5–125.0); p < 0.001) and the AIP (0.77 ± 0.23 vs. 0.14 ± 0.52; p < 0.001) values were higher in the deceased group (Table 2 ).
Table 2.
Lipid profiles and the atherogenic index of plasma values of the patients with COVID-19
| Variable | Total (n = 139) | Deceased (n = 26) | Survivors (n = 113) | p-value |
|---|---|---|---|---|
| Total cholesterol (mg/dL) | 144.5 ± 38.4 | 121.0 ± 46.6 | 150.7 ± 33.7 | 0.004 |
| Triglyceride (mg/dL) | 69.0 (39.2–131.5) | 136.0 (113.0–198.0) | 64.0 (38.5–125.0) | < 0.001 |
| HDL-C (mg/dL) | 39.0 (30.5–74.5) | 28.5 (21.5–32.0) | 44.0 (32.5–77.0) | < 0.001 |
| LDL-C (mg/dL) | 112.0 ± 53.6 | 63.2 ± 39.8 | 118.1 ± 52.1 | < 0.001 |
| AIP | 0.21 ± 0.53 | 0.77 ± 0.23 | 0.14 ± 0.52 | < 0.001 |
Data are presented as mean±standart deviation or median (interquartile range) unless otherwise stated.
Abb. AIP: The atherogenic index of plasma; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol.
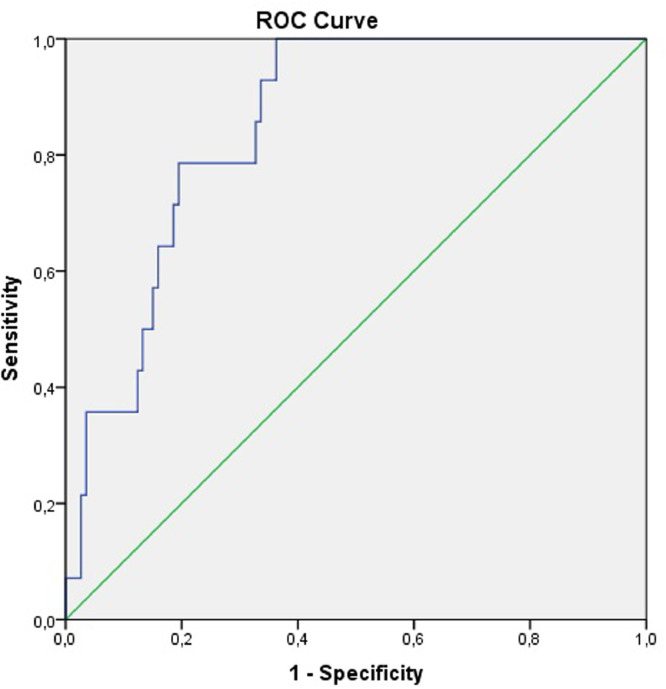
ROC curve is constructed to evaluate predictive value of in-hospital mortality of the AIP in patients with COVID-19 (Fig. 1 ). The area under the curve of the AIP is 0.850 (95% confidence interval (CI): 0.772–0.928). The optimal cut-off value is calculated as 0.6285 with a sensitivity of 78.6% and specificity of 80.5%.
Fig. 1.
Receiver operator characteristic curve for the atherogenic index of plasma to predict mortality
The AIP value is statistically higher in confirmed pneumonia patients compared to patients without pneumonia (p = 0.002). Patients who were intubated during hospitalization have higher AIP values (p < 0.001). Patients who were followed in intensive care unit also have higher AIP values than patients followed in normal hospital room (p < 0.001) (Table 3 ).
Table 3.
The Atherogenic index of plasma values of patients with and without pneumonia; intubated patients and patients without intubation period; patient followed in intensive care and patients followed in normal patient room.
| Patients with pneumonia (n = 87) | Patients without pneumonia (n = 52) | p | |
|---|---|---|---|
| AIP | 0.33 ± 0.58 | 0.04 ± 0.39 | 0.002 |
| Intubated patients (n = 26) | Patients without intubation period (n = 113) | p | |
|---|---|---|---|
| AIP | 0.78 ± 0.23 | 0.14 ± 0.52 | < 0.001 |
| Patients followed in intensive care (n = 27) | Patients followed in normal patient room (n = 112) | p | |
|---|---|---|---|
| AIP | 0.76 ± 0.24 | 0.14 ± 0.52 | < 0.001 |
Abb. AIP: The atherogenic index of plasma
Logistic regression analysis was performed with the AIP, gender, age and comorbidities of the patients to evaluate the explanatory power of the variables on mortality. Regression analysis showed that age and the AIP are independently associated with mortality (Table 4 ).
Table 4.
Logistic regression analysis of baseline characteristics of the study population and AIP on mortality
| Variable | Odds Ratio | 95% Confidence Interval | p-value |
|---|---|---|---|
| Age | 1.131 | 1.026–1.247 | 0.013 |
| The Atherogenic Index of Plasma | 1253.371 | 1.547–1015343.021 | 0.037 |
| Gender | 1.212 | 0.128–11.460 | 0.866 |
| Hypertension | 1.149 | 0.000–80.731 | 0.545 |
| Diabetes mellitus | 0.335 | 0.040–2.840 | 0.316 |
| Cardiovascular diseases | 0.290 | 0.001–164.884 | 0.702 |
| COPD/Asthma | 1.718 | 0.054–54.939 | 0.759 |
| Hypotiroidism | 0.077 | 0.002–3.631 | 0.192 |
| Chronic renal insufficiency | 0.141 | 0.000–80.731 | 0.545 |
| Atrial fibrillation | 0.000 | 0.000-. | 1.000 |
| Malignancy | 0.139 | 0.004–4.455 | 0.264 |
| Cerebrovascular diseases | 118789.095 | 0.000-. | 1.000 |
| Constant | 232.544 | 1.000 |
Abb. COPD; chronic obstructive pulmonary disease
Since there was a significant difference between age and underlying diseases in the deceased and survivor groups, a new analysis was performed by excluding random patients from both study groups. In this analysis, the deceased and survivor groups consisted of 21 patients each, and these two groups did not differ in terms of age, gender and underlying disease status (Table 5 ). This statistical analysis showed similar results with the analysis of the whole study population. Triglyceride levels were higher [163.0 (110.0–207.0) and 77.0 (36.0–119.5); p = 0.001] and HDL-C (27.0 (21.5–33.0) and 40.0 (33.0–73.0); p < 0.001) and LDL-C (65.9 ± 42.7 and 115.8 ± 47.4; p = 0.005) levels were lower in deceased group compared to the survivor group (Table 6 ).
Table 5.
Age, sex and underlying disease matched baseline characteristics of the patients with COVID-19
| Variable | Deceased (n, %) | Survivors (n, %) | p-value |
|---|---|---|---|
| Patients | 21 | 21 | |
| Age years | 72.4 ± 7.4 | 70.9 ± 9.2 | 0.570 |
| Gender | |||
| Male | 19 (73.1) | 15 (71.4) | 0.204 |
| Underlying diseases | |||
| Hypertension | 10 (47.6) | 13 (61.9) | 0.352 |
| Diabetes mellitus | 7 (33.3) | 6 (28.6) | 0.739 |
| Cardiovascular diseases | 4 (19.0) | 1 (4.8) | 0.153 |
| COPD/Asthma | 1 (4.8) | 3 (1.4) | 0.293 |
| Hypotiroidism | 1 (4.8) | 1 (4.8) | 0.756 |
| Chronic renal insufficiency | 3 (1.4) | 0 (0) | 0.072 |
| Atrial fibrillation | 2 (9.5) | 0 (0) | 0.147 |
| Malignancy | 2 (9.5) | 1 (4.8) | 0.549 |
| Cerebrovascular diseases | 0 (0) | 0 (0) | - |
Data are presented as n, mean±standart deviation or n (%) unless otherwise stated.
Table 6.
Age, sex and underlying disease matched lipid profiles and the atherogenic index of plasma values of the patients with COVID-19
| Variable | Deceased (n = 21) | Survivors (n = 21) | p-value |
|---|---|---|---|
| Total cholesterol (mg/dL) | 125.4 ± 50.4 | 147.3 ± 34.4 | 0.215 |
| Triglyceride (mg/dL) | 163.0 (110.0–207.0) | 77.0 (36.0–119.5) | 0.001 |
| HDL-C (mg/dL) | 27.0 (21.5–33.0) | 40.0 (33.0–73.0) | < 0.001 |
| LDL-C (mg/dL) | 65.9 ± 42.7 | 115.8 ± 47.4 | 0.005 |
| AIP | 0.79 ± 0.24 | 0.12 ± 0.51 | < 0.001 |
Data are presented as mean±standart deviation or median (interquartile range) unless otherwise stated.
Abb. AIP: The atherogenic index of plasma; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol.
Discussion
Our study states that patients that are deceased due to COVID-19 have higher AIP and triglyceride values and lower total cholesterol, HDL-C, LDL-C values compared to survivors of COVID-19. The AIP values are also higher in patients with pneumonia, intubated patients, patients followed in intensive care unit for COVID-19.
Although SARS-CoV-2 entered our lives only recently in 2019, the coronavirus family has longer history among humans; passing from animal to human and causing serious infections among humans.23 SARS-CoV had spread to 26 countries in 2002 and killed 774 people; MERS-CoV had spread to 27 countries in 2012 resulting in 659 related deaths.24 , 25 The Coronaviridae family is an enveloped virus family containing non-segmented single-stranded, positive-sense RNA genome.26 All viruses in the Coronaviridae family require host cells for viral replication and the host cell must increase metabolism in order to cope with the virus.27
The lipid metabolism is also affected in viral infections.28 Lipids are part of both viral and cellular membranes.11 Viruses need to regulate the lipid synthesis of the host cell to produce the lipids needed for their own membranes and envelopes.28 , 29 Especially cholesterol and fatty acids are an important component of the viral membranes and these lipids are required for the replication of the viruses. Yan et al.12 showed that viruses can modulate the lipid metabolism of the host cell for its optimal viral replication. The data for SARS-CoV-2 and its effect on the lipid metabolism is limited since its emergence is relatively recent. In our study total cholesterol, HDL-C and LDL-C was lower at the patients who are deceased. We may speculate that the viral load may be higher in these patients and SARS-CoV-2 may have used up the required lipid for their metabolism and replication. Heaton et al.30 reports that Dengue virus, which is also a RNA virus, causes an autophagy-dependent processing of lipid droplets to release fatty acids. This causes increase in β-oxidation and ATP formation which are used for viral replication. HIV-infected patients were shown to have lower total cholesterol and LDL-C and higher triglyceride values.13 Constans et al.14 showed that triglycerides increase at a late stage of the disease in HIV positive patients. The study explains this alteration on lipid metabolism with lipid peroxidation. Tumor necrosis factor-α regulates the plasma lipoprotein peroxidation in HIV positive patients and stimulates the reactive oxygen species production.31 Also the lipoprotein lipases and the hepatic lipases decrease their function in HIV positive patients and this may cause increase in triglycerides.32, 33, 34
In this study we have not compared the COVID-19 patients with non-infected population, but rather we compared patients who have survived the disease with patients who have deceased. The deceased patients had higher triglyceride levels which shows patients with worse prognosis has higher triglyceride levels. Even though the previously mentioned studies are on other RNA viruses, results seem similar, hence an identical mechanism may play a role on lipid metabolism of the host.
High triglyceride and low HDL-C levels are both risk factors for cardiovascular diseases.35 The AIP combines these two risk factors and several studies performed on this subject and showed that the AIP is associated with atherosclerosis, cardiovascular diseases, diabetes mellitus, hypertension, vascular and endothelial damage16, 17, 18, 19, 20. SARS-CoV-2 affects cardiovascular system on different levels. The patients may have myocardial injury, myocarditis, heart failure and it may cause acute myocardial infarction due to increased hypercoagulability and inflammation.26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38 In our study, the deceased patient group had higher AIP levels when compared to the survivor group. This finding suggests that higher AIP is related to worse prognosis in COVID-19 patients. Even though patients with in-hospital death outcome had lower total cholesterol and LDL-C values, they are at higher risk of atherosclerosis compared to survivor group according to our results. Myocardial injury is very common at COVID-19 patients and it is a predictor of mortality.38 , 39 Myocardial injury is caused by cardiac physiologic stress or hypoxia in COVID-19 patients but increased atherosclerotic tendency may be one of the reasons for increased troponin I levels, myocardial injury and mortality in COVID-19 patients.36 , 40 , 41 Interestingly, similar results are seen in HIV positive patients. The AIP values were higher in acute HIV infection which shows that the relationship between viral load and the AIP.21 Previous reports on 2002 SARS-CoV epidemic shows that higher viral load of SARS-CoV is associated with overall worse prognosis.42, 43, 44 Although there are major differences between the disease caused by SARS-CoV and the pandemic driven by SARS-CoV-2, studies show that viral load of SARS-CoV-2 is also related to worse outcomes.10 Huang et al.45 show that viral load is higher in patients who died compared to patients whom recovered during COVID-19 pandemic. There are also studies that show the correlation between viral load and biochemical and hematological markers. For example, higher LDH, troponin I and lower lymphocyte levels are associated with viral load.45, 46, 47, 48 Likewise, the patients in the deceased group, patients with pneumonia, intubation history and intensive care need have higher AIP values in our study. These results show that patients with worse outcomes have higher AIP. Based on these results, we suggest that elevated AIP may be associated with increased viral load and an indicator for worse prognosis in COVID-19 patients.
We found out that an AIP level of 0.6285 can be used as a cut-off value for mortality with a sensitivity of 78.6% and specificity of 80.5%. As far as we know this is the first study to evaluate the relationship between the AIP and mortality in COVID-19 patients. The AIP values are also higher in patients with pneumonia, intubated patients and patients in intensive care unit. This results show that along with mortality, higher AIP values also predicts pneumonia incidence, intensive care unit and intubation need in COVID-19 patients.
Several chronic disorders like cardiovascular diseases, hypertension, diabetes mellitus, atrial fibrillation and chronic renal insufficiency are associated with worse outcomes in COVID-19 patients.28 , 49, 50, 51 In our study, patients in the deceased group have higher rates of cardiovascular diseases, hypertension, diabetes mellitus, chronic renal insufficiency and atrial fibrillation history than survivor group. As already discussed, previous studies support these findings as well. Finally, patients with pre-existing morbidities should be evaluated more carefully to prevent worse outcomes and mortality in these patients.
Limitations
Major limitation of our study is the small sample size. A multicenter study with a larger sample size should be performed to strengthen and improve upon the findings in this study. Additionally, majority of the confirmed cases did not have lipid profile values due to the retrospective design of the study. The exclusion of these patients at the beginning further reduces the size of the patients enrolled in the study. Furthermore, our study only includes hospitalized patients. It would be superior to perform the study on every confirmed COVID-19 case.
Conclusion
COVID-19 quickly became a global pandemic and still continues to cause death among people especially with previous comorbidities.1, 2, 3 , 28 , 49, 50, 51 Several hematological and biochemical parameters have been shown to be related to mortality among COVID-19 patients.45, 46, 47, 48 Our study showed that the AIP may be used as a biomarker for mortality. The AIP on admission greater than 0.6285 value can be an indicator of in-hospital mortality in patients with COVID-19. Patients with higher AIP values should be treated carefully and followed up for clinically worse outcomes. In addition, higher triglyceride level and lower total cholesterol, LDL-C and HDL-C values are observed in COVID-patients who were deceased in the process which may be an effect of the SARS-CoV-2 on lipid metabolism of the host subject.
Declaration of Competing Interest
No funding was taken for this study. The authors declare that they have no conflict of interest.
References
- 1.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected Pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu JT, Leung K, Leung GM. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study [published correction appears in Lancet. 2020 Feb 4] Lancet. 2020;395(10225):689–697. doi: 10.1016/S0140-6736(20)30260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turgay Yıldırım Ö. The use of Angiotensin converting enzyme (ACE) inhibitors and Angiotensin Receptor Blockers (ARB) in COVID-19 Pandemic. J Cukurova Anesth Surg. 2020;3(1):47–52. doi: 10.36516/jocass.2020.32. [DOI] [Google Scholar]
- 5.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 Cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsuyama S, Nao N, Shirato K, et al. Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc Natl Acad Sci U S A. 2020;117(13):7001–7003. doi: 10.1073/pnas.2002589117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bansal M. Cardiovascular disease and COVID-19. Diabetes Metab Syndr. 2020;14(3):247–250. doi: 10.1016/j.dsx.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaim S, Chong JH, Sankaranarayanan V, Harky A. COVID-19 and multiorgan response. Curr Probl Cardiol. 2020;45(8) doi: 10.1016/j.cpcardiol.2020.100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J, Zhang S, Wu Z, et al. Clinical outcomes of COVID-19 in Wuhan, China: a large cohort study. Ann Intensive Care. 2020;10(1):99. doi: 10.1186/s13613-020-00706-3. Published 2020 Jul 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rao SN, Manissero D, Steele VR, Pareja J. A narrative systematic review of the clinical utility of cycle threshold values in the context of COVID-19 [published online ahead of print, 2020 Jul 28] Infect Dis Ther. 2020:1–14. doi: 10.1007/s40121-020-00324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lorizate M, Kräusslich HG. Role of lipids in virus replication. Cold Spring Harb Perspect Biol. 2011;3(10) doi: 10.1101/cshperspect.a004820. a004820. Published 2011 Oct 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan B, Zou Z, Chu H, et al. Lipidomic profiling reveals significant perturbations of intracellular lipid homeostasis in enterovirus-infected cells. Int J Mol Sci. 2019;20(23):5952. doi: 10.3390/ijms20235952. Published 2019 Nov 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguemaïm NF, Mbuagbaw J, Nkoa T, et al. Serum lipid profile in highly active antiretroviral therapy-naïve HIV-infected patients in Cameroon: a case-control study. HIV Med. 2010;11(6):353–359. doi: 10.1111/j.1468-1293.2009.00784.x. [DOI] [PubMed] [Google Scholar]
- 14.Constans J, Pellegrin JL, Peuchant E, et al. Plasma lipids in HIV-infected patients: a prospective study in 95 patients. Eur J Clin Invest. 1994;24(6):416–420. doi: 10.1111/j.1365-2362.1994.tb02185.x. [DOI] [PubMed] [Google Scholar]
- 15.Dobiásová M, Frohlich J. The plasma parameter log (TG/HDL-C) as an atherogenic index: correlation with lipoprotein particle size and esterification rate in apoB-lipoprotein-depleted plasma (FER(HDL)) Clin Biochem. 2001;34(7):583–588. doi: 10.1016/s0009-9120(01)00263-6. [DOI] [PubMed] [Google Scholar]
- 16.Onat A, Can G, Kaya H, Hergenç G. Atherogenic index of plasma" (log10 triglyceride/high-density lipoprotein-cholesterol) predicts high blood pressure, diabetes, and vascular events. J Clin Lipidol. 2010;4(2):89–98. doi: 10.1016/j.jacl.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Yildiz G, Duman A, Aydin H, et al. Evaluation of association between atherogenic index of plasma and intima-media thickness of the carotid artery for subclinic atherosclerosis in patients on maintenance hemodialysis. Hemodial Int. 2013;17(3):397–405. doi: 10.1111/hdi.12041. [DOI] [PubMed] [Google Scholar]
- 18.Dobiásová M, Frohlich J, Sedová M, Cheung MC, Brown BG. Cholesterol esterification and atherogenic index of plasma correlate with lipoprotein size and findings on coronary angiography. J Lipid Res. 2011;52(3):566–571. doi: 10.1194/jlr.P011668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niroumand S, Khajedaluee M, Khadem-Rezaiyan M, et al. Atherogenic Index of Plasma (AIP): A marker of cardiovascular disease. Med J Islam Repub Iran. 2015;29:240. Published 2015 Jul 25. [PMC free article] [PubMed] [Google Scholar]
- 20.Turgay Yıldırım Ö, Akşit E, Aydın F, Hüseyinoğlu Aydın A. Evaluation of atherogenic index of plasma levels at hypertensive patients. ACEM. 2019;4(2):72–75. doi: 10.25000/acem.563986. [DOI] [Google Scholar]
- 21.Wang Q, Ding H, Xu J, et al. Lipids profile among ART-naïve HIV infected patients and men who have sex with men in China: a case control study. Lipids Health Dis. 2016;15(1):149. doi: 10.1186/s12944-016-0297-1. Published 2016 Sep 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Q, Zhou L, Sun X, et al. Altered lipid metabolism in recovered SARS patients twelve years after infection. Sci Rep. 2017;7(1):9110. doi: 10.1038/s41598-017-09536-z. Published 2017 Aug 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China [published correction appears in Lancet. 2020 Jan 30] Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christian MD, Poutanen SM, Loutfy MR, Muller MP, Low DE. Severe acute respiratory syndrome. Clin Infect Dis. 2004;38(10):1420–1427. doi: 10.1086/420743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arabi YM, Balkhy HH, Hayden FG, et al. Middle east respiratory syndrome. N Engl J Med. 2017;376(6):584–594. doi: 10.1056/NEJMsr1408795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern [published correction appears in Lancet. 2020 Jan 29] Lancet. 2020;395(10223):470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gualdoni GA, Mayer KA, Kapsch AM, et al. Rhinovirus induces an anabolic reprogramming in host cell metabolism essential for viral replication. Proc Natl Acad Sci U S A. 2018;115(30):E7158–E7165. doi: 10.1073/pnas.1800525115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abu-Farha M, Thanaraj TA, Qaddoumi MG, Hashem A, Abubaker J, Al-Mulla F. The role of lipid metabolism in COVID-19 Virus infection and as a drug target. Int J Mol Sci. 2020;21(10):3544. doi: 10.3390/ijms21103544. Published 2020 May 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murillo A, Vera-Estrella R, Barkla BJ, Méndez E, Arias CF. Identification of host cell factors associated with astrovirus replication in Caco-2 Cells. J Virol. 2015;89(20):10359–10370. doi: 10.1128/JVI.01225-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heaton NS, Randall G. Dengue virus-induced autophagy regulates lipid metabolism. Cell Host Microbe. 2010;8(5):422–432. doi: 10.1016/j.chom.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDonagh J, Fossel ET, Kradin RK, Dubinett SM, Laposata M, Hallaq YA. Effect of Tumor Necrosis Factor-a on peroxidation of plasma lipoprotein lipids in experimental animals and patients. Blood. 1992;80:3217–3226. doi: 10.1182/blood.V80.12.3217.bloodjournal80123217. [PubMed] [Google Scholar]
- 32.Grunfeld C, Pang M, Doerrler W, Shigenaga JK, Jensen P, Feingold KR. Lipids, lipoproteins, triglyceride clearance, and cytokines in human immunodeficiency virus infection and the acquired immunodeficiency syndrome. J Clin Endocrinol Metab. 1992;74(5):1045–1052. doi: 10.1210/jcem.74.5.1373735. [DOI] [PubMed] [Google Scholar]
- 33.Teto G, Kanmogne GD, Torimiro JN, et al. Lipid peroxidation and total cholesterol in HAART-naïve patients infected with circulating recombinant forms of human immunodeficiency virus type-1 in Cameroon. PLoS One. 2013;8(6):e65126. doi: 10.1371/journal.pone.0065126. Published 2013 Jun 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weinroth SE, Parenti DM, Simon GL. Wasting syndrome in AIDS: pathophysiologic mechanisms and therapeutic approaches. Infect Agents Dis. 1995;4(2):76–94. [PubMed] [Google Scholar]
- 35.Consensus conference NIH. Triglyceride, high-density lipoprotein, and coronary heart disease. NIH Consensus Development Panel on Triglyceride, High-Density Lipoprotein, and Coronary Heart Disease. JAMA. 1993;269(4):505–510. [PubMed] [Google Scholar]
- 36.Driggin E, Madhavan MV, Bikdeli B, et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 Pandemic. J Am Coll Cardiol. 2020;75(18):2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Welt FGP, Shah PB, Aronow HD, et al. Catheterization laboratory considerations during the Coronavirus (COVID-19) Pandemic: from the ACC's Interventional Council and SCAI. J Am Coll Cardiol. 2020;75(18):2372–2375. doi: 10.1016/j.jacc.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Long B, Brady WJ, Koyfman A, Gottlieb M. Cardiovascular complications in COVID-19. Am J Emerg Med. 2020;38(7):1504–1507. doi: 10.1016/j.ajem.2020.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Du RH, Liang LR, Yang CQ, et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020;55(5) doi: 10.1183/13993003.00524-2020. Published 2020 May 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen C, Zhou Y, Wang DW. SARS-CoV-2: a potential novel etiology of fulminant myocarditis. Herz. 2020;45(3):230–232. doi: 10.1007/s00059-020-04909-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients With COVID-19 in Wuhan, China [published online ahead of print, 2020 Mar 25] JAMA Cardiol. 2020;5(7):802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng VC, Hung IF, Tang BS, et al. Viral replication in the nasopharynx is associated with diarrhea in patients with severe acute respiratory syndrome. Clin Infect Dis. 2004;38(4):467–475. doi: 10.1086/382681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ng EK, Hui DS, Chan KC, et al. Quantitative analysis and prognostic implication of SARS coronavirus RNA in the plasma and serum of patients with severe acute respiratory syndrome. Clin Chem. 2003;49(12):1976–1980. doi: 10.1373/clinchem.2003.024125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chu CM, Poon LL, Cheng VC, et al. Initial viral load and the outcomes of SARS. CMAJ. 2004;171(11):1349–1352. doi: 10.1503/cmaj.1040398rao. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang JT, Ran RX, Lv ZH, et al. Chronological Changes of Viral Shedding in Adult Inpatients with COVID-19 in Wuhan, China [published online ahead of print, 2020 May 23] Clin Infect Dis. 2020:ciaa631. doi: 10.1093/cid/ciaa631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu Y, Liao W, Wan L, Xiang T, Zhang W. Correlation between relative nasopharyngeal Virus RNA load and lymphocyte count disease severity in patients with COVID-19 [published online ahead of print, 2020 Apr 10] Viral Immunol. 2020 doi: 10.1089/vim.2020.0062. 10.1089/vim.2020.0062. [DOI] [PubMed] [Google Scholar]
- 47.Yuan C, Zhu H, Yang Y, et al. Viral loads in throat and anal swabs in children infected with SARS-CoV-2. Emerg Microbes Infect. 2020;9(1):1233–1237. doi: 10.1080/22221751.2020.1771219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Azzi L, Carcano G, Gianfagna F, et al. Saliva is a reliable tool to detect SARS-CoV-2. J Infect. 2020;81(1) doi: 10.1016/j.jinf.2020.04.005. e45-e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nishiga M, Wang DW, Han Y, Lewis DB, Wu JC. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives [published online ahead of print, 2020 Jul 20] Nat Rev Cardiol. 2020:1–16. doi: 10.1038/s41569-020-0413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Atkins JL, Masoli JAH, Delgado J, et al. Preexisting Comorbidities Predicting COVID-19 and Mortality in the UK Biobank Community Cohort [published online ahead of print, 2020 Jul 20] J Gerontol A Biol Sci Med Sci. 2020:glaa183. doi: 10.1093/gerona/glaa183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bajwa H, Riaz Y, Ammar M, Farooq S, Yousaf A. The dilemma of renal involvement in COVID-19: a systematic review. Cureus. 2020;12(6):e8632. doi: 10.7759/cureus.8632. Published 2020 Jun 15. [DOI] [PMC free article] [PubMed] [Google Scholar]