Abstract
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has affected millions of people worldwide and the pandemic has yet to wane. Despite its associated significant morbidity and mortality, there are no definitive cures and no fully preventative measures to combat SARS-CoV-2. Hence, the urgency to identify the pathobiological mechanisms underlying increased risk for and the severity of SARS-CoV-2 infection is mounting. One contributing factor, the accumulation of damage-associated molecular pattern molecules, is a leading trigger for the activation of nuclear factor-kB and the IRF (interferon regulatory factors), such as IRF7. Activation of these pathways, particularly in the lung and other organs, such as the heart, contributes to a burst of cytokine release, which predisposes to significant tissue damage, loss of function, and mortality. The receptor for advanced glycation end products (RAGE) binds damage-associated molecular patterns is expressed in the lung and heart, and in priming organs, such as the blood vessels (in diabetes) and adipose tissue (in obesity), and transduces the pathological signals emitted by damage-associated molecular patterns. It is proposed that damage-associated molecular pattern-RAGE enrichment in these priming tissues, and in the lungs and heart during active infection, contributes to the widespread tissue damage induced by SARS-CoV-2. Accordingly, the RAGE axis might play seminal roles in and be a target for therapeutic intervention in SARS-CoV-2 infection.
Keywords: angiotensin-converting enzyme 2, COVID-19, endothelial cells, interferon regulatory factors, pandemics
Highlights.
Severe acute respiratory syndrome coronavirus 2 is associated with significant morbidity and mortality; yet, there are no definitive cures and no fully preventative measures to combat severe acute respiratory syndrome coronavirus 2.
Activation of damage-associated molecular pattern pathways in severe acute respiratory syndrome coronavirus 2, in the lung and other organs, contributes to a burst of cytokine release, which predisposes to significant tissue damage, loss of function, and mortality.
This Review puts for the proposal that damage-associated molecular pattern interaction with their central receptor, receptor for advanced glycation end products, contributes both to the increased vulnerability of obese/diabetic tissues to severity of severe acute respiratory syndrome coronavirus 2 and to the widespread tissue damage induced by this infection in the lung and other organs.
COVID-19: Properties and Cellular Interactions
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causing coronavirus disease 2019 (COVID-19) has affected >58 million persons worldwide with nearly 1.4 million deaths, as of late November, 2020.1 SARS-CoV-2 is an enveloped, nonsegmented positive-sense RNA virus that uses an enzyme RdRp (RNA-dependent RNA polymerase).2 Cellular infection is dependent on the binding of the SARS-Cov-2 spike protein (S protein) to ACE2 (angiotensin-converting enzyme 2) on the surface of cells; proteases such as the serine protease TMPRSS2 prime the S protein to facilitate entry into the host cells.3 ACE2 is expressed on a broad range of cells, such as lung alveolar epithelial cells (type I and type II), enterocytes, endothelial cells, smooth muscle cells, the basal cell layer of the epidermis, and proximal tubule cells in the kidney.4
Among the most prominent and best-described consequences of SARS-CoV-2 are those that affect the lung. In a multi-center study of lung tissue examination at autopsy from patients succumbing to SARS-CoV-2 infection in Italy and New York, frequent tracheobronchitis and significant pulmonary vascular involvement, with noted presence of large vessel thrombi, capillary microthrombi, endothelial swelling, and inflammation was observed.5
Beyond the lung alveolar epithelial cells, ACE2 is also expressed on such cells as cardiomyocytes and adipocytes.6,7 Evidence has linked complications of SARS-CoV-2 to the heart.8,9 At presentation of COVID-19 disease, elevated levels of cardiac troponin have been observed, which are indicators of cardiac involvement and harbingers of poor outcome.10 SARS-CoV-2 may directly infect human cardiomyocytes.11 Beyond the indirect effects to the heart consequent to the severe respiratory consequences12 and the yet-to-be-fully explained disorders of coagulation, exemplified in part by high levels of the D-dimer,13,14 extensive damage to the heart may ensue from direct infection through necrosis and myocarditis.9
In other cell types, the expression of ACE2 on adipocytes may link SARS-CoV-2 to obesity, as ACE2 expression in murine adipocytes was regulated by high-fat diet (HFD) feeding.6 Advanced age and elevation of body mass index have been linked to increased risk for SARS-CoV-2 severity15 and others reported that dysglycemia, such as in metabolic syndrome or diabetes (type 1 or type 2), also heightens risk for SARS-Cov-2 severity.16–18
Collectively, these considerations lead to a key question, are there common threads linking both the enhanced risk for and the severity of SARS-Cov-2 in human infection? Numerous nodes in the pulmonary-cardiac-vascular-immunometabolic SARS-Cov-2 network point to potential roles for the receptor for advanced glycation end products (RAGE) in COVID-19. In the sections to follow, evidence for a 2-part story for RAGE, both in enhanced risk for and increased severity of SARS-Cov-2 pathobiology will be presented.
RAGE—From Its Discovery in the Endothelium and Onwards
This story begins with a brief summary of RAGE. RAGE is a member of the immunoglobulin superfamily of cell surface receptors; it is composed of 5 principal domains. There are 3 extracellular domains, one immunoglobulin (Ig)-like Variable (V) domain followed by 2 Ig-like Constant (C)-type domains (C1 and C2); these are followed by a single transmembrane spanning domain and a short highly charged cytoplasmic domain that contributes to RAGE signaling.19 RAGE was discovered as a receptor for the advanced glycation end products (AGEs) based on its identification from bovine aortic endothelial cells.20 AGEs form and accumulate in diverse settings such as in diabetes, aging, oxidative stress, in highly processed foods, renal failure, inflammation, and obesity.21–25 Profiling of the expression of human RAGE in adult tissues revealed that RAGE was most highly expressed in the lung, particularly in the type I alveolar epithelial cells, and it has been suggested that RAGE is expressed in the type II alveolar epithelial cells and alveolar macrophages as well.26,27 In distinct settings of immunometabolic perturbation, RAGE expression is upregulated in organs such as the heart and coronary arteries, adipose tissue, liver, kidney and the brain, and in immune cells, such as monocytes, macrophages, T and B lymphocytes, and dendritic cells.24,28–31
These considerations and tissue expression patterns for RAGE suggested its involvement in a diverse group of disorders; this concept was supported by the discovery that RAGE was a multi-ligand receptor. Its ability to transduce the effects of multiple members of non-AGE ligand families, such as the S100/calgranulin family, HMGB1 (high-mobility group box 1), amyloid-beta peptide, lysophosphatidic acid, phosphatidylserine, C1q and Mac-1,32–38 underscored the pleiotropic nature of the ligand-RAGE interaction consequences. It was on account of this multi-ligand nature of RAGE and its association with chronic disease that led to its inclusion as a damage-associated molecular pattern (DAMP) receptor.39
Recent reports in COVID-19 have cemented putative links to RAGE and its ligands. First, levels of RAGE ligands S100A8, S100A9, S100A11, and EN-RAGE (S100A12) were highly expressed in lung and serum of fatal versus less severe cases of COVID-19.40 Second, levels of plasma S100A12 correlated with disease severity and increased bacterial products in patients with COVID-19.41 Third, serum S100B levels were associated with increased disease severity and COVID-19 score in affected patients.42 Fourth, in extracellular vesicles, significantly higher levels of S100A12 were observed in patients with severe versus moderate COVID-19.43 Fifth, higher levels of S100A8/A9 and a distinct DAMP RAGE ligand, HMGB1, were found to associate with higher risk of intensive care unit admission and death in patients with COVID-19.44 Sixth, HMGB1 was reported to induce NETosis (neutrophil extracellular traps)45 and to induce ACE2 expression, critical for SARS-CoV-2 entry into cells.46
Collectively, these considerations, particularly the prominent expression of DAMPs and RAGE in the lung and in alveolar pneumocytes, its role as a DAMP receptor, and their upregulation and activities in COVID-19 and in disorders of immunometabolism pinpoint DAMPs-RAGE as a perfect storm for contributions both to SARS-Cov-2 infection and to conditions that predispose to increased severity of COVID-19. In the sections to follow, evidence will be presented for the concept of the RAGE 2 part story in COVID-19, that is, direct RAGE roles in SARS-CoV-2 infection and in the underlying conditions that predispose to increased risk for COVID-19 severity.
Part 1: Direct Roles for RAGE in COVID-19 Infection
RAGE and the Lung—Activity in a Range of Lung Disorders, Especially Acute Respiratory Disease Syndrome
Evidence from human subjects and animal models has shown strong links between RAGE and lung disorders, such as allergic airway inflammation and asthma, pulmonary fibrosis, lung cancer, chronic obstructive pulmonary disease, acute lung injury, pneumonia, cystic fibrosis (CF), and bronchopulmonary dysplasia.47 In CF, studies from the French CF Gene Modifier Study fortified the association between RAGE and the severity of CF, as the AGER promoter variant, −429C, was reported to be associated with increased expression of RAGE and the potential for increased lung inflammation and lung disease.48 Roles for the RAGE ligand S100A12 have also been implicated in mucin overproduction by epithelial cells in CF in a pathway involving activation of NF-kB (nuclear factor-kappa B).49 Other studies linked the AGER polymorphism rs2070600T (Ser82) to lung function in smokers.50
In addition to genetic variants, distinct biomarkers for tracking the activity of the RAGE pathway include the measurement of soluble RAGEs (sRAGE). In addition to cell surface forms of RAGE that bind and transduce the effects of RAGE ligand signaling, soluble forms of RAGE have been identified. In the measurement of total plasma sRAGE in human subjects, ≈80% of the sRAGE is the result of cleavage of the cell surface receptor through the actions of ADAM10 and MMPs (matrix metalloproteinases); the remaining 20% of total sRAGE results from a splice variant of RAGE, called endogenous or esRAGE (endogenous secretory RAGE).51 Multiple studies have deployed measurements of sRAGE to gauge associations for the RAGE pathway in disorders in which its ligands accumulate and the response to therapeutic intervention.51 In this context, levels of plasma/serum and bronchoalveolar lavage fluid sRAGE have demonstrated strong associations with acute respiratory disease syndrome and other forms of lung disease.52,53
Is there evidence for roles for RAGE in acute lung injuries from animal model studies? Multiple studies have addressed this concept. For example, in an animal model of acute respiratory disease syndrome induced by intratracheal instillation of acid, mice were treated with an anti-RAGE monoclonal antibody or with sRAGE. The authors assessed lung injury by a number of functional, histological, and molecular mediators and reported that both anti-RAGE pathway therapeutics reduced lung injury and alveolar inflammation and improved arterial oxygenation.54 In another set of studies, cecal ligation and puncture was performed to induce severe polymicrobial sepsis with survival as an end point; mice devoid of Ager and wild-type mice treated with an anti-RAGE antibody displayed significantly higher survival and reduced inflammation and lung pathology versus the respective controls.55 In that same study, intravenous treatment with Listeria monocytogenes was also induced to mediate severe sepsis; mice devoid of Ager or treated with the anti-RAGE antibody demonstrated improved survival and less organ damage than the respective controls.55 In distinct work, in lung injury induced by World Trade Center particulate matter, exposure of mice to the particulate matter resulted in striking upregulation of inflammation and reduction of lung function; this was prevented in mice devoid of Ager.56 Of note, in that study, firefighters exposed during the aftermath of the World Trade Center disaster with the greatest risk for lung injury displayed high levels of circulating sRAGE, in parallel with high levels of CRP (C-reactive protein) and low levels of MMP-9.56
In summary, RAGE expression in the lung, particularly in alveolar pneumocytes and alveolar macrophages, together with the findings in animal models that deletion or antagonism of RAGE attenuates acute respiratory disease syndrome-like and lung injury pathologies due to distinct stimuli, such as prolific accumulation of DAMPs, may signal potential roles for the receptor in SARS-Cov-2 infection manifestations in the lung. In the cardiopulmonary sphere of dysfunction, the heart is also a direct and indirect target of this infection. Much evidence has shown that RAGE contributes to the cardiac complications of diabetes, ischemia/reperfusion, and viral infections and suggest that the study of this receptor pathway for SARS-Cov-2 impact in the heart is logical.
RAGE and the Heart—Mediator of COVID-19 Injury to the Heart
RAGE is expressed in the heart in cells such as cardiomyocytes, vascular cells and resident, and infiltrating immune cells.57,58 Global deletion of Ager or pharmacological antagonism of RAGE, either in the isolated perfused heart model (ex vivo) or in the in vivo infarction model (ligation of the left anterior descending coronary artery) imparted protection from the adverse consequences of diabetes or ischemia/reperfusion injury, at least in part through protection from aberrant JAK/STAT (Janus-associated kinase/signal transducer and activator of transcription) and GSK3 (glycogen synthase kinase 3) signaling.59,60 Experiments implicated roles for RAGE in endothelial cells or monocytes/macrophages in these processes.61 Furthermore, distinct work suggested roles for RAGE in inflammatory heart disease, such as that induced by viruses (such as Coxsackievirus B3) and by autoimmune stimuli.62–65
Hence, in the context of cardiopulmonary dysfunction, such as that which might be induced by these above stimuli or SARS-Cov-2, what might be the underlying RAGE-dependent mechanisms? It is known that dying lung cells (endogenous and infiltrated immune cells) and dying cardiac cells may release DAMPs, such as HMGBI and S100/calgranulins, some of which may bind to RAGE and activate highly inflammatory cascades, such as NF-kB,66 which may exacerbate the early phases of tissue damage. Beyond the DAMPs, recent work has linked both RAGE and SARS-Cov-2 to neutrophil extracellular traps (NETs), which will be considered in the section to follow.
DAMPs, RAGE, and SARS-Cov-2: Casting a Wide Net
Recent studies underscore the link between NETs and COVID-19 pathology; for example, it was shown that when compared with healthy control subjects, in 32 hospitalized patients with COVID-19, the concentration of NETs was increased in plasma, tracheal aspirates, and lung autopsy tissues.67 These NETs were derived from SARS-CoV-2-infected neutrophils; in in vitro studies, the NETs mediated lung epithelial cell death,67 which was proposed to be an important mediator of lung pathology in this disease.
How might these considerations relate to RAGE? A growing body of evidence links RAGE to NET formation and consequences. For example, NETs induce platelet aggregation through RAGE68; NET-derived HMGB1 induces macrophage pyroptosis through RAGE69; disulfide HMGB1 facilitates prothrombotic NET formation via RAGE70; HMGB1 facilitates NET formation in part via RAGE71; RAGE facilitates NET formation in pancreatic cancer72; and platelet-derived HMGB1 facilitates NET formation via RAGE.73 If and by what means RAGE biology intersects with that of SARS-CoV-2 in the context of NET formation and its prothrombotic and other consequences, especially in the lungs, remains to be tested.
In summary, there are multiple putative mechanisms by which RAGE might play key roles in the pathogenesis of lung-triggered infection, inflammation, and the consequent inflammatory burst that may trigger systemic complications and severe local tissue damage.
Beyond direct RAGE roles in the host response to SARS-CoV-2 infection, it is also plausible that the accumulation of DAMPs in chronic immunometabolic perturbations such as diabetes and obesity may contribute, in part through RAGE, to the enhanced risk and severity of COVID-19. In this part 2 of the putative RAGE story in COVID-19, the following sections will present the evidence supporting this premise.
Part 2: Roles for RAGE in Immunometabolic Disorders That Exacerbate COVID-19 Infection
Diabetes—The Cascade of Consequences Triggered by Elevated Blood Glucose in SARS-CoV-2 Infection
Studies have shown that diabetes renders patients with SARS-CoV-2 at greater risk of worse prognosis and death.74–78 While a myriad of mechanisms may underlie these findings, it is well-established that hyperglycemia perturbs fundamental homeostatic properties of blood vessels that cause increased oxidative stress, higher prothrombotic potential, increased inflammation, including upregulation of adhesion molecules and matrix metalloproteinases, and disruption of the glycocalyx, to name just a few.79–83 Some of these, in fact, have parallels in the pathobiology of SARS-CoV-2 infection. In the case of the RAGE axis, the first of the RAGE ligands to be discovered, the AGEs, are increased in diabetes, and their formation is a direct consequence of high levels of blood glucose. AGE-RAGE interaction in endothelial cells enhances vascular permeability, upregulates vascular cell adhesion molecule-1, and increases hypoxia-mediated upregulation of Egr1 (early growth response-1).20,84,85 Critically, beyond AGES, distinct DAMP RAGE ligands, such as HMGB1 and multiple members of the S100/calgranulin family, are also upregulated in the circulation and tissues in types 1 and 2 diabetes and often associate with the severity of disease and complications.86–92
In this context, extensive evidence has indicated that the ligand-RAGE pathway contributes to the pathogenesis of both microvascular and macrovascular complications of diabetes.93–95 In experiments using genetic and pharmacological approaches, modulation of RAGE signaling protects from many of the adverse complications of long-term diabetes.93–95 In the case of AGE-RAGE dynamics and the direct impact on the vasculature, when mice with targeted expression of dominant negative-RAGE in endothelial cells were bred into the atherosclerosis-prone Apoe-deficient background, decreased atherosclerosis, reduced endothelial inflammation and suppression of proatherogenic signal transduction was observed when compared with control Apoe null mice.84
In addition to direct vascular cell damage by AGE-RAGE interactions, especially in diabetes, the biology of RAGE has important links to the renin angiotensin system, which is dysregulated in diabetes.96 ACE2, the receptor for SARS-CoV-2, normally functions to convert Ang II (angiotensin II) into Ang (1–7), thereby opposing the inflammatory and vascular injury-provoking effects of Ang II.97,98 In diabetes, reductions in ACE2 favor unchecked actions of Ang II, which may potentiate diabetes-mediated injury.99,100 It has been suggested that such dysregulation of the renin angiotensin system in diabetes might contribute to poor outcome in SARS-CoV-2 infection.101
Previous research linked RAGE to the renin angiotensin system. For example, in spontaneously hypertensive rats, treatment with sRAGE reduced ACE activity, enhanced ACE2 expression, reduced oxidative and inflammatory stress, and limited activation of NF-kB in vascular tissues.102 Furthermore, others showed that activation of the AT1 receptor by Ang II transactivated the RAGE cytoplasmic domain, leading to proinflammatory effects.103 Collectively, these considerations suggest that components of the Ang II/ATI and ACE2 axis may be impacted by RAGE, especially in diabetes and, therefore, may contribute to perturbations upon SARS-CoV-2 infection.
Are cells beyond vascular cells affected by hyperglycemia, thereby modulating the impact of SARS-CoV-2 in the infected subject? Indeed, a recent study examined the effects of high levels of glucose on monocytes/macrophage properties in response to this infection. In that work, the authors used publicly available single-cell RNA sequencing data from BAL (bronchoalveolar lavage) fluid of patients with mild and severe COVID-19 and controls, and they identified that several genes associated with IFN (interferon) α/β signaling pathway were upregulated in patients with mild and severe COVID-19 versus controls; this was observed in all 6 clusters of monocytes.104 It was further shown that SARS-CoV-2 infects peripheral blood monocytes and enhances the expression of ACE2, thereby increasing SARS-CoV-2 infection. SARS-CoV-2-infected monocytes expressed higher levels of a range of proinflammatory factors, such as IFNα, β, and λ and higher levels of TNFα (tumor necrosis factor alpha), IL (interleukin) 1β, and IL6. Importantly, in environments characterized by high levels of glucose, sustained aerobic glycolysis in monocytes, through HIF-1α, promoted viral replication, cytokine production, and mediated the subsequent T-cell dysfunction and lung epithelial cell death observed in SARS-CoV-2-infected lung. These findings thus identify a direct molecular mechanism by which high levels of glucose modulate monocyte/macrophage metabolism, which imparts significant consequences on inflammation and cellular survival in COVID-19.
On account of the wide range of cellular targets in hyperglycemia, it is likely that multiple insights will continue to emerge regarding the modifying effects of high levels of glucose on the host response to SARS-CoV-2 infection. If and how such perturbations may relate to RAGE signaling remain to be tested.
RAGE and Obesity—Does Fat Harbor DAMPs That Predispose to Exaggerated Immune Responses in SARS-CoV-2 Infection?
Adipocytes express ACE2 and ongoing investigations are addressing the question of whether or not adipose tissue is a reservoir for SARS-CoV-2 and, if so, does this serve as a means to amplify systemic viral load?105,106 These considerations notwithstanding, it is established that obese adipose tissue may harbor DAMPs, such as AGEs, HMGB1, and S100/calgranulins.24,107,108 RAGE is expressed in human and murine adipose tissue, in adipocytes as well as in other cells such as immune cells.24,109,110 Mice bearing global deletion of Ager were subjected to HFD feeding; compared with wild-type mice fed HFD, mice with loss of Ager, although consuming equivalent amounts of food, were significantly protected from diet-induced obesity. In parallel, the HFD-fed mice devoid of Ager displayed improved glucose and insulin tolerance.109 The epididymal visceral adipose tissue (eWAT) of these mice fed the HFD revealed that the total CD11B+/F4/80+ macrophage content was reduced in the Ager null eWAT versus the wild-type eWAT. Furthermore, the population of CD11B+/F4/80+/CD11C+ cells in the eWAT, which are speculated to be more inflammatory, were also significantly lower in the Ager-deficient versus Ager-expressing eWAT.109 The mice devoid of Ager and fed the HFD displayed significantly higher energy expenditure than those mice expressing Ager despite no differences in food intake. The underlying RAGE-dependent mechanisms were traced to its expression in adipocytes.
Mice with adipocyte-specific deletion of Ager (in both white and brown adipocytes; using the Adipoq cre recombinase approach) were employed. AgerFlox/Flox Adipoq Cre+/wt (adipocyte-specific deletion of Ager) and their floxed controls (AgerFlox/Flox Adipoq Crewt/wt) were fed a HFD; compared with the floxed control mice, those mice with adipocyte-specific deletion of Ager displayed significant protection from diet-induced obesity and improvements in glucose and insulin tolerance.110 These mice displayed significantly higher energy expenditure despite no major differences in food intake or physical activity; their brown and subcutaneous white adipose tissues (iBAT [interscapular brown adipose tissue] and iWAT [inguinal white adipose tissue (subcutaneous)], respectively) displayed significantly higher expression of genes linked to thermogenesis such as Ucp.110 Further, the eWAT of the adipocyte Ager-deleted mice fed HFD experiments traced the mechanism to RAGE ligand-dependent downregulation of protein kinase A activities on lipolysis and regulation of thermogenic gene programs.110
These intriguing findings uncovered potential innate functions for RAGE in conservation of energy mechanisms; in the lean state, the quantity of DAMPs and pathological ligands in adipose tissue is low. However, in over-nutrition and obesity, the hoarding of energy in fat cells appears to correspond to plentiful accumulation of proinflammatory ligands. If and how the basal upregulation of RAGE ligands including the DAMPs in adipose tissue depots might raise the risk for severity of infection by viruses such as SARS-Cov-2 remains to be tested.
It is notable that obesity may not only portend adverse outcomes in SARS-Cov-2 but in other viral infections, as well. It was reported that during the influenza A subtype H1N1 pandemic, obesity was found to correlate with worse outcome and death compared with lean persons based on the stratification by body mass index.111
Hence, at least in a subset of viral infections, the increased adipose and immune cell inflammation may, by yet to be defined mechanisms, facilitate excessively exuberant host responses to discrete infections, thereby leading to adverse clinical outcomes. If and how DAMP accumulation, and potential roles in such mechanisms as trained immunity, may contribute to such vulnerability needs to be studied.
In summary, the 2 part hypothesized story for RAGE in both severity of and enhanced risk for more severe COVID-19 was recently buttressed by new insights into roles for RAGE in interferon biology, as presented in the section to follow.
A New Twist: RAGE Meets IRF7
An unexpected facet of RAGE biology was recently unearthed in studies probing mechanisms by which RAGE suppressed regression of diabetic atherosclerosis in a murine model.112 In parallel with accelerated regression of atherosclerosis in diabetic mice devoid of Ager, RNA sequencing studies revealed that Ager null macrophages retrieved from the regressing atherosclerotic plaques demonstrated significant reduction in the interferon signaling pathway, and, in particular, in expression of Irf7. In in vitro studies, in primary bone marrow derived macrophages, RAGE ligands or serum from Western diet-fed mice devoid of the low density lipoprotein receptor (Ldlr; 2%) upregulated Irf7 in a manner that was reduced by genetic deletion of Ager or by siRNA-targeted reduction of Ager in wild-type bone marrow derived macrophages. Furthermore, the plasma of diabetic Ager null mice undergoing regression of diabetic atherosclerosis revealed significantly lower levels of IFN-γ versus levels observed in the control wild-type diabetic mice.112
IRF7 (interferon regulatory factor 7) is considered a master regulator of the type 1 interferon response, as mice devoid of Irf7 display severe vulnerability to viral infections and reduction in IFNα/β.113 IRF7 is also expressed on nonimmune cells, such as smooth muscle cells.114,115 In the context of SARS-CoV-2, ongoing research is revealing that the biology of interferon and type 1 IFN immunity is complex. It has been suggested that patients with severe and life-threatening COVID-19 display inborn errors of type 1 IFN immunity.116 An enrichment of rare variants predicted to cause loss of function at 13 human loci known to govern TLR3 (toll-like receptor 3) and IRF7-dependent type 1 IFN immunity to influenza virus was noted in 659 patients with life-threatening severe COVID-19 pneumonia when compared with 534 subjects with very mild or asymptomatic SARS-CoV-2 infection. In vitro, human fibroblasts bearing these mutations displayed increased vulnerability to SARS-CoV-2 infection.116 In other studies, it was shown that 101 of 987 patients with life-threatening COVID-19 displayed neutralizing antibodies against IFN-ω (and in some cases IFN-α) or both. It was reported that these auto-antibodies neutralized the ability of the relevant IFNs to block SARS-CoV-2 infection in vitro.117 Collectively, these findings suggest beneficial and critical roles for IFNs in the response to SARS-CoV-2; the situation, however, may be more complex and requires further investigation.
Recent evidence suggests that there may be distinct time-dependent differences in the roles of IFNs in SARS-CoV-2 infection. It is possible that whereas early rises in IFNs may be protective, later stage rises in IFNs may actually cause hyperinflammatory responses in part via the accumulation of monocytes and macrophages in the lung.118,119 Hence, despite the success of a number of recent IFN-β trials, it was noted that care needed to be taken to consider issues of timing and patients subgroups.119 Insights into this complexity emerged from studies in mouse models.
Channappanavar et al120 showed that delayed IFN1 signaling in SARS-CoV-infected mice resulted in the pathological accumulation of inflammatory monocytes/macrophages and consequent elevation of lung cytokine levels, vascular leakage and impaired virus-specific T-cell responses. In mice expressing human ACE2 (adenovirus), these authors showed that whereas IFNs did not mediate major changes in viral replication, they did cause significant pathological activation of immune cells.121 The authors, using wild-type mice and mice devoid of the IFN-α receptor (Ifnar−/−) or devoid of both Irf3/Irf7, suggested that type 1 IFNs may be important drivers of pathological sequelae in SARS-CoV-2 infection. Perhaps, it is all about the timing, the distinct cellular milieus, and the potential priming effects of preexisting and comorbid conditions associated with increased severity of COVID-19. It will be essential to dissect these potential time-dependent effects of IFNs in COVID-19 to maximize the possible benefits of IFN treatment for this disease.
These concepts lead to the premise that if, how, and in what settings the DAMP receptor RAGE might modulate the timing of IFN responses, perhaps through IRF7, remains to be tested. In this context, studies have tested roles for RAGE in viral infections and revealed complex relationships. In influenza A infection, deletion of Ager resulted in improved survival, improved viral clearance, and enhanced T-cell responses and neutrophil activation.122 In Newcastle disease virus infection, HMGB1-RAGE interaction increased cytokine production in cellular models.123 In cellular models of the blood brain barrier, RAGE and CC7 enhanced the transmigration of Zika-infected monocytes through the barrier.124 In cellular models, RAGE, along with TLRs 2 and 4, promoted cytokine production induced by porcine reproductive and respiratory syndrome virus.125 Mice devoid of Ager or mice treated with sRAGE were protected from respiratory syncytial virus A2 strain-induced weight loss and inflammation126). In a distinct study, mice devoid of Ager displayed impaired antiviral immunity when infected with pneumonia virus of mice strain J3666, which is a murine analogue of RSV (respiratory syncytial virus).127 The reasons for these divergent RAGE-dependent responses were not elucidated from those studies.
However, antiviral responses are also mediated through distinct DAMP receptors, the TLRs. In the section to follow, the interplay between RAGE and TLRs in the context of COVID-19 is considered.
RAGE and TLR in SARS-CoV-2: Partners or Detours for RAGE From the Toll Road?
The discovery that RAGE ligands upregulated Irf7 in bone marrow derived macrophages raises the key issue of possible links between RAGE and the TLRs in the response to SARS-CoV-2 infection. Infection of the human lung epithelial cell line, A549, with SARS-CoV-2 upregulated a number of pathways; by gene ontology analysis, upregulation of the TLR pathway was observed.128 Among the genes identified relevant to this pathway were Irf7, Ccl5, Stat1, Cxcl8, Tlr8, and Fos. TLRs transmit the biological signals emitted by pathogen-associated molecular patterns, such as those relevant to viral infections.129 Although much work needs to be done to dissect the potential specific contributions of the multiple members of the TLR family, it is speculated that TLR7 may be key to driving the effects of single stranded RNA, relevant to SARS-CoV-2, which leads to activation of the type I IFN response.130 In in vitro studies, it was suggested that incubation of human lung macrophages with SARS-CoV-2 triggered TLR4-mediated cytokine release.131 Full elucidation of the beneficial versus antagonist roles for specific TLRs may lead to novel therapeutic opportunities for COVID-19.
In this context, as discussed above, RAGE also activates NF-kB and plays roles in macrophages in regulation of IRF7. It was previously shown that RAGE may bind to DNA.132 In other studies, it was reported that RAGE bound RNA and that RAGE enhanced cellular RNA uptake into endosomes.133 With respect to intersection with the TLRs, it was also shown that RAGE increased the sensitivity of the single strand RNA sensing TLRs, such as TLR7, 8, and 13.133 Atop these considerations that both RAGE and TLRs may bind DNA and RNA, additional molecular bridges between these 2 pathways ensue from the ability of both RAGE and TLRs to bind HMGB1.134
Hence, it is likely that there may be relationships between RAGE and TLRs in the lung and other tissues such as the heart, upon infection and contact with SARS-CoV-2. It is important to point out that nature and evolutionary forces may have had divergent expectations and plans for the functions of TLRs versus RAGE, or, perhaps RAGE evolved to complement and fortify at least some of the functions of the TLRs. On the one hand, the TLRs may be traced to orthologs in Drosphila.135 In contrast, AGER, located on chromosome 6 in the MHC (major histocompatibility complex) III humans,136 first appeared in Laurasiatheria.137 Laurasiatheria is a superorder of placental mammals that is part of the larger group of mammals classified as Eutheria, which are mammalian clades that date to 160 million years ago.138 In the Eutherians, a key property is the expression of UCP1 (uncoupling protein 1), which is linked to nonshivering thermogenesis.139
Do the distinct origins of the TLR and RAGE networks have implications for responses to viral infections? At this time, in SARS-CoV-2 infection, it is clear that much work needs to be done to identify if interactions between RAGE and the TLRs exist and the timing of their involvement in priming and active infection in COVID-19. Studies in animal models are likely to shed light on the nature of the host responses that orchestrate the (patho)biological response to SARS-CoV-2 infection. This will be considered in the section to follow.
Animal Models of SARS-CoV-2 Infection and Consequence: the Pros and Cons
Multiple species of animals have been considered for the study of SARS-CoV-2 infection; in naturally vulnerable species such as hamsters, ferrets, cats, and nonhuman primates, disease associated with this infection is often mild and not progressive but, in some species, age-related increases in severity have been observed.140 Efforts have been made to study SARS-CoV-2 infection in mice models on account of the more ready availability of mouse models for testing roles for specific genes. As the murine ACE2 may have reduced affinity for the SARS-CoV-2 Spike protein, humanized models of the ACE2 have been generated to overcome this limitation.141–143 Examples include clustered regularly interspaced short palindromic repeats/clustered regularly interspaced short palindromic repeat (CRISPR-Cas)-mediated knock-in of the hACE2 (human angiotensin-1 converting enzyme 2)141; expression of hACE2 under control of the murine Ace2 promoter142; and K18 transgenic hACE2 mouse, in which hACE2 is under control of the cytokeratin-18 gene promoter.143 In each case, there are pros and cons; for example, in the K18-hACE2 mouse, although SARS-CoV-2-infected animals display weight loss, severe pulmonary involvement and mortalities, the expression of hACE2 is artificially driven and not under the natural control mechanisms for the gene.143
These considerations notwithstanding, such murine models, may facilitate understanding of the mediators of injury directly upon COVID-19 infection and may aid in uncovering how underlying conditions such as obesity or diabetes may exacerbate the impact of SARS-CoV-2 infection in the host. Such knowledge may then lead to targets amenable to therapeutic testing. In this context, based on the hypothesized part 1 (direct infection) and part 2 (conditions amplifying COVID-19 severity) roles for RAGE, the potential benefits of antagonism of RAGE may be probed. In the section to follow, means to antagonize RAGE will be considered.
Identification of Potential Therapeutic Strategies Targeting RAGE
Multiple efforts have been made to target RAGE using small molecules antagonists of the extracellular domains, antibodies, aptamers, and sRAGEs, as examples.51,144–146 However, the extracellular domains of RAGE are complex; they are composed of 3 extracellular Ig-like domains that bind the ligands of RAGE at distinct sites within the V and C1 and C2 domains.147,148 Accordingly, it is possible that blockade of RAGE at discrete sites on these extracellular domains may fail to capture the pathobiology of all of its relevant ligands. Toward this end, targeting RAGE through blockade of its cellular signaling pathways has been proposed as a more comprehensive strategy.
The cytoplasmic domain or tail of RAGE, although essential for RAGE signaling, requires its interaction with adapter molecules to transduce the consequences of RAGE ligand extracellular binding and to initiate signaling cascades. In this context, the cytoplasmic domain or tail of RAGE binds to the FH1 (formin homology 1) domain of DIAPH1 (Diaphanous 1) and DIAPH1 is important for RAGE-mediated signaling.149 The specific amino acids in cytoplasmic domain or tail of RAGE required for this interaction are R5Q6; upon their mutation to alanine residues, NMR-based spectroscopic evidence of their interaction is attenuated and in smooth muscle cells bearing the A5/A6 mutant, RAGE ligand-triggered signal transduction (activation of AKT [protein kinase B]) was attenuated versus that observed in the R5/Q6 wild-type construct.150
This interaction site provided a potential opportunity for the development of small molecule antagonists; screening of a >59 000 compound small molecule library resulted in the identification of 13 small molecules that blocked cytoplasmic domain or tail of RAGE-DIAPH1 interaction.151 In cellular systems and in vivo, after infusion of RAGE ligands into wild-type mice, administration of small molecule antagonists suppressed inflammation in liver and kidney tissue compared with mice receiving vehicle.151
The observation that the RAGE-DIAPH1 axis represents a key signal transduction scaffold that is amenable to therapeutic interruption, unveils an entirely new set of hypotheses regarding potential roles for this signaling axis, RAGE-DIAPH1, in vascular and immune cell biology. To date, multiple studies in mice devoid of Diaph1 reveal striking similarities to the findings observed in Ager null mice, thereby linking these 2 pathways in biological systems beyond NMR spectroscopy and cell culture to rigorous hypothesis-testing in vivo.152–154
In summary, given the availability of a range of animal model species of SARS-CoV-2 infection, the targeting of extracellular or intracellular RAGE in SARS-CoV-2 infection is feasible.
Summary and Perspectives
Research has advanced knowledge of RAGE, from its earliest identified high level of basal expression in the lung, to its role as a conduit for transducing the effects of AGEs and other DAMPs, to one whose intracellular domain engages a formin, DIAPH1, thereby connecting RAGE to signaling scaffolds mediating biological and pathobiological functions through the actin cytoskeleton, Rho GTPase signaling and regulation of SRF (serum response factor)-dependent genes—each key pieces in vascular and immune cell perturbations (Figure).155–157 Atop these observations, the recent discovery of RAGE ligand-dependent regulation of IRF7 in macrophages raises new and unanticipated possibilities—is RAGE involved in the host response to viral infections; does DIAPH1 participate; does RAGE-dependent activation of NF-kB and IRF7 contribute to the cytokine barrage after SARS-CoV-2 infection; might antagonism of RAGE/DIAPH attenuate the aggressive inflammatory barrage triggered at least in some patients infected with COVID-19?
Figure.
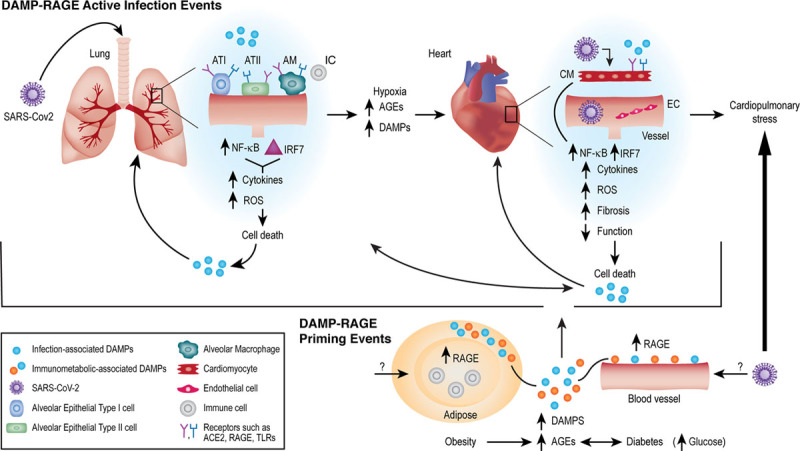
Receptor for advanced glycation end products (RAGE) and the many roads to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Recent studies from SARS-CoV-2-infected human subjects have demonstrated that elevation of damage-associated molecular patterns (DAMP) ligands of RAGE, such as HMGB1, S100A8, S100A9, S100A11, S100A12, and S100B in the lung tissue, and plasma/serum are associated with disease severity and risk of death. Hence, in active infection, in part I of the hypothesized RAGE response in acute infection (top), these DAMP ligands of RAGE may exacerbate the local responses to infection in organs such as the lung and heart, leading to severe cell stress/death. The known sequelae of RAGE activities, in part through the actions of NF-kB (nuclear factor-kappa B), lead to endothelial dysfunction (permeability, prothrombotic and proinflammatory state); immune cell activation, oxidative stress, and upregulation of distinct factors such as EGR1 (early growth response 1), which coordinates much of the adverse responses to hypoxia and ischemia. The recent observation that RAGE ligands upregulate IRF7 (interferon regulatory factor 7) in immune cells suggests that DAMP RAGE ligands might aggravate disease in SARS-CoV-2 infection. Within the sphere of RAGE biology, many of these same DAMP ligands also accumulate in chronic inflammatory and metabolic disorders. In part 2 of the hypothesized RAGE priming mechanisms in diabetes/hyperglycemia and obesity (bottom), the inexorable accumulation of advanced glycation end products (AGEs) and other DAMP RAGE ligands relevant to cardiometabolic perturbation may prime the organs for amplification of inflammatory and tissue-damaging mechanisms upon SARS-CoV-2 infection. Hence, blockade of RAGE, during immunometabolic priming in diabetes/obesity, or during active SARS-CoV-2 infection, might be efficacious in tempering the damage from acute infection and in preventing diabetes/obesity-mediated amplification of coronavirus disease 2019 (COVID-19) severity. These hypotheses are open questions amenable to investigation. EC indicates endothelial cell; and TLR, toll-like receptor.
Finally, as clinical studies have identified that disorders of metabolism (diabetes and obesity) amplify the risk for severe manifestations of COVID-19, is it plausible that the increased production and accumulation of DAMPs in metabolic and vascular tissues, and through their interactions with RAGE, raise basal signaling and inflammatory stress via circulating immune cell-host cell communications, thereby priming the tissues throughout the diabetic or obese host, thus amplifying the response to SARS-Cov-2 infection? Undoubtedly, optimal prevention and control of SARS-CoV-2 infection will require a multi-pronged approach. If and to what extent RAGE might be a key component within the COVID-19 armamentarium of therapeutic strategies is under investigation as the journey continues.
Acknowledgments
We are grateful to Latoya Woods and Renee Lucas for their assistance in the preparation of this article.
Sources of Funding
A.M. Schmidt and R. Ramasamy are supported, in part, by funds from the Diabetes Research Program, NYU Grossman School of Medicine and grants from the US Public Health Service P01HL143697, R01HL132516, R01DK109675 (to A.M. Schmidt and R. Ramasamy), and P01HL131481 (A.M. Schmidt and R. Ramasamy, Fisher, E (PI) and American Heart Association (17SFRN33520045). D. Roy was supported by a training grant for medical students (T35 DK007421, R. Ramasamy [PI]).
Disclosures
A.M. Schmidt and R. Ramasamy hold patents and patent applications related to antagonism of receptor for advanced glycation end products. The other author reports no conflict.
Nonstandard Abbreviations and Acronyms
- ACE2
- angiotensin-converting enzyme 2
- AGEs
- advanced glycation end products
- Ang II
- angiotensin II
- CF
- cystic fibrosis
- COVID-19
- coronavirus disease 2019
- CRP
- C-reactive protein
- DAMP
- damage-associated molecular patterns
- DIAPH1
- diaphanous 1
- eWAT
- epididymal visceral adipose tissue
- FH1
- formin homology 1
- HFD
- high-fat diet
- HMGB1
- high-mobility group box 1
- IFN
- interferon
- IRF
- interferon regulatory factor
- MHC
- major histocompatibility complex
- MMPs
- matrix metalloproteinases
- NET
- neutrophil extracellular trap
- RAGE
- receptor for advanced glycation end products
- RdRP
- RNA-dependent RNA polymerase
- SARS-CoV-2
- severe acute respiratory syndrome coronavirus 2
- sRAGE
- soluble RAGE
- TLR
- toll-like receptor
This article was sent to William C. Sessa, PhD, Consulting Editor, for review by expert referees, editorial decision, and final disposition.
For Sources of Funding and Disclosures, see page 623.
Contributor Information
Divya Roy, Email: droy@nyit.edu.
Ravichandran Ramasamy, Email: ravichandran.ramasamy@nyumc.org.
References
- 1.Johns Hopkins University School of Medicine. Coronavirus Resource Center. 2020. https://coronavirus.jhu.edu/map.html. November 21, 2020.
- 2.Gaurav A, Al-Nema M. Gupta SP, ed. Chapter 10 - polymerases of coronaviruses: structure, function, and inhibitors. In: Viral Polymerases. 2019. Academic Press; 271–300. [Google Scholar]
- 3.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borczuk AC, Salvatore SP, Seshan SV, Patel SS, Bussel JB, Mostyka M, Elsoukkary S, He B, Del Vecchio C, Fortarezza F, et al. Covid-19 pulmonary pathology: a multi-institutional autopsy cohort from italy and new york city. Mod Pathol. 2020;33:2156–2168. doi: 10.1038/s41379-020-00661-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupte M, Boustany-Kari CM, Bharadwaj K, Police S, Thatcher S, Gong MC, English VL, Cassis LA. ACE2 is expressed in mouse adipocytes and regulated by a high-fat diet. Am J Physiol Regul Integr Comp Physiol. 2008;295:R781–R788. doi: 10.1152/ajpregu.00183.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicin L, Abplanalp WT, Mellentin H, Kattih B, Tombor L, John D, Schmitto JD, Heineke J, Emrich F, Arsalan M, et al. Cell type-specific expression of the putative SARS-CoV-2 receptor ACE2 in human hearts. Eur Heart J. 2020;41:1804–1806. doi: 10.1093/eurheartj/ehaa311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kadosh BS, Garshick MS, Gaztanaga J, Moore KJ, Newman JD, Pillinger M, Ramasamy R, Reynolds HR, Shah B, Hochman J, et al. COVID-19 and the heart and vasculature: novel approaches to reduce virus-induced inflammation in patients with cardiovascular disease. Arterioscler Thromb Vasc Biol. 2020;40:2045–2053. doi: 10.1161/ATVBAHA.120.314513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Topol EJ. COVID-19 can affect the heart. Science. 2020;370:408–409. doi: 10.1126/science.abe2813 [DOI] [PubMed] [Google Scholar]
- 10.Tersalvi G, Vicenzi M, Calabretta D, Biasco L, Pedrazzini G, Winterton D. Elevated troponin in patients with coronavirus disease 2019: possible mechanisms. J Card Fail. 2020;26:470–475. doi: 10.1016/j.cardfail.2020.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bojkova D, Wagner JUG, Shumliakivska M, Aslan GS, Saleem U, Hansen A, Luxán G, Günther S, Pham MD, Krishnan J, et al. SARS-CoV-2 infects and induces cytotoxic effects in human cardiomyocytes. Cardiovasc Res. 2020;116:2207–2215. doi: 10.1093/cvr/cvaa267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borges do Nascimento IJ, von Groote TC, O’Mathúna DP, Abdulazeem HM, Henderson C, Jayarajah U, Weerasekara I, Poklepovic Pericic T, Klapproth HEG, Puljak L, et al. Clinical, laboratory and radiological characteristics and outcomes of novel coronavirus (SARS-COV-2) infection in humans: a systematic review and series of meta-analyses. PLoS One. 2020;15:e0239235 doi: 10.1371/journal.pone.0239235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mackman N, Antoniak S, Wolberg AS, Kasthuri R, Key NS. Coagulation abnormalities and thrombosis in patients infected with SARS-CoV-2 and other pandemic viruses. Arterioscler Thromb Vasc Biol. 2020;40:2033–2044. doi: 10.1161/ATVBAHA.120.314514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berger JS, Kunichoff D, Adhikari S, Ahuja T, Amoroso N, Aphinyanaphongs Y, Cao M, Goldenberg R, Hindenburg A, Horowitz J, et al. Prevalence and outcomes of D-dimer elevation in hospitalized patients with COVID-19. Arterioscler Thromb Vasc Biol. 2020;40:2539–2547. doi: 10.1161/ATVBAHA.120.314872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petrilli CM, Jones SA, Yang J, Rajagopalan H, O’Donnell L, Chernyak Y, Tobin KA, Cerfolio RJ, Francois F, Horwitz LI. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966 doi: 10.1136/bmj.m1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rao S, Lau A, So HC. Exploring diseases/traits and blood proteins causally related to expression of ACE2, the putative receptor of SARS-CoV-2: a mendelian randomization analysis highlights tentative relevance of diabetes-related traits. Diabetes Care. 2020;43:1416–1426. doi: 10.2337/dc20-0643 [DOI] [PubMed] [Google Scholar]
- 17.Hayden MR. Endothelial activation and dysfunction in metabolic syndrome, type 2 diabetes and coronavirus disease 2019. J Int Med Res. 2020;48:300060520939746 doi: 10.1177/0300060520939746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coppelli A, Giannarelli R, Aragona M, Penno G, Falcone M, Tiseo G, Ghiadoni L, Barbieri G, Monzani F, Virdis A, et al. ; Pisa COVID-19 Study Group. Hyperglycemia at hospital admission is associated with severity of the prognosis in patients hospitalized for COVID-19: the pisa COVID-19 study. Diabetes Care. 2020;43:2345–2348. doi: 10.2337/dc20-1380 [DOI] [PubMed] [Google Scholar]
- 19.Yan SF, Ramasamy R, Schmidt AM. Soluble RAGE: therapy and biomarker in unraveling the RAGE axis in chronic disease and aging. Biochem Pharmacol. 2010;79:1379–1386. doi: 10.1016/j.bcp.2010.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wautier JL, Schmidt AM. Protein glycation: a firm link to endothelial cell dysfunction. Circ Res. 2004;95:233–238. doi: 10.1161/01.RES.0000137876.28454.64 [DOI] [PubMed] [Google Scholar]
- 21.Uribarri J, del Castillo MD, de la Maza MP, Filip R, Gugliucci A, Luevano-Contreras C, Macías-Cervantes MH, Markowicz Bastos DH, Medrano A, Menini T, et al. Dietary advanced glycation end products and their role in health and disease. Adv Nutr. 2015;6:461–473. doi: 10.3945/an.115.008433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henning C, Glomb MA. Pathways of the Maillard reaction under physiological conditions. Glycoconj J. 2016;33:499–512. doi: 10.1007/s10719-016-9694-y [DOI] [PubMed] [Google Scholar]
- 23.Maessen DE, Stehouwer CD, Schalkwijk CG. The role of methylglyoxal and the glyoxalase system in diabetes and other age-related diseases. Clin Sci (Lond). 2015;128:839–861. doi: 10.1042/CS20140683 [DOI] [PubMed] [Google Scholar]
- 24.Gaens KH, Goossens GH, Niessen PM, van Greevenbroek MM, van der Kallen CJ, Niessen HW, Rensen SS, Buurman WA, Greve JW, Blaak EE, et al. Nε-(carboxymethyl)lysine-receptor for advanced glycation end product axis is a key modulator of obesity-induced dysregulation of adipokine expression and insulin resistance. Arterioscler Thromb Vasc Biol. 2014;34:1199–1208. doi: 10.1161/ATVBAHA.113.302281 [DOI] [PubMed] [Google Scholar]
- 25.Anderson MM, Requena JR, Crowley JR, Thorpe SR, Heinecke JW. The myeloperoxidase system of human phagocytes generates Nepsilon-(carboxymethyl)lysine on proteins: a mechanism for producing advanced glycation end products at sites of inflammation. J Clin Invest. 1999;104:103–113. doi: 10.1172/JCI3042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fehrenbach H, Kasper M, Tschernig T, Shearman MS, Schuh D, Müller M. Receptor for advanced glycation endproducts (RAGE) exhibits highly differential cellular and subcellular localisation in rat and human lung. Cell Mol Biol (Noisy-le-grand). 1998;44:1147–1157. [PubMed] [Google Scholar]
- 27.Katsuoka F, Kawakami Y, Arai T, Imuta H, Fujiwara M, Kanma H, Yamashita K. Type II alveolar epithelial cells in lung express receptor for advanced glycation end products (RAGE) gene. Biochem Biophys Res Commun. 1997;238:512–516. doi: 10.1006/bbrc.1997.7263 [DOI] [PubMed] [Google Scholar]
- 28.Burke AP, Kolodgie FD, Zieske A, Fowler DR, Weber DK, Varghese PJ, Farb A, Virmani R. Morphologic findings of coronary atherosclerotic plaques in diabetics: a postmortem study. Arterioscler Thromb Vasc Biol. 2004;24:1266–1271. doi: 10.1161/01.ATV.0000131783.74034.97 [DOI] [PubMed] [Google Scholar]
- 29.Gaens KH, Niessen PM, Rensen SS, Buurman WA, Greve JW, Driessen A, Wolfs MG, Hofker MH, Bloemen JG, Dejong CH, et al. Endogenous formation of Nε-(carboxymethyl)lysine is increased in fatty livers and induces inflammatory markers in an in vitro model of hepatic steatosis. J Hepatol. 2012;56:647–655. doi: 10.1016/j.jhep.2011.07.028 [DOI] [PubMed] [Google Scholar]
- 30.Prasad K. AGE-RAGE stress: a changing landscape in pathology and treatment of Alzheimer’s disease. Mol Cell Biochem. 2019;459:95–112. doi: 10.1007/s11010-019-03553-4 [DOI] [PubMed] [Google Scholar]
- 31.Tanji N, Markowitz GS, Fu C, Kislinger T, Taguchi A, Pischetsrieder M, Stern D, Schmidt AM, D’Agati VD. Expression of advanced glycation end products and their cellular receptor RAGE in diabetic nephropathy and nondiabetic renal disease. J Am Soc Nephrol. 2000;11:1656–1666. [DOI] [PubMed] [Google Scholar]
- 32.Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, Avila C, Kambham N, Bierhaus A, Nawroth P, et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. doi: 10.1016/s0092-8674(00)80801-6 [DOI] [PubMed] [Google Scholar]
- 33.Hori O, Brett J, Slattery T, Cao R, Zhang J, Chen JX, Nagashima M, Lundh ER, Vijay S, Nitecki D. The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and co-expression of rage and amphoterin in the developing nervous system. J Biol Chem. 1995;270:25752–25761. doi: 10.1074/jbc.270.43.25752 [DOI] [PubMed] [Google Scholar]
- 34.Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, Slattery T, Zhao L, Nagashima M, Morser J, et al. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer’s disease. Nature. 1996;382:685–691. doi: 10.1038/382685a0 [DOI] [PubMed] [Google Scholar]
- 35.He M, Kubo H, Morimoto K, Fujino N, Suzuki T, Takahasi T, Yamada M, Yamaya M, Maekawa T, Yamamoto Y, et al. Receptor for advanced glycation end products binds to phosphatidylserine and assists in the clearance of apoptotic cells. EMBO Rep. 2011;12:358–364. doi: 10.1038/embor.2011.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rai V, Touré F, Chitayat S, Pei R, Song F, Li Q, Zhang J, Rosario R, Ramasamy R, Chazin WJ, et al. Lysophosphatidic acid targets vascular and oncogenic pathways via RAGE signaling. J Exp Med. 2012;209:2339–2350. doi: 10.1084/jem.20120873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma W, Rai V, Hudson BI, Song F, Schmidt AM, Barile GR. RAGE binds C1q and enhances C1q-mediated phagocytosis. Cell Immunol. 2012;274:72–82. doi: 10.1016/j.cellimm.2012.02.001 [DOI] [PubMed] [Google Scholar]
- 38.Orlova VV, Choi EY, Xie C, Chavakis E, Bierhaus A, Ihanus E, Ballantyne CM, Gahmberg CG, Bianchi ME, Nawroth PP, et al. A novel pathway of HMGB1-mediated inflammatory cell recruitment that requires Mac-1-integrin. EMBO J. 2007;26:1129–1139. doi: 10.1038/sj.emboj.7601552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rubartelli A, Lotze MT. Inside, outside, upside down: damage-associated molecular-pattern molecules (DAMPs) and redox. Trends Immunol. 2007;28:429–436. doi: 10.1016/j.it.2007.08.004 [DOI] [PubMed] [Google Scholar]
- 40.Wu M, Chen Y, Xia H, Wang C, Tan CY, Cai X, Liu Y, Ji F, Xiong P, Liu R, et al. Transcriptional and proteomic insights into the host response in fatal COVID-19 cases. Proc Natl Acad Sci U S A. 2020;117:28336–28343. doi: 10.1073/pnas.2018030117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arunachalam PS, Wimmers F, Mok CKP, Perera RAPM, Scott M, Hagan T, Sigal N, Feng Y, Bristow L, Tak-Yin Tsang O, et al. Systems biological assessment of immunity to mild versus severe COVID-19 infection in humans. Science. 2020;369:1210–1220. doi: 10.1126/science.abc6261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aceti A, Margarucci LM, Scaramucci E, Orsini M, Salerno G, Di Sante G, Gianfranceschi G, Di Liddo R, Valeriani F, Ria F, et al. Serum S100B protein as a marker of severity in Covid-19 patients. Sci Rep. 2020;10:18665 doi: 10.1038/s41598-020-75618-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krishnamachary B, Cook C, Spikes L, Chalise P, Dhillon NK.The potential role of extracellular vesicles in covid-19 associated endothelial injury and pro-inflammation. 2020. medRxiv. doi: 10.1101/2020.08.27.20182808. [DOI] [PMC free article] [PubMed]
- 44.Chen L, Long X, Xu Q, Tan J, Wang G, Cao Y, Wei J, Luo H, Zhu H, Huang L, et al. Elevated serum levels of S100A8/A9 and HMGB1 at hospital admission are correlated with inferior clinical outcomes in COVID-19 patients. Cell Mol Immunol. 2020;17:992–994. doi: 10.1038/s41423-020-0492-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cicco S, Cicco G, Racanelli V, Vacca A. Neutrophil extracellular traps (NETs) and damage-associated molecular patterns (DAMPs): two potential targets for COVID-19 treatment. Mediators Inflamm. 2020;2020:7527953 doi: 10.1155/2020/7527953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wei J, Alfajaro MM, DeWeirdt PC, Hanna RE, Lu-Culligan WJ, Cai WL, Strine MS, Zhang SM, Graziano VR, Schmitz CO, et al. Genome-wide crispr screens reveal host factors critical for SARS-COV-2 infection [published online October 20, 2020]. Cell. doi: 10.1016/j.cell.2020.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oczypok EA, Perkins TN, Oury TD. All the “RAGE” in lung disease: The receptor for advanced glycation endproducts (RAGE) is a major mediator of pulmonary inflammatory responses. Paediatr Respir Rev. 2017;23:40–49. doi: 10.1016/j.prrv.2017.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beucher J, Boëlle PY, Busson PF, Muselet-Charlier C, Clement A, Corvol H; French C F Modifier Gene Study Investigators. AGER -429T/C is associated with an increased lung disease severity in cystic fibrosis. PLoS One. 2012;7:e41913 doi: 10.1371/journal.pone.0041913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kang JH, Hwang SM, Chung IY. S100A8, S100A9 and S100A12 activate airway epithelial cells to produce MUC5AC via extracellular signal-regulated kinase and nuclear factor-κB pathways. Immunology. 2015;144:79–90. doi: 10.1111/imm.12352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller S, Henry AP, Hodge E, Kheirallah AK, Billington CK, Rimington TL, Bhaker SK, Obeidat M, Melén E, Merid SK, et al. The Ser82 RAGE variant affects lung function and serum RAGE in smokers and sRAGE production in vitro. PLoS One. 2016;11:e0164041 doi: 10.1371/journal.pone.0164041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmidt AM. Soluble RAGEs - prospects for treating & tracking metabolic and inflammatory disease. Vascul Pharmacol. 2015;72:1–8. doi: 10.1016/j.vph.2015.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yerkovich ST, Chang AB, Carroll ML, Petsky HL, Scrivener G, Upham JW. Soluble receptor for advanced glycation end products (sRAGE) is present at high concentrations in the lungs of children and varies with age and the pattern of lung inflammation. Respirology. 2012;17:841–846. doi: 10.1111/j.1440-1843.2012.02174.x [DOI] [PubMed] [Google Scholar]
- 53.Kamo T, Tasaka S, Tokuda Y, Suzuki S, Asakura T, Yagi K, Namkoong H, Ishii M, Hasegawa N, Betsuyaku T. Levels of soluble receptor for advanced glycation end products in bronchoalveolar lavage fluid in patients with various inflammatory lung diseases. Clin Med Insights Circ Respir Pulm Med. 2015;9suppl 1147–154. doi: 10.4137/CCRPM.S23326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blondonnet R, Audard J, Belville C, Clairefond G, Lutz J, Bouvier D, Roszyk L, Gross C, Lavergne M, Fournet M, et al. RAGE inhibition reduces acute lung injury in mice. Sci Rep. 2017;7:7208 doi: 10.1038/s41598-017-07638-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lutterloh EC, Opal SM, Pittman DD, Keith JC, Jr, Tan XY, Clancy BM, Palmer H, Milarski K, Sun Y, Palardy JE, et al. Inhibition of the RAGE products increases survival in experimental models of severe sepsis and systemic infection. Crit Care. 2007;11:R122 doi: 10.1186/cc6184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Caraher EJ, Kwon S, Haider SH, Crowley G, Lee A, Ebrahim M, Zhang L, Chen LC, Gordon T, Liu M, et al. Receptor for advanced glycation end-products and World Trade Center particulate induced lung function loss: a case-cohort study and murine model of acute particulate exposure. PLoS One. 2017;12:e0184331 doi: 10.1371/journal.pone.0184331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramasamy R, Schmidt AM. Receptor for advanced glycation end products (RAGE) and implications for the pathophysiology of heart failure. Curr Heart Fail Rep. 2012;9:107–116. doi: 10.1007/s11897-012-0089-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bucciarelli LG, Kaneko M, Ananthakrishnan R, Harja E, Lee LK, Hwang YC, Lerner S, Bakr S, Li Q, Lu Y, et al. Receptor for advanced-glycation end products: key modulator of myocardial ischemic injury. Circulation. 2006;113:1226–1234. doi: 10.1161/CIRCULATIONAHA.105.575993 [DOI] [PubMed] [Google Scholar]
- 59.Aleshin A, Ananthakrishnan R, Li Q, Rosario R, Lu Y, Qu W, Song F, Bakr S, Szabolcs M, D’Agati V, et al. RAGE modulates myocardial injury consequent to LAD infarction via impact on JNK and STAT signaling in a murine model. Am J Physiol Heart Circ Physiol. 2008;294:H1823–H1832. doi: 10.1152/ajpheart.01210.2007 [DOI] [PubMed] [Google Scholar]
- 60.Shang L, Ananthakrishnan R, Li Q, Quadri N, Abdillahi M, Zhu Z, Qu W, Rosario R, Touré F, Yan SF, et al. RAGE modulates hypoxia/reoxygenation injury in adult murine cardiomyocytes via JNK and GSK-3beta signaling pathways. PLoS One. 2010;5:e10092 doi: 10.1371/journal.pone.0010092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bucciarelli LG, Ananthakrishnan R, Hwang YC, Kaneko M, Song F, Sell DR, Strauch C, Monnier VM, Yan SF, Schmidt AM, et al. RAGE and modulation of ischemic injury in the diabetic myocardium. Diabetes. 2008;57:1941–1951. doi: 10.2337/db07-0326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Müller I, Vogl T, Pappritz K, Miteva K, Savvatis K, Rohde D, Most P, Lassner D, Pieske B, Kühl U, et al. Pathogenic role of the damage-associated molecular patterns s100a8 and s100a9 in coxsackievirus b3-induced myocarditis. Circ Heart Fail. 201710, e004125 doi: 10.1161/CIRCHEARTFAILURE.117.004125 [DOI] [PubMed] [Google Scholar]
- 63.Ziegler T, Horstkotte M, Lange P, Ng J, Bongiovanni D, Hinkel R, Laugwitz KL, Sperandio M, Horstkotte J, Kupatt C. Endothelial RAGE exacerbates acute postischaemic cardiac inflammation. Thromb Haemost. 2016;116:300–308. doi: 10.1160/TH15-11-0898 [DOI] [PubMed] [Google Scholar]
- 64.Bangert A, Andrassy M, Müller AM, Bockstahler M, Fischer A, Volz CH, Leib C, Göser S, Korkmaz-Icöz S, Zittrich S, et al. Critical role of RAGE and HMGB1 in inflammatory heart disease. Proc Natl Acad Sci U S A. 2016;113:E155–E164. doi: 10.1073/pnas.1522288113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang WI, Lee D, Lee DL, Hong SY, Lee SH, Kang SM, Choi DH, Jang Y, Kim SH, Park S. Blocking the receptor for advanced glycation end product activation attenuates autoimmune myocarditis. Circ J. 2014;78:1197–1205. doi: 10.1253/circj.cj-13-1235 [DOI] [PubMed] [Google Scholar]
- 66.Andersson U, Ottestad W, Tracey KJ. Extracellular HMGB1: a therapeutic target in severe pulmonary inflammation including COVID-19? Mol Med. 2020;26:42 doi: 10.1186/s10020-020-00172-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Veras FP, Pontelli MC, Silva CM, Toller-Kawahisa JE, de Lima M, Nascimento DC, Schneider AH, Caetité D, Tavares LA, Paiva IM, et al. Sars-cov-2–triggered neutrophil extracellular traps mediate covid-19 pathology. J Exp Med. 2020;217:e20201129 doi: 10.1084/jem.20201129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boone BA, Murthy P, Miller-Ocuin J, Doerfler WR, Ellis JT, Liang X, Ross MA, Wallace CT, Sperry JL, Lotze MT, et al. Chloroquine reduces hypercoagulability in pancreatic cancer through inhibition of neutrophil extracellular traps. BMC Cancer. 2018;18:678 doi: 10.1186/s12885-018-4584-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen L, Zhao Y, Lai D, Zhang P, Yang Y, Li Y, Fei K, Jiang G, Fan J. Neutrophil extracellular traps promote macrophage pyroptosis in sepsis. Cell Death Dis. 2018;9:597 doi: 10.1038/s41419-018-0538-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stark K, Philippi V, Stockhausen S, Busse J, Antonelli A, Miller M, Schubert I, Hoseinpour P, Chandraratne S, von Brühl ML, et al. Disulfide HMGB1 derived from platelets coordinates venous thrombosis in mice. Blood. 2016;128:2435–2449. doi: 10.1182/blood-2016-04-710632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ma YH, Ma TT, Wang C, Wang H, Chang DY, Chen M, Zhao MH. High-mobility group box 1 potentiates antineutrophil cytoplasmic antibody-inducing neutrophil extracellular traps formation. Arthritis Res Ther. 2016;18:2 doi: 10.1186/s13075-015-0903-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boone BA, Orlichenko L, Schapiro NE, Loughran P, Gianfrate GC, Ellis JT, Singhi AD, Kang R, Tang D, Lotze MT, et al. The receptor for advanced glycation end products (RAGE) enhances autophagy and neutrophil extracellular traps in pancreatic cancer. Cancer Gene Ther. 2015;22:326–334. doi: 10.1038/cgt.2015.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maugeri N, Campana L, Gavina M, Covino C, De Metrio M, Panciroli C, Maiuri L, Maseri A, D’Angelo A, Bianchi ME, et al. Activated platelets present high mobility group box 1 to neutrophils, inducing autophagy and promoting the extrusion of neutrophil extracellular traps. J Thromb Haemost. 2014;12:2074–2088. doi: 10.1111/jth.12710 [DOI] [PubMed] [Google Scholar]
- 74.Apicella M, Campopiano MC, Mantuano M, Mazoni L, Coppelli A, Del Prato S. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020;8:782–792. doi: 10.1016/S2213-8587(20)30238-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, et al. ; China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, Akdis CA, Gao YD. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730–1741. doi: 10.1111/all.14238 [DOI] [PubMed] [Google Scholar]
- 77.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fadini GP, Morieri ML, Longato E, Avogaro A. Prevalence and impact of diabetes among people infected with SARS-CoV-2. J Endocrinol Invest. 2020;43:867–869. doi: 10.1007/s40618-020-01236-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Paton RC, Passa P. Platelets and diabetic vascular disease. Diabete Metab. 1983;9:306–312. [PubMed] [Google Scholar]
- 80.Tooke JE. Microcirculation and diabetes. Br Med Bull. 1989;45:206–223. doi: 10.1093/oxfordjournals.bmb.a072313 [DOI] [PubMed] [Google Scholar]
- 81.Ruderman NB, Williamson JR, Brownlee M. Glucose and diabetic vascular disease. FASEB J. 1992;6:2905–2914. doi: 10.1096/fasebj.6.11.1644256 [DOI] [PubMed] [Google Scholar]
- 82.King GL, Brownlee M. The cellular and molecular mechanisms of diabetic complications. Endocrinol Metab Clin North Am. 1996;25:255–270. doi: 10.1016/s0889-8529(05)70324-8 [DOI] [PubMed] [Google Scholar]
- 83.Yan SD, Stern D, Schmidt AM. What’s the RAGE? The receptor for advanced glycation end products (RAGE) and the dark side of glucose. Eur J Clin Invest. 1997;27:179–181. doi: 10.1046/j.1365-2362.1996.00072.x [DOI] [PubMed] [Google Scholar]
- 84.Harja E, Bu DX, Hudson BI, Chang JS, Shen X, Hallam K, Kalea AZ, Lu Y, Rosario RH, Oruganti S, et al. Vascular and inflammatory stresses mediate atherosclerosis via RAGE and its ligands in apoE-/- mice. J Clin Invest. 2008;118:183–194. doi: 10.1172/JCI32703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xu Y, Toure F, Qu W, Lin L, Song F, Shen X, Rosario R, Garcia J, Schmidt AM, Yan SF. Advanced glycation end product (AGE)-receptor for AGE (RAGE) signaling and up-regulation of Egr-1 in hypoxic macrophages. J Biol Chem. 2010;285:23233–23240. doi: 10.1074/jbc.M110.117457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dong N, Shi H, Xu B, Cai Y. Increased plasma S100A12 levels are associated with diabetic retinopathy and prognostic biomarkers of macrovascular events in type 2 diabetic patients. Invest Ophthalmol Vis Sci. 2015;56:4177–4185. doi: 10.1167/iovs.15-16470 [DOI] [PubMed] [Google Scholar]
- 87.Anguita-Ruiz A, Mendez-Gutierrez A, Ruperez AI, Leis R, Bueno G, Gil-Campos M, Tofe I, Gomez-Llorente C, Moreno LA, Gil Á, et al. The protein S100A4 as a novel marker of insulin resistance in prepubertal and pubertal children with obesity. Metabolism. 2020;105:154187 doi: 10.1016/j.metabol.2020.154187 [DOI] [PubMed] [Google Scholar]
- 88.Basta G, Sironi AM, Lazzerini G, Del Turco S, Buzzigoli E, Casolaro A, Natali A, Ferrannini E, Gastaldelli A. Circulating soluble receptor for advanced glycation end products is inversely associated with glycemic control and S100A12 protein. J Clin Endocrinol Metab. 2006;91:4628–4634. doi: 10.1210/jc.2005-2559 [DOI] [PubMed] [Google Scholar]
- 89.Kosaki A, Hasegawa T, Kimura T, Iida K, Hitomi J, Matsubara H, Mori Y, Okigaki M, Toyoda N, Masaki H, et al. Increased plasma S100A12 (EN-RAGE) levels in patients with type 2 diabetes. J Clin Endocrinol Metab. 2004;89:5423–5428. doi: 10.1210/jc.2003-032223 [DOI] [PubMed] [Google Scholar]
- 90.Wang H, Qu H, Deng H. Plasma HMGB-1 levels in subjects with obesity and type 2 diabetes: a cross-sectional study in China. PLoS One. 2015;10:e0136564 doi: 10.1371/journal.pone.0136564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nin JW, Ferreira I, Schalkwijk CG, Jorsal A, Prins MH, Parving HH, Tarnow L, Rossing P, Stehouwer CD. Higher plasma high-mobility group box 1 levels are associated with incident cardiovascular disease and all-cause mortality in type 1 diabetes: a 12 year follow-up study. Diabetologia. 2012;55:2489–2493. doi: 10.1007/s00125-012-2622-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guzmán-Ruiz R, Ortega F, Rodríguez A, Vázquez-Martínez R, Díaz-Ruiz A, Garcia-Navarro S, Giralt M, Garcia-Rios A, Cobo-Padilla D, Tinahones FJ, et al. Alarmin high-mobility group B1 (HMGB1) is regulated in human adipocytes in insulin resistance and influences insulin secretion in β-cells. Int J Obes (Lond). 2014;38:1545–1554. doi: 10.1038/ijo.2014.36 [DOI] [PubMed] [Google Scholar]
- 93.Yamagishi SI. Role of advanced glycation endproduct (AGE)-receptor for advanced glycation endproduct (RAGE) axis in cardiovascular disease and its therapeutic intervention. Circ J. 2019;83:1822–1828. doi: 10.1253/circj.CJ-19-0618 [DOI] [PubMed] [Google Scholar]
- 94.Shekhtman A, Ramasamy R, Schmidt AM. Glycation & the RAGE axis: targeting signal transduction through DIAPH1. Expert Rev Proteomics. 2017;14:147–156. doi: 10.1080/14789450.2017.1271719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Litwinoff E, Hurtado Del Pozo C, Ramasamy R, Schmidt AM. Emerging targets for therapeutic development in diabetes and its complications: the RAGE signaling pathway. Clin Pharmacol Ther. 2015;98:135–144. doi: 10.1002/cpt.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rein J, Bader M. Renin-angiotensin system in diabetes. Protein Pept Lett. 2017;24:833–840. doi: 10.2174/0929866524666170728144357 [DOI] [PubMed] [Google Scholar]
- 97.Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem. 2000;275:33238–33243. doi: 10.1074/jbc.M002615200 [DOI] [PubMed] [Google Scholar]
- 98.Douglas GC, O’Bryan MK, Hedger MP, Lee DK, Yarski MA, Smith AI, Lew RA. The novel angiotensin-converting enzyme (ACE) homolog, ACE2, is selectively expressed by adult Leydig cells of the testis. Endocrinology. 2004;145:4703–4711. doi: 10.1210/en.2004-0443 [DOI] [PubMed] [Google Scholar]
- 99.Tikellis C, Pickering R, Tsorotes D, Du XJ, Kiriazis H, Nguyen-Huu TP, Head GA, Cooper ME, Thomas MC. Interaction of diabetes and ACE2 in the pathogenesis of cardiovascular disease in experimental diabetes. Clin Sci (Lond). 2012;123:519–529. doi: 10.1042/CS20110668 [DOI] [PubMed] [Google Scholar]
- 100.Patel VB, Parajuli N, Oudit GY. Role of angiotensin-converting enzyme 2 (ACE2) in diabetic cardiovascular complications. Clin Sci (Lond). 2014;126:471–482. doi: 10.1042/CS20130344 [DOI] [PubMed] [Google Scholar]
- 101.Obukhov AG, Stevens BR, Prasad R, Li Calzi S, Boulton ME, Raizada MK, Oudit GY, Grant MB. SARS-CoV-2 infections and ACE2: clinical outcomes linked with increased morbidity and mortality in individuals with diabetes. Diabetes. 2020;69:1875–1886. doi: 10.2337/dbi20-0019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu Y, Yu M, Zhang L, Cao Q, Song Y, Liu Y, Gong J. Soluble receptor for advanced glycation end products mitigates vascular dysfunction in spontaneously hypertensive rats. Mol Cell Biochem. 2016;419:165–176. doi: 10.1007/s11010-016-2763-5 [DOI] [PubMed] [Google Scholar]
- 103.Pickering RJ, Tikellis C, Rosado CJ, Tsorotes D, Dimitropoulos A, Smith M, Huet O, Seeber RM, Abhayawardana R, Johnstone EK, et al. Transactivation of RAGE mediates angiotensin-induced inflammation and atherogenesis. J Clin Invest. 2019;129:406–421. doi: 10.1172/JCI99987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Codo AC, Davanzo GG, Monteiro LB, de Souza GF, Muraro SP, Virgilio-da-Silva JV, Prodonoff JS, Carregari VC, de Biagi Junior CAO, Crunfli F, et al. Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1α/glycolysis-dependent axis. Cell Metab. 2020;32:498–499. doi: 10.1016/j.cmet.2020.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kruglikov IL, Shah M, Scherer PE. Obesity and diabetes as comorbidities for covid-19: underlying mechanisms and the role of viral-bacterial interactions. eLife. 2020;9:e61330 doi: 10.7554/eLife.61330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ryan PM, Caplice NM. Is adipose tissue a reservoir for viral spread, immune activation, and cytokine amplification in coronavirus disease 2019? Obesity (Silver Spring). 2020;28:1191–1194. doi: 10.1002/oby.22843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang J, Zhang L, Zhang S, Yu Q, Xiong F, Huang K, Wang CY, Yang P. HMGB1, an innate alarmin, plays a critical role in chronic inflammation of adipose tissue in obesity. Mol Cell Endocrinol. 2017;454:103–111. doi: 10.1016/j.mce.2017.06.012 [DOI] [PubMed] [Google Scholar]
- 108.Riuzzi F, Chiappalupi S, Arcuri C, Giambanco I, Sorci G, Donato R. S100 proteins in obesity: liaisons dangereuses. Cell Mol Life Sci. 2020;77:129–147. doi: 10.1007/s00018-019-03257-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Song F, Hurtado del Pozo C, Rosario R, Zou YS, Ananthakrishnan R, Xu X, Patel PR, Benoit VM, Yan SF, Li H, et al. RAGE regulates the metabolic and inflammatory response to high-fat feeding in mice. Diabetes. 2014;63:1948–1965. doi: 10.2337/db13-1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hurtado Del Pozo C, Ruiz HH, Arivazhagan L, Aranda JF, Shim C, Daya P, Derk J, MacLean M, He M, Frye L, et al. A receptor of the immunoglobulin superfamily regulates adaptive thermogenesis. Cell Rep. 2019;28:773–791.e7. doi: 10.1016/j.celrep.2019.06.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tsatsanis C, Margioris AN, Kontoyiannis DP. Association between H1N1 infection severity and obesity-adiponectin as a potential etiologic factor. J Infect Dis. 2010;202:459–460. doi: 10.1086/653842 [DOI] [PubMed] [Google Scholar]
- 112.Senatus L, López-Díez R, Egaña-Gorroño L, Liu J, Hu J, Daffu G, Li Q, Rahman K, Vengrenyuk Y, Barrett TJ, et al. Rage impairs murine diabetic atherosclerosis regression and implicates irf7 in macrophage inflammation and cholesterol metabolism. JCI Insight. 2020;5:e137289 doi: 10.1172/jci.insight.137289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Honda K, Yanai H, Negishi H, Asagiri M, Sato M, Mizutani T, Shimada N, Ohba Y, Takaoka A, Yoshida N, et al. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434:772–777. doi: 10.1038/nature03464 [DOI] [PubMed] [Google Scholar]
- 114.Zhao GN, Jiang DS, Li H. Interferon regulatory factors: at the crossroads of immunity, metabolism, and disease. Biochim Biophys Acta. 2015;1852:365–378. doi: 10.1016/j.bbadis.2014.04.030 [DOI] [PubMed] [Google Scholar]
- 115.Huang L, Zhang SM, Zhang P, Zhang XJ, Zhu LH, Chen K, Gao L, Zhang Y, Kong XJ, Tian S, et al. Interferon regulatory factor 7 protects against vascular smooth muscle cell proliferation and neointima formation. J Am Heart Assoc. 2014;3:e001309 doi: 10.1161/JAHA.114.001309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang Q, Bastard P, Liu Z, Le Pen J, Moncada-Velez M, Chen J, Ogishi M, Sabli IKD, Hodeib S, Korol C, et al. Inborn errors of type I IFN immunity in patients with life-threatening covid-19. Science (New York, N.Y.). 2020;370:eabd4570 doi: 10.1126/science.abd4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bastard P, Rosen LB, Zhang Q, Michailidis E, Hoffmann HH, Zhang Y, Dorgham K, Philippot Q, Rosain J, Béziat V, et al. Auto-antibodies against type I IFNS in patients with life-threatening covid-19. Science (New York, N.Y.). 2020;370:eabd4585 doi: 10.1126/science.abd4585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Acharya D, Liu G, Gack MU. Dysregulation of type I interferon responses in COVID-19. Nat Rev Immunol. 2020;20:397–398. doi: 10.1038/s41577-020-0346-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lee JS, Shin EC. The type I interferon response in COVID-19: implications for treatment. Nat Rev Immunol. 2020;20:585–586. doi: 10.1038/s41577-020-00429-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Channappanavar R, Fehr AR, Vijay R, Mack M, Zhao J, Meyerholz DK, Perlman S. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe. 2016;19:181–193. doi: 10.1016/j.chom.2016.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Israelow B, Song E, Mao T, Lu P, Meir A, Liu F, Alfajaro MM, Wei J, Dong H, Homer RJ, et al. Mouse model of SARS-COV-2 reveals inflammatory role of type i interferon signaling. J Exp Med. 2020;217:e20201241 doi: 10.1084/jem.20201241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.van Zoelen MA, van der Sluijs KF, Achouiti A, Florquin S, Braun-Pater JM, Yang H, Nawroth PP, Tracey KJ, Bierhaus A, van der Poll T. Receptor for advanced glycation end products is detrimental during influenza A virus pneumonia. Virology. 2009;391:265–273. doi: 10.1016/j.virol.2009.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Qu Y, Zhan Y, Yang S, Ren S, Qiu X, Rehamn ZU, Tan L, Sun Y, Meng C, Song C, et al. Newcastle disease virus infection triggers HMGB1 release to promote the inflammatory response. Virology. 2018;525:19–31. doi: 10.1016/j.virol.2018.09.001 [DOI] [PubMed] [Google Scholar]
- 124.de Carvalho GC, Borget MY, Bernier S, Garneau D, da Silva Duarte AJ, Dumais N. RAGE and CCR7 mediate the transmigration of Zika-infected monocytes through the blood-brain barrier. Immunobiology. 2019;224:792–803. doi: 10.1016/j.imbio.2019.08.007 [DOI] [PubMed] [Google Scholar]
- 125.Duan E, Wang D, Luo R, Luo J, Gao L, Chen H, Fang L, Xiao S. Porcine reproductive and respiratory syndrome virus infection triggers HMGB1 release to promote inflammatory cytokine production. Virology. 2014;468470:1–9. doi: 10.1016/j.virol.2014.07.046 [DOI] [PubMed] [Google Scholar]
- 126.Miller AL, Sims GP, Brewah YA, Rebelatto MC, Kearley J, Benjamin E, Keller AE, Brohawn P, Herbst R, Coyle AJ, et al. Opposing roles of membrane and soluble forms of the receptor for advanced glycation end products in primary respiratory syncytial virus infection. J Infect Dis. 2012;205:1311–1320. doi: 10.1093/infdis/jir826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Arikkatt J, Ullah MA, Short KR, Zhang V, Gan WJ, Loh Z, Werder RB, Simpson J, Sly PD, Mazzone SB, et al. Rage deficiency predisposes mice to virus-induced paucigranulocytic asthma. eLife. 2017;6:e21199 doi: 10.7554/eLife.21199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Soremekun OS, Omolabi KF, Soliman MES. Identification and classification of differentially expressed genes reveal potential molecular signature associated with SARS-CoV-2 infection in lung adenocarcinomal cells. Inform Med Unlocked. 2020;20:100384 doi: 10.1016/j.imu.2020.100384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.An H, Yu Y, Zhang M, Xu H, Qi R, Yan X, Liu S, Wang W, Guo Z, Guo J, et al. Involvement of ERK, p38 and NF-kappaB signal transduction in regulation of TLR2, TLR4 and TLR9 gene expression induced by lipopolysaccharide in mouse dendritic cells. Immunology. 2002;106:38–45. doi: 10.1046/j.1365-2567.2002.01401.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Petes C, Odoardi N, Gee K. The toll for trafficking: toll-like receptor 7 delivery to the endosome. Front Immunol. 2017;8:1075 doi: 10.3389/fimmu.2017.01075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Onofrio L, Caraglia M, Facchini G, Margherita V, Placido S, Buonerba C. Toll-like receptors and Covid-19: a two-faced story with an exciting ending. Future Sci OA. 2020;6:Fso605 doi: 10.2144/fsoa-2020-0091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sirois CM, Jin T, Miller AL, Bertheloot D, Nakamura H, Horvath GL, Mian A, Jiang J, Schrum J, Bossaller L, et al. RAGE is a nucleic acid receptor that promotes inflammatory responses to DNA. J Exp Med. 2013;210:2447–2463. doi: 10.1084/jem.20120201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bertheloot D, Naumovski AL, Langhoff P, Horvath GL, Jin T, Xiao TS, Garbi N, Agrawal S, Kolbeck R, Latz E. RAGE enhances TLR responses through binding and internalization of RNA. J Immunol. 2016;197:4118–4126. doi: 10.4049/jimmunol.1502169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yang QW, Lu FL, Zhou Y, Wang L, Zhong Q, Lin S, Xiang J, Li JC, Fang CQ, Wang JZ. HMBG1 mediates ischemia-reperfusion injury by TRIF-adaptor independent Toll-like receptor 4 signaling. J Cereb Blood Flow Metab. 2011;31:593–605. doi: 10.1038/jcbfm.2010.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature. 2017;542:177–185. doi: 10.1038/nature21363 [DOI] [PubMed] [Google Scholar]
- 136.Wu X, Wu J, Thompson CW, Li Y. Adaptive evolution of the MHC class III-encoded receptor RAGE in primates and murine rodents. Int J Immunogenet. 2015;42:461–468. doi: 10.1111/iji.12230 [DOI] [PubMed] [Google Scholar]
- 137.Sessa L, Gatti E, Zeni F, Antonelli A, Catucci A, Koch M, Pompilio G, Fritz G, Raucci A, Bianchi ME. The receptor for advanced glycation end-products (RAGE) is only present in mammals, and belongs to a family of cell adhesion molecules (CAMs). PLoS One. 2014;9:e86903 doi: 10.1371/journal.pone.0086903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gerkema MP, Davies WI, Foster RG, Menaker M, Hut RA. The nocturnal bottleneck and the evolution of activity patterns in mammals. Proc Biol Sci. 2013;280:20130508 doi: 10.1098/rspb.2013.0508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hughes DA, Jastroch M, Stoneking M, Klingenspor M. Molecular evolution of UCP1 and the evolutionary history of mammalian non-shivering thermogenesis. BMC Evol Biol. 2009;9:4 doi: 10.1186/1471-2148-9-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cleary SJ, Pitchford SC, Amison RT, Carrington R, Robaina Cabrera CL, Magnen M, Looney MR, Gray E, Page CP. Animal models of mechanisms of SARS-CoV-2 infection and COVID-19 pathology. Br J Pharmacol. 2020;177:4851–4865. doi: 10.1111/bph.15143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sun SH, Chen Q, Gu HJ, Yang G, Wang YX, Huang XY, Liu SS, Zhang NN, Li XF, Xiong R, et al. A mouse model of SARS-CoV-2 infection and pathogenesis. Cell Host Microbe. 2020;28:124–133.e4. doi: 10.1016/j.chom.2020.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bao L, Deng W, Huang B, Gao H, Liu J, Ren L, Wei Q, Yu P, Xu Y, Qi F, et al. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature. 2020;583:830–833. doi: 10.1038/s41586-020-2312-y [DOI] [PubMed] [Google Scholar]
- 143.Winkler ES, Bailey AL, Kafai NM, Nair S, McCune BT, Yu J, Fox JM, Chen RE, Earnest JT, Keeler SP, et al. SARS-CoV-2 infection of human ACE2-transgenic mice causes severe lung inflammation and impaired function. Nat Immunol. 2020;21:1327–1335. doi: 10.1038/s41590-020-0778-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Burstein AH, Sabbagh M, Andrews R, Valcarce C, Dunn I, Altstiel L. Development of Azeliragon, an oral small molecule antagonist of the receptor for advanced glycation endproducts, for the potential slowing of loss of cognition in mild Alzheimer’s disease. J Prev Alzheimers Dis. 2018;5:149–154. doi: 10.14283/jpad.2018.18 [DOI] [PubMed] [Google Scholar]
- 145.Flyvbjerg A, Denner L, Schrijvers BF, Tilton RG, Mogensen TH, Paludan SR, Rasch R. Long-term renal effects of a neutralizing RAGE antibody in obese type 2 diabetic mice. Diabetes. 2004;53:166–172. doi: 10.2337/diabetes.53.1.166 [DOI] [PubMed] [Google Scholar]
- 146.Reverdatto S, Rai V, Xue J, Burz DS, Schmidt AM, Shekhtman A. Combinatorial library of improved peptide aptamers, CLIPs to inhibit RAGE signal transduction in mammalian cells. PLoS One. 2013;8:e65180 doi: 10.1371/journal.pone.0065180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Xue J, Rai V, Singer D, Chabierski S, Xie J, Reverdatto S, Burz DS, Schmidt AM, Hoffmann R, Shekhtman A. Advanced glycation end product recognition by the receptor for AGEs. Structure. 2011;19:722–732. doi: 10.1016/j.str.2011.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Leclerc E, Fritz G, Vetter SW, Heizmann CW. Binding of S100 proteins to RAGE: an update. Biochim Biophys Acta. 2009;1793:993–1007. doi: 10.1016/j.bbamcr.2008.11.016 [DOI] [PubMed] [Google Scholar]
- 149.Hudson BI, Kalea AZ, Del Mar Arriero M, Harja E, Boulanger E, D’Agati V, Schmidt AM. Interaction of the RAGE cytoplasmic domain with diaphanous-1 is required for ligand-stimulated cellular migration through activation of Rac1 and Cdc42. J Biol Chem. 2008;283:34457–34468. doi: 10.1074/jbc.M801465200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rai V, Maldonado AY, Burz DS, Reverdatto S, Yan SF, Schmidt AM, Shekhtman A. Signal transduction in receptor for advanced glycation end products (RAGE): solution structure of C-terminal rage (ctRAGE) and its binding to mDia1. J Biol Chem. 2012;287:5133–5144. doi: 10.1074/jbc.M111.277731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Manigrasso MB, Pan J, Rai V, Zhang J, Reverdatto S, Quadri N, DeVita RJ, Ramasamy R, Shekhtman A, Schmidt AM. Small molecule inhibition of ligand-stimulated RAGE-DIAPH1 signal transduction. Sci Rep. 2016;6:22450 doi: 10.1038/srep22450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Touré F, Fritz G, Li Q, Rai V, Daffu G, Zou YS, Rosario R, Ramasamy R, Alberts AS, Yan SF, et al. Formin mDia1 mediates vascular remodeling via integration of oxidative and signal transduction pathways. Circ Res. 2012;110:1279–1293. doi: 10.1161/CIRCRESAHA.111.262519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.O’Shea KM, Ananthakrishnan R, Li Q, Quadri N, Thiagarajan D, Sreejit G, Wang L, Zirpoli H, Aranda JF, Alberts AS, et al. The formin, DIAPH1, is a key modulator of myocardial ischemia/reperfusion injury. EBioMedicine. 2017;26:165–174. doi: 10.1016/j.ebiom.2017.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Manigrasso MB, Friedman RA, Ramasamy R, D’Agati V, Schmidt AM. Deletion of the formin Diaph1 protects from structural and functional abnormalities in the murine diabetic kidney. Am J Physiol Renal Physiol. 2018;315:F1601–F1612. doi: 10.1152/ajprenal.00075.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Arnold TR, Stephenson RE, Miller AL. Rho GTPases and actomyosin: partners in regulating epithelial cell-cell junction structure and function. Exp Cell Res. 2017;358:20–30. doi: 10.1016/j.yexcr.2017.03.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kühn S, Geyer M. Formins as effector proteins of Rho GTPases. Small GTPases. 2014;5:e29513 doi: 10.4161/sgtp.29513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Young KG, Copeland JW. Formins in cell signaling. Biochim Biophys Acta. 2010;1803:183–190. doi: 10.1016/j.bbamcr.2008.09.017 [DOI] [PubMed] [Google Scholar]


