Supplemental Digital Content is available in the text.
Keywords: coronavirus, COVID-19, extracellular vesicles, fibrinogen, thrombosis
Objective:
Patients with coronavirus disease 2019 (COVID-19) have a high rate of thrombosis. We hypothesized that severe acute respiratory syndrome coronavirus 2 infection leads to induction of TF (tissue factor) expression and increased levels of circulating TF-positive extracellular vesicles (EV) that may drive thrombosis.
Approach and Results:
We measured levels of plasma EV TF activity in 100 patients with COVID-19 with moderate and severe disease and 28 healthy controls. Levels of EV TF activity were significantly higher in patients with COVID-19 compared with controls. In addition, levels of EV TF activity were associated with disease severity and mortality. Finally, levels of EV TF activity correlated with several plasma markers, including D-dimer, which has been shown to be associated with thrombosis in patients with COVID-19.
Conclusions:
Our results indicate that severe acute respiratory syndrome coronavirus 2 infection induces the release of TF-positive EVs into the circulation that are likely to contribute to thrombosis in patients with COVID-19. EV TF activity was also associated with severity and mortality.
Highlights.
Extracellular vesicle tissue factor activity is increased in patients with coronavirus disease 2019 (COVID-19).
Extracellular vesicle tissue factor activity is associated with severity.
Extracellular vesicle tissue factor activity is associated with mortality.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection leads to coronavirus disease 2019 (COVID-19) and has caused a global pandemic. Strikingly, patients with COVID-19 have a high rate of thrombotic events.1 There has been much speculation about the mechanism of thrombosis in patients with COVID-19, including activation of coagulation and platelets, endothelial cell activation, release of neutrophil extracellular traps, inflammation, and activation of the complement system.2 TF (tissue factor) is the main activator of the coagulation cascade.3 Two recent reviews speculated that induction of TF expression may play a significant role in COVID-19-related thrombosis.2,4
We developed an assay to measure levels of extracellular vesicle (EV) TF activity in plasma.5 EVs are also referred to as microparticles or microvesicles and are small membrane vesicles released from activated cells.6 We and others found that circulating EV TF activity is increased in a variety of diseases that are associated with activation of coagulation and thrombosis, including cancer, endotoxemia, and bacterial and viral infection.7 Importantly, we showed that EV TF activity is increased with several viral infections, including influenza A virus/H1N1 (hemagglutinin type 1 and neuraminidase type 1), Puumala orthohantavirus, Sin Nombre Virus, and Ebola virus.8–12 Notably, levels of EV TF activity are associated with thrombosis in patients with pancreatic cancer and patients infected with Puumala orthohantavirus.9,13,14 This suggests that EV TF activity can be used as a biomarker to assess induction of TF expression in patients and thrombotic risk.
In this study, we measured levels of circulating EV TF activity in patients with COVID-19 and determined if they were associated with severity and mortality.
Materials and Methods
Study Population
One hundred patients with COVID-19 admitted to Danderyd Hospital, Stockholm, Sweden between April 9 and June 8, 2020 were included in the study. Patients were diagnosed with COVID-19 using reverse-transcriptase polymerase chain reaction viral detection of oropharyngeal or nasopharyngeal swabs (n=96) or clinical presentation (n=4). Eighty-five patients received anticoagulation (prophylactic low molecular weight heparin, n=57; prophylactic high dose low molecular weight heparin, n=24; oral anticoagulant, n=4; therapeutic anticoagulation, n=0). Twelve patients received glucocorticoids. No antivirals or hydroxychloroquine were used in these patients.
Exclusion criteria were age <18 years and thrombosis before blood sampling (1 deep vein thrombosis and 3 pulmonary embolism). Demographic data, comorbidities, medications, respiratory support, and mortality (<45 days from admission) were obtained from medical records. Median age for the patients was 60 (50–69) years (65% male). Patients were divided into 2 groups based on the WHO-OSCI (World Health Organization-Ordinal Scale for Clinical Improvement; https://www.who.int/blueprint/priority-diseases/key-action/COVID-19_Treatment_Trial_Design_Master_Protocol_synopsis_Final_18022020.pdf). Ninety-six patients had a score of <5 and 4 had a score of ≥5. Due to the low number of patients in the more severe group, we also divided the patients into 2 groups based on the level of respiratory support at the time of sampling: none/low level (≤5 L oxygen on cannula, n=83) and high level of respiratory support (>5 L oxygen on cannula, noninvasive respiratory support or intubation, n=17). Samples from 28 healthy individuals were used as controls with a median age of 71 (71–73) years (79% male). The study complied with the declaration of Helsinki, and informed consent was obtained from all healthy controls and patients or their next-of-kin. The study was approved by the Swedish Ethical Review Board (COMMUNITY study [Covid-19 Biomarker and Immunity] dnr 2020-01653).
Preparation of Plasma
Blood samples were collected within 7 days of admission via venipuncture (98 patients) or preexisting arterial lines (2 patients) into sodium citrate (9:1 vol/vol) vacuum tubes. Platelet poor plasma was prepared from whole blood within 3 hours of sampling by centrifugation (2000×g, 20 minutes, room temperature) and stored at −80 °C. Blood samples were collected from controls via venipuncture.
Measurement of EV TF Activity and Other Biomarkers
EV TF activity was measured as described.5 Briefly, EVs are pelleted from plasma (100 μL) at 20 000g for 15 minutes at 4 °C. EVs are washed to remove coagulation factors in the plasma and resuspended in Hepes buffer saline with BSA. TF activity was measured by adding human factor VIIa (4.8 nmol/L [final concentration: 2.4 nmol/L] from Enzyme Research Laboratories, South Bend, IN), human factor X (46.4 nmol/L [final concentration: 73.2 nmol/L] from Enzyme Research Laboratories), and calcium chloride (final concentration: 5 mmol/L) in the presence of either an antihuman TF antibody (clone: HTF-1, BD Biosciences, San Jose, CA) or a control IgG (Sigma Aldrich. MO). After 2 hours of incubation at 37 °C, the reaction was stopped with EDTA and factor Xa measured using a chromogenic substrate (Pefachrome FXa 8595 [final concentration: 0.67 mmol/L] from Enzyme Research Laboratories). The absorbance at 405 nm was measured using a plate reader. We measured levels of a variety of other biomarkers and functional assays described below in a separate study.15 Prothrombin time and plasma levels of fibrinogen, prothrombin, antithrombin, factor VIII, and D-dimer were measured using an automated coagulation analyzer (STACompact 3, Stago, Breda, the Netherlands). Levels of thrombin-antithrombin complexes were measured using a commercial assay (TAT; Siemens, Erlangen, Germany). We used a commercially available ELISA from R&D systems (Minneapolis, MN) to measure plasma levels of plasminogen activator inhibitor type 1. Plasma levels of plasmin-antiplasmin complexes were measured with a commercially available ELISA (Technozyme, Technoclone, Vienna, Austria). An in-house ELISA with a commercially available polyclonal antibodies against von Willebrand factor (DAKO, Glostrup, Denmark) was used to measure plasma levels of von Willebrand factor. We used the FRETS-VWF73 assay (Peptanova, Sandhausen, Germany) to measure plasma levels of a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 activity. Thrombin generation assay was performed with thrombomodulin using the fluorimetric method as described.16 We measured the fibrinolytic capacity of the plasma samples by assessing changes in turbidity during clot formation and lysis of a tissue factor-induced clot by exogenous tissue plasminogen activator.17
Statistical Analysis
Comparisons of continuous variables were performed using Mann-Whitney U test and categorical variables with Fisher exact test (GraphPad Software). Median and interquartile range (IQR) are reported for nonparametric data. Statistical analyses were performed using SPSS statistics, version 26 (IBM inc., Chicago, IL), GraphPad Prism (GraphPad Software Inc, San Diego, CA) and Stata 16 (StataCorp, Houston, TX). Median and IQR are reported for nonparametric data for EV TF activity. A receiver operating characteristic curve was plotted, and a maximum Youden’s index yielded a cutoff value for EV TF activity of 0.565 pg/mL. Kaplan-Meier curves were generated, and survival analysis were performed using this cutoff. The relationship between EV TF activity and other biomarkers was evaluated using the Spearman’s rho. Univariate and multivariate Cox proportional hazards models were constructed to determine the hazard ratio of death for EV TF activity. We adjusted for D-dimer levels in a multivariate analysis. All tests were 2-sided, and P<0.05 was considered statistically significant.
Results and Discussion
Levels of EV TF activity were significantly higher in patients with COVID-19 compared with healthy controls (Figure [A]). It is notable that 2 of the patients had high levels of EV TF activity (3.03 and 3.37 pg/mL). These 2 patients were 2 of 4 patients with WHO-OSCI scores of ≥5 indicating severe disease. We have observed similar high values for EV TF activity in individual patients with pancreatic cancer (0.94, 1.23, 4.4, and 5.5)18,19 and in patients with severe influenza virus A/H1N1 infection (3.80±0.9, mean±SD, n=15).10
Figure.
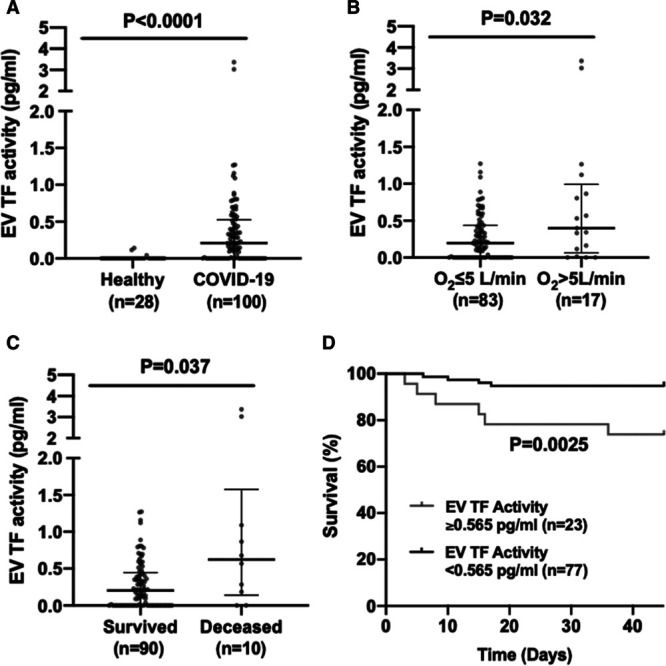
Levels of extracellular vesicle (EV) tissue factor activity in patients with coronavirus disease 2019 (COVID-19). A, EV TF activity in healthy individuals (n=28) and patients with COVID-19 (n=100). B, EV TF activity in patients with COVID-19 with low respiratory support (O2 ≤5 L/min; n=83) compared with patients with COVID-19 with high respiratory support (O2 >5 L/min; n=17). C, EV TF activity in patients with COVID-19 who survived (n=90) compared with patients with COVID-19 who died (n=10). Data is presented as median with interquartile range. D, Kaplan-Meier survival curve of patients with COVID-19 with high (≥0.565 pg/mL) or low (<0.565 pg/mL) EV TF activity.
We divided the patients into 2 groups based on WHO-OSCI and found that patients with a score of ≥5 had significantly higher levels of EV TF activity than patients with a score of <5 (median, 2.1 [IQR, 0.71–3.3], n=4 versus 0.20 [0–0.46], n=96; P=0.0002). Due to the low numbers of severely ill patients using the WHO-OSIC score, we also stratified patients with respect to respiratory support and observed significantly higher levels of EV TF activity in patients with O2 >5 L/min compared with those with O2 ≤5 L/min (Figure [B]). Furthermore, levels of EV TF activity were associated with mortality with significantly higher levels being observed with patients who died compared with levels in patients who survived (Figure [C]). In unadjusted Cox regression, EV TF activity had a hazard ratio of 3.4 (95% CI, 1.9–6.0) for death (P<0.001). After adjustment for D-dimer levels, the hazard ratio for death was 4.6 (95% CI, 2.0–10.4) for EV TF activity (P<0.001). These results indicate a strong association between EV TF activity and short-term mortality. Finally, significantly more patients who died had EV TF activity above the threshold of 0.565 pg/mL (6/10, 60%) compared with patients that survived (19/90, 21%; P<0.05, Fisher exact test). Using a cutoff of 0.565 pg/mL, we found that patients with ≥0.565 pg/mL of EV TF activity had significantly higher mortality compared with patients with <0.565 pg/mL (Figure [D]).
Levels of EV TF activity were not associated with use of anticoagulation (0.15 [0–0.56], n=15 versus 0.22 [0–0.52], n=85; P=0.44), the use of prophylactic versus prophylactic high-dose anticoagulation (0.20 [0–0.43], n=57 versus 0.34 [0.11–0.66], n=24; P=0.09), or by treatment with corticosteroids (0.24 [0.03–0.51], n=12 versus 0.20 [0–0.53], n=88; P=0.93). We would not expect anticoagulants to affect levels of EV TF activity because EV production should not be reduced by treatment with anticoagulants, and EV are isolated from plasma for measurement of TF activity.
At present, we do not know the cellular source of the circulating TF+ EVs in patients with COVID-19. We and others cannot detect TF+EVs by flow cytometry.20 We speculate the TF+EVs are derived from activated monocytes and endothelial cells as well as perivascular cells.3,21
We determined if EV TF activity correlated with other biomarkers and coagulation and fibrinolysis assays in patients with COVID-19. EV TF activity was significantly correlated with D-dimer, prothrombin time, and international normalized ratio but not with thrombin-antithrombin complexes (Table). D-dimer has a considerably longer half-life than thrombin-antithrombin complexes and is used clinically.22 Interestingly, EV TF activity was significantly correlated with the lagtime in the thrombin generation assay but not with other parameters (Table). The positive correlation between EV TF activity and lagtime is surprising because we have shown that TF decreases lagtime.23 EV TF activity was significantly correlated with levels of prothrombin, fibrinogen, and antithrombin (Table). In terms of biomarkers of fibrinolysis, EV TF activity significantly correlated with plasmin-antiplasmin complexes but not with total plasminogen activator inhibitor type 1 or the clot lysis time (Table). EV TF activity positively correlated with von Willebrand factor levels and negatively correlated with a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (Table). These results indicate a link between EV TF, coagulation, fibrinolysis, and endothelial activation.
Table.
Correlation of Extracellular Vesicle Tissue Factor Activity With Other Biomarkers in Patients With COVID-19
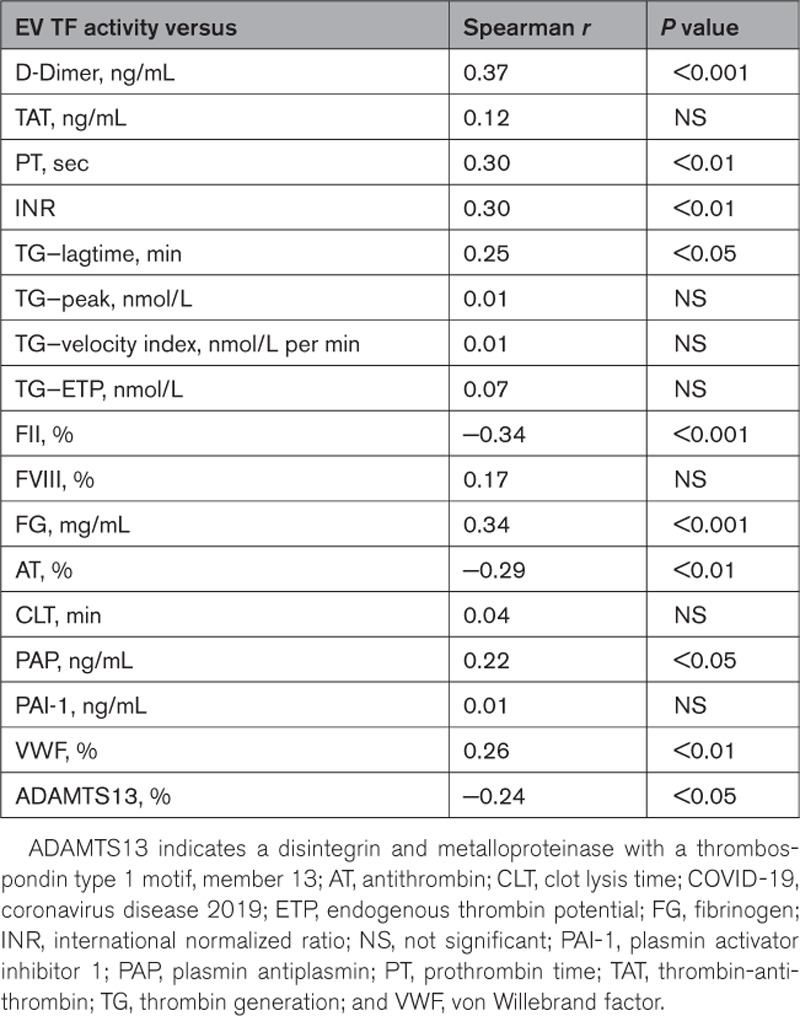
Our studies with patients with cancer found a stronger correlation between EV TF activity and D-dimer in patients with pancreatic cancer (rs=0.35 and rs=0.51) compared with a general cancer population (rs=0.145).24–26 Importantly, D-dimer has been shown to be associated with thrombosis in patients with COVID-19.1 The current study suggests that the increase in EV TF activity reflects an induction of TF expression in patients with COVID-19 and release of TF-positive EVs into the circulation. It is likely that TF-positive EVs also contribute to thrombosis in patients with COVID-19.
A limitation of the study is that the number of severely ill patients with COVID-19 in our cohort at the time of sample collection was small (n=4). Two of these severely ill patients had high levels of EV TF activity that were similar to the level observed in patients with severe influenza A virus/H1N1 infection.10
We and others have shown that EV TF activity is associated with mortality in a general cancer population and also in patients with pancreatic cancer.25–29 In addition, levels of EV TF activity are associated mortality in severe influenza A/H1N1 infection.10 The current study indicates that EV TF activity is also a prognostic marker in patients with COVID-19.
Acknowledgments
We would like to acknowledge the patients who participated in this study and Lena Gabrielsson, Ann-Christin Salomonson, Nina Greilert, and Eva Isaksson at Danderyd Hospital for administration and blood sampling.
Sources of Funding
The work was funded by an National Institutes of Health R01 HL119523 (N. Mackman), a University of North Carolina at Chapel Hill School of Medicine and NCTraCS ECBR pilot grant (N. Mackman) and funding from the Region Stockholm, and Knut & Alice Wallenberg foundation (C. Thålin).
Disclosures
None.
Supplementary Material
Nonstandard Abbreviations and Acronyms
- COVID-19
- coronavirus disease 2019
- EV
- extracellular vesicle
- SARS-CoV-2
- severe acute respiratory syndrome coronavirus 2
- TF
- tissue factor
These authors contributed equally as to this article as co-first authors.
These authors contributed equally to this article as co-senior authors.
This article was sent to William C. Sessa, Consulting Editor, for review by expert referees, editorial decision, and final disposition.
For Sources of Funding and Disclosures, see page 882.
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/ATVBAHA.120.315547.
Contributor Information
Axel Rosell, Email: axel.rosell@sll.se.
Sebastian Havervall, Email: sebastian.havervall@sll.se.
Fien von Meijenfeldt, Email: f.a.von.meijenfeldt@umcg.nl.
Yohei Hisada, Email: yohei_hisada@med.unc.edu.
Katherina Aguilera, Email: katherina.aguilera-gatica@sll.se.
Steven P. Grover, Email: steven_grover@med.unc.edu.
Ton Lisman, Email: j.a.lisman@umcg.nl.
Charlotte Thålin, Email: charlotte.thalin@sll.se.
References
- 1.Berger JS, Kunichoff D, Adhikari S, Ahuja T, Amoroso N, Aphinyanaphongs Y, Cao M, Goldenberg R, Hindenburg A, Horowitz J, et al. Prevalence and outcomes of d-dimer elevation in hospitalized patients with covid-19. Arterioscler Thromb Vasc Biol. 2020;40:2539–2547. doi: 10.1161/ATVBAHA.120.314872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mackman N, Antoniak S, Wolberg AS, Kasthuri R, Key NS. Coagulation abnormalities and thrombosis in patients infected with SARS-CoV-2 and other pandemic viruses. Arterioscler Thromb Vasc Biol. 2020;40:2033–2044. doi: 10.1161/ATVBAHA.120.314514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grover SP, Mackman N. Tissue factor: an essential mediator of hemostasis and trigger of thrombosis. Arterioscler Thromb Vasc Biol. 2018;38:709–725. doi: 10.1161/ATVBAHA.117.309846 [DOI] [PubMed] [Google Scholar]
- 4.Bautista-Vargas M, Bonilla-Abadía F, Cañas CA. Potential role for tissue factor in the pathogenesis of hypercoagulability associated with in COVID-19. J Thromb Thrombolysis. 2020;50:479–483. doi: 10.1007/s11239-020-02172-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hisada Y, Mackman N. Measurement of tissue factor activity in extracellular vesicles from human plasma samples. Res Pract Thromb Haemost. 2019;3:44–48. doi: 10.1002/rth2.12165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coumans FAW, Brisson AR, Buzas EI, Dignat-George F, Drees EEE, El-Andaloussi S, Emanueli C, Gasecka A, Hendrix A, Hill AF, et al. Methodological guidelines to study extracellular vesicles. Circ Res. 2017;120:1632–1648. doi: 10.1161/CIRCRESAHA.117.309417 [DOI] [PubMed] [Google Scholar]
- 7.Hisada Y, Alexander W, Kasthuri R, Voorhees P, Mobarrez F, Taylor A, McNamara C, Wallen H, Witkowski M, Key NS, et al. Measurement of microparticle tissue factor activity in clinical samples: a summary of two tissue factor-dependent FXa generation assays. Thromb Res. 2016;139:90–97. doi: 10.1016/j.thromres.2016.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tatsumi K, Hisada Y, Connolly AF, Buranda T, Mackman N. Patients with severe orthohantavirus cardiopulmonary syndrome due to Sin Nombre Virus infection have increased circulating extracellular vesicle tissue factor and an activated coagulation system. Thromb Res. 2019;179:31–33. doi: 10.1016/j.thromres.2019.04.032 [DOI] [PubMed] [Google Scholar]
- 9.Schmedes CM, Grover SP, Hisada YM, Goeijenbier M, Hultdin J, Nilsson S, Thunberg T, Ahlm C, Mackman N, Fors Connolly AM. Circulating extracellular vesicle tissue factor activity during orthohantavirus infection is associated with intravascular coagulation. J Infect Dis. 2020;222:1392–1399. doi: 10.1093/infdis/jiz597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rondina MT, Tatsumi K, Bastarache JA, Mackman N. Microvesicle tissue factor activity and interleukin-8 levels are associated with mortality in patients with influenza A/H1N1 infection. Crit Care Med. 2016;44:e574–e578. doi: 10.1097/CCM.0000000000001584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hodowanec AC, Lee RD, Brady KE, Gao W, Kincaid S, Plants J, Bahk M, Mackman N, Landay AL, Huhn GD. A matched cross-sectional study of the association between circulating tissue factor activity, immune activation and advanced liver fibrosis in hepatitis C infection. BMC Infect Dis. 2015;15:190 doi: 10.1186/s12879-015-0920-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenberg A, Huber BR, Liu DX, Logue JP, Hischak AMW, Hart RJ, Abbott M, Isic N, Hisada YM, Mackman N, et al. Quantification of viral and host biomarkers in the liver of rhesus macaques: a longitudinal study of zaire Ebolavirus Strain Kikwit (EBOV/Kik). Am J Pathol. 2020;190:1449–1460. doi: 10.1016/j.ajpath.2020.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hisada Y, Mackman N. Update from the laboratory: mechanistic studies of pathways of cancer-associated venous thrombosis using mouse models. Hematology Am Soc Hematol Educ Program. 2019;2019:182–186. doi: 10.1182/hematology.2019000025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geddings JE, Mackman N. Tumor-derived tissue factor-positive microparticles and venous thrombosis in cancer patients. Blood. 2013;122:1873–1880. doi: 10.1182/blood-2013-04-460139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Meijenfeldt FA, Havervall S, Adelmeijer J, Lundström A, Rudberg A, Magnusson M, Mackman N, Thalin C, Lisman T. Prothrombotic changes in patients with COVID-19 are associated with disease severity and mortality [published online December 6, 2020]. Res Pract Thromb Haemost. doi: 10.1002/rth2.12462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hemker HC, Giesen P, Al Dieri R, Regnault V, de Smedt E, Wagenvoord R, Lecompte T, Béguin S. Calibrated automated thrombin generation measurement in clotting plasma. Pathophysiol Haemost Thromb. 2003;33:4–15. doi: 10.1159/000071636 [DOI] [PubMed] [Google Scholar]
- 17.Meltzer ME, Lisman T, Doggen CJ, de Groot PG, Rosendaal FR. Synergistic effects of hypofibrinolysis and genetic and acquired risk factors on the risk of a first venous thrombosis. PLoS Med. 2008;5:e97 doi: 10.1371/journal.pmed.0050097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khorana AA, Francis CW, Menzies KE, Wang JG, Hyrien O, Hathcock J, Mackman N, Taubman MB. Plasma tissue factor may be predictive of venous thromboembolism in pancreatic cancer. J Thromb Haemost. 2008;6:1983–1985. doi: 10.1111/j.1538-7836.2008.03156.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kasthuri RS, Hisada Y, Ilich A, Key NS, Mackman N. Effect of chemotherapy and longitudinal analysis of circulating extracellular vesicle tissue factor activity in patients with pancreatic and colorectal cancer. Res Pract Thromb Haemost. 2020;4:636–643. doi: 10.1002/rth2.12317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee RD, Barcel DA, Williams JC, Wang JG, Boles JC, Manly DA, Key NS, Mackman N. Pre-analytical and analytical variables affecting the measurement of plasma-derived microparticle tissue factor activity. Thromb Res. 2012;129:80–85. doi: 10.1016/j.thromres.2011.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ansari SA, Keshava S, Pendurthi UR, Rao LVM. Oxidative stress product, 4-hydroxy-2-nonenal, induces the release of tissue factor-positive microvesicles from perivascular cells into circulation. Arterioscler Thromb Vasc Biol. 2021;41:250–265. doi: 10.1161/ATVBAHA.120.315187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rühl H, Berens C, Winterhagen A, Müller J, Oldenburg J, Pötzsch B. Label-free kinetic studies of hemostasis-related biomarkers including D-dimer using autologous serum transfusion. PLoS One. 2015;10:e0145012 doi: 10.1371/journal.pone.0145012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ollivier V, Wang J, Manly D, Machlus KR, Wolberg AS, Jandrot-Perrus M, Mackman N. Detection of endogenous tissue factor levels in plasma using the calibrated automated thrombogram assay. Thromb Res. 2010;125:90–96. doi: 10.1016/j.thromres.2009.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manly DA, Wang J, Glover SL, Kasthuri R, Liebman HA, Key NS, Mackman N. Increased microparticle tissue factor activity in cancer patients with venous thromboembolism. Thromb Res. 2010;125:511–512. doi: 10.1016/j.thromres.2009.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thaler J, Ay C, Mackman N, Bertina RM, Kaider A, Marosi C, Key NS, Barcel DA, Scheithauer W, Kornek G, et al. Microparticle-associated tissue factor activity, venous thromboembolism and mortality in pancreatic, gastric, colorectal and brain cancer patients. J Thromb Haemost. 2012;10:1363–1370. doi: 10.1111/j.1538-7836.2012.04754.x [DOI] [PubMed] [Google Scholar]
- 26.Bharthuar A, Khorana AA, Hutson A, Wang JG, Key NS, Mackman N, Iyer RV. Circulating microparticle tissue factor, thromboembolism and survival in pancreaticobiliary cancers. Thromb Res. 2013;132:180–184. doi: 10.1016/j.thromres.2013.06.026 [DOI] [PubMed] [Google Scholar]
- 27.Tesselaar ME, Romijn FP, Van Der Linden IK, Prins FA, Bertina RM, Osanto S. Microparticle-associated tissue factor activity: a link between cancer and thrombosis? J Thromb Haemost. 2007;5:520–527. doi: 10.1111/j.1538-7836.2007.02369.x [DOI] [PubMed] [Google Scholar]
- 28.Tesselaar ME, Romijn FP, van der Linden IK, Bertina RM, Osanto S. Microparticle-associated tissue factor activity in cancer patients with and without thrombosis. J Thromb Haemost. 2009;7:1421–1423. doi: 10.1111/j.1538-7836.2009.03504.x [DOI] [PubMed] [Google Scholar]
- 29.Hisada Y, Thålin C, Lundström S, Wallén H, Mackman N. Comparison of microvesicle tissue factor activity in non-cancer severely ill patients and cancer patients. Thromb Res. 2018;165:1–5. doi: 10.1016/j.thromres.2018.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


